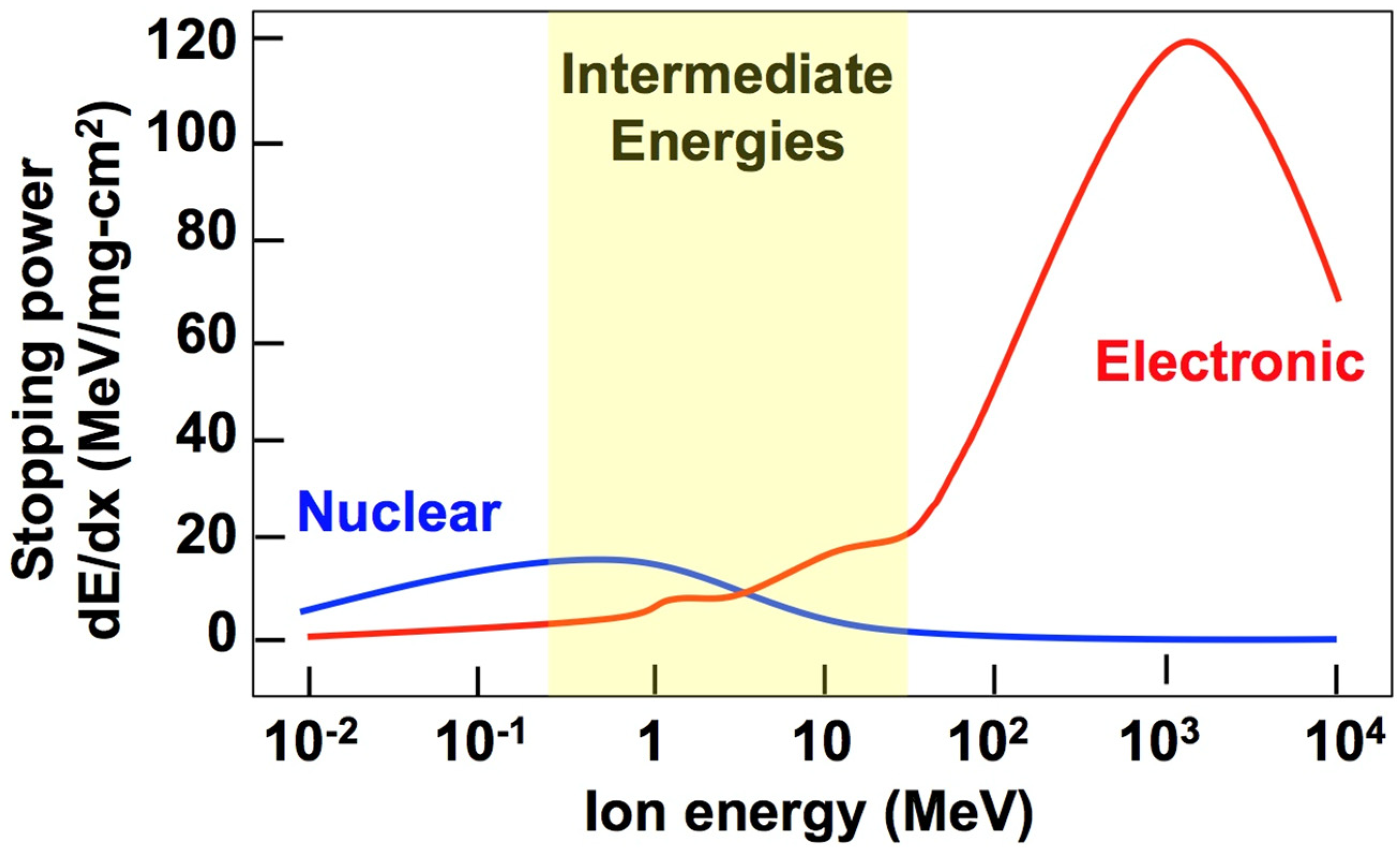
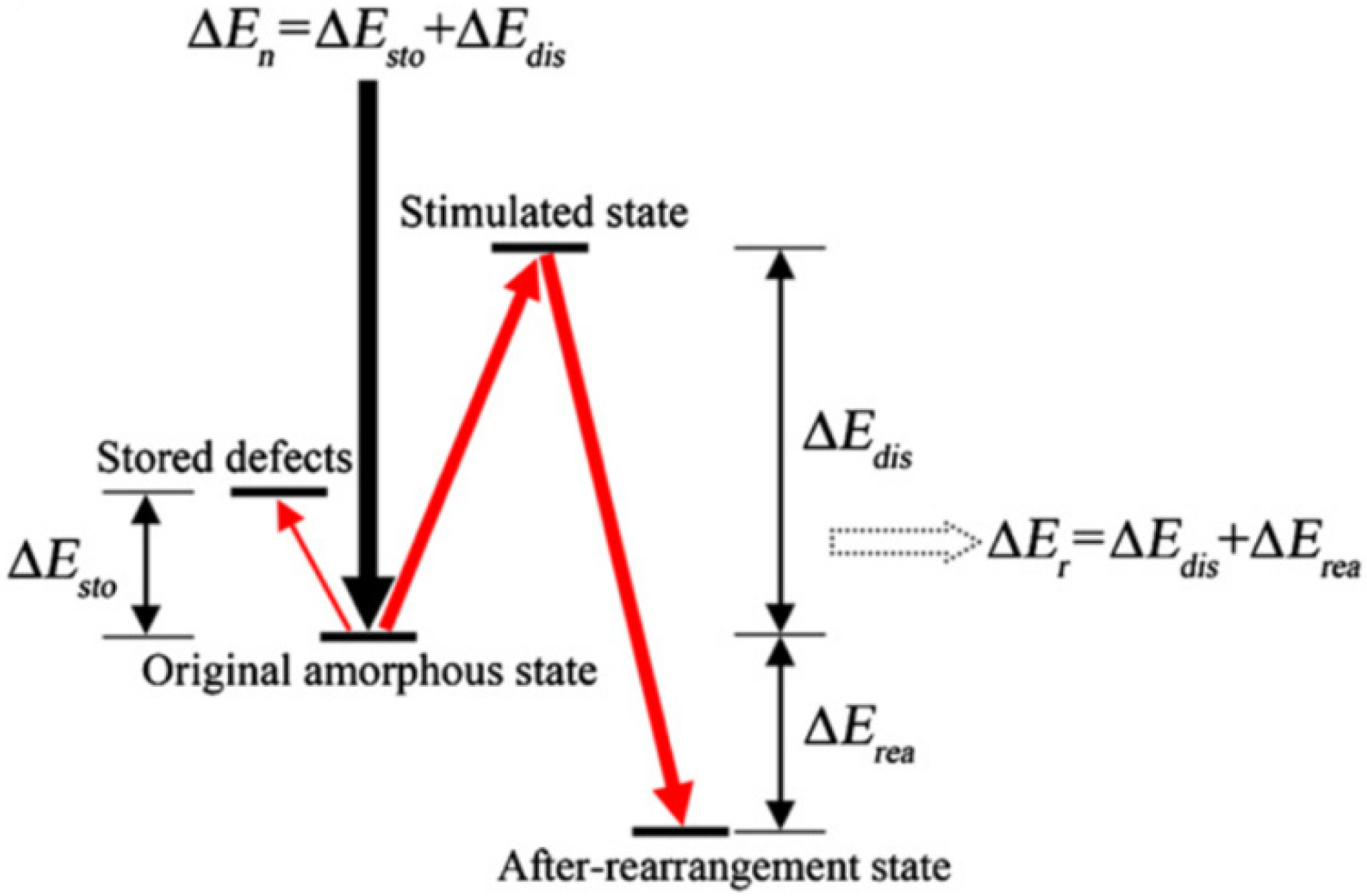
Materials wherein a-to-c transformations have been observed in a variety of compositions and under a variety of irradiating conditions are summarized in
Table 1. Important parameters that likely affect the observed a-to-c transformation, such as the target material, irradiating particle species, the energy of the irradiating beam, fluence, and irradiating temperature, are listed in
Table 1 where relevant. The a-to-c transformations can be broadly categorized into three types: (1) athermal, resulting exclusively from either electron or ion irradiation of amorphous material, (2) resulting from the combined effects of heating and either ion or electron irradiation, or (3) epitaxial growth or recrystallization of an amorphous oxide due to ion or electron irradiation. The a-to-c transformations are usually observed in target materials that are amorphous as-prepared [
40,
41,
42,
43,
44,
45,
46,
47]. Less commonly, crystalline or single crystal materials are first amorphized by one type of irradiation, and then the a-to-c transformation is accomplished by irradiating the material in a subsequent experiment [
48,
49,
50,
51]. This section summarizes reported a-to-c transformations in ceramic materials, including zirconium oxide, titanium oxide, aluminum oxide, silicon oxide materials, silicon carbide, silicates, and other ceramic materials.
3.1. Zirconium Dioxide
Roddatis et al. demonstrated that transmission electron microscopy (TEM) beam irradiation of amorphous ZrO
2 films prepared via a self-assembled monolayer method can result in an a-to-c transformation [
40]. At a cryogenic temperature of 4.2 K, a 160 kV TEM electron beam at a current density of 0.5 A/cm
2 was able to form crystallites in the as-prepared amorphous film. The size and number of crystallites increased with irradiation exposure time. After 20 min of TEM electron beam irradiation, the crystallites grew to ~10 nm in size, and fast Fourier transform (FFT) analysis of the high-resolution TEM (HR-TEM) images confirmed that the crystallites were structured as tetragonal ZrO
2. To assess whether there was a change in the coordination and film composition as a result of TEM electron irradiation, electron energy loss spectroscopy (EELS) was performed on an as-prepared amorphous sample, a sample irradiated for 5 min with TEM electrons, a tetragonal ZrO
2 powder, and a monoclinic ZrO
2 powder. From the oxygen K-edge spectra, it was found that the amorphous sample lacked a distinct peak at 534 eV, but such a peak was present in both the irradiated sample and the reference standards. Therefore, the EELS results provided evidence for a change in coordination in the amorphous film due to electron irradiation-induced a-to-c transformation. It was also found that the a-to-c transformation occurred faster when amorphous films were irradiated with 200 kV electrons at room temperature compared to with 160 kV electrons at 4.2 K. However, crystallization at room temperature was faster with 200 kV than with 400 kV electron irradiation. While the effects of temperature and electron energy could not be deconvoluted, the authors explained that this seemingly strange result might be due to inelastic scattering being less probable at higher electron energies and that inelastic electron scattering of electrons on the sample surface plays a role in the electron irradiation-induced structural changes observed [
40].
Naguib and Kelly irradiated amorphous ZrO
2 films with Kr ions at energies ranging from 2–35 keV [
48]. The amorphous films were prepared by sputtering crystalline ZrO
2 films on Zr foil with Kr or O
2 ions at 6 keV for 75 min, yielding an amorphized layer of ZrO
2. The as-prepared films were confirmed to be amorphous by TEM selected area electron diffraction (SAED). They first investigated the thermally-induced crystallization of amorphous ZrO
2 by TEM in situ pulse heating over 400–540 °C. This was found to result in recrystallization of the amorphous film, and SAED analysis indicated that the resulting crystallites were mainly cubic, with some monoclinic character (inset image in
Figure 2a) [
48]. As a control, amorphous films were heated in a tube furnace until crystallization was achieved. Samples achieved full crystallization when heated at temperatures between 520–540 °C for 5 min or in air at 400–425 °C for 360 min and showed similar SAED patterns to the pulse-heated sample.
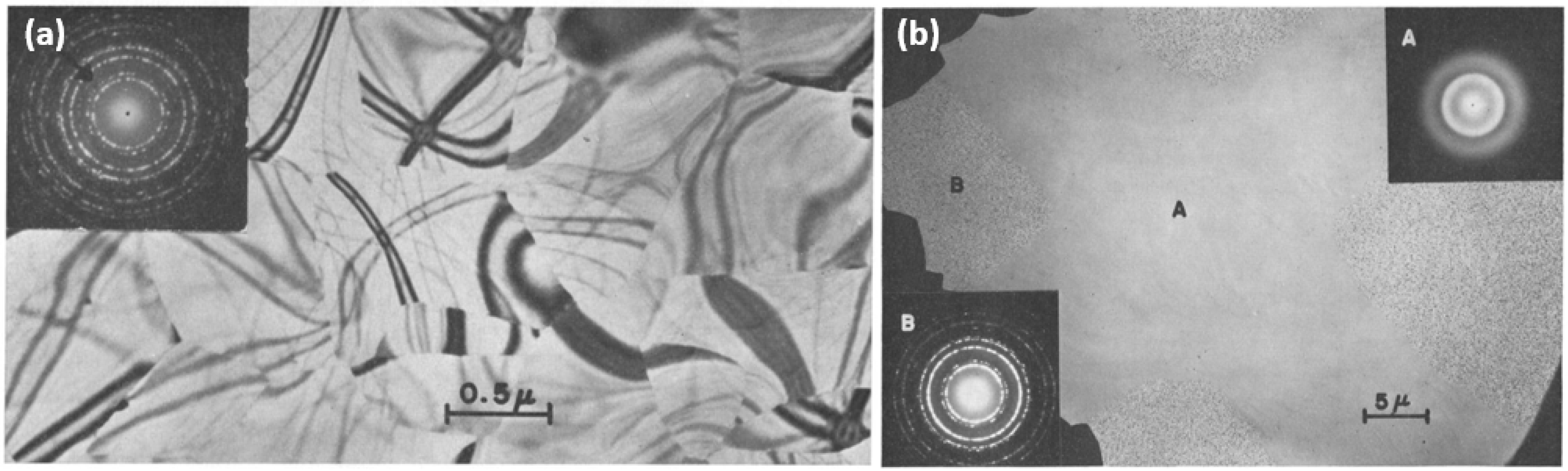
Subsequently, Naguib and Kelly observed athermal irradiation-induced a-to-c transformations as a result of Kr ion bombardment at fluxes ranging from 1–20 µA/cm
2. As the dose rate (i.e., flux) decreased, the corresponding threshold ion fluence required to induce an a-to-c transformation increased dramatically. The crystallites produced by ion bombardment were found to produce SAED patterns similar to those produced in crystalline films formed by heating alone and yielded crystallite sizes of 80–100 nm and ~700 nm, respectively (
Figure 2b).
To determine whether the observed crystallization was primarily influenced by local heating from the ion beam, a 400-mesh Cu grid was placed loosely on the surface of an amorphous sample prior to irradiation (
Figure 2b). At low flux (<7 µA/cm
2), TEM and SAED analysis showed in the top right inset of
Figure 2b that the regions shielded from ion bombardment (region ‘A’) remained amorphous while the unshielded regions underwent the expected a-to-c transformation. This is evidenced by the shielded region (
Figure 2b, region marked ‘A’) producing a SAED pattern with diffuse and indistinct rings, while the unshielded region (
Figure 2b, region marked ‘B’) exhibited a distinct diffraction pattern that could be attributed to cubic ZrO
2. It was also found that irradiation at higher fluxes (20 µA/cm
2) resulted in crystallization of both the shielded and unshielded regions. Crystallites in the unshielded region ‘B’ were larger than those in the shielded region and resembled the crystallites induced by pulse heating (e.g., those in
Figure 2a) [
48]. This implied that at low flux, the a-to-c transformation is primarily athermal and caused by ion bombardment alone, whereas, at higher flux, the influence of local heating may be more significant.
In a similar study, Leteurtre and Soullard created amorphous ZrO
2 films prepared by sputtering crystalline ZrO
2 with O
2 ions [
49]. The resulting amorphous films were then irradiated with 1 MeV Kr ions. While these irradiation conditions did not consistently result in a homogeneous a-to-c transformation, TEM analysis of the irradiated amorphous films exhibited distinct diffraction spot patterns in regions having higher electron transmission, indicating at least partial a-to-c transformation. The authors attributed this to the effects of collision cascades creating zones with differentiated order.
In a more recent study, Lian et al. reported ion irradiation-induced a-to-c transformation in thin bilayer films of nanocrystalline cubic and amorphous ZrO
2 layers [
41]. Room temperature TEM in situ ion irradiation was performed using 1 MeV Kr
+ ions at a flux of 6.25 × 10
10 ions/cm
2·s to various total fluence levels. TEM and SAED analysis of the bilayer provided evidence of an amorphous-to-tetragonal phase transformation in the original amorphous layer (
Figure 3). Specifically, evidence of recrystallization was observed from TEM analysis after irradiating the film to a fluence of 3.13 × 10
14 ions/cm
2 and a
d102 ring corresponding to tetragonal ZrO
2 was observed after irradiating to a fluence of 3.13 × 10
14 ions/cm
2, 1.88 × 10
15 ions/cm
2, and 3.13 × 10
15 ions/cm
2 [
41]. Because this a-to-c transformation occurred at room temperature and no significant ion beam heating of the sample occurred under the irradiation conditions used, the authors suggested that the a-to-c transformation might be more energetically favorable under non-equilibrium conditions than under thermal conditions alone [
41]. They also reported that irradiation-induced grain growth was more prominent for tetragonal crystallites that formed in the amorphous layer than for cubic grains in the nanocrystalline layer, but that the grain growth in both layers was strongly dependent on irradiation fluence [
41].
3.2. Titanium Dioxide
Kern et al. conducted experiments in which electrolytically deposited amorphous TiO
2 films on polished AISI 316 steel substrate were irradiated with a scanning electron microscope (SEM) electron beam at 20 kV under beam currents of 24 µA, 250 nA, 30 nA, and 10 nA [
42]. The samples were mounted to a heating stage that was either held at room temperature or 250 °C, and the irradiation time was varied between 1 and 600 s. Results showed evidence of a decrease in film thickness by ~20 nm when irradiated at 10 nA and by ~40 nm when irradiated at 30 nA or higher beam current. The change in film thickness was the result of electron-stimulated O desorption in the amorphous TiO
2 film and reduction of TiO
2 to TiO [
42]. Some of the irradiation conditions resulted in a phase transformation from an amorphous state to anatase TiO
2 as identified via Raman spectroscopy (
Figure 4). Specifically, the amorphous-to-anatase transformation occurred locally after irradiation with 24 µA for 600 s at room temperature. Increasing temperature accelerated the rate of transformation; when the sample stage was preheated to 250 °C, the transformation occurred after only 60 s at the same beam current. Both experimental temperatures were below the 440 °C thermally-induced crystallization temperature of electrolytically deposited amorphous TiO
2 films [
42,
62]. Local heating from the electron beam may have contributed to, but could not completely explain, the lower phase transformation temperatures under irradiation.
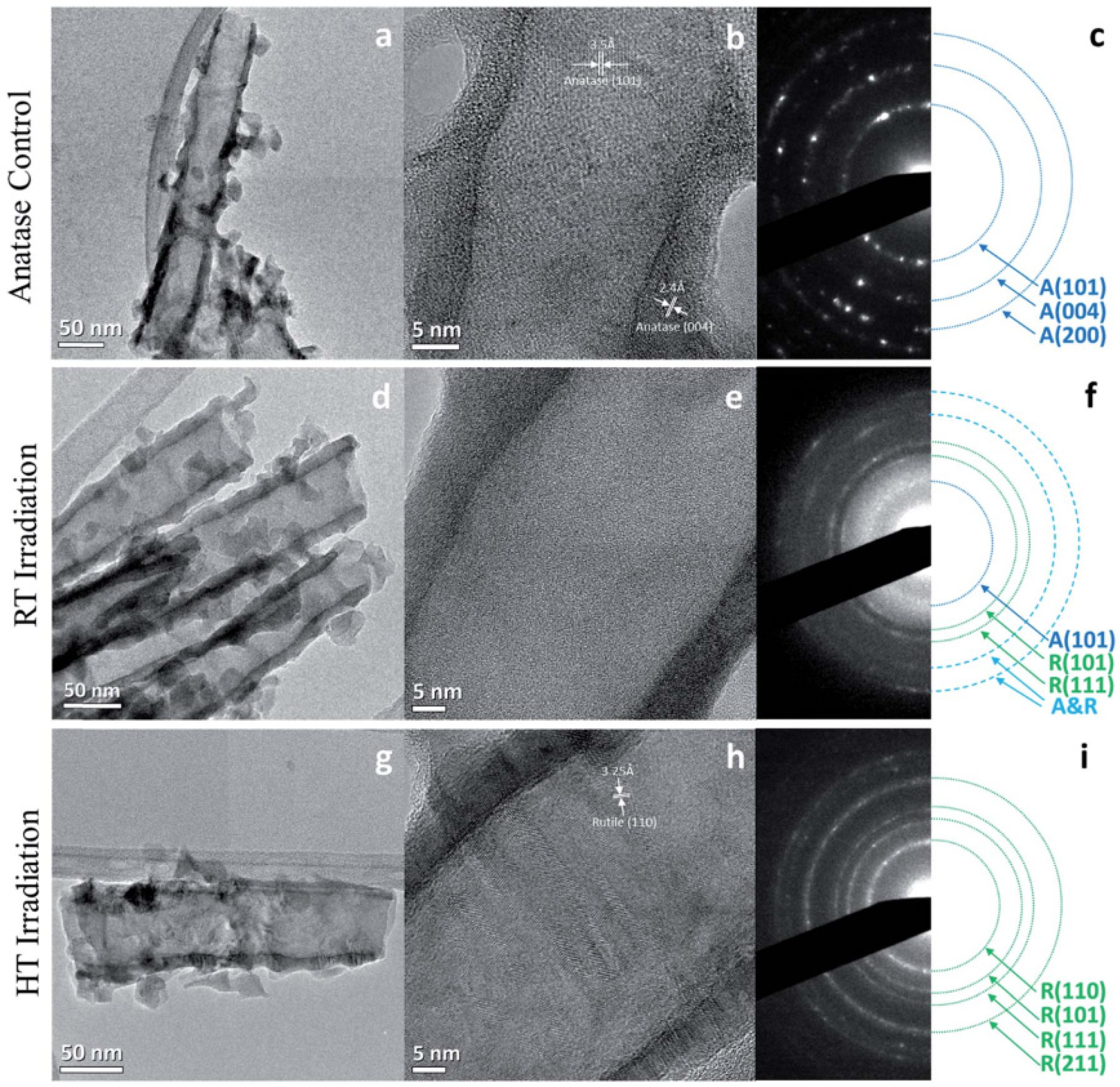
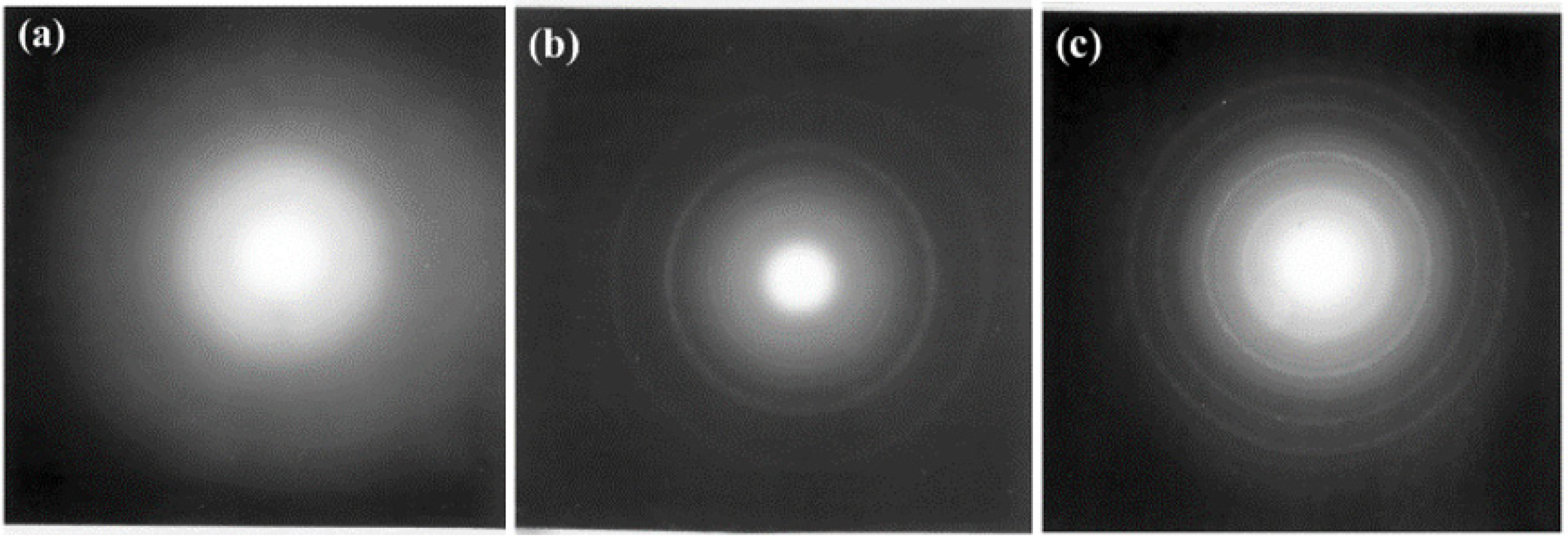
Smith and coworkers investigated the effects of proton irradiation on amorphous TiO
2 nanotubes and found that 200 keV proton irradiation at a fluence of 2.18 × 10
17 ions/cm
2 at both room temperature (RT) and a higher temperature (HT) of 250 °C resulted in varying degrees of a-to-c phase transformations [
43]. TEM and SAED analysis (
Figure 5) showed that irradiation at RT resulted in a partial phase transformation of the amorphous material to anatase and rutile TiO
2, while irradiation at 250 °C resulted in the formation of only the disordered rutile phase. Rings corresponding to anatase (101) and rutile (101) and (111) were indexed in the RT irradiated sample (
Figure 5f), while only rings corresponding to the rutile phase were visible in the SAED for the HT sample (
Figure 5i). The presence of a disordered rutile phase was also confirmed by Raman spectroscopy and EELS. Based on the phase diagram of TiO
2, the crystallization to the anatase phase generally requires thermal annealing temperatures > 400 °C, and the rutile phase requires temperatures > 600 °C [
43,
63]. In a sense, this corroborates Kern’s [
42] finding that increasing irradiation temperature accelerated the a-to-c transformation. Additionally, thermal annealing of TiO
2 nanotubes at >600 °C is generally not a viable method for synthesizing rutile TiO
2 nanotubes because it results in the collapse of the nanotube structure [
43]. However, SEM analysis showed that the proton irradiation did not significantly change the morphology of the nanotubes (
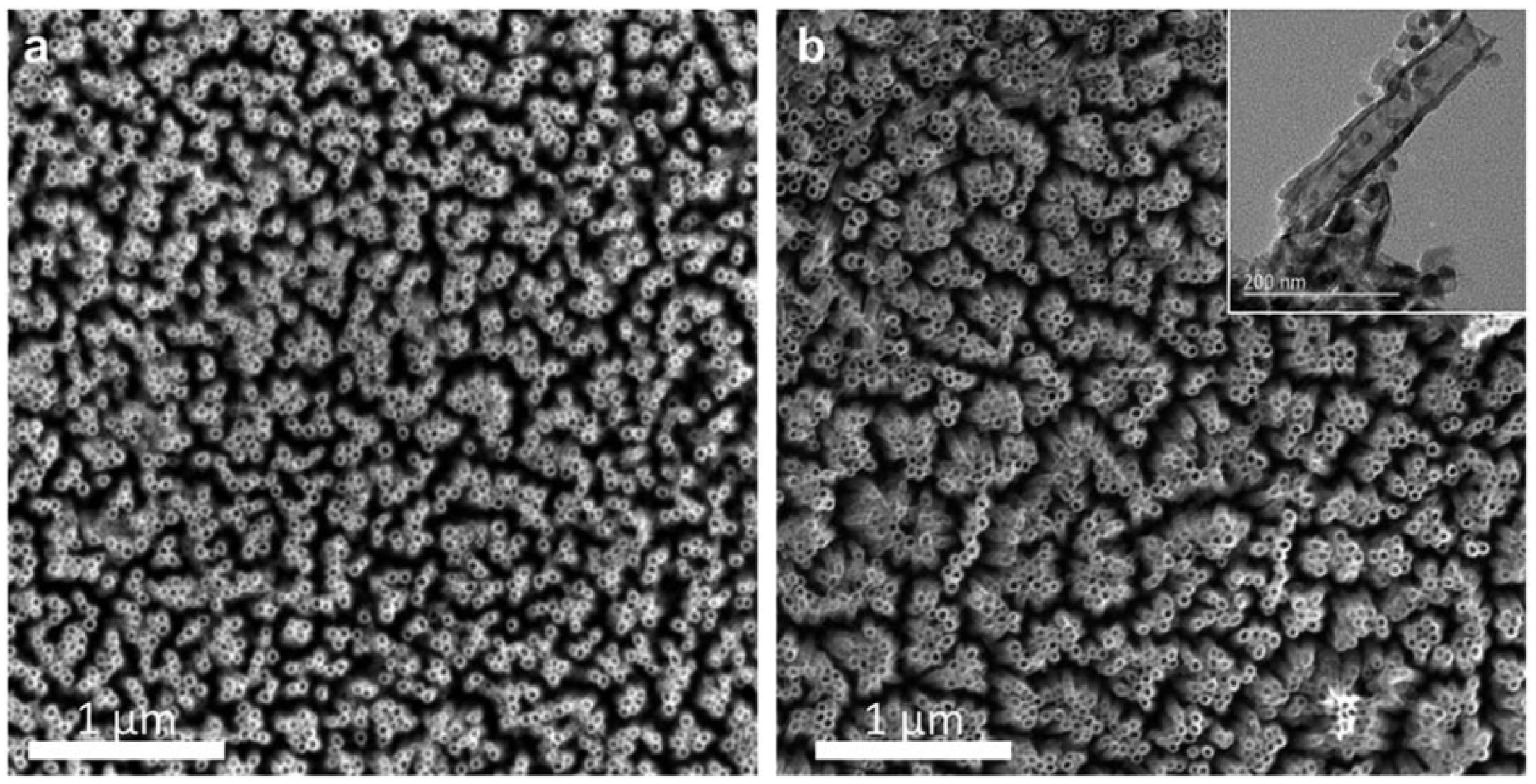
Figure 6). Therefore, this work highlights a new avenue to create crystalline nanostructures through ion irradiation on amorphous starting materials.
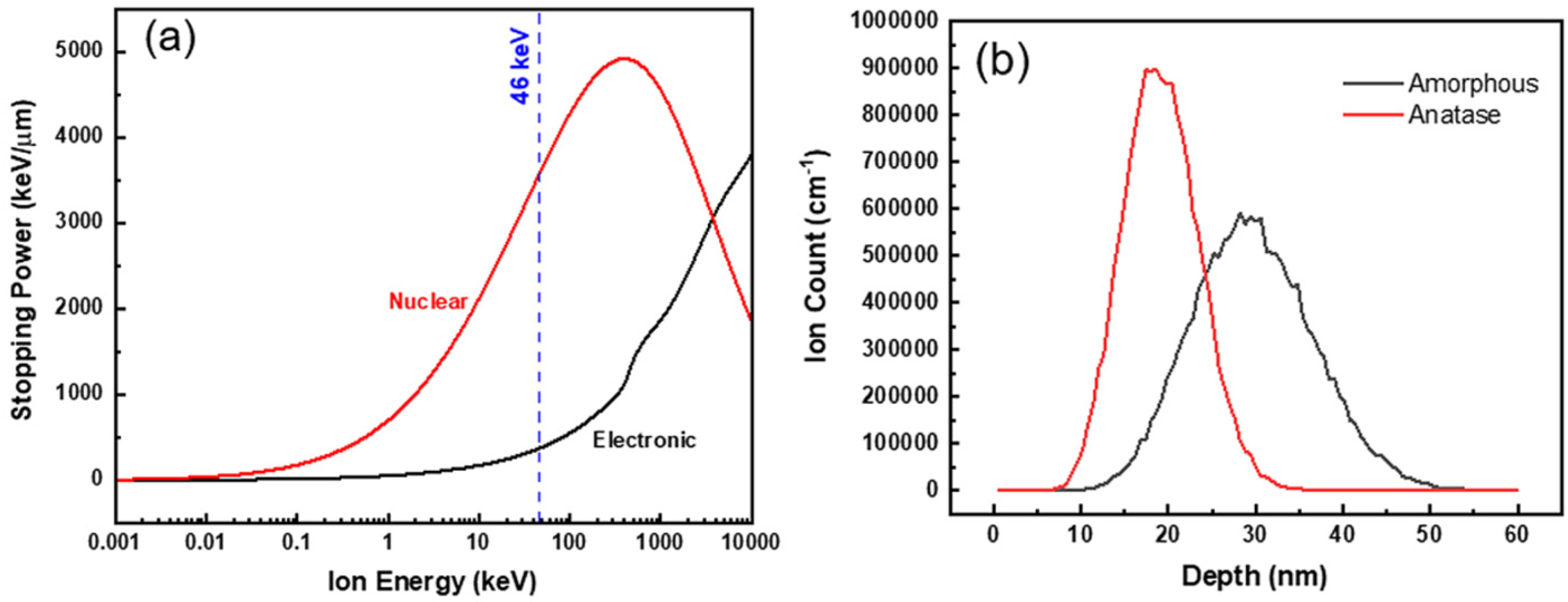
In another study, Yang and coworkers [
44] investigated the effects of TEM in situ 46 keV Au
– ion irradiation on amorphous and anatase TiO
2 nanotubes at ambient temperature to a fluence of 2.3 × 10
14 ions/cm
2. In some amorphous TiO
2 nanotubes, it was observed that bending of the nanotubes occurred as a result of irradiation of Au
– ions under these conditions, while anatase nanotubes exhibited no significant change in morphology (
Figure 7). While some changes in contrast were observed on the anatase nanotubes during irradiation (
Figure 7b, areas circled in red), the features were too small to identify as defects or phase transformations.
This morphological change of the initially amorphous nanotubes was attributed to partial irradiation-induced a-to-c transformation, as observed via HRTEM. Sites of partial a-to-c transformation in the amorphous TiO
2 nanotubes tended to be more prevalent near areas where the nanotubes were bent. The authors proposed that the heterogeneous formation of nanocrystal domains rather than complete crystallization could be best explained by inspection of energy loss mechanisms and ion range profiles for TiO
2 (
Figure 8).
The extent of irradiation-induced crystallization, and therefore the size of resulting crystallites, decreased with increasing electronic stopping power. Additionally, the magnitude of either nuclear or electronic stopping power varied depending on the orientation of nanotubes relative to the incident ion beam since the nanotubes were randomly distributed on the TEM grid (
Figure 8a). Furthermore, stopping and range of ions in matter (SRIM) simulations predicted that peak Au ion implantation occurred at a depth of about 30 nm, and since the thickness of the nanotube walls was about 10 nm, many incident ions would pass through the nanotubes if they were oriented normal to the beam direction (
Figure 8b) [
44].
3.3. Aluminum Oxide
Liu et al. investigated the effects of electron irradiation of amorphous Al
2O
3 that led to an a-to-c transformation [
45,
46]. The as-prepared samples were 300 nm thick layers of amorphous Al
2O
3 on dislocation-free
B-doped Si wafers. Electron irradiation was conducted using a TEM electron beam operating at 100 keV at a dose rate of 100 mA/cm
2. When the exposure time was varied over 1–16 min at a dose rate of 100 mA/cm
2, near-spherical trigonal Al
2O
3 crystallites formed in the bulk layer of Al
2O
3, and dendrites formed at the Al
2O
3/Si boundary. As irradiation time increased, the size and number of crystallites in the amorphous layer increased, while the dendrites remained unchanged. The size of crystallites began to approach a steady state after about 5 min, and the crystallite density plateaued after an irradiation time of 10 min. A critical electron beam dose rate was required for the a-to-c transformation to occur. Specifically, when the particles were irradiated at 10 mA/cm
2, no a-to-c transformation occurred [
45]. The authors initially thought that thermal activation from the electron beam due to local heating was the main cause of the a-to-c transformation [
45]. However, in a follow-up publication, the estimated temperature contribution was found to not be significant enough to be a factor in the mechanism for the observed a-to-c transformation [
46]. The authors subsequently proposed that electron irradiation-induced defects were a more likely cause for the a-to-c transformation [
46].
Zhou and Sood used two ion irradiation conditions to first form an amorphous layer in an α-axis oriented single crystal sapphire (α-Al
2O
3), then subsequently induce epitaxial crystallization in that amorphous layer [
50]. Amorphous layers on single crystal α-Al
2O
3 samples were generated by irradiating with 100 keV In ions to a fluence between 0.7 and 2.7 × 10
16 ions/cm
2. The amorphized layers were then irradiated with 1.5 MeV Si ions to a fluence of 3 × 10
16 ions/cm
2 at 400 °C. Rutherford backscattering and channeling (RBSC) measurements showed that a thin amorphous layer was successfully created on the surface of the α-Al
2O
3 after the initial In ion irradiation. RBSC spectra after the 400 °C Si irradiation, then showed signs of recrystallization to a γ-Al
2O
3 phase, consistent with that formed through 700 °C thermal annealing. These results demonstrated that epitaxial recrystallization of amorphous Al
2O
3 is possible at lower temperatures when used in conjunction with Si ion irradiation.
In a more recent study of amorphous films on single crystal (0001) α-Al
2O
3, Ning et al. found that epitaxial growth of γ-Al
2O
3 in the amorphous layer could be induced via irradiation with either 360 keV Ar
2+ ions or 180 keV O
+ ions [
47] at various temperatures. RBSC spectra of the amorphous films before and after irradiation indicated that epitaxial recrystallization of γ-Al
2O
3 had taken place, and this was confirmed by cross-sectional TEM and SAED. When comparing SAED patterns of the unirradiated and O ion irradiated films, a diffraction pattern appeared that was consistent with orientation-related epitaxial growth of γ-Al
2O
3 from the original single crystal layer [
47]. The ion beam-induced epitaxial growth between 400 and 600 °C produced films with higher epitaxial quality than those produced by annealing alone at 800 °C. This improvement in quality was attributed to the elimination of {111} twin boundaries in the γ-Al
2O
3 layer due to ion channeling effects during the irradiation.
Finally, Sina et al. showed that epitaxial crystallization of γ-Al
2O
3 on α-Al
2O
3 could be accomplished via two-step ion irradiation at room temperature [
51]. In their study, α-Al
2O
3 samples were irradiated with Zr
+ ions at 175 keV to fluences ranging from 7.5 × 10
15 to 1.5 × 10
16 ions/cm
2 and then subsequently irradiated with O
+ ions at 55 keV to fluences that corresponded with 25% of the maximum displacement damage dose produced by the initial Zr
+ ion irradiation. The lower Zr
+ ion fluence (7.5 × 10
15 ions/cm
2) specimens had buried polycrystalline (but not amorphous) regions at depths that corresponded to the maximum Zr
+ ion range calculated by SRIM; subsequent irradiation with O
+ ions changed little. Meanwhile, in samples that were irradiated to higher Zr
+ ion fluences of 1.5 × 10
16 ions/cm
2, a sub-surface amorphous layer appeared at depths of 20–65 nm, which corresponded to the peak damage calculated by SRIM simulations (
Figure 9) [
51].
Subsequent irradiation with O
+ ions showed evidence of epitaxial recrystallization at the interface between the buried Zr
+ irradiation-induced amorphous region and the crystalline surface region (
Figure 9). Analysis was conducted of the crystalline near-surface region, the buried amorphous region, and the bottom-most crystalline region using nano-beam electron diffraction (NBED). The NBED analysis found that the bottom-most region was consistent with the diffraction pattern for α-Al
2O
3. The buried amorphous region exhibited diffuse rings, consistent with an amorphous phase. However, the near-surface region contained diffraction patterns consistent with both α-Al
2O
3 and γ-Al
2O
3, suggesting that epitaxial γ-Al
2O
3 can form through two-step irradiation-induced amorphization and subsequent recrystallization.
3.4. Silicon Oxide
Upon ion irradiation, a-to-c transformations have been observed in the Si regions of SiO after SiO transformed into both Si and SiO
2 [
52,
53]. Rodichev et al. [
52] irradiated SiO at room temperature with 575 MeV Ni and 863 MeV Pb ions to fluences of 10
11 ions/cm
2 and 10
13 ions/cm
2, respectively. They observed the transition of SiO to Si and SiO
2 through infrared (IR) spectroscopy, in which the Si-O bond for SiO at 1000 cm
−1 shifted to 1035–1055 cm
−1, which was consistent with the Si-O bond for SiO
2. TEM images of the irradiated samples confirmed these results with two distinct regions observed: one with light-contrasting amorphous zones and the other being localized dark-contrasting zones attributed to high-Z number contrast. Si nanocrystals were believed to be present due to the lattice fringes in the dark-contrast regions within the HR-TEM images. These darker regions were located in a track within the material in a crystal-like arrangement. The number of dark regions increases with ion fluence. The authors concluded that two types of transformations occurred. The first involved short-range diffusion where SiO converted to Si and SiO
2, wherein the Si aggregates were small and mixed with SiO
2 and were thus unresolvable in TEM. The second type of transformation that occurred was diffusion over several atomic distances close to the ion track, which accounted for the oxygen concentration near the core of the ion track and rearrangement of Si atoms into nanocrystals.
When amorphous SiO was irradiated with 80 keV He ions at a fluence of 7 × 10
20 ions/cm
2, small circular precipitates were observed by Walters et al. [
53]. The precipitates exhibited an electron diffraction pattern matching closely with crystallized Si. During irradiation, the sample reached 850 °C. To ensure that the crystallization was an effect of irradiation and not thermal annealing, the researchers thermally annealed the samples at 900 °C. Following 60 min exposure to 900 °C, there were no changes, suggesting that the crystallites formed as a result of irradiation. Walters and coworkers also found that the crystallization as a function of dose was non-linear and instead followed an S-shaped curve described by the Avrami equation. Below a flux of 2 × 10
18 ions/m
2·s, the authors state that, similar to other non-metallic materials, amorphous SiO requires extended diffusion to form the observed precipitates.
SiO
2 has been investigated with neutral atom, ion, and electron irradiation [
12,
13], and a-to-c transformations were found with neutral atom and electron irradiation. When Mizutani et al. irradiated SiO
2 with 400 eV Ne neutral atoms or Ne ions to a fluence of 2 × 10
17 atoms/cm
2 or ions/cm
2, RBS showed that O was preferentially sputtered by the Ne ion, creating an O-deficient, Si-rich layer on the surface of the ion irradiated sample [
12]. By contrast, neutral Ne atom irradiation did not create a chemical deficiency or enrichment at the surface. Auger electron spectroscopy (AES) showed that the neutral Ne atom irradiated sample changed to a different phase, although its chemical composition was maintained, whereas the Ne ion irradiated sample showed AES peaks associated with Si and SiO
x, indicating chemical separation.
When neutral Ne atom irradiation was conducted at a lower energy of 350 eV and to a lower fluence of 1 × 1017 atoms/cm2, TEM analysis exhibited darker regions, suggesting crystalline phases were formed, though electron diffraction patterns could not be obtained due to the small size of the crystallites. Using Ne ions at the same energy of 350 eV to the same fluence of 1 × 1017 ions/cm2, however, no layers or regions having different contrast were found, even with the Si-rich surface layer. Furthermore, following neutral Ne and Kr atom irradiation (both at 350 eV, 1 × 1017 atoms/cm2), reflection high-energy electron diffraction (RHEED) analysis showed diffraction rings associated with α-cristobalite and α-quartz, respectively. These results suggested that both Ne and Kr neutral atom irradiations caused a-to-c transformations, whereas the ion irradiations did not. The authors suggested that a greater extent of crystal growth may have occurred due to successive neutral irradiations. They also noted that since the two crystalline phases (α-cristobalite and α-quartz) were not found in the same sample, but rather one phase induced by Kr and one induced by Ne, the neutral beam irradiation conditions determine the pressure and temperature processes governing which crystalline phase will form. They also found that the preferential sputtering of O with the ionic counterparts (e.g., Kr+ and Ne+) prevented crystallinity changes due to the higher density of Si in the samples. They suggested that with neutral beam irradiation, local hotspots were formed by the bombardment of neutral atoms on the surface atoms.
Other work by Du et al. using 200 kV electron irradiation of amorphous SiO
2 to a dose of 10
18 C/m
2 showed that rather than SiO
2 crystallizing, Si crystallized in two steps [
13]. Initially, in the amorphous SiO
2 sample, amorphous Si formed and then eventually crystallized with continued electron irradiation. The authors stated that the bonds were broken by creating an O vacancy from the knock-on displacement as a result of energy transferred from the electron irradiation. Because of the O vacancies, the Si atoms then aggregated towards one another. They then argued that the beam heating decreased the nucleation barrier, while knock-on displacement increased the potential energy above the ground state, thus leading to the crystallization of Si.
3.5. Silicon Carbide
Silicon carbide is another ceramic material that has also demonstrated a-to-c transformations upon irradiation with Xe and Si ions. Amorphous SiC samples were prepared by Zhang et al. and then irradiated with 5 MeV Xe to a fluence of 1.15 × 10
16 ions/cm
2 at 700 K [
54]. The amorphous film of SiC formed randomly oriented crystalline grains homogeneously distributed throughout the material, as shown in
Figure 10a. While amorphous regions remain, the crystalline grains are of the 3C-SiC polymorph with a cubic structure consistent with the known zinc-blende structure of the 3C-SiC polymorph [
64], as shown in
Figure 10b. SAED patterns demonstrating the cubic crystalline structure formed are shown as an inset in
Figure 10b. The crystalline grains contained stacking faults, identified by the shoulder peak at ~33.7° in the grazing incidence X-ray diffraction (GIXRD) spectrum and confirmed by TEM and Raman spectroscopy. The authors confirmed that the a-to-c transformation was irradiation-induced by conducting thermal annealing at 700 K and finding this was too low a thermal annealing temperature to induce crystalline grain nucleation and growth without irradiation.
The amorphous SiC to crystalline 3C-SiC transformation was also seen under 300 keV Si irradiation to a fluence of 1 × 10
17 ions/cm
−2 at 500 °C and 1050 °C by Heera et al. [
55]. In their study, a series of samples were investigated at 500 °C, where one was thermally annealed, and the other was Si ion irradiated. The thermally annealed sample showed no changes from its original amorphous structure, whereas the ion irradiated sample demonstrated both epitaxial and polycrystalline crystallization. Although the ion irradiation-induced crystallization was not homogenous, neither epitaxial nor polycrystalline crystallization could be achieved at 500 °C through thermal annealing alone. The authors suggested that the polycrystalline layer consisted of two main zones, one of 6H-SiC and the other of 3C-SiC, resulting from ion beam-induced random nucleation and subsequent ion beam-enhanced grain growth. A series of samples were also studied at 1050 °C, and it was shown that the sample became fully crystalline following thermal annealing alone. The crystalline thermally annealed sample consisted of columns of hexagonal SiC, aligning with the underlying 6H substrate, though slightly inclined with respect to the substrate. The crystallinity under ion irradiation at 1050 °C was substantially improved, showing almost perfect epitaxial 6H-SiC with a near-surface columnar structured layer and aligned 3C-SiC grains. The overall number of 3C-SiC grains in the ion irradiated sample was smaller than the thermally annealed sample at elevated temperatures.
The effects of 690 keV Xe ion irradiation at dose levels of 10
15, 5 × 10
15, and 10
16 ions/cm
2 on amorphous MoSi
2/SiC nanolayered composites were investigated by Lu et al. [
56]. Initial electron diffraction showed that the alternating layers of MoSi
2/SiC were amorphous. Following Xe
+ irradiation, the layers lost their structural integrity, MoSi
2 turned into a metastable hexagonal C40 phase, and the SiC began to spheroidize. This behavior occurred at all doses, although the changes were more pronounced with increasing dose. HRTEM showed that the C40 MoSi
2 grains were nanocrystalline, while the SiC spheroidization indicated microstructural coarsening. These results from the irradiated sample indicated that the energy of the Xe ions enhanced diffusion similar to thermal annealing at 500 °C, although the irradiating particle energy is not sufficient to fully crystallize the SiC layer.
3.6. Silicates
Silicates are classified as any derivative of A
xB
ySiO
z excluding SiO and SiO
2 and have exhibited a-to-c transformations under both electron and ion irradiation. Electron irradiation has been shown to induce compositional changes and crystallization in some silicates [
2,
57], while others exhibited epitaxial recrystallization [
58]. Meldrum et al. studied the recrystallization of zircon (ZrSiO
4) using two-stage irradiations [
3]. First, amorphous ZrSiO
4 was created by irradiating a natural crystalline zircon with 1.5 MeV Kr to a dose of 9 × 10
14 ions/cm
2 to ensure complete amorphization. Then, 200 keV electron irradiation at a beam current of 90 A/cm
2 induced crystallization zones among the amorphous regions. Upon recrystallization, the diffraction patterns showed that the crystallites adopted cubic or tetragonal ZrO
2 structures. When lowering the beam energy below 200 keV, zircon was unable to crystallize within a reasonable experimental time. The authors determined the maximum temperature increase due to beam heating to be only 19 °C, suggesting that thermal effects have little contribution to the observed crystallization [
65].
While Meldrum’s work did not observe a compositional change associated with the amorphization or subsequent a-to-c transformation, Carrez et al. saw a compositional change followed by crystallization during 300 keV electron irradiation of MgSiO
3 at room temperature [
57]. Initial SAED showed diffuse rings indicative of amorphous samples, but as the fluence increased above 5 × 10
16 electrons/cm
2, small structures began forming on the surface of the sample too small for SAED identification, as shown in
Figure 11. Above a fluence of 3 × 10
17 electrons/cm
2, the material crystallized to MgO as indicated by new diffraction rings (
Figure 11b). With increasing electron fluence, the rings became progressively sharper, indicating growth of the initial nuclei and newly formed crystallites. The newly formed structure was associated with periclase MgO, though as the fluence increased to 5 × 10
18 electrons/cm
2, the periclase rings disappeared, and new rings attributed to forsterite MgO appeared (
Figure 11c). As the crystallites formed, so did the number of MgO-rich and MgO-poor domains. The authors suggested that SiO
2 and MgO were not miscible under the experimental conditions, and the oxide separation is unsurprising as the phase diagram displayed a liquid immiscibility at high temperatures within the composition range of interest. They suggested that the sample went through two stages during irradiation. In the first stage, the sample was unstable and decomposed into MgO and SiO
2; in the second stage, nanosized MgO crystallites formed. The nucleation and growth processes were activated by radiation-enhanced diffusion as a result of ionization processes as well as a thermodynamic driving force for crystallization. The paper did not report on the phase evolution of the SiO
2 under irradiation.
Epitaxial recrystallization was observed by Bae et al. using two-step irradiation on an initially crystalline Sr
2Nd
8(SiO
4)
6O
2. The amorphous Sr
2Nd
8(SiO
4)
6O
2 was generated by irradiating the sample with 1.0 MeV Au to a fluence of 5 × 10
13 ions/cm
2 at 300 K, then subsequently irradiated with 200 keV electrons at a current of 0.29 A/cm
2 at room temperature [
58]. Following 840 s of exposure to an electron fluence of 1.53 × 10
21 electrons/cm
2, recrystallization was observed from both the amorphous/crystalline interface and surface, as shown in
Figure 12. The SAED pattern in
Figure 12a shows the initial amorphous diffraction pattern, and following electron irradiation, the SAED patterns in
Figure 12d show distinct rings that correspond with recrystallization. In
Figure 12d, the top right SAED pattern corresponds to the recrystallization occurring at the surface, and the bottom left SAED corresponds to the recrystallization at the interface. The recrystallization proceeded at the surface and the interface at roughly the same rates, with the recrystallized regions finally connecting in the center of the specimen after 3600 s of electron irradiation. This result demonstrated epitaxial growth from the electron beam irradiation due to the substrate acting as a template material on which recrystallization may occur. By utilizing the Bethe–Bloche equation, the authors calculated a maximum temperature increase of only 7 K from the electron beam. Further studies with thermal annealing were conducted, and the authors found no evidence of crystallization. Hence, they concluded that the recrystallization was an effect of electron irradiation alone. The authors stated that the ionization-induced processes caused by the electrons transferring their energy to the target atoms via inelastic interactions led to localized electronic excitations playing a role in the recrystallization. These excitations disturbed local atomic bonds and structures by lowering the energy barrier for defect recovery and recrystallization. Consequently, these electronic excitations lowered the energy barrier for defect recovery and recrystallization.
Other silicates which displayed crystallization as a result of Kr and Xe ion irradiation were ZrSiO
4, ThSiO
4, and HfSiO
4, as reported by Meldrum et al. [
2]. ThSiO
4 and HfSiO
4 were irradiated with 800 keV Kr ions, and ZrSiO
4 was irradiated with 800 keV Xe ions. When ZrSiO
4 was irradiated at temperatures above 900 K, the single crystal samples initially became amorphous and then, with increasing dose, decomposed into its component oxides: tetragonal ZrO
2 and amorphous SiO
2. This decomposition was similar to their previous results attained by electron irradiation [
3], possibly demonstrating the immiscible properties of ZrO
2 and SiO
2. HfSiO
4 similarly decomposed when heated to above 950 K into tetragonal HfO
2 and amorphous SiO
2. With ThSiO
4, a similar irradiation-induced decomposition occurred at 1000 K into monoclinic ThSiO
4 containing a network of distorted huttonite and randomly oriented ThO
2. However, this decomposition process in ThSiO
4 occurred with slower kinetics than the decompositions in ZrSiO
4 and HfSiO
4, and regions demonstrated more intense electron diffraction rings rather than rapid and complete crystallization. To determine whether the decomposition occurred due to thermal annealing alone, the ion beam amorphized samples were heated to 1100 K and held for 30 min without irradiation; neither crystallization nor decomposition occurred, so the authors concluded that the decomposition was associated with ion irradiation.
3.7. Other Ceramic Materials
Jiang et al. demonstrated that an a-to-c transformation could be induced in amorphous ZrC by irradiating with 30 keV He
+ ions at an ion flux of 1.79 × 10
13 ions/cm
2-s to various fluences at 593 K [
59]. The sample was prepared by depositing amorphous ZrC on a layer of crystalline ZrC using magnetron sputtering. At a fluence of 1.08 × 10
16 ions/cm
2, nanocrystals were visible in TEM cross-sections. At fluences ranging from 3.23 to 6.45 × 10
16 ions/cm
2, SAED analysis showed evidence of increased crystallization in the amorphous ZrC layer; i.e., diffuse rings corresponding to crystalline ZrC domains appeared at a fluence of 1.08 × 10
16 ions/cm
2 and the SAED patterns became more defined as the fluence increased. Additionally, irradiated samples were annealed at a temperature of 723 K to determine if heating at elevated temperatures would affect the morphology or crystallinity of irradiation-induced nanocrystals, but no significant changes were observed. The mechanism for the a-to-c transformation was primarily explained as a diffusion-assisted mechanism via the thermal spike model. Essentially, He
+ irradiation transfers much of its energy to electrons in the target material and causes a sharp increase in temperature along the ion path, causing adjacent atoms to diffuse more rapidly. A related correlation between atomic distributions of Zr and C and the irradiation-induced crystallization was observed using EDS analysis of the sample cross-sections. This analysis found that the observed a-to-c transformation tended to occur at depths where the relative concentration of C was lower, and this concentration gradient occurred near the interface between the amorphous and crystalline layers of ZrC. Therefore, He
+ irradiation seemed to preferentially form ZrC crystals near the amorphous-to-crystalline interphase where the C concentration was lower, and irradiation enhanced diffusion of Zr and C atoms would cause crystallites to gradually develop closer to the sample surface as the C to Zr ratio was decreased as a result of ion irradiation [
59].
Allen and coworkers studied the effects of Kr ion irradiation on the crystallization of CoSi
2 thin films [
60]. The samples were partially crystallized from TEM electron beam irradiation operated at 300 kV prior to ion irradiation. Further crystallization was observed after 1.5 MeV Kr
+ irradiation to a fluence of 8.5 × 10
14 ions/cm
2 at 300 K. The authors suggested that the prior electron irradiation led to a profuse number of crystallites with sizes that fell within the critical to supercritical size range that enabled the crystallites to continue to grow via subsequent ion irradiation-assisted recrystallization. The authors then irradiated another as-deposited film with 1.5 MeV Kr at a lower fluence of 3.4 × 10
14 ions/cm
2 at 300 K, then thermally crystallized the sample at 450 K. The irradiated portion of the sample showed higher crystallization rates, about double those of the non-irradiated portion of the sample. Additionally, when the ion dose was lower, specifically 3.4 × 10
13 ions/cm
2 at 300 K, the number density of crystallites was 30% lower, but the crystallite growth rates in the irradiated and non-irradiated portions of the sample were the same. This suggested that low-dose ion irradiation prior to thermal crystallization sufficiently induced critical or supercritical-sized crystallite nuclei that assisted in thermal crystallization.
Som et al. used two-step ion implantation and irradiation to induce recrystallization of an implanted Si
3N
4 layer in Si [
61]. Initial implantation of 100 keV N ions into a Si wafer at 300 °C to a fluence of 8 × 10
17 ions/cm
2 created an amorphous Si
3N
4 layer while the Si above and below this layer remained crystalline. Subsequent irradiation with 100 MeV Ag ions was conducted to a fluence of 10
14 ions/cm
2. The implanted layer remained amorphous when the Ag ion irradiation was carried out at 150 °C, but the implanted layer crystallized when the Ag ion irradiation was carried out at 200 °C or higher. Using HRTEM, the
d-spacing of the implanted then irradiation-crystallized layer matched with that of the hexagonal α-Si
2N
4 phase. This implanted layer
d-spacing did not match that of the crystalline Si layers above and below it, so the authors suggested that the Ag ion irradiation caused the crystallization. The crystallization was explained by irradiation defect creation in the Si substrate. That is, as the Ag ions penetrated through the multi-layered structure and deposited in the substrate, they created vacancies and interstitials in the crystalline Si. These point defects were available at the amorphous/crystalline (a/c) interfaces between the layers and migrated through thermal diffusion. The vacancies in the amorphous layer enabled thermal vibrations of Si and N atoms to occur more freely and unconstrained, thus inducing redistribution that resulted in the crystallization of one monolayer at the a/c interface. These monolayers then progressed inward from both a/c interfaces bounding the amorphous layer.
Finally, Meldrum et al. used two-step irradiation to induce recrystallization of phosphates, namely ScPO
4 and LaPO
4 specimens [
3]. The amorphous ScPO
4 and LaPO
4 were made by first irradiating single crystals with 1.5 MeV Kr to fluences of 4 × 10
14 ions/cm
2 and 2 × 10
15 ions/cm
2, respectively, to ensure complete amorphization. The amorphized specimens were subsequently irradiated with a 200 keV electron beam at a beam current of 1.45 A/cm
2 at ambient temperature. The LaPO
4 showed facile crystallization in mere seconds under the electron beam, whereas it took about 10 times as long for ScPO
4 to fully recrystallize under the same electron beam conditions. When the electron beam current was increased, the crystallite size decreased for both phosphates, though crystallites in ScPO
4 were consistently about 50% larger than those in LaPO
4. When both materials were subjected to thermal annealing over a temperature range of 200–300 °C, solid phase epitaxy was achieved over a period of 1–3 h. To investigate the temperature increase as a result of electron beam heating, the Fisher model was utilized and showed that the temperature increased within the range of 4–40°C [
65]. By contrast, temperatures in excess of 400 °C are needed to thermally anneal the amorphized samples for recrystallization.