Photothermal-Controlled Release of IL-4 in IL-4/PDA-Immobilized Black Titanium Dioxide (TiO2) Nanotubes Surface to Enhance Osseointegration: An In Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. RNA Extraction, DNA Microarray, GO Enrichment Analysis
2.2. Fabrication of B-TNT
2.3. Fabrication of B-TNT/PDA/IL-4
2.4. Surface Characterization
2.5. Photothermal Performance
2.6. Drug Release Test
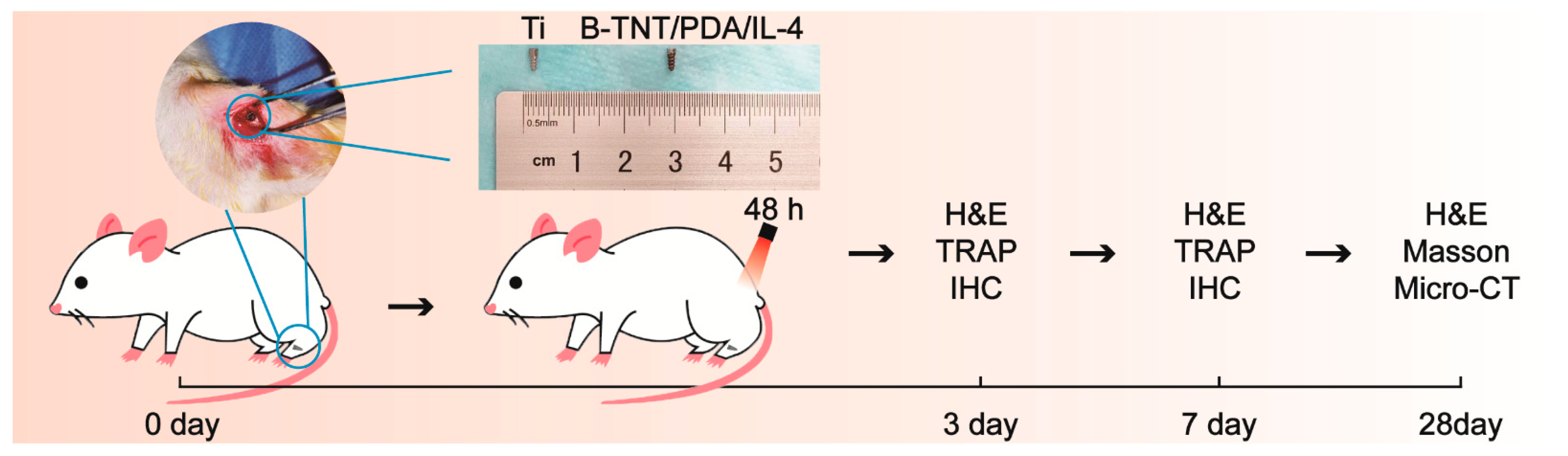
2.7. In Vivo Experiments
2.7.1. Surgical Procedure
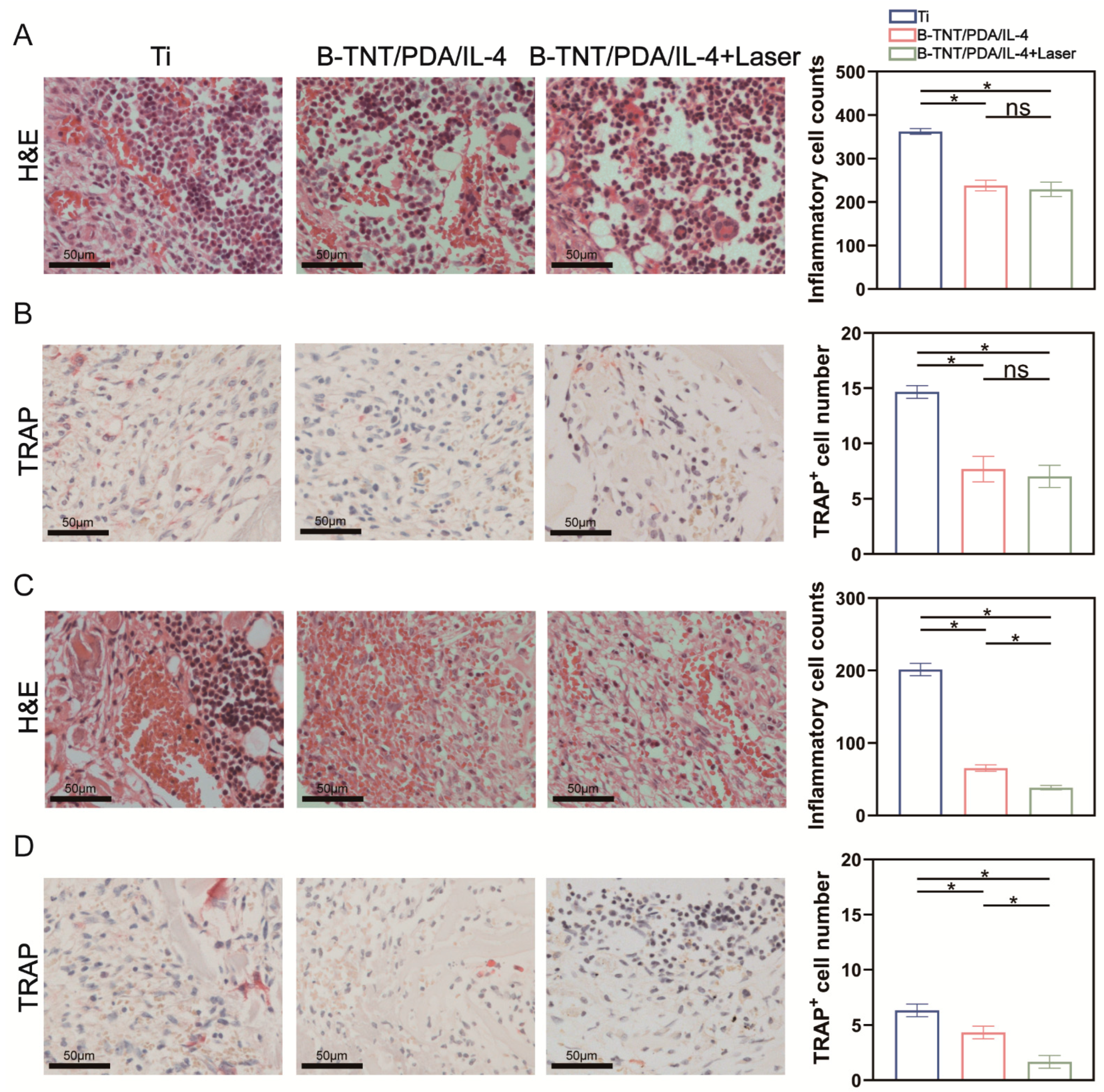
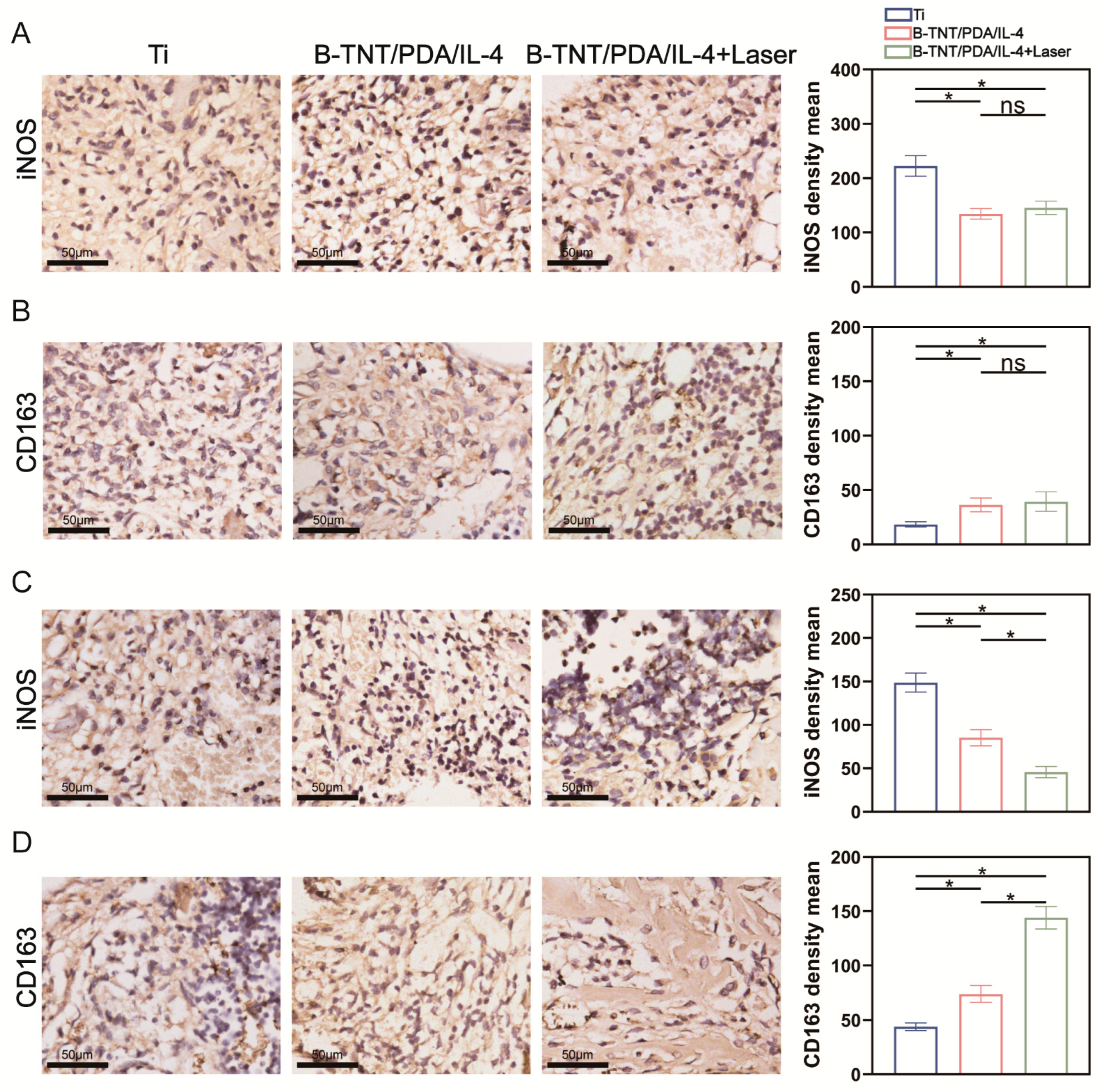
2.7.2. H&E, iNOS, CD163 and TRAP Staining on 3- and 7-Days Post-Implantation
2.7.3. Micro-CT Analysis, H&E and Masson Staining on day 28 Post-Implantation
2.8. Statistical Analysis
3. Results
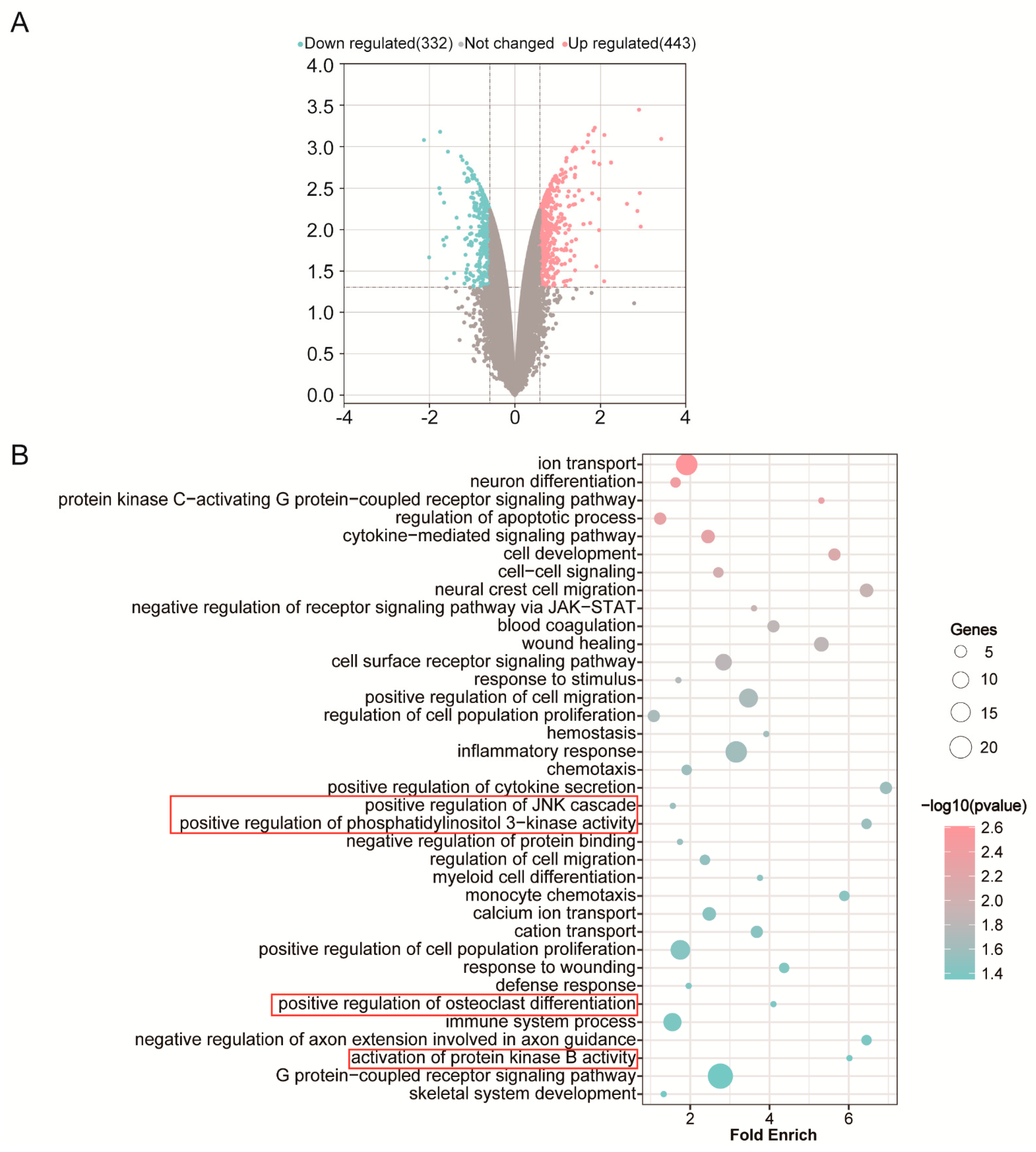
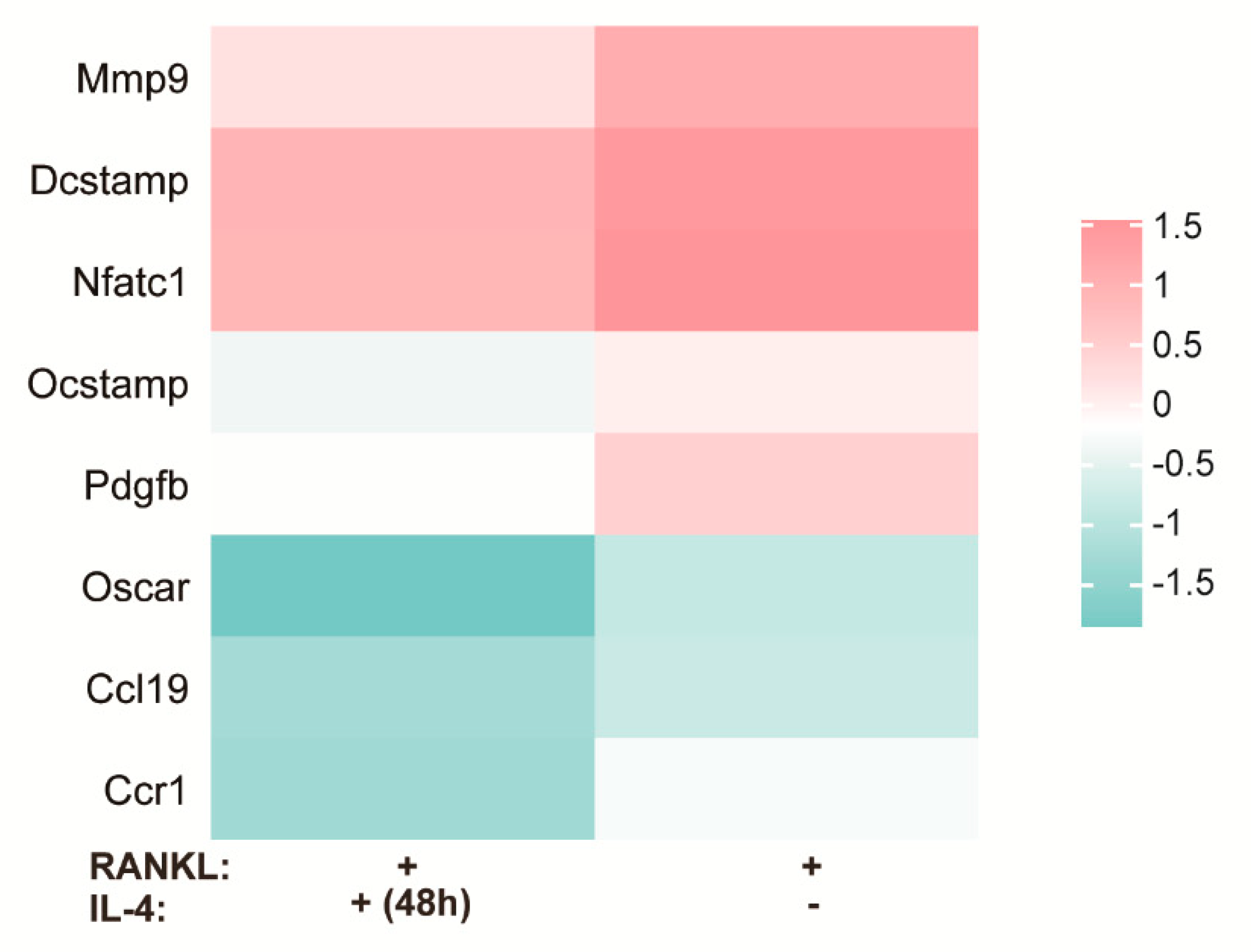
3.1. DNA Microarray Analysis
3.2. Surface Characterization and Drug Release Behavior Detection
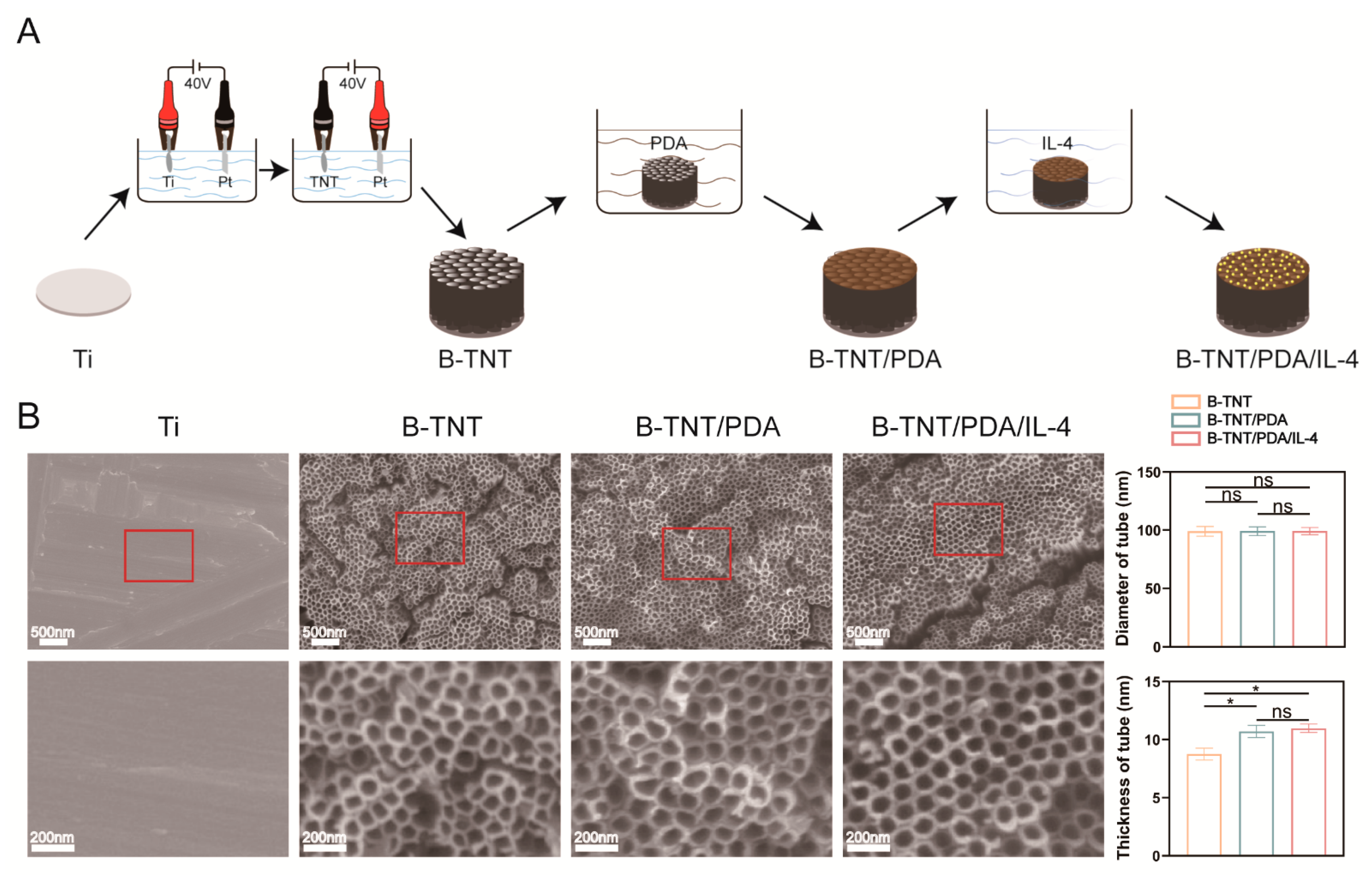
3.2.1. Surface Morphology Characterization
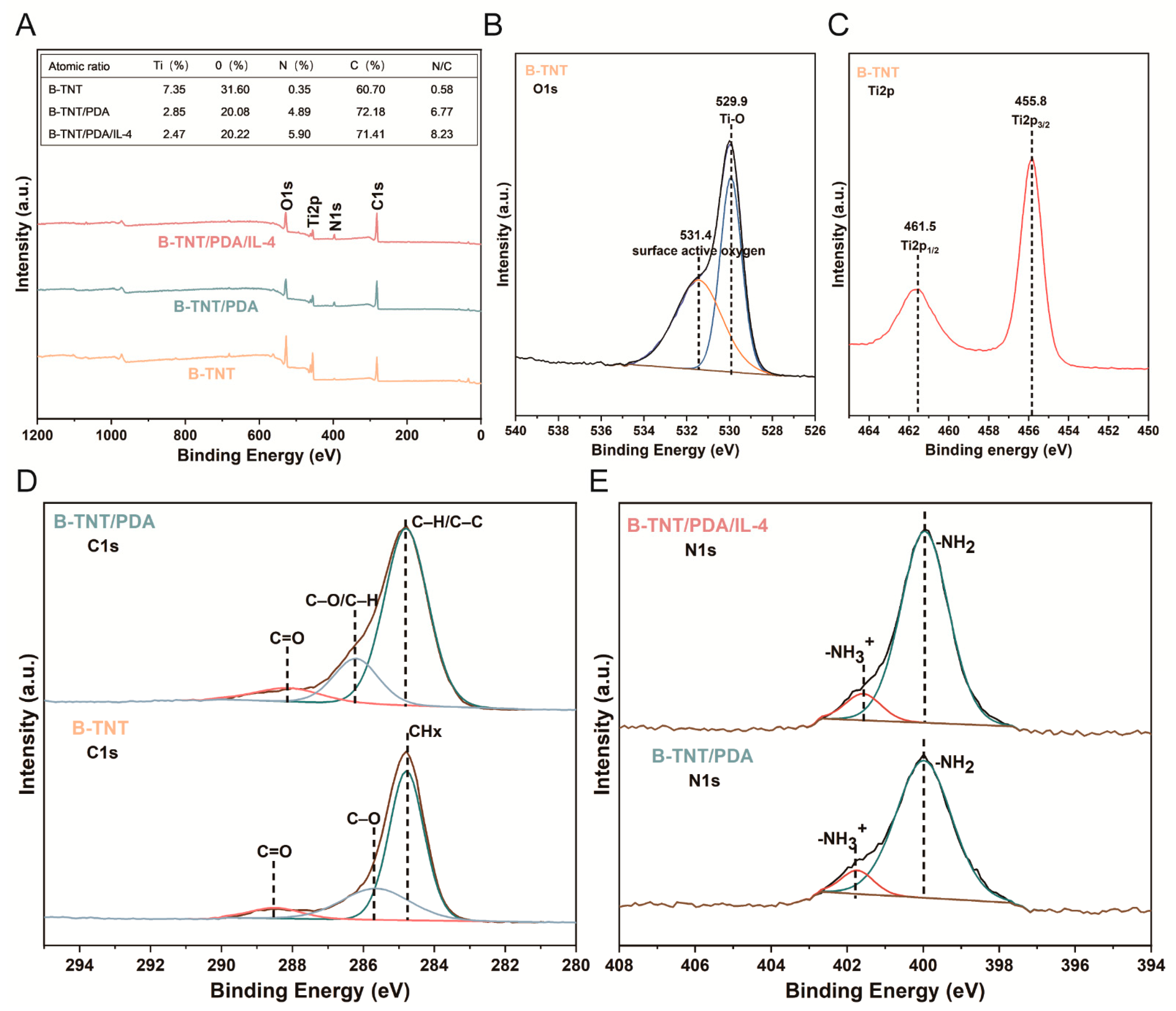
3.2.2. Surface Chemical Composition Characterization
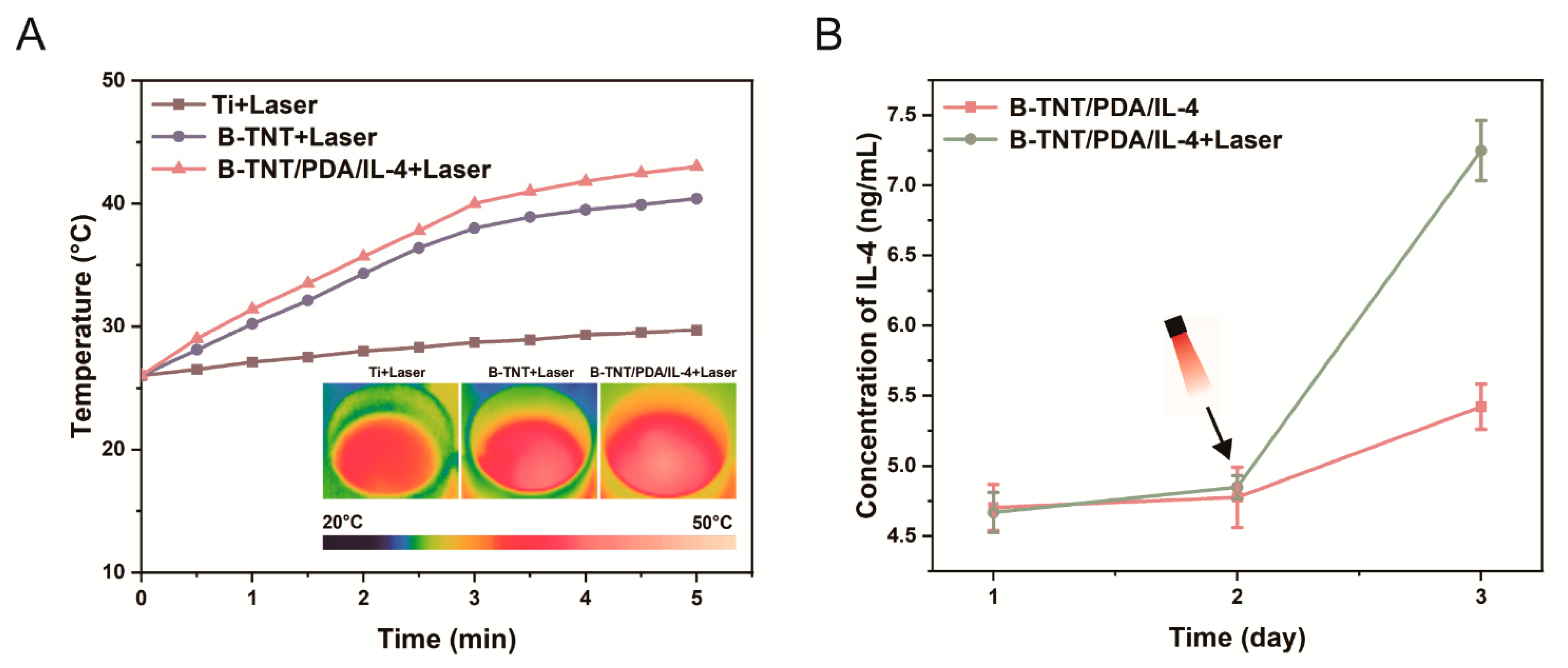
3.2.3. Photothermal Performance and Drug Release Profile
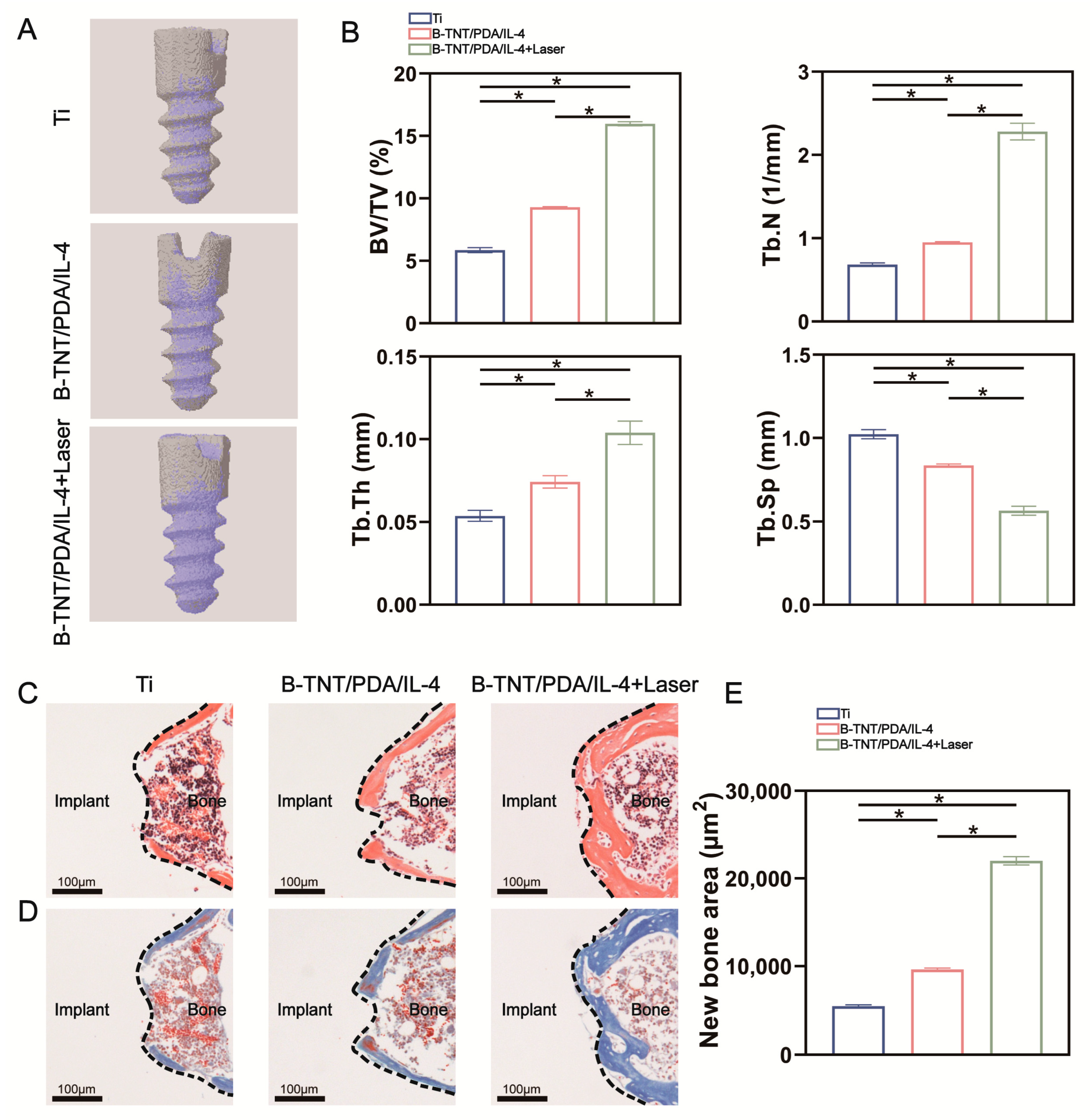
3.3. In Vivo Effect Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, L.; Wang, M.; Sun, H.; Li, P.; Wang, K.; Ren, F.; Lu, X. Porous titanium scaffolds with self-assembled micro/nano-hierarchical structure for dual functions of bone regeneration and anti-infection. J. Biomed. Mater. Res. A 2017, 105, 3482–3492. [Google Scholar] [CrossRef]
- Dayan, A.; Babin, G.; Ganoth, A.; Kayouf, N.S.; Nitoker Eliaz, N.; Mukkala, S.; Tsfadia, Y.; Fleminger, G. The involvement of coordinative interactions in the binding of dihydrolipoamide dehydrogenase to titanium dioxide-Localization of a putative binding site. J. Mol. Recognit. 2017, 30, e2617. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Wang, X.; Xiao, S.; Zhang, G.; Li, M.; Wang, P.; Shi, Q.; Qiao, P.; Lingling, E.; Liu, H. Osseointegration of layer-by-layer polyelectrolyte multilayers loaded with IGF1 and coated on titanium implant under osteoporotic condition. Int. J. Nanomed. 2017, 12, 7709–7720. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, M.; Radtke, A.; Roszek, K.; Jędrzejewski, T.; Piszczek, P. Assessment of Titanate Nanolayers in Terms of Their Physicochemical and Biological Properties. Materials 2021, 14, 806. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Arruda, I.R.S.; Silva, G.M.M.; Machado, G.; Coelho, L.; Correia, M.T.S. Functionalization of titanium dioxide nanotubes with biomolecules for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, J.Y.; Li, H.Q.; Chen, Z.; Zhao, A.Z.; Wang, Y.; Zhang, K.Q.; Sun, H.T.; Al-Deyab, S.S.; Lai, Y.K. TiO2 nanotube platforms for smart drug delivery: A review. Int. J. Nanomed. 2016, 11, 4819–4834. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Li, J.; Fu, X.; Li, H.; Wang, H.; Xin, S.; Zhou, L.; Liang, C.; Li, C. Influence of nanostructures on the biological properties of Ti implants after anodic oxidation. J. Mater. Sci. Mater. Med. 2014, 25, 199–205. [Google Scholar] [CrossRef]
- Li, B.E.; Li, Y.; Min, Y.; Hao, J.Z.; Liang, C.Y.; Li, H.P.; Wang, G.C.; Liu, S.M.; Wang, H.S. Synergistic effects of hierarchical hybrid micro/nanostructures on the biological properties of titanium orthopaedic implants. RSC Adv. 2015, 5, 49552–49558. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Li, J.; Fu, X.; Li, C.; Wang, H.; Liu, S.; Guo, L.; Xin, S.; Liang, C.; et al. Improvement of biological properties of titanium by anodic oxidation and ultraviolet irradiation. Appl. Surf. Sci. 2014, 307, 202–208. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Song, Y.; Ma, A.; Li, C.; Zhang, X.; Li, H.; Zhang, Q.; Zhang, K. Improved osteoblast adhesion and osseointegration on TiO2 nanotubes surface with hydroxyapatite coating. Dent. Mater. J. 2019, 38, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Song, Y.; Ma, A.; Li, C. Surface Immobilization of TiO2 Nanotubes with Bone Morphogenetic Protein-2 Synergistically Enhances Initial Preosteoblast Adhesion and Osseointegration. BioMed Res. Int. 2019, 2019, 5697250. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; You, Y.; Ma, A.; Song, Y.; Jiao, J.; Song, L.; Shi, E.; Zhong, X.; Li, Y.; Li, C. Zn-Incorporated TiO2 Nanotube Surface Improves Osteogenesis Ability Through Influencing Immunomodulatory Function of Macrophages. Int. J. Nanomed. 2020, 15, 2095–2118. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, P.; Mazare, A.; Schmuki, P.; Cimpean, A. Attenuation of the macrophage inflammatory activity by TiO₂ nanotubes via inhibition of MAPK and NF-κB pathways. Int. J. Nanomed. 2015, 10, 6455–6467. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Zhang, C.; Gao, B.; Tan, P.; Mi, B.; Zhang, Y.; Cheng, H.; Liao, H.; Huo, K.; et al. The Dimension of Titania Nanotubes Influences Implant Success for Osteoclastogenesis and Osteogenesis Patients. J. Nanosci. Nanotechnol. 2015, 15, 4136–4142. [Google Scholar] [CrossRef]
- Bai, L.; Chen, P.; Zhao, Y.; Hang, R.; Yao, X.; Tang, B.; Liu, C.; Xiao, Y.; Hang, R. A micro/nano-biomimetic coating on titanium orchestrates osteo/angio-genesis and osteoimmunomodulation for advanced osseointegration. Biomaterials 2021, 278, 121162. [Google Scholar] [CrossRef]
- Chen, Z.; Klein, T.; Murray, R.Z.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y.J.M.T. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today 2016, 19, 304–321. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, H.; Liu, X.; Wu, J.; Yang, C.; Wong, T.M.; Kwan, K.Y.H.; Cheung, K.M.C.; Wu, S.; Yeung, K.W.K. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci. Adv. 2021, 7, eabf6654. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Risser, G.E.; Spiller, K.L. Sequential drug delivery to modulate macrophage behavior and enhance implant integration. Adv. Drug Deliv. Rev. 2019, 149–150, 85–94. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, S.; Han, S.; Crawford, R.; Lu, S.; Wei, F.; Chang, J.; Wu, C.; Xiao, Y. Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages. Nanoscale 2017, 9, 706–718. [Google Scholar] [CrossRef]
- Li, M.; Gao, L.; Chen, J.; Zhang, Y.; Wang, J.; Lu, X.; Duan, K.; Weng, J.; Feng, B. Controllable release of interleukin-4 in double-layer sol-gel coatings on TiO2 nanotubes for modulating macrophage polarization. Biomed. Mater. 2018, 13, 45008. [Google Scholar] [CrossRef]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.W.; Zuo, K.Q.; Wang, K.; Sun, Z.Y.; Lu, Y.P.; Cheng, L.; Xiao, G.Y.; Liu, C. Interleukin-4 assisted calcium-strontium-zinc-phosphate coating induces controllable macrophage polarization and promotes osseointegration on titanium implant. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111512. [Google Scholar] [CrossRef] [PubMed]
- Schlundt, C.; Fischer, H.; Bucher, C.H.; Rendenbach, C.; Duda, G.N.; Schmidt-Bleek, K. The multifaceted roles of macrophages in bone regeneration: A story of polarization, activation and time. Acta Biomater. 2021, 133, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Y.; Ji, L.; Geng, Z.; Wang, J.; Liu, C. Calcium phosphate-based materials regulate osteoclast-mediated osseointegration. Bioact. Mater. 2021, 6, 4517–4530. [Google Scholar] [CrossRef]
- Wang, Q.; Ge, G.; Liang, X.; Bai, J.; Wang, W.; Zhang, W.; Zheng, K.; Yang, S.; Wei, M.; Yang, H.; et al. Punicalagin ameliorates wear-particle-induced inflammatory bone destruction by bi-directional regulation of osteoblastic formation and osteoclastic resorption. Biomater. Sci. 2020, 8, 5157–5171. [Google Scholar] [CrossRef]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef]
- Hachim, D.; LoPresti, S.T.; Rege, R.D.; Umeda, Y.; Iftikhar, A.; Nolfi, A.L.; Skillen, C.D.; Brown, B.N. Distinct macrophage populations and phenotypes associated with IL-4 mediated immunomodulation at the host implant interface. Biomater. Sci. 2020, 8, 5751–5762. [Google Scholar] [CrossRef]
- Moreno, J.L.; Kaczmarek, M.; Keegan, A.D.; Tondravi, M. IL-4 suppresses osteoclast development and mature osteoclast function by a STAT6-dependent mechanism: Irreversible inhibition of the differentiation program activated by RANKL. Blood 2003, 102, 1078–1086. [Google Scholar] [CrossRef]
- Fujii, T.; Kitaura, H.; Kimura, K.; Hakami, Z.W.; Takano-Yamamoto, T. IL-4 inhibits TNF-α-mediated osteoclast formation by inhibition of RANKL expression in TNF-α-activated stromal cells and direct inhibition of TNF-α-activated osteoclast precursors via a T-cell-independent mechanism in vivo. Bone 2012, 51, 771–780. [Google Scholar] [CrossRef]
- Toosi, S.; Behravan, J. Osteogenesis and bone remodeling: A focus on growth factors and bioactive peptides. Biofactors 2020, 46, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, N.; Zamanian, A.; Massoudi, A. Mussel-inspired surface modification of titania nanotubes as a novel drug delivery system. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Huang, Y.; You, S.; Xiang, Y.; Cai, E.; Mao, R.; Pan, W.; Tong, X.; Dong, W.; Ye, F.; et al. Engineering Robust Ag-Decorated Polydopamine Nano-Photothermal Platforms to Combat Bacterial Infection and Prompt Wound Healing. Adv. Sci. 2022, 9, e2106015. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Duan, Y.; Duan, Y. Application of polydopamine in tumor targeted drug delivery system and its drug release behavior. J. Control. Release 2018, 290, 56–74. [Google Scholar] [CrossRef]
- Yan, X.; Shen, K.; Tang, Q.; Fang, X.; Zhang, C.; Zhu, Z.; Hou, Y.; Lai, M. IL-4 functionalized titanium dioxide nanotubes modulate the inflammatory response of macrophages. J. Biomater. Sci. Polym. Ed. 2020, 31, 2238–2251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, H.; Miron, R.J.; Zhang, Y. Modulating macrophage polarization on titanium implant surface by poly(dopamine)-assisted immobilization of IL4. Clin. Implant Dent. Relat. Res. 2019, 21, 977–986. [Google Scholar] [CrossRef]
- Sun, X.; Li, L.; Zhang, H.; Dong, M.; Wang, J.; Jia, P.; Bu, T.; Wang, X.; Wang, L. Near-Infrared Light-Regulated Drug-Food Homologous Bioactive Molecules and Photothermal Collaborative Precise Antibacterial Therapy Nanoplatform with Controlled Release Property. Adv. Healthc. Mater. 2021, 10, e2100546. [Google Scholar] [CrossRef]
- Yu, G.; Yang, Z.; Fu, X.; Yung, B.C.; Yang, J.; Mao, Z.; Shao, L.; Hua, B.; Liu, Y.; Zhang, F.; et al. Polyrotaxane-based supramolecular theranostics. Nat. Commun. 2018, 9, 766. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, W.; Wang, X.; Zhong, L.; Song, X.; Wang, W.; Zhao, Y.; Dong, X. Multishell Nanoparticles with “Linkage Mechanism” for Thermal Responsive Photodynamic and Gas Synergistic Therapy. Adv. Healthc. Mater. 2021, 10, e2002038. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, J.; Chen, X.; Chen, Y.; Hou, S.; Qian, H.; Zhang, L.; Tang, G.; Chen, Z.; Ping, Y.; et al. Mesoporous polydopamine with built-in plasmonic core: Traceable and NIR triggered delivery of functional proteins. Biomaterials 2020, 238, 119847. [Google Scholar] [CrossRef]
- Xu, X.; Bai, B.; Wang, H.; Suo, Y. A Near-Infrared and Temperature-Responsive Pesticide Release Platform through Core-Shell Polydopamine@PNIPAm Nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 6424–6432. [Google Scholar] [CrossRef] [PubMed]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 anatase with a bandgap in the visible region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Gulzar, A.; Yang, G.; Gai, S.; He, F.; Dai, Y.; Zhong, C.; Yang, P. Au Nanoclusters Sensitized Black TiO2−X Nanotubes for Enhanced Photodynamic Therapy Driven by Near-Infrared Light. Small 2017, 13, 1703007. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, N.; Chen, J.; Chen, C.; San, H.; Chen, J.; Cheng, Z. Betavoltaic Enhancement Using Defect-Engineered TiO2 Nanotube Arrays through Electrochemical Reduction in Organic Electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 22174–22181. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, J.; Li, K.; Zhao, J.; Ma, H.; Wu, C.; Zhang, C.; Xie, Y.; Yang, F.; Zheng, X. A hydrogenated black TiO2 coating with excellent effects for photothermal therapy of bone tumor and bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 458–470. [Google Scholar] [CrossRef]
- Hua, P.; Cui, H.; Xu, J.; Cai, R.; She, Z.; Gu, Q. Diaporisoindole E inhibits RANKL-induced osteoclastogenesis via suppression of PI3K/AKT and MAPK signal pathways. Phytomed. Int. J. Phytother. Phytopharm. 2020, 75, 153234. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Zheng, Y.; Li, C.; Kwok Yeung, K.W.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Wu, S. Ag3PO4 decorated black urchin-like defective TiO2 for rapid and long-term bacteria-killing under visible light. Bioact. Mater. 2021, 6, 1575–1587. [Google Scholar] [CrossRef]
- Xu, X.; Cai, J.; Zhou, M.; Du, X.; Zhang, Y. Photoelectrochemical degradation of 2,4-dichlorophenoxyacetic acid using electrochemically self-doped Blue TiO(2) nanotube arrays with formic acid as electrolyte. J. Hazard. Mater. 2020, 382, 121096. [Google Scholar] [CrossRef]
- Kim, C.; Kim, S.; Lee, J.; Kim, J.; Yoon, J. Capacitive and oxidant generating properties of black-colored TiO2 nanotube array fabricated by electrochemical self-doping. ACS Appl. Mater. Interfaces 2015, 7, 7486–7491. [Google Scholar] [CrossRef]
- Li, L.; Xie, C.; Xiao, X. Polydopamine modified TiO2 nanotube arrays as a local drug delivery system for ibuprofen. J. Drug Deliv. Sci. Technol. 2020, 56, 101537. [Google Scholar] [CrossRef]
- Saidin, S.; Chevallier, P.; Abdul Kadir, M.R.; Hermawan, H.; Mantovani, D. Polydopamine as an intermediate layer for silver and hydroxyapatite immobilisation on metallic biomaterials surface. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4715–4724. [Google Scholar] [CrossRef] [PubMed]
- Abaricia, J.O.; Farzad, N.; Heath, T.J.; Simmons, J.; Morandini, L.; Olivares-Navarrete, R. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 2021, 133, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Loi, F.; Córdova, L.A.; Zhang, R.; Pajarinen, J.; Lin, T.H.; Goodman, S.B.; Yao, Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res. Ther. 2016, 7, 15. [Google Scholar] [CrossRef]
- Lampiasi, N.; Russo, R.; Kireev, I.; Strelkova, O.; Zhironkina, O.; Zito, F. Osteoclasts Differentiation from Murine RAW 264.7 Cells Stimulated by RANKL: Timing and Behavior. Biology 2021, 10, 117. [Google Scholar] [CrossRef]
- Liu, J.; Tang, Y.; Yang, W.; Tao, B.; He, Y.; Shen, X.; Shen, T.; Lin, C.; Cai, K. Functionalization of titanium substrate with multifunctional peptide OGP-NAC for the regulation of osteoimmunology. Biomater. Sci. 2019, 7, 1463–1476. [Google Scholar] [CrossRef]
- Yin, X.; Li, Y.; Yang, C.; Weng, J.; Wang, J.; Zhou, J.; Feng, B. Alginate/chitosan multilayer films coated on IL-4-loaded TiO2 nanotubes for modulation of macrophage phenotype. Int. J. Biol. Macromol. 2019, 133, 503–513. [Google Scholar] [CrossRef]
- Zito, F.; Lampiasi, N.; Kireev, I.; Russo, R. United we stand: Adhesion and molecular mechanisms driving cell fusion across species. Eur. J. Cell Biol. 2016, 95, 552–562. [Google Scholar] [CrossRef]
- Li, N.; Li, X.; Zheng, K.; Bai, J.; Zhang, W.; Sun, H.; Ge, G.; Wang, W.; Wang, Z.; Gu, Y.; et al. Inhibition of Sirtuin 3 prevents titanium particle-induced bone resorption and osteoclastsogenesis via suppressing ERK and JNK signaling. Int. J. Biol. Sci. 2021, 17, 1382–1394. [Google Scholar] [CrossRef]
- Gómez-Cerezo, N.; Casarrubios, L.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Sánchez-Margallo, F.M.; Portolés, M.T.; et al. Mesoporous bioactive glass/ε-polycaprolactone scaffolds promote bone regeneration in osteoporotic sheep. Acta Biomater. 2019, 90, 393–402. [Google Scholar] [CrossRef]
- Song, C.; Yang, X.; Lei, Y.; Zhang, Z.; Smith, W.; Yan, J.; Kong, L. Evaluation of efficacy on RANKL induced osteoclast from RAW264.7 cells. J. Cell. Physiol. 2019, 234, 11969–11975. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Chon, S.; Jung, W.W.; Choi, E.M. Effects of methylglyoxal on RANKL-induced osteoclast differentiation in RAW264.7 cells. Chem. Biol. Interact. 2018, 296, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wang, M.W.; Teitelbaum, S.L.; Ross, F.P. Interleukin-4 reversibly inhibits osteoclastogenesis via inhibition of NF-kappa B and mitogen-activated protein kinase signaling. J. Biol. Chem. 2002, 277, 6622–6630. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, J.; Shi, Z.; Xu, D.; Luo, S.; Siegal, G.P.; Feng, X.; Wei, S. Interleukin-4 inhibits RANKL-induced NFATc1 expression via STAT6: A novel mechanism mediating its blockade of osteoclastogenesis. J. Cell. Biochem. 2011, 112, 3385–3392. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, Y.; Song, Y.; Bachus, C.; Sun, C.; Sheshbaradaran, H.; Glogauer, M. AP-002: A novel inhibitor of osteoclast differentiation and function without disruption of osteogenesis. Eur. J. Pharmacol. 2020, 889, 173613. [Google Scholar] [CrossRef]
- Zeng, X.Z.; He, L.G.; Wang, S.; Wang, K.; Zhang, Y.Y.; Tao, L.; Li, X.J.; Liu, S.W. Aconine inhibits RANKL-induced osteoclast differentiation in RAW264.7 cells by suppressing NF-κB and NFATc1 activation and DC-STAMP expression. Acta Pharmacol. Sin. 2016, 37, 255–263. [Google Scholar] [CrossRef]
- Dairaghi, D.J.; Oyajobi, B.O.; Gupta, A.; McCluskey, B.; Miao, S.; Powers, J.P.; Seitz, L.C.; Wang, Y.; Zeng, Y.; Zhang, P.; et al. CCR1 blockade reduces tumor burden and osteolysis in vivo in a mouse model of myeloma bone disease. Blood 2012, 120, 1449–1457. [Google Scholar] [CrossRef]
- Li, D.Q.; Wan, Q.L.; Pathak, J.L.; Li, Z.B. Platelet-derived growth factor BB enhances osteoclast formation and osteoclast precursor cell chemotaxis. J. Bone Miner. Metab. 2017, 35, 355–365. [Google Scholar] [CrossRef]
- Lee, J.; Park, C.; Kim, H.J.; Lee, Y.D.; Lee, Z.H.; Song, Y.W.; Kim, H.H. Stimulation of osteoclast migration and bone resorption by C-C chemokine ligands 19 and 21. Exp. Mol. Med. 2017, 49, e358. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, J.; Wu, X.; Xie, Y.; Yuan, H.; Shi, Y. Preparation of Well-Aligned TiO₂ Nanotubes with High Length-Diameter Aspect Ratio by Anodic Oxidation Method. J. Nanosci. Nanotechnol. 2018, 18, 5810–5816. [Google Scholar] [CrossRef]
- Ma, A.; You, Y.; Chen, B.; Wang, W.; Liu, J.; Qi, H.; Liang, Y.; Li, Y.; Li, C. Icariin/Aspirin Composite Coating on TiO2 Nanotubes Surface Induce Immunomodulatory Effect of Macrophage and Improve Osteoblast Activity. Coatings 2020, 10, 427. [Google Scholar] [CrossRef]
- Mazare, A.; Park, J.; Simons, S.; Mohajernia, S.; Hwang, I.; Yoo, J.E.; Schneider, H.; Fischer, M.J.; Schmuki, P. Black TiO2 nanotubes: Efficient electrodes for triggering electric field-induced stimulation of stem cell growth. Acta Biomater. 2019, 97, 681–688. [Google Scholar] [CrossRef]
- Yu, X.; Kim, B.; Kim, Y.K. Highly Enhanced Photoactivity of Anatase TiO2 Nanocrystals by Controlled Hydrogenation-Induced Surface Defects. ACS Catal. 2013, 3, 2479–2486. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, H.; Han, H.; Fu, Y.; Ma, C.; Yu, Z.; Dong, X. Black TiO(2) nanotube arrays fabricated by electrochemical self-doping and their photoelectrochemical performance. RSC Adv. 2018, 8, 18992–19000. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Tsang, C.-K.; Li, Z.; Ran, X.; Lee, C.; Nie, B.; Zheng, L.; Tf, H.; Lu, J. Electrochemical doping of anatase TiO2 in organic electrolytes for high-performance supercapacitors and photocatalysts. J. Mater. Chem. A 2014, 2, 229–236. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Y.; Wang, F.; Dai, J. Preparation of ZnO/two-layer self-doped black TiO(2) nanotube arrays and their enhanced photochemical properties. RSC Adv. 2021, 11, 2307–2314. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef]
- Tong, L.; Liao, Q.; Zhao, Y.; Huang, H.; Gao, A.; Zhang, W.; Gao, X.; Wei, W.; Guan, M.; Chu, P.K.; et al. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials 2019, 193, 1–11. [Google Scholar] [CrossRef]
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138. [Google Scholar] [CrossRef]
- Galarraga-Vinueza, M.E.; Obreja, K.; Ramanauskaite, A.; Magini, R.; Begic, A.; Sader, R.; Schwarz, F. Macrophage polarization in peri-implantitis lesions. Clin. Oral Investig. 2021, 25, 2335–2344. [Google Scholar] [CrossRef]
- Hachim, D.; LoPresti, S.T.; Yates, C.C.; Brown, B.N. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials 2017, 112, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Hu, Y.; Li, Y.; Zhou, Q.; Lv, X. Involvement of JNK signaling in IL4-induced M2 macrophage polarization. Exp. Cell Res. 2017, 357, 155–162. [Google Scholar] [CrossRef]
- Sun, J.; Huang, Y.; Zhao, H.; Niu, J.; Ling, X.; Zhu, C.; Wang, L.; Yang, H.; Yang, Z.; Pan, G.; et al. Bio-clickable mussel-inspired peptides improve titanium-based material osseointegration synergistically with immunopolarization-regulation. Bioact. Mater. 2022, 9, 1–14. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Liang, Y.; Song, Y.; Liang, Y.; Jiao, J.; Bai, H.; Li, Y. Photothermal-Controlled Release of IL-4 in IL-4/PDA-Immobilized Black Titanium Dioxide (TiO2) Nanotubes Surface to Enhance Osseointegration: An In Vivo Study. Materials 2022, 15, 5962. https://doi.org/10.3390/ma15175962
Chen B, Liang Y, Song Y, Liang Y, Jiao J, Bai H, Li Y. Photothermal-Controlled Release of IL-4 in IL-4/PDA-Immobilized Black Titanium Dioxide (TiO2) Nanotubes Surface to Enhance Osseointegration: An In Vivo Study. Materials. 2022; 15(17):5962. https://doi.org/10.3390/ma15175962
Chicago/Turabian StyleChen, Bo, Yu Liang, Yunjia Song, Yunkai Liang, Jian Jiao, Hong Bai, and Ying Li. 2022. "Photothermal-Controlled Release of IL-4 in IL-4/PDA-Immobilized Black Titanium Dioxide (TiO2) Nanotubes Surface to Enhance Osseointegration: An In Vivo Study" Materials 15, no. 17: 5962. https://doi.org/10.3390/ma15175962
APA StyleChen, B., Liang, Y., Song, Y., Liang, Y., Jiao, J., Bai, H., & Li, Y. (2022). Photothermal-Controlled Release of IL-4 in IL-4/PDA-Immobilized Black Titanium Dioxide (TiO2) Nanotubes Surface to Enhance Osseointegration: An In Vivo Study. Materials, 15(17), 5962. https://doi.org/10.3390/ma15175962






