Eco-Sustainable Magnesium Oxychloride Cement Pastes Containing Waste Ammonia Soda Residue and Fly Ash
Abstract
:1. Introduction
2. Role of Fly Ash in MOC Pastes
2.1. Characteristics of FA
2.2. Specimen Preparation
2.3. Workability
2.4. Strength
2.5. Shrinkage
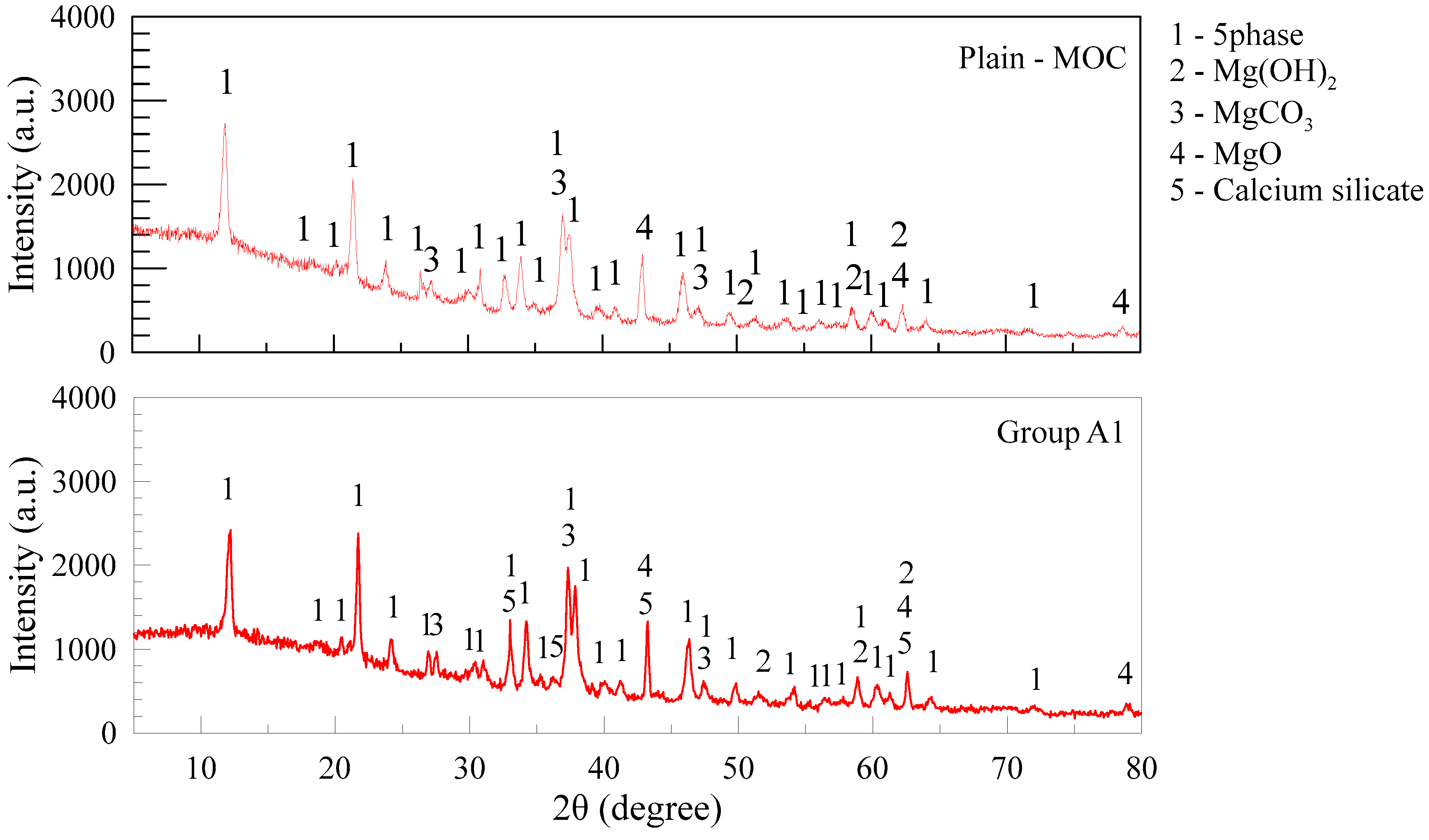
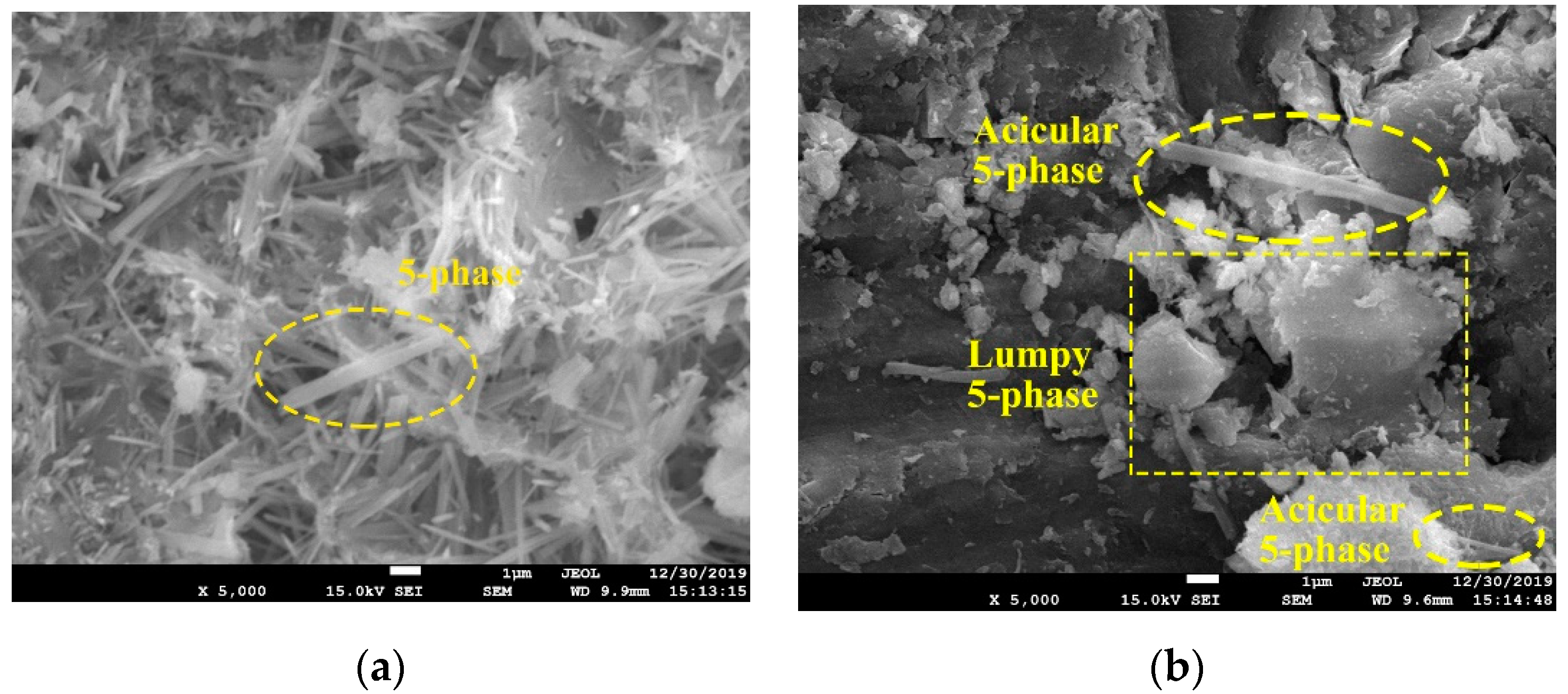
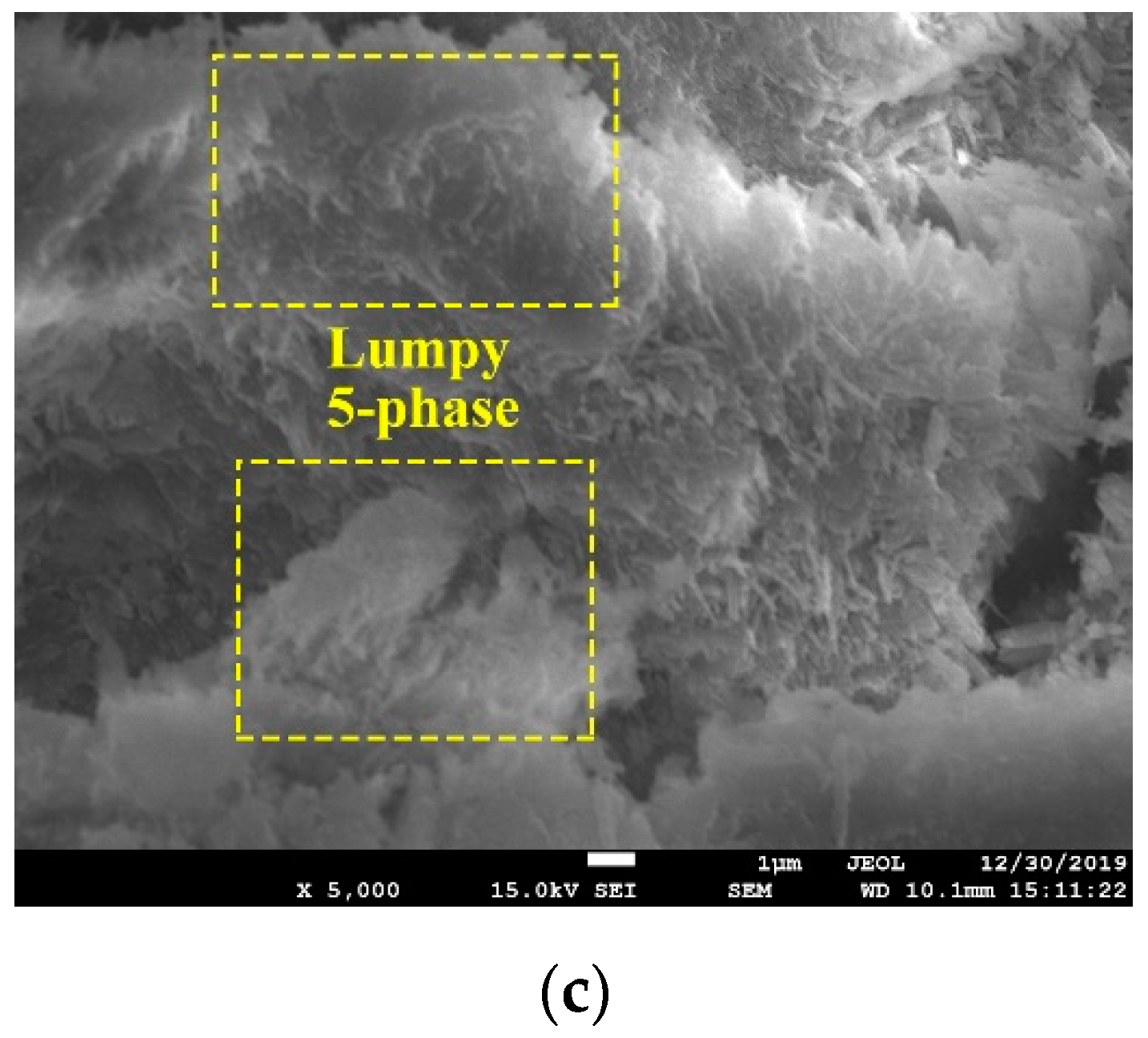
2.6. Microstructure Changes
3. MOC Pastes with FA and ASR
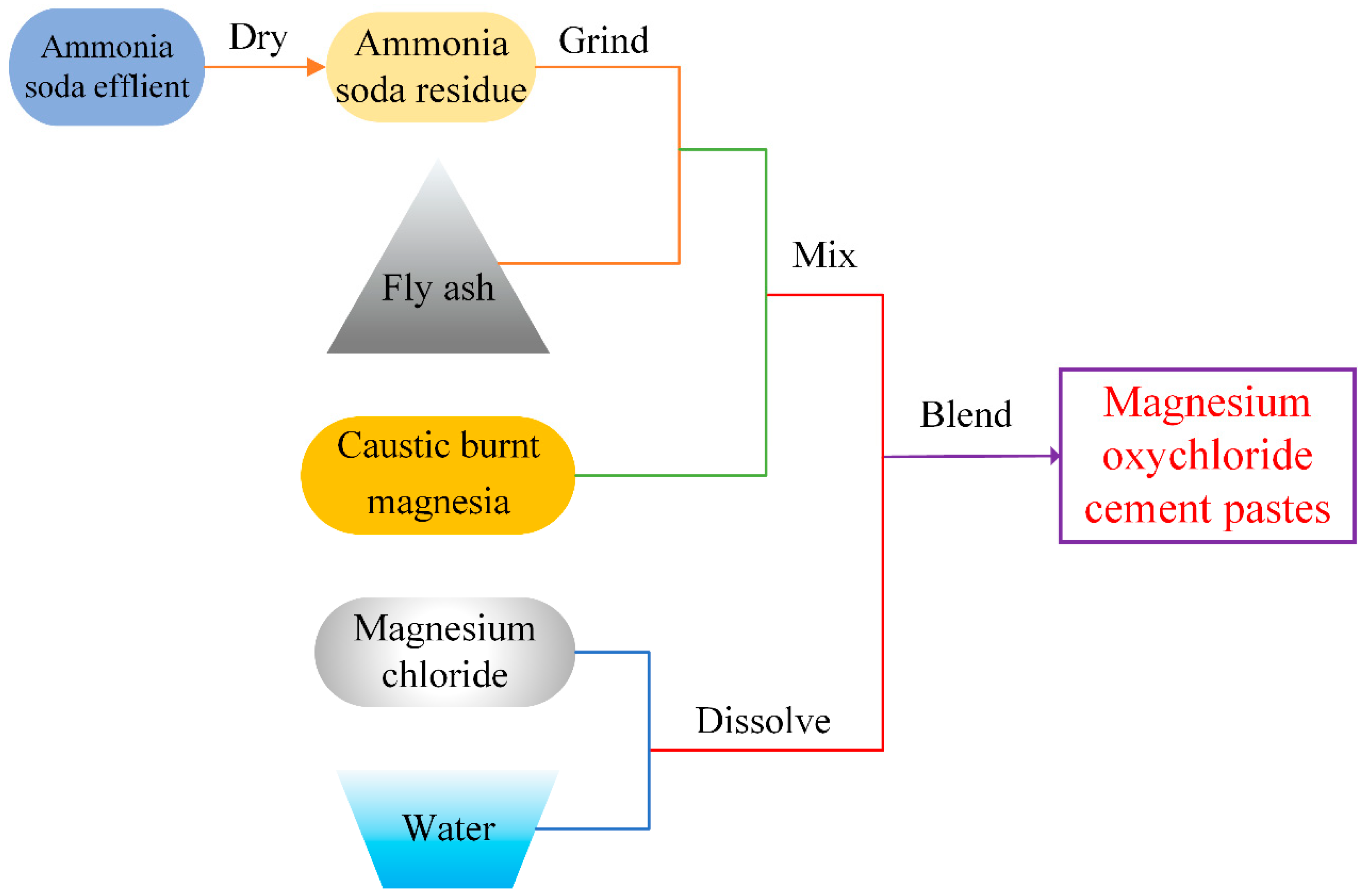
3.1. Mix Proportions and Preparation
3.2. Properties of MOC with Industrial Waste
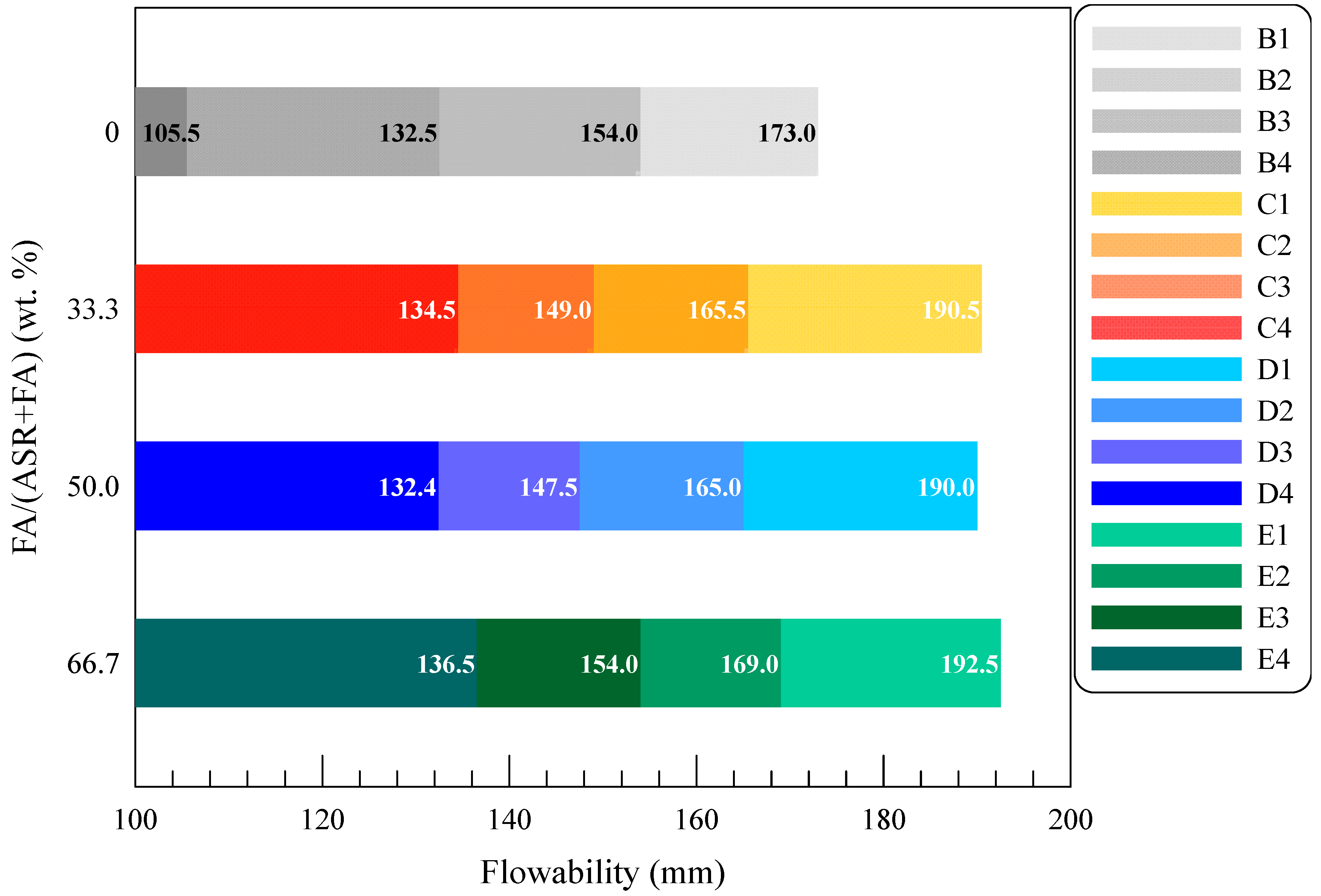
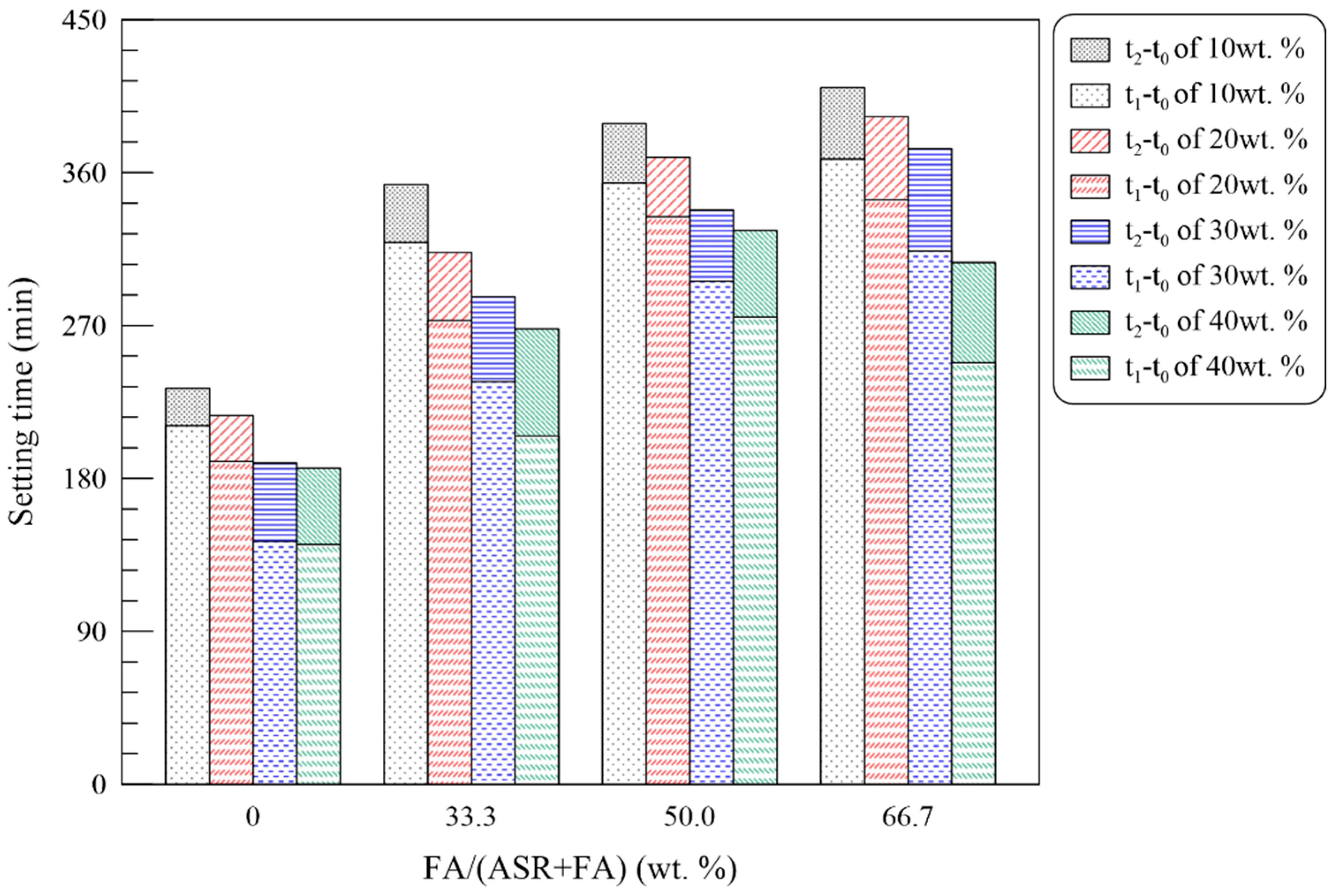
3.2.1. Fresh State Performance
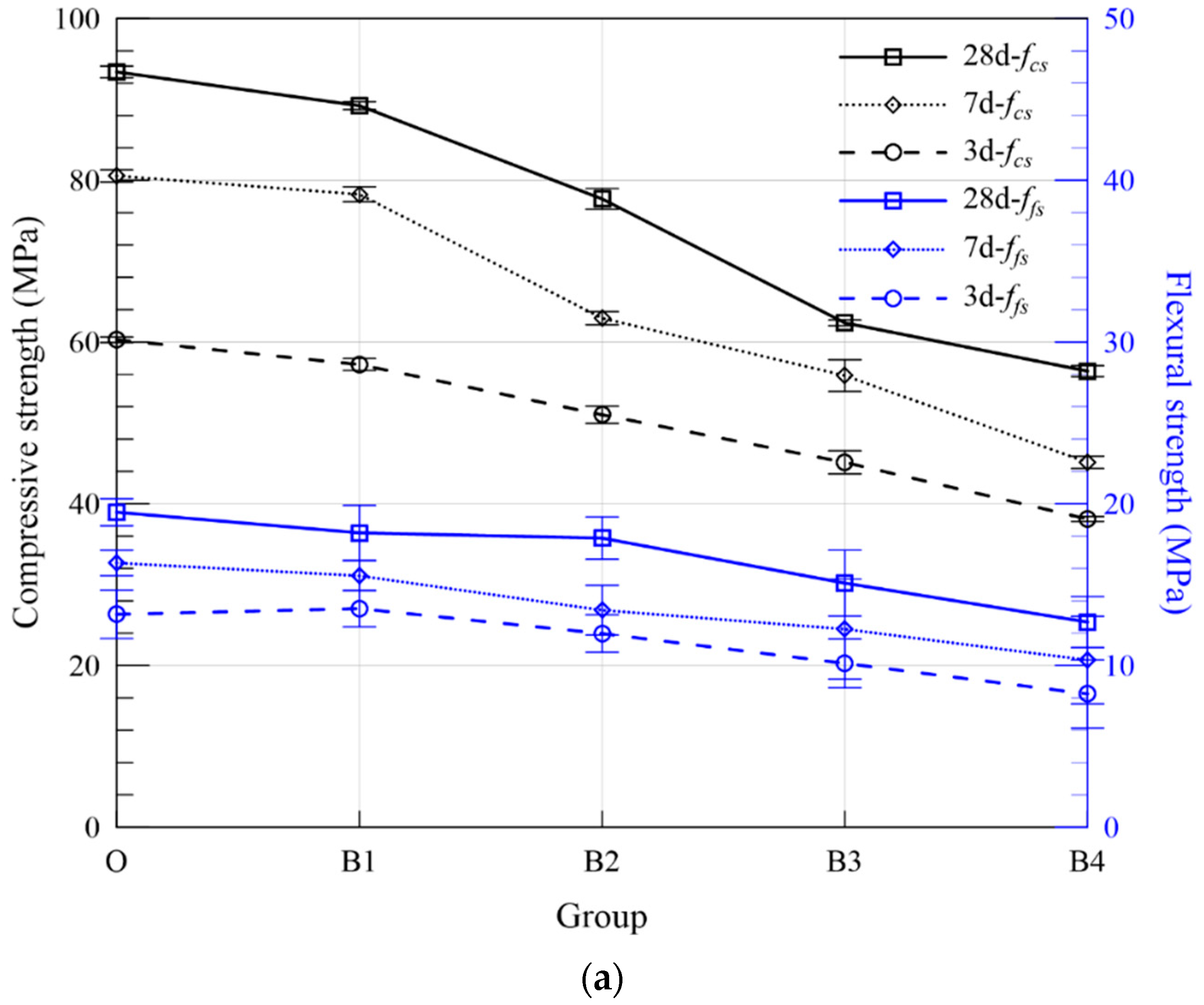
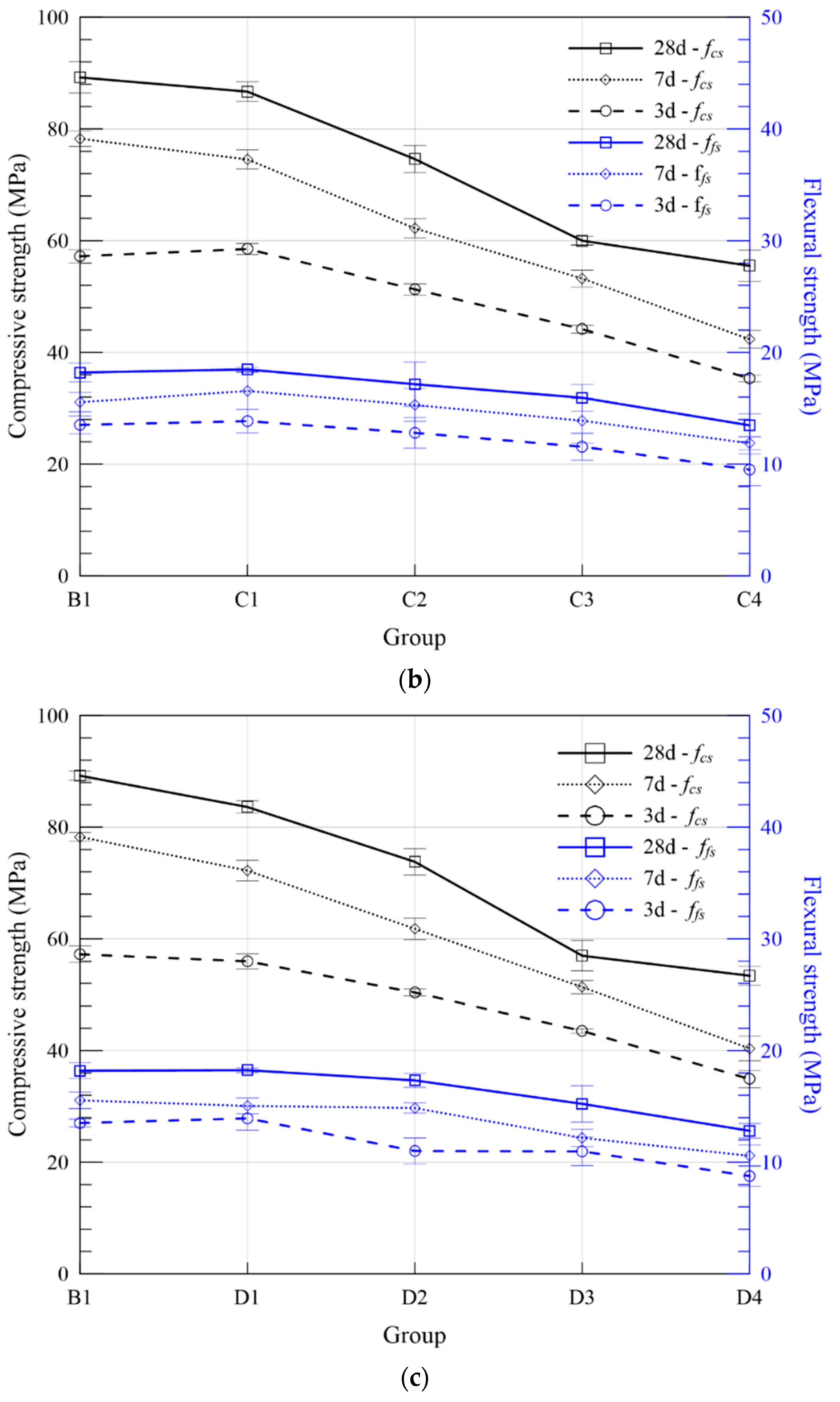
3.2.2. Flexural and Compressive Strength
3.2.3. Volume Stability
3.2.4. Microstructure of Blended MOC Pastes
4. Analyses and Discussions
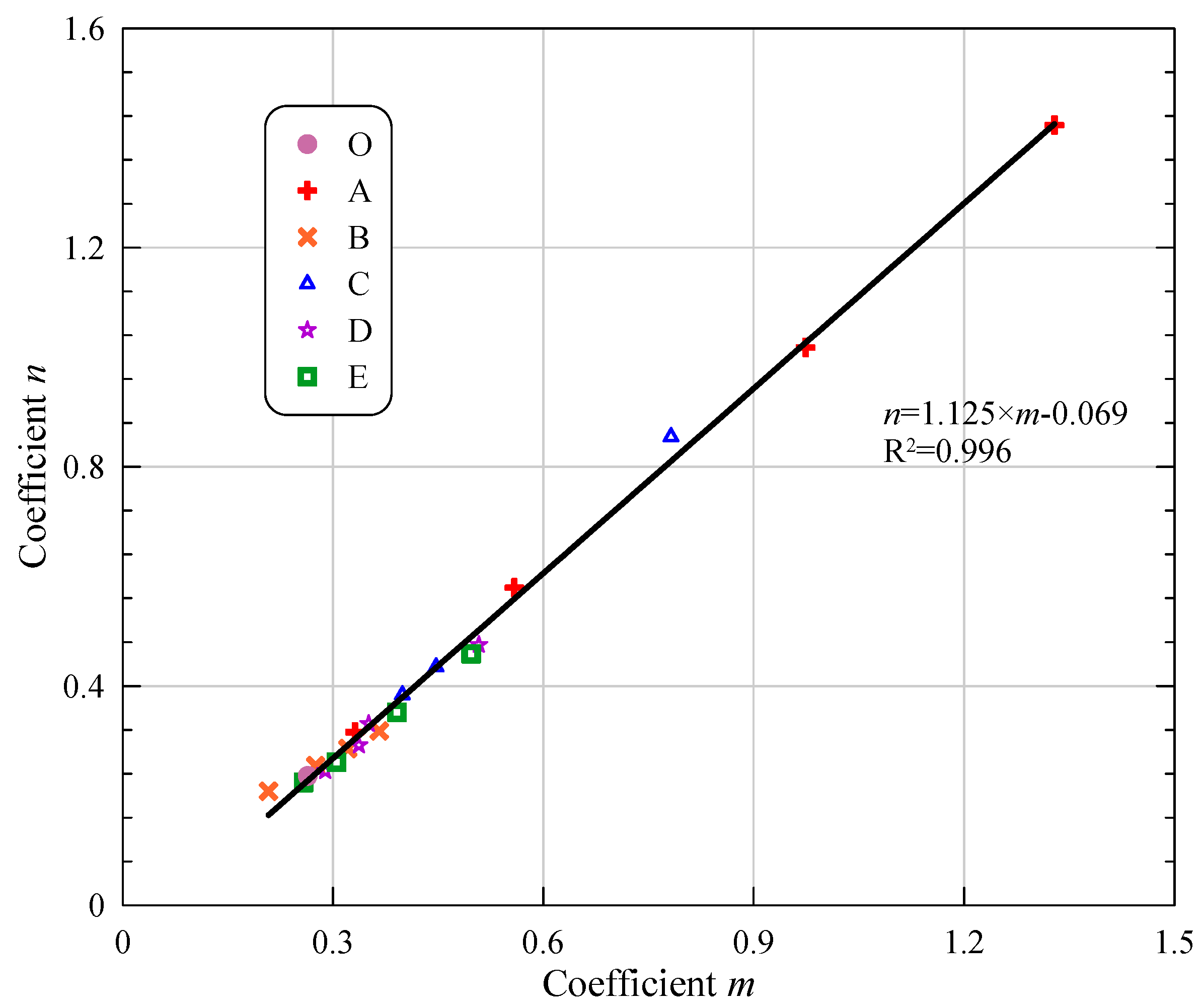
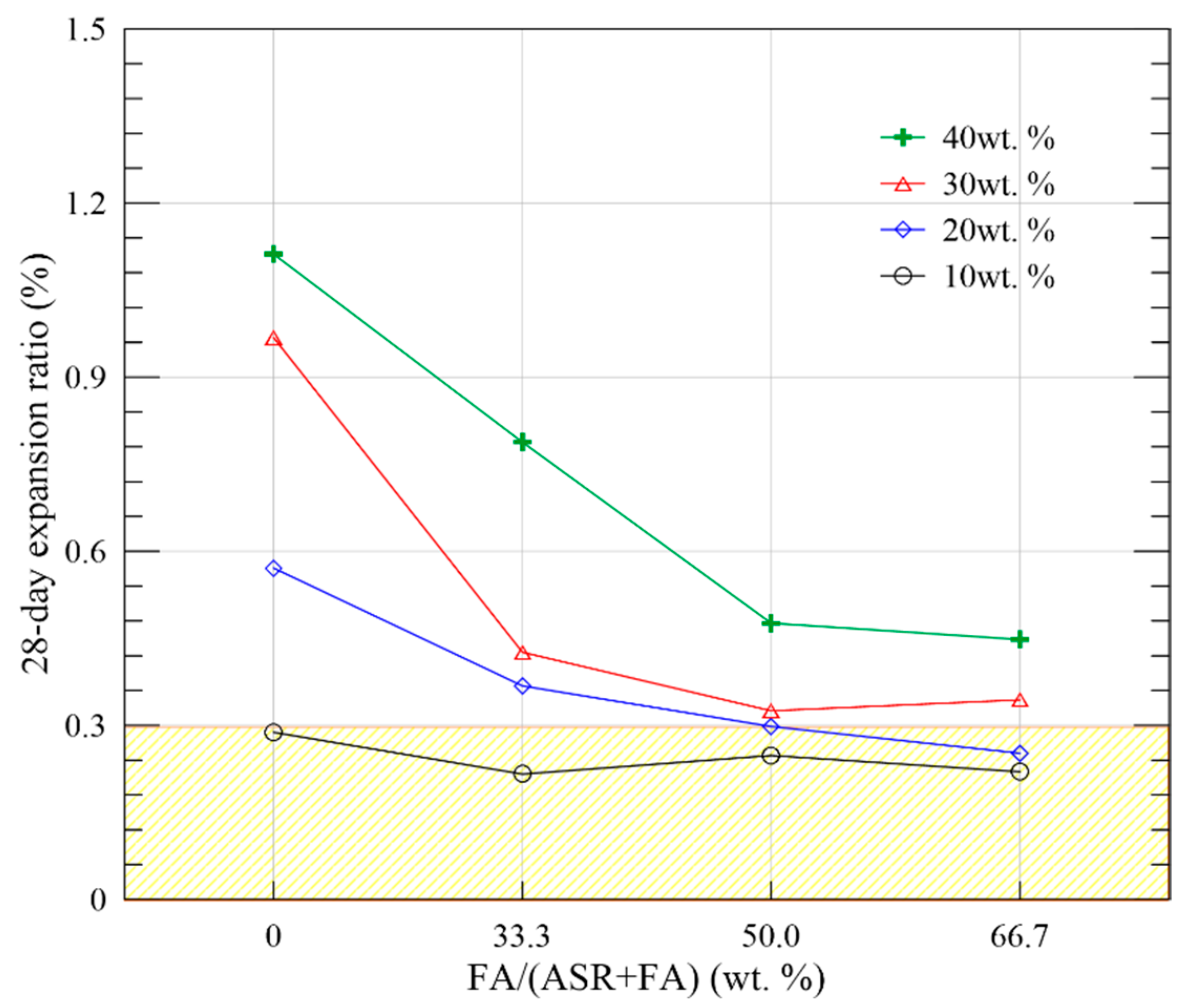
4.1. Expansion Ratio
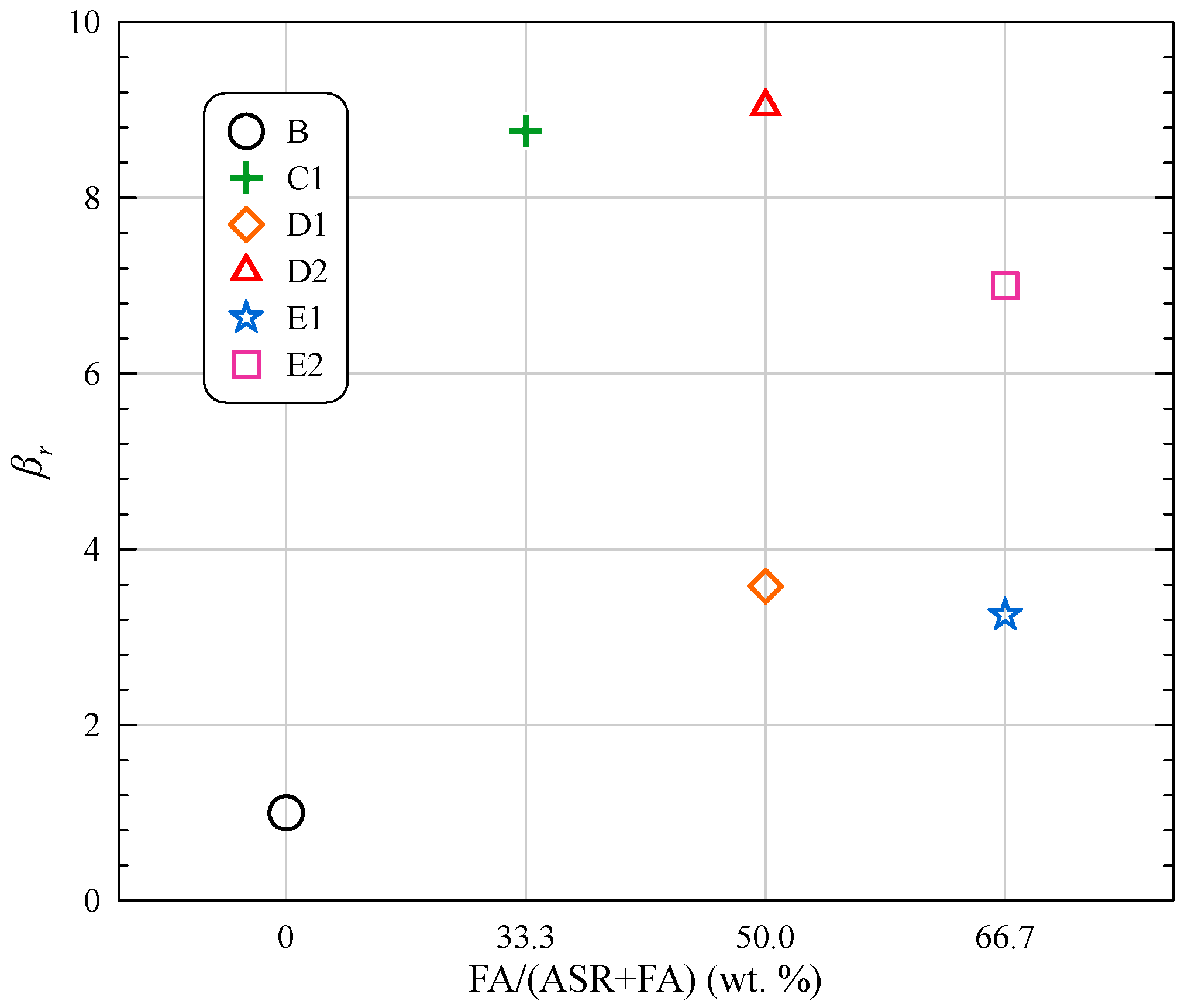
4.2. Simultaneous Expansion-Strength Performance
5. Conclusions
- Recycling solid waste FA and ASR in MOC paste is technically feasible.
- Inferior flowability and short setting process in the ASR-MOC system caused by irregular ASR can be improved by adding FA.
- FA would improve the volume stability of MOC, but the maximum dosage is suggested to be lower than 30% by weight.
- XRD curves indicate that FA affects the intensity of characteristic peaks of 5-phase and Mg(OH)2, which well explains the strength and volume stability of ASR-MOC pastes.
- Through simultaneous expansion-strength performance analysis, up to 20% of solid wastes in weight (10% FA + 10% ASR) can be recycled in MOC products within relevant specifications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasikowski, T.; Buczkowski, R.; Lemanowska, E. Cleaner production in the ammonia-soda industry: An ecological and economic study. J. Environ. Manag. 2004, 73, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, G. Cleaner production in the Solvay Process: General strategies and recent developments. J. Clean. Prod. 2008, 16, 833–841. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Tewari, A. Effect of soda ash industry effluent on agarophytes, alginophytes and carrageenophyte of west coast of India. J. Hazard. Mater. 2009, 162, 498–502. [Google Scholar] [CrossRef]
- Wang, X.B.; Yan, X.; Li, X.Y. Environmental risk for application of ammonia-soda white mud in soils in China. J. Integr. Agric. 2020, 19, 601–611. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Yao, G.; Zhu, X.; Hu, S.; Qiu, J.; Chen, P.; Lyu, X. Characterization of the mechanical properties and microcosmic mechanism of Portland cement prepared with soda residue. Constr. Build. Mater. 2020, 241, 117994. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Wang, L.; Zuo, L.; Zhu, Q.; Ma, W. Physical and mechanical properties and micro characteristics of fly ash-based geopolymers incorporating soda residue. Cem. Concr. Compos. 2019, 98, 125–136. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Zuo, L.; Zhu, Q.; Ma, W.; Liu, Y. Preparation and characterization of press-formed fly ash cement incorporating soda residue. Mater. Lett. 2020, 259, 126852. [Google Scholar] [CrossRef]
- Li, C.B.; Liang, Y.H.; Jiang, L.F.; Zhang, C.M.; Wang, Q. Characteristics of ammonia-soda residue and its reuse in magnesium oxychloride cement pastes. Constr. Build. Mater. 2021, 300, 123981. [Google Scholar] [CrossRef]
- Gong, W.; Wang, N.; Zhang, N. Effect of fly ash and metakaolin on the macroscopic and microscopic characterizations of magnesium oxychloride cement. Constr. Build. Mater. 2021, 267, 120957. [Google Scholar] [CrossRef]
- Huang, Q.; Zheng, W.; Xiao, X.; Dong, J.; Wen, J.; Chang, C. Effects of fly ash, phosphoric acid, and nano-silica on the properties of magnesium oxychloride cement. Ceram. Int. 2021, 47, 34341–34351. [Google Scholar] [CrossRef]
- GB/T 33544; Glass Fiber and Magnesium Cement Board. China Standard Press: Beijing, China, 2017.
- Neville, A.M. Properties of Concrete, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Chalangaran, N.; Farzampour, A.; Paslar, N.; Fatem, H. Experimental investigation of sound transmission loss in concrete containing recycled rubber crumbs. Adv. Concr. Constr. 2021, 11, 447–454. [Google Scholar]
- Chalangaran, N.; Farzampour, A.; Paslar, N. Nano Silica and Metakaolin Effects on the Behavior of Concrete Containing Rubber Crumbs. CivilEng 2020, 1, 264–274. [Google Scholar] [CrossRef]
- Mansouri, I.; Shahheidari, F.S.; Hashemi, S.M.A.; Farzampour, A. Investigation of steel fiber effects on concrete abrasion resistance. Adv. Concr. Constr. 2020, 9, 367–374. [Google Scholar]
- Farzampour, A. Compressive behavior of concrete under environmental effects. In Compressive Strength of Concrete; Intech Open: London, UK, 2019. [Google Scholar]
- Farzampour, A. Temperature and humidity effects on behavior of grouts. Adv. Concr. Constr. 2017, 5, 659–669. [Google Scholar]
- Wang, L.; Guo, F.; Lin, Y.; Yang, H.; Tang, S.W. Comparison between the effects of phosphorous slag and fly ash on the C-S-H structure, long-term hydration heat and volume deformation of cement-based materials. Constr. Build. Mater. 2020, 250, 118807. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.J.; Zhang, Z.H. Influence of fly ash on the pore structure and shrinkage characteristics of metakaolin-based geopolymer pastes and mortars. Constr. Build. Mater. 2017, 153, 284–293. [Google Scholar] [CrossRef]
- Kristiawan, S.A.; Aditya, M.T.M. Effect of High Volume Fly Ash on Shrinkage of Self-compacting Concrete. Procedia Eng. 2015, 125, 705–712. [Google Scholar] [CrossRef]
- Marvila, M.T.; de Azevedo, A.R.G.; de Matos, P.R.; Monteiro, S.N.; Vieira, C.M.F. Materials for Production of High and Ultra-High Performance Concrete: Review and Perspective of Possible Novel Materials. Materials 2021, 14, 4304. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, H.F.; Gong, W.; Liu, T.; Wang, N.; Tan, Y.S.; Wu, C.Y. Effects of low- and high-calcium fly ash on the water resistance of magnesium oxysulfate cement. Constr. Build. Mater. 2020, 230, 116951. [Google Scholar] [CrossRef]
- Xu, D.; Fu, P.F.; Hui, W.; Wang, Q.H.; Li, K.Q. Characterization and Hydration Mechanism ofAmmonia Soda Residue and Portland Cement Composite Cementitious Material. Materials 2021, 14, 4794. [Google Scholar] [CrossRef]
- QB/T 2605; Industrial Magnesium Chloride. China Standard Press: Beijing, China, 2018.
- ISO 679; Cement—Test Methods—Determination of Strength. IX-ISO: Geneva, Switzerland, 2009.
- JC/T 313; Test Method for Determinig Expansive Ratio of Expansive Cement. China Standard Press: Beijing, China, 2009.
- GB/T 8077; Methods for Testing Uniformity of Concrete Admixture. China Standard Press: Beijing, China, 2012.
- Yin, S.; Yan, Z.; Chen, X.; Wang, L. Effect of fly-ash as fine aggregate on the workability and mechanical properties of cemented paste backfill. Case Stud. Constr. Mater. 2022, 16, e01039. [Google Scholar] [CrossRef]
- Sua-iam, G.; Chatveera, B. A study on workability and mechanical properties of eco-sustainable self-compacting concrete incorporating PCB waste and fly ash. J. Clean. Prod. 2021, 329, 129523. [Google Scholar] [CrossRef]
- Chau, C.K.; Chan, J.; Li, Z. Influences of fly ash on magnesium oxychloride mortar. Cem. Concr. Compos. 2009, 31, 250–254. [Google Scholar] [CrossRef]
- GB/T 1346; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. China Standard Press: Beijing, China, 2011.
- Guo, Y.; Zhang, Y.X.; Soe, K.; Wuhrer, R.; Hutchison, W.D.; Timmers, H. Development of magnesium oxychloride cement with enhanced water resistance by adding silica fume and hybrid fly ash-silica fume. J. Clean. Prod. 2021, 313, 127682. [Google Scholar] [CrossRef]
- Zdeb, T.; Tracz, T.; Adamczyk, M. Physical, mechanical properties and durability of cement mortars containing fly ash from the sewage sludge incineration process. J. Clean. Prod. 2022, 345, 131055. [Google Scholar] [CrossRef]
- Yang, K.H.; Mun, J.S.; Shim, H.J. Shrinkage of heavyweight magnetite concrete with and without fly ash. Constr. Build. Mater. 2013, 47, 56–65. [Google Scholar] [CrossRef]
- Termkhajornkit, P.; Nawa, T.; Nakai, M.; Saito, T. Effect of fly ash on autogenous shrinkage. Cem. Concr. Res. 2005, 35, 473–482. [Google Scholar] [CrossRef]
- Liu, J.; Zha, F.; Xu, L.; Kang, B.; Yang, C.; Feng, Q.; Zhang, W.; Zhang, J. Strength and microstructure characteristics of cement-soda residue solidified/stabilized zinc contaminated soil subjected to freezing–thawing cycles. Cold Reg. Sci. Technol. 2020, 172, 102992. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, J.; Zhao, S. Experimental study on shrinkage of HPC containing fly ash and ground granulated blast-furnace slag. Constr. Build. Mater. 2017, 155, 145–153. [Google Scholar] [CrossRef]
- Ma, C.; Chen, G.; Cao, L.; Zhou, H.; Ren, W. Effects and mechanisms of waste gypsum influencing the mechanical properties and durability of magnesium oxychloride cement. J. Clean. Prod. 2022, 339, 130679. [Google Scholar] [CrossRef]
- Xu, W.; Song, Z.; Guo, M.Z.; Jiang, L.; Chu, H. Improvement in water resistance of magnesium oxychloride cement via incorporation of dredged sediment. J. Clean. Prod. 2022, 356, 131830. [Google Scholar] [CrossRef]
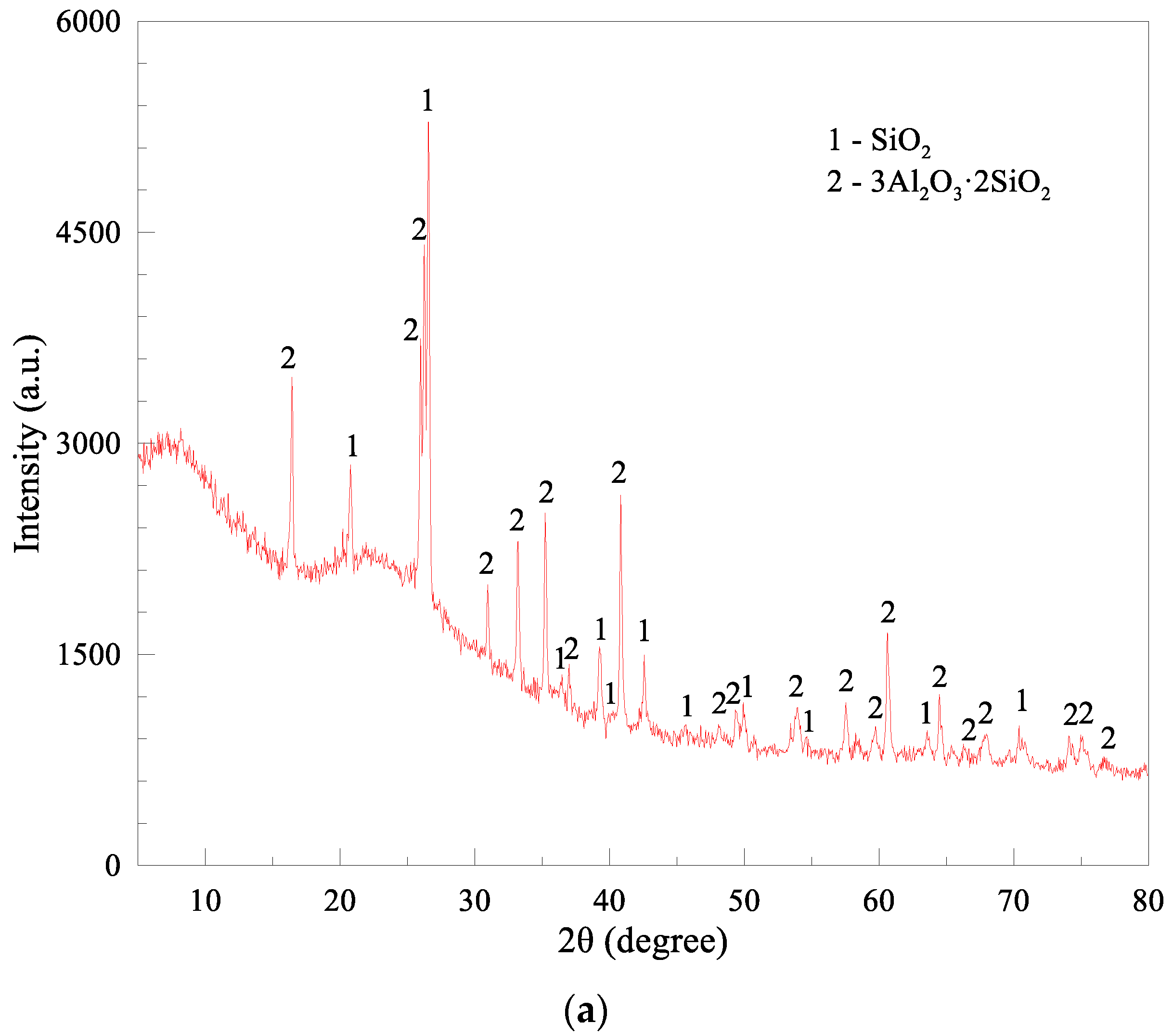
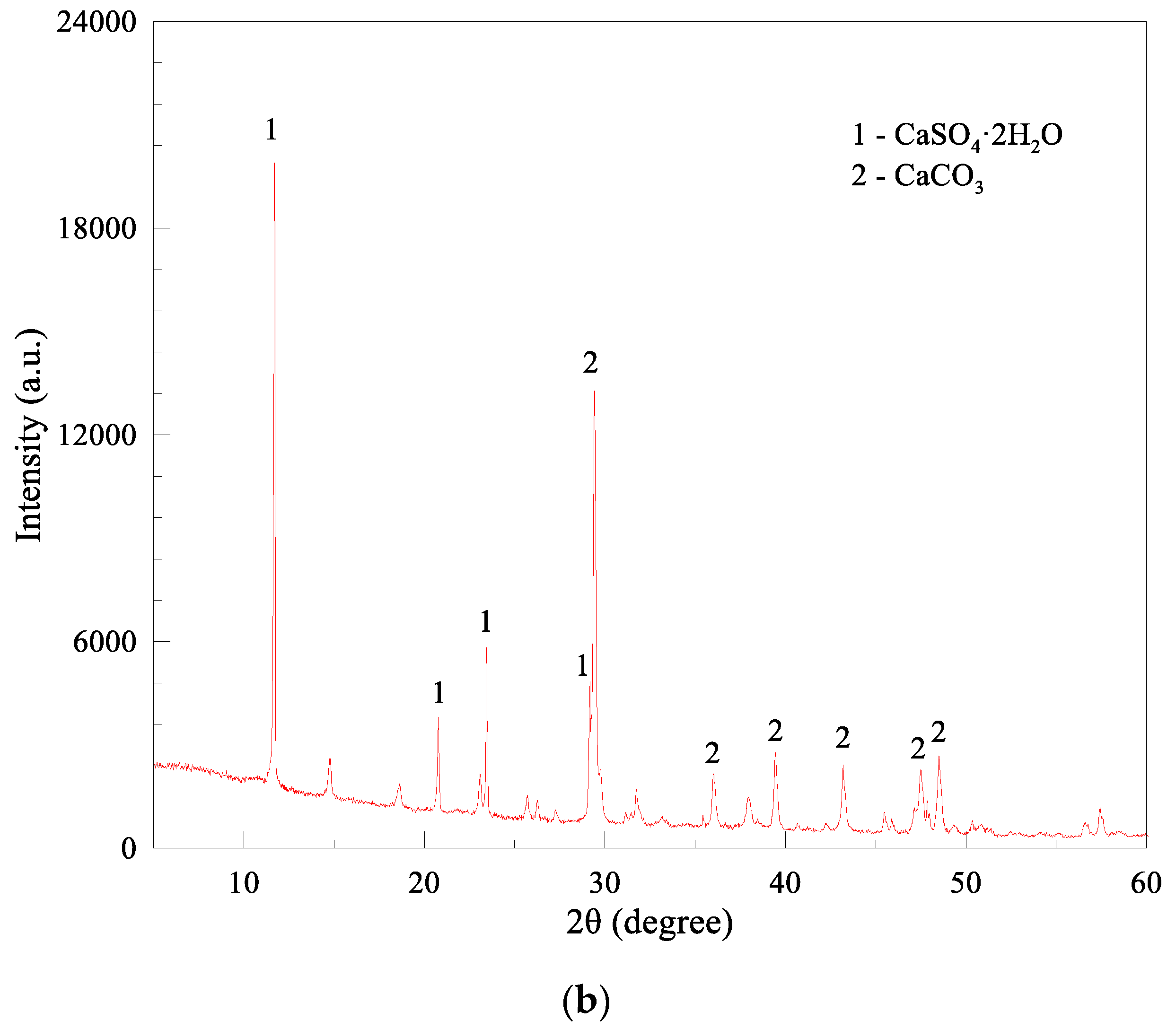






| Compositions | CaCO3 | CaSO4· 2H2O | Mg(OH)2 | CaCl2·2H2O | SiO2 | Al2O3 | Fe2O3 | SO3 | Others |
|---|---|---|---|---|---|---|---|---|---|
| ASR | 56.10 | 25.50 | 9.60 | 3.70 | 1.60 | 1.00 | 0.80 | - | 0.60 |
| FA | - | - | - | - | 57.47 | 26.56 | 6.18 | 0.41 | 4.01 |
| No. | Series | Groups | FA (wt. %) | FA (vol. %) | MgO (vol. %) | ·6H2O (vol. %) | H2O (vol. %) |
|---|---|---|---|---|---|---|---|
| 1 | O | - | 0 | 0 | 27.34 | 39.57 | 33.10 |
| 2 | A | A1 | 10 | 3.89 | 26.27 | 38.03 | 31.81 |
| 3 | A2 | 20 | 7.48 | 25.29 | 36.61 | 30.62 | |
| 4 | A3 | 30 | 10.81 | 24.38 | 35.30 | 29.52 | |
| 5 | A4 | 40 | 13.92 | 23.53 | 34.06 | 28.49 |
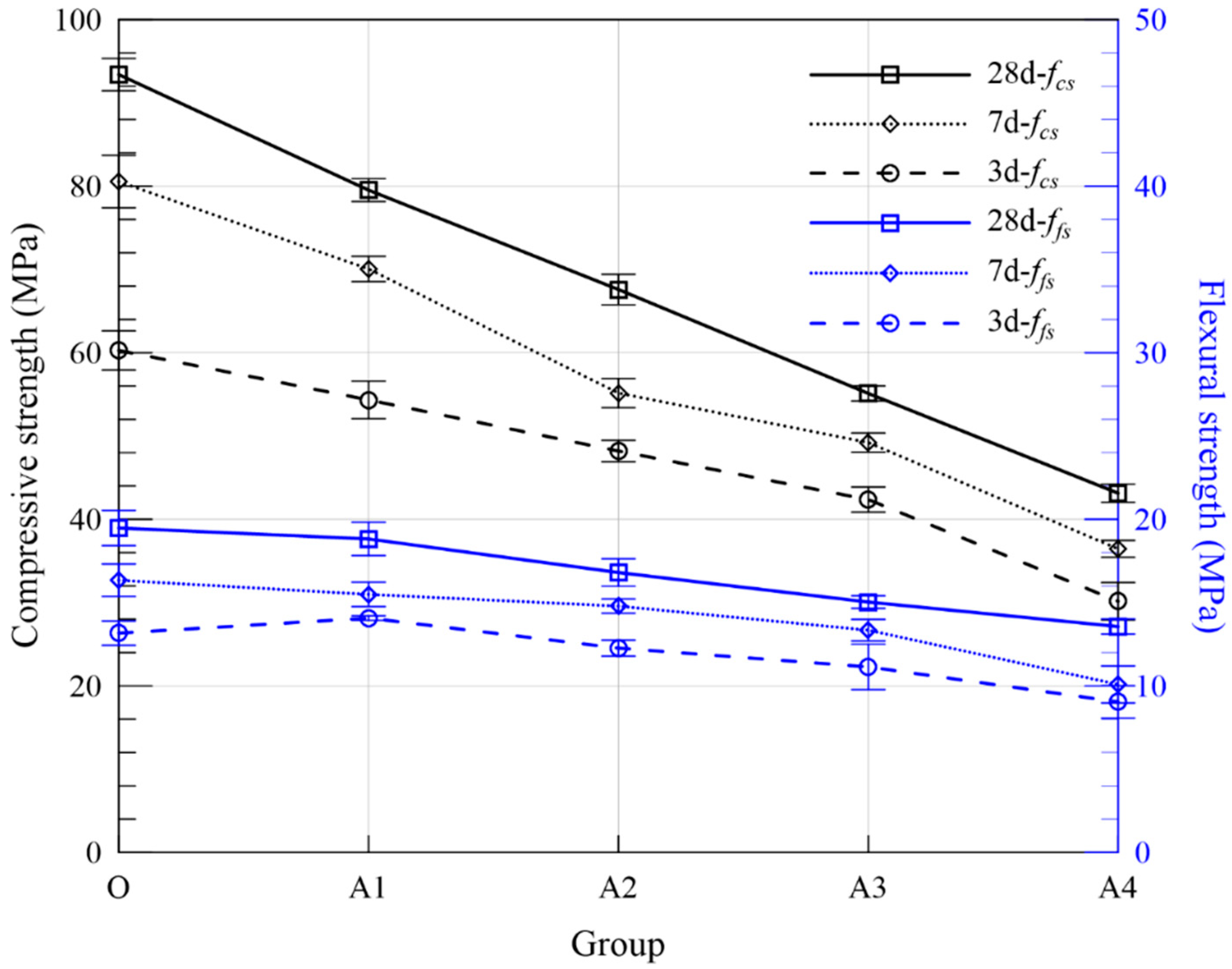
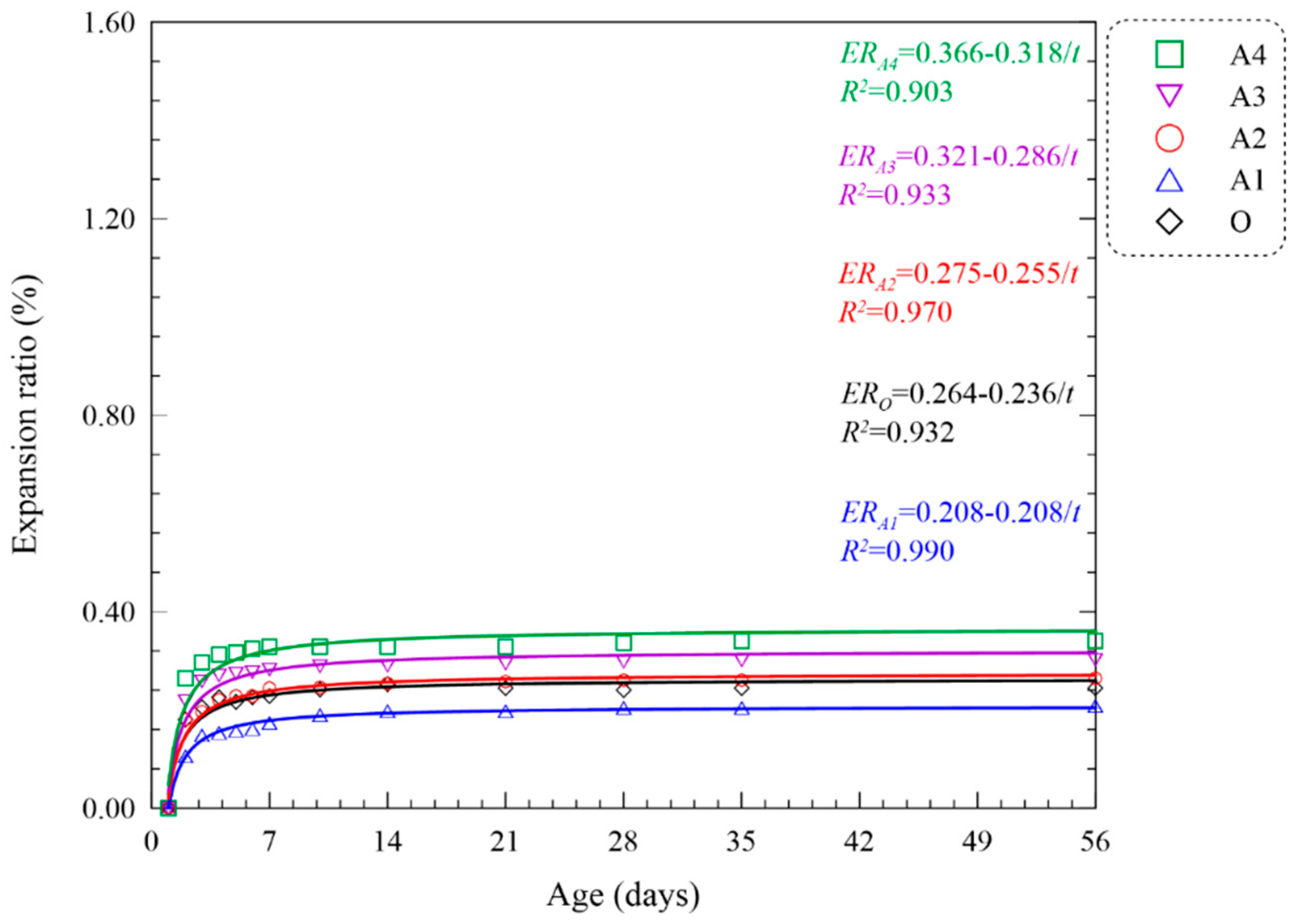
| Series | Groups | Flowability (mm) | Setting Time (min) | Compressive Strength (MPa) | Flexural Strength (MPa) | Expansion Ratio (%) | Fitting Curve | R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t1–t0 | t2–t0 | fc,3 | fc,7 | fc,28 | ff,3 | ff,7 | ff,28 | 28d | 56d | |||||
| O | - | 220 | 265 | 299 | 60.28 | 80.55 | 93.39 | 13.16 | 16.34 | 19.48 | 0.244 | 0.244 | ERO = 0.264 − 0.236/t | 0.932 |
| A | A1 | 192.5 | 435 | 479 | 54.33 | 70.06 | 79.52 | 14.07 | 15.49 | 18.82 | 0.204 | 0.208 | ERA1 = 0.208 − 0.208/t | 0.990 |
| A2 | 171.5 | 421 | 474 | 48.20 | 55.16 | 67.59 | 12.27 | 14.79 | 16.80 | 0.260 | 0.264 | ERA2 = 0.275 − 0.255/t | 0.970 | |
| A3 | 155.5 | 403 | 458 | 42.37 | 49.22 | 55.11 | 11.14 | 13.34 | 15.03 | 0.298 | 0.302 | ERA3 = 0.321 − 0.286/t | 0.933 | |
| A4 | 137.5 | 376 | 446 | 30.16 | 36.45 | 43.13 | 9.04 | 10.09 | 13.56 | 0.336 | 0.340 | ERA4 = 0.366 − 0.318/t | 0.903 | |
| No. | Series | Description (in Weight) | Groups | ASR + FA (wt. %) | FA/(FA + ASR) | ASR (vol. %) | FA (vol. %) | MgO (vol. %) | ·6H2O (vol. %) | H2O (vol. %) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | B | 100% ASR + 0% FA | B1 | 10 | 0 | 3.46 | 0 | 26.39 | 38.20 | 31.95 |
| 2 | B2 | 20 | 0 | 6.70 | 0 | 25.51 | 36.92 | 30.88 | ||
| 3 | B3 | 30 | 0 | 9.72 | 0 | 24.68 | 35.72 | 29.88 | ||
| 4 | B4 | 40 | 0 | 12.55 | 0 | 23.91 | 34.60 | 28.94 | ||
| 5 | C | 66.7%ASR + 33.3%FA | C1 | 10 | 0.33 | 2.31 | 1.30 | 26.35 | 38.14 | 31.90 |
| 6 | C2 | 20 | 0.33 | 4.45 | 2.51 | 25.43 | 36.82 | 30.79 | ||
| 7 | C3 | 30 | 0.33 | 6.45 | 3.64 | 24.58 | 35.58 | 29.76 | ||
| 8 | C4 | 40 | 0.33 | 8.32 | 4.69 | 23.78 | 34.42 | 28.79 | ||
| 9 | D | 50%ASR + 50%FA | D1 | 10 | 0.50 | 1.73 | 1.95 | 26.33 | 38.11 | 31.88 |
| 10 | D2 | 20 | 0.50 | 3.33 | 3.76 | 25.40 | 36.76 | 30.75 | ||
| 11 | D3 | 30 | 0.50 | 4.83 | 5.44 | 24.53 | 35.50 | 29.70 | ||
| 12 | D4 | 40 | 0.50 | 6.23 | 7.02 | 23.72 | 34.33 | 28.71 | ||
| 13 | E | 33.3%ASR + 66.7%FA | E1 | 10 | 0.67 | 1.15 | 2.60 | 26.31 | 38.09 | 31.86 |
| 14 | E2 | 20 | 0.67 | 2.22 | 5.00 | 25.36 | 36.71 | 30.71 | ||
| 15 | E3 | 30 | 0.67 | 3.21 | 7.24 | 24.48 | 35.43 | 29.64 | ||
| 16 | E4 | 40 | 0.67 | 4.14 | 9.33 | 23.65 | 34.24 | 28.64 |
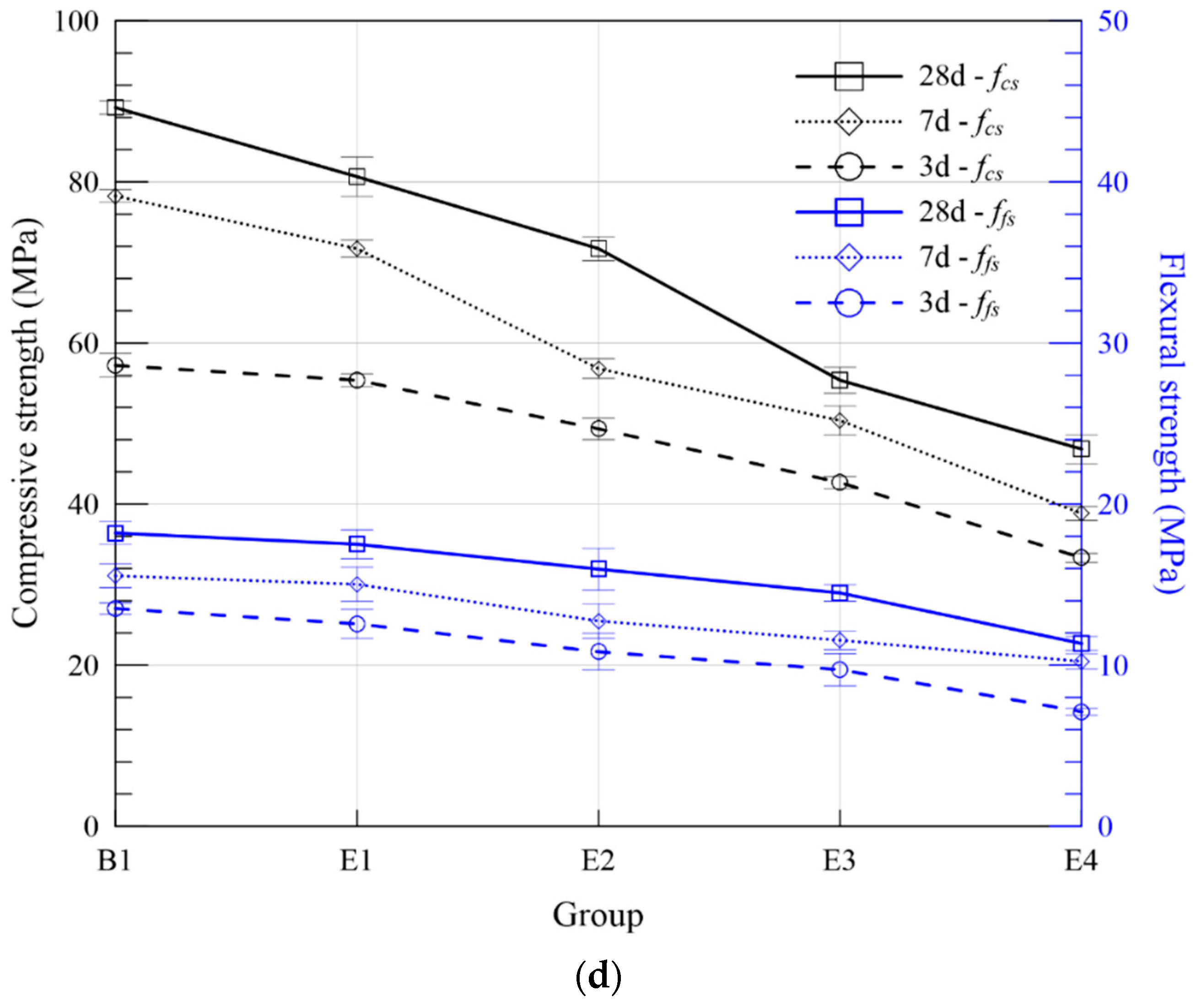
| Series | Group | ASR + FA (wt. %) | Compressive Strength (MPa) | Flexural Strength (MPa) | Expansion Ratio (%) | Fitting Curve | R2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fc,3 | fc,7 | fc,28 | ff,3 | ff,7 | ff,28 | 28d | 56d | |||||
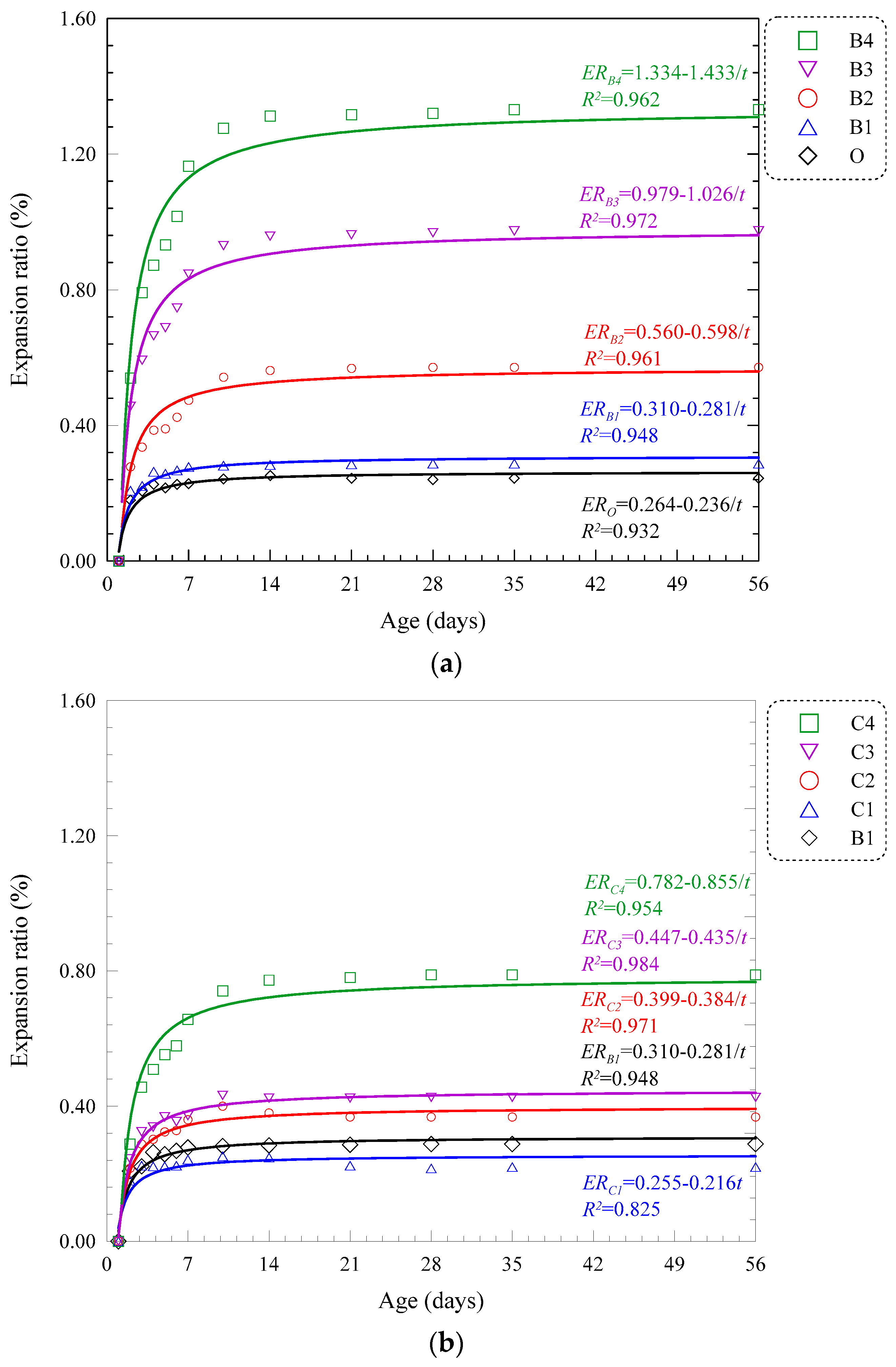
| B | B1 | 10 | 57.21 | 78.26 | 89.22 | 13.52 | 15.55 | 18.20 | 0.288 | 0.288 | ERB1 = 0.310 − 0.281/t | 0.948 |
| B2 | 20 | 51.00 | 62.95 | 77.69 | 11.98 | 13.42 | 17.88 | 0.571 | 0.571 | ERB2 = 0.560 − 0.598/t | 0.961 | |
| B3 | 30 | 45.12 | 55.85 | 62.37 | 10.13 | 12.25 | 15.09 | 0.968 | 0.973 | ERB3 = 0.979 − 1.026/t | 0.972 | |
| B4 | 40 | 38.11 | 45.12 | 56.40 | 8.24 | 10.34 | 12.69 | 1.312 | 1.331 | ERB4 = 1.334 − 1.433/t | 0.962 | |
| C | C1 | 10 | 58.51 | 74.54 | 86.67 | 13.85 | 16.55 | 18.48 | 0.216 | 0.220 | ERC1 = 0.255 − 0.216/t | 0.825 |
| C2 | 20 | 51.28 | 62.21 | 74.61 | 12.80 | 15.30 | 17.16 | 0.368 | 0.368 | ERC2 = 0.399 − 0.384/t | 0.971 | |
| C3 | 30 | 44.17 | 53.22 | 59.99 | 11.56 | 13.89 | 15.95 | 0.426 | 0.426 | ERC3 = 0.447 − 0.435/t | 0.984 | |
| C4 | 40 | 35.33 | 42.34 | 55.51 | 9.51 | 11.87 | 13.48 | 0.788 | 0.788 | ERC4 = 0.782 − 0.855/t | 0.954 | |
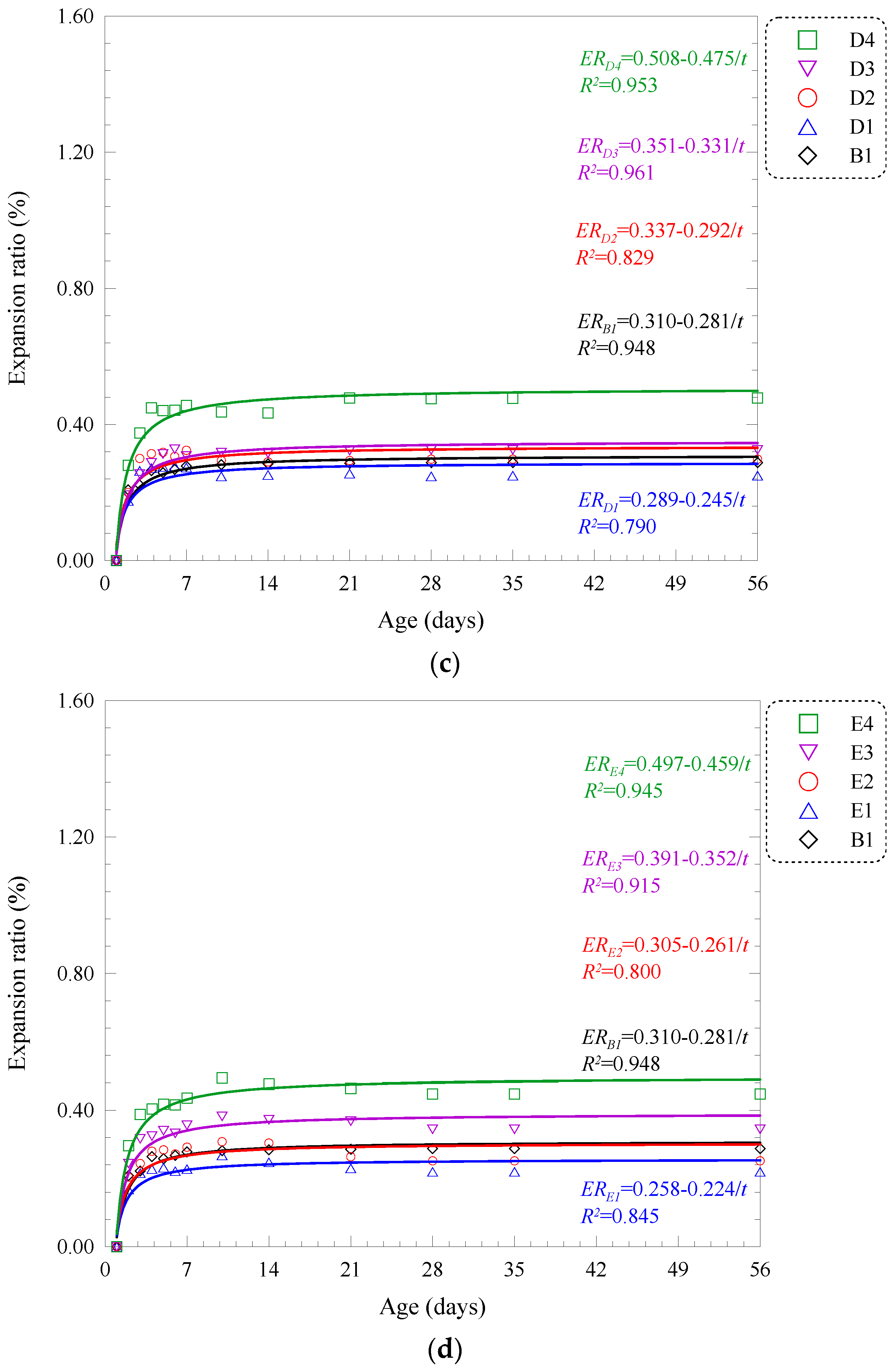
| D | D1 | 10 | 55.95 | 72.22 | 83.61 | 13.94 | 15.05 | 18.25 | 0.248 | 0.250 | ERD1 = 0.289 − 0.245/t | 0.790 |
| D2 | 20 | 50.37 | 61.78 | 73.79 | 11.01 | 14.86 | 17.34 | 0.298 | 0.298 | ERD2 = 0.337 − 0.292/t | 0.829 | |
| D3 | 30 | 43.52 | 51.34 | 56.95 | 10.98 | 12.19 | 15.23 | 0.325 | 0.325 | ERD3 = 0.351 − 0.331/t | 0.961 | |
| D4 | 40 | 34.91 | 40.39 | 53.37 | 8.77 | 10.58 | 12.82 | 0.476 | 0.478 | ERD4 = 0.508 − 0.475/t | 0.953 | |
| E | E1 | 10 | 55.37 | 71.71 | 80.65 | 12.56 | 15.02 | 17.51 | 0.220 | 0.220 | ERE1 = 0.258 − 0.224/t | 0.845 |
| E2 | 20 | 49.34 | 56.81 | 71.71 | 10.83 | 12.74 | 15.96 | 0.252 | 0.252 | ERE2 = 0.305 − 0.261/t | 0.800 | |
| E3 | 30 | 42.68 | 50.37 | 55.37 | 9.72 | 11.55 | 14.48 | 0.344 | 0.344 | ERE3 = 0.391 − 0.352/t | 0.915 | |
| E4 | 40 | 33.33 | 38.83 | 46.83 | 7.11 | 10.23 | 11.34 | 0.448 | 0.448 | ERE4 = 0.497 − 0.459/t | 0.945 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Huang, W.; Liang, Y.; Li, C.; Lai, M.; Sun, J. Eco-Sustainable Magnesium Oxychloride Cement Pastes Containing Waste Ammonia Soda Residue and Fly Ash. Materials 2022, 15, 5941. https://doi.org/10.3390/ma15175941
Wang Q, Huang W, Liang Y, Li C, Lai M, Sun J. Eco-Sustainable Magnesium Oxychloride Cement Pastes Containing Waste Ammonia Soda Residue and Fly Ash. Materials. 2022; 15(17):5941. https://doi.org/10.3390/ma15175941
Chicago/Turabian StyleWang, Qing, Wenjie Huang, Yuhang Liang, Congbo Li, Mianheng Lai, and Jing Sun. 2022. "Eco-Sustainable Magnesium Oxychloride Cement Pastes Containing Waste Ammonia Soda Residue and Fly Ash" Materials 15, no. 17: 5941. https://doi.org/10.3390/ma15175941
APA StyleWang, Q., Huang, W., Liang, Y., Li, C., Lai, M., & Sun, J. (2022). Eco-Sustainable Magnesium Oxychloride Cement Pastes Containing Waste Ammonia Soda Residue and Fly Ash. Materials, 15(17), 5941. https://doi.org/10.3390/ma15175941






