Improving the DC Dielectric Properties of XLPE with Appropriate Content of Dicumyl Peroxide for HVDC Cables Insulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Structural Analysis
2.3. DC Performance Measurements
3. Results and Discussion
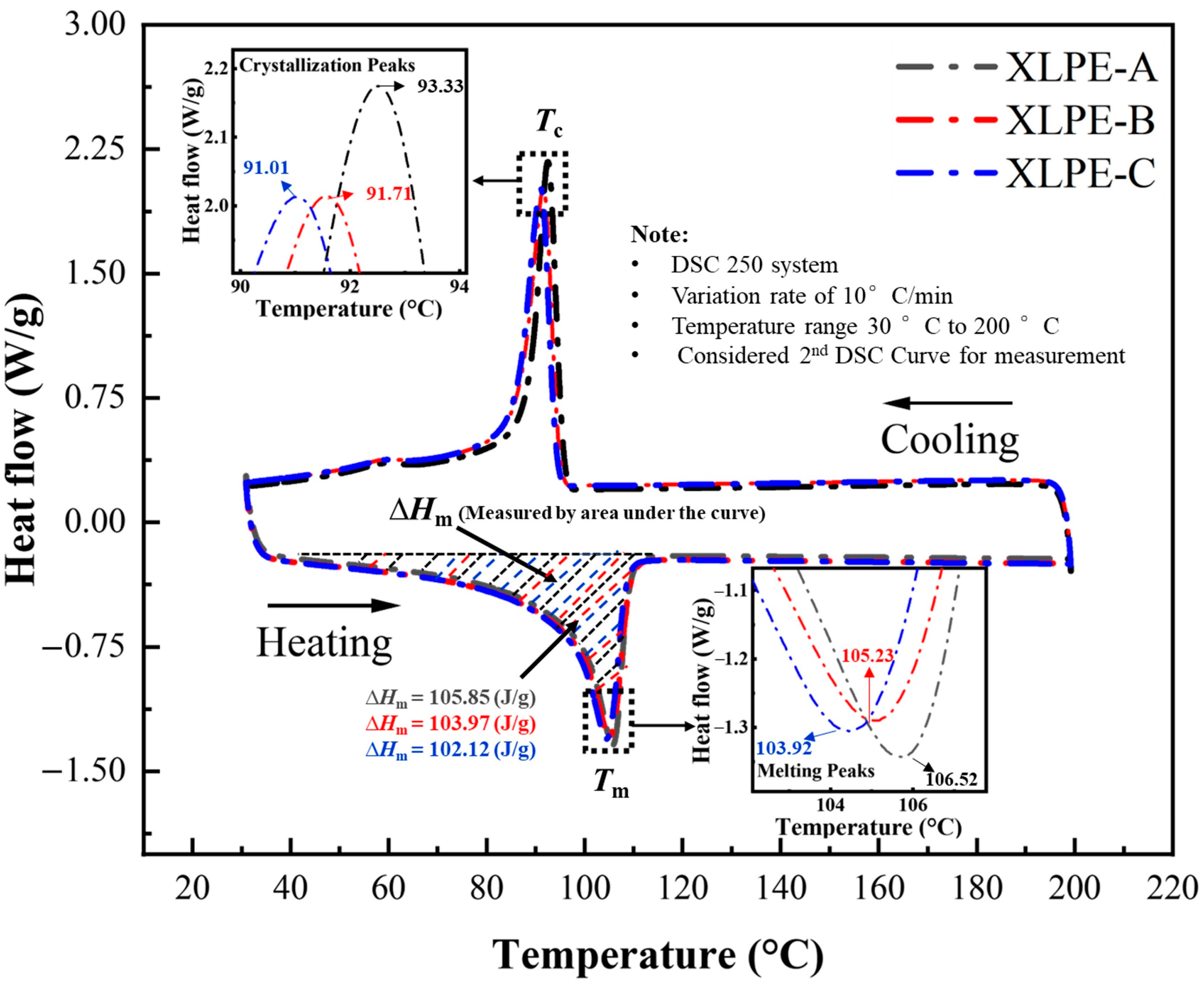
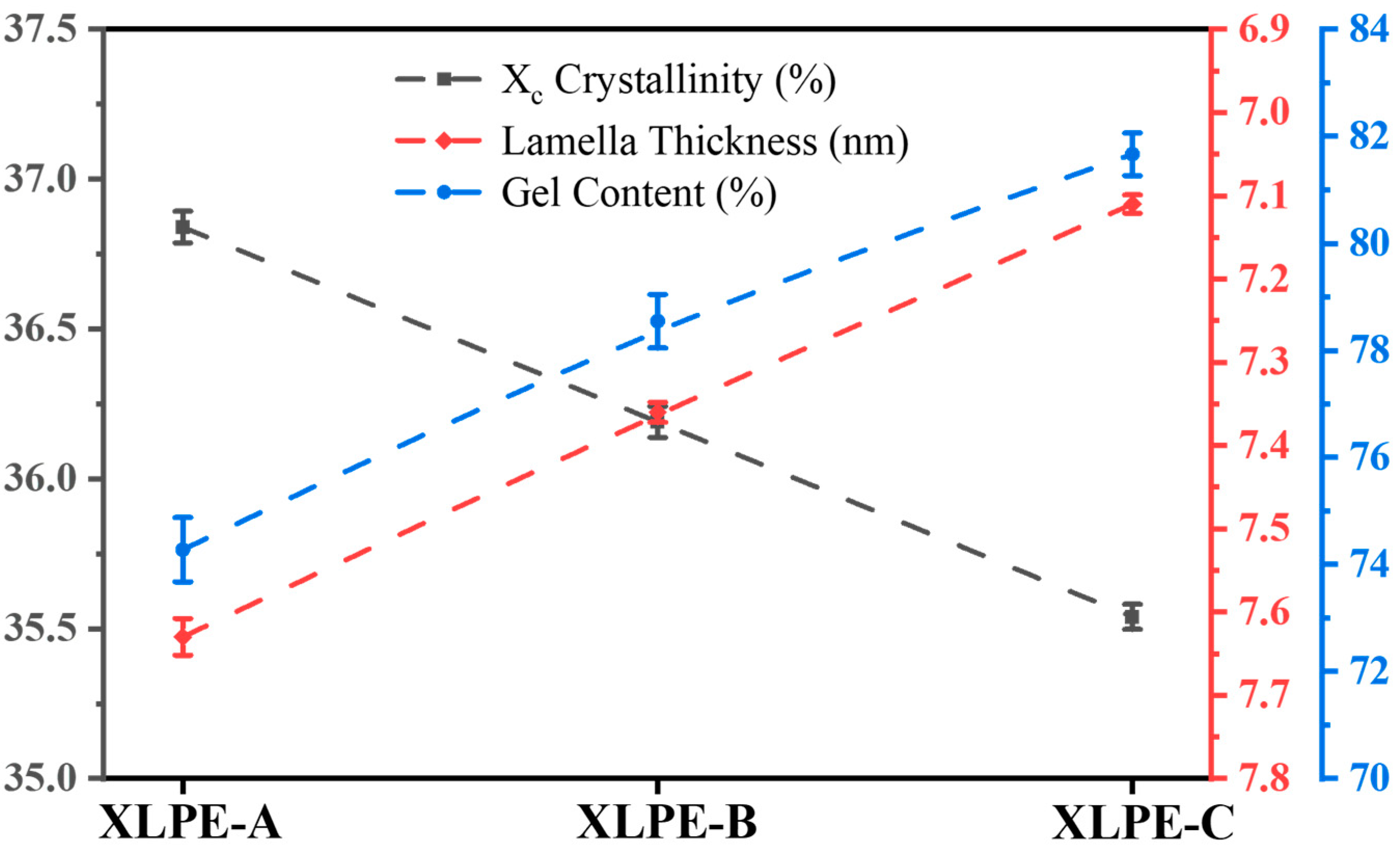
3.1. Microstructural Analysis
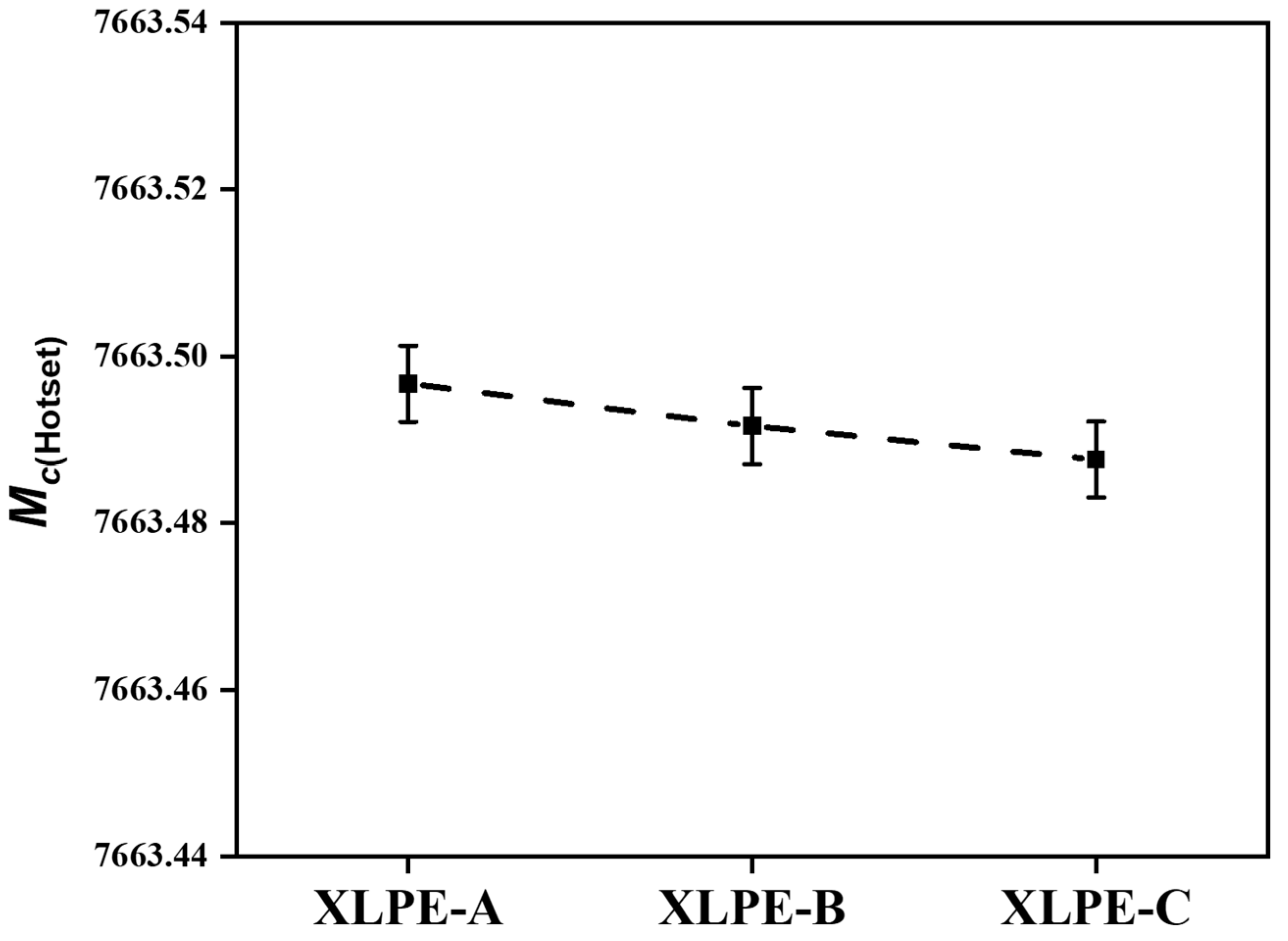
3.2. Molecular Network Density Analysis
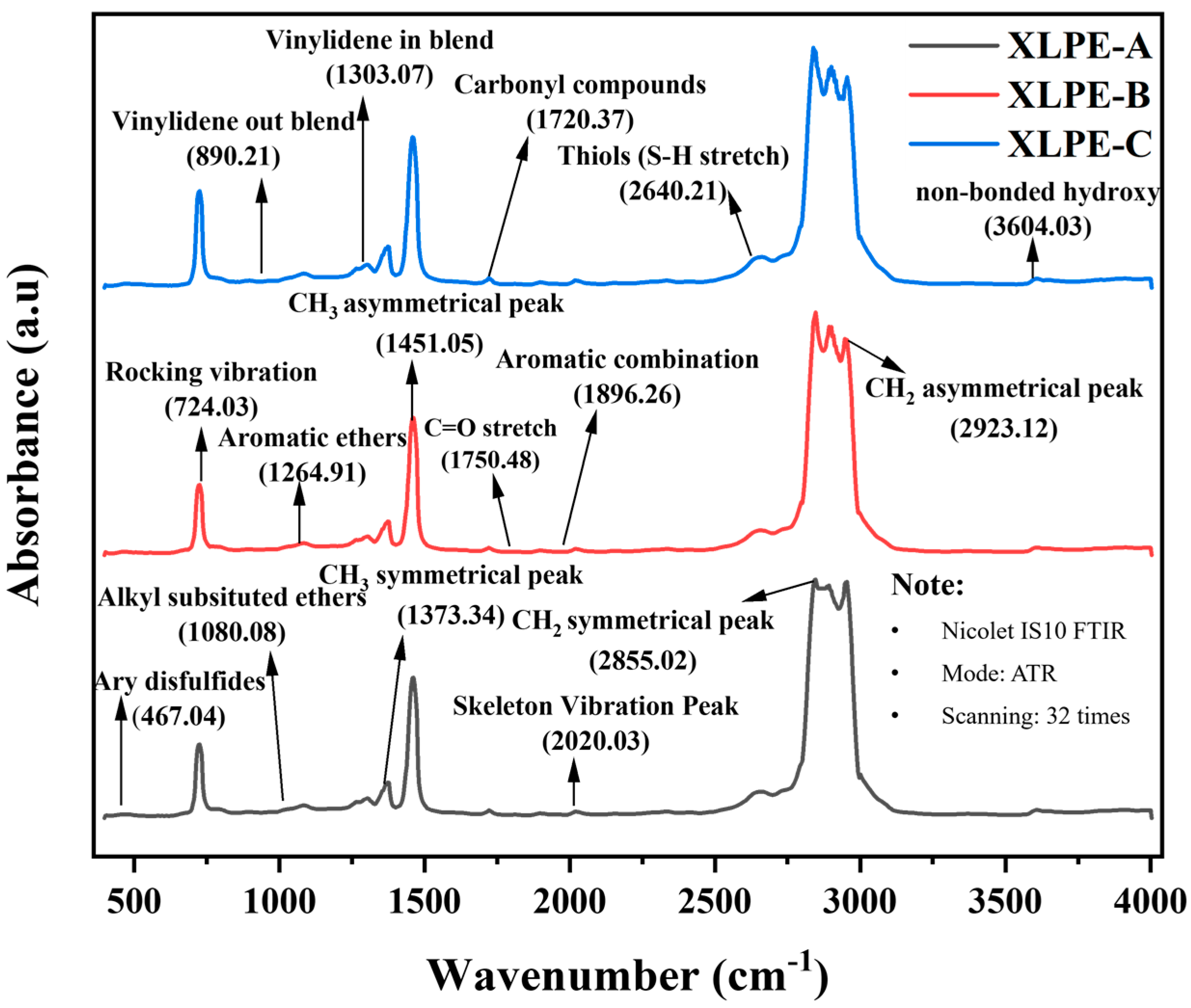
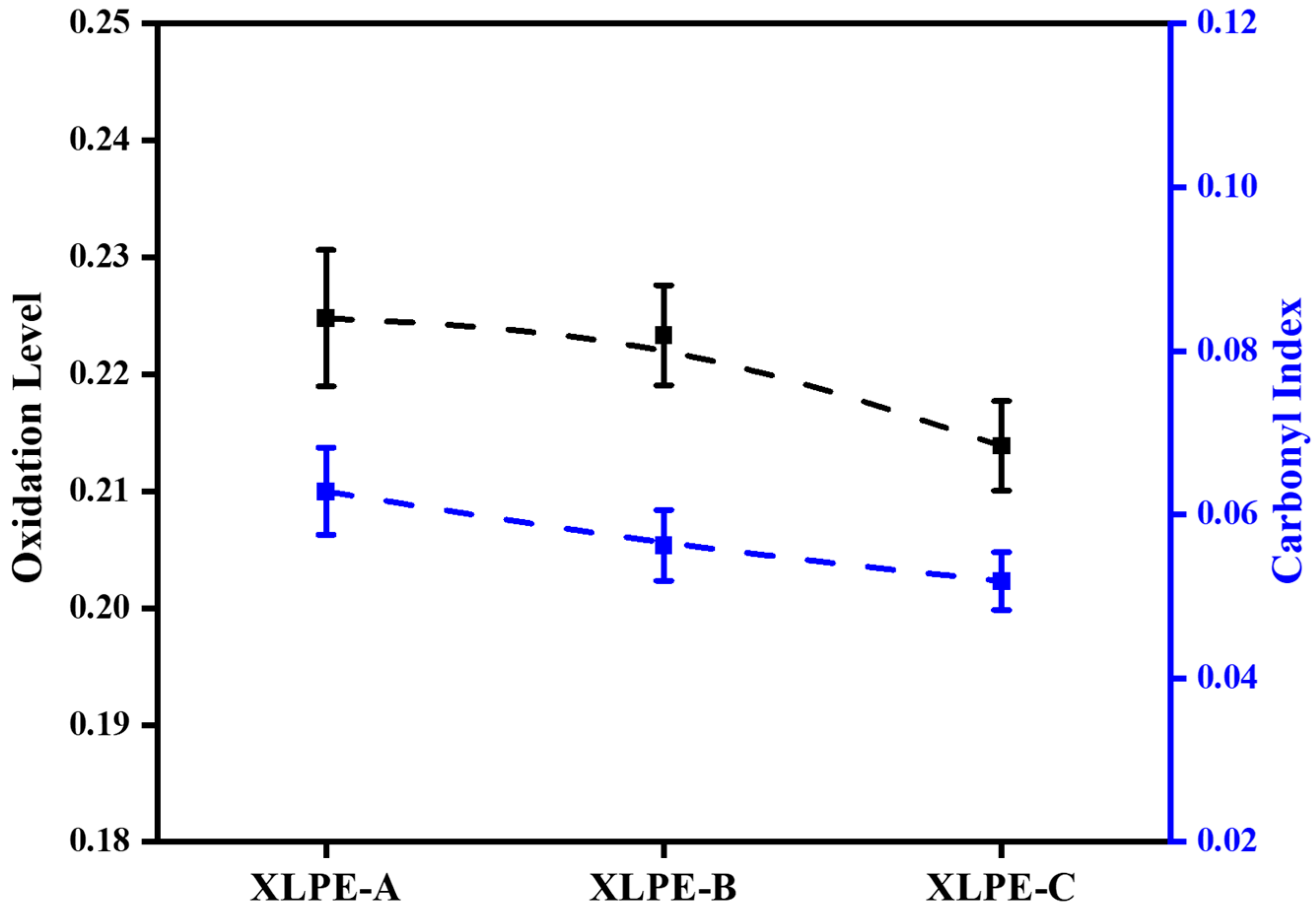
3.3. Chemical Composition Analysis
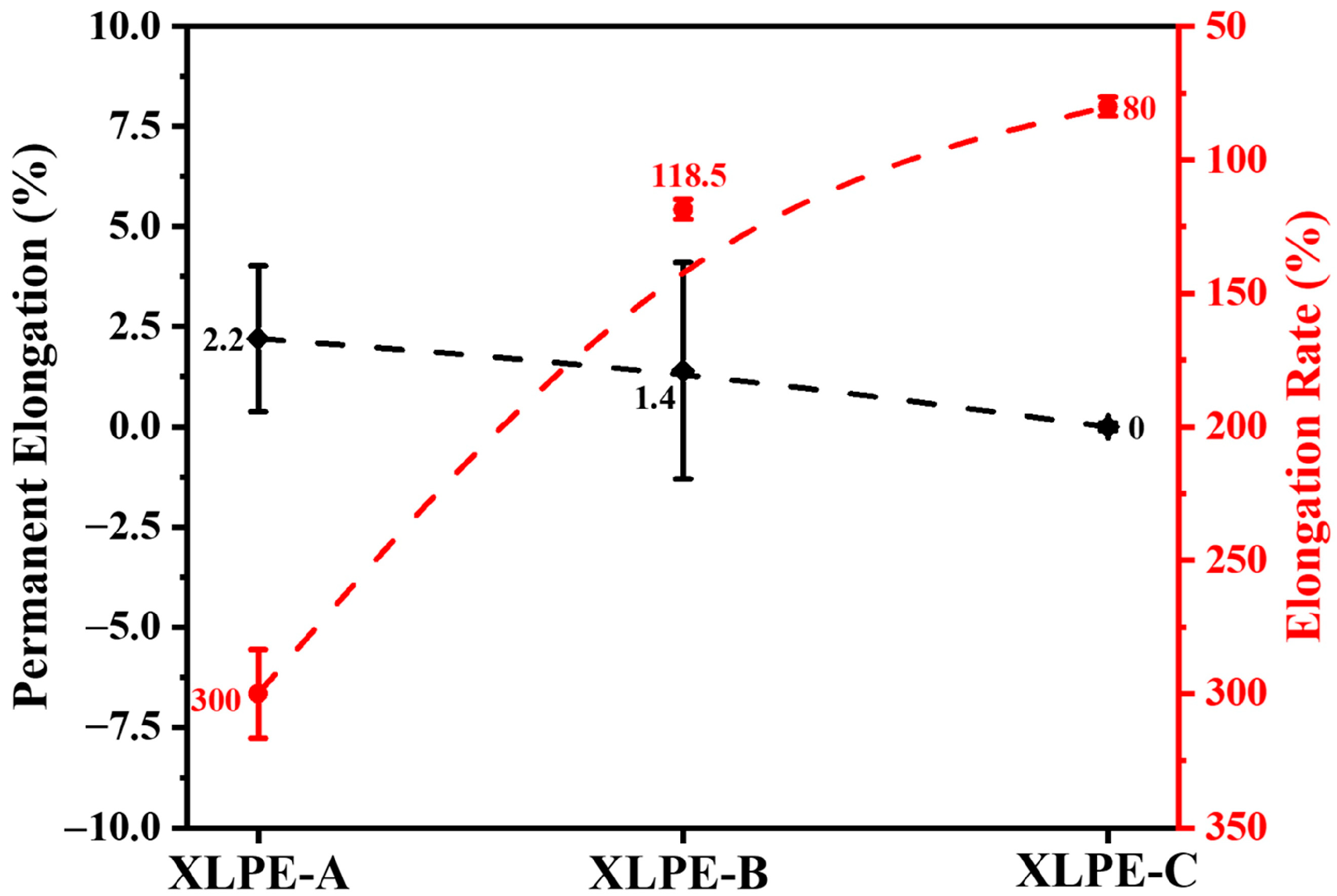
3.4. Mechanical Analysis
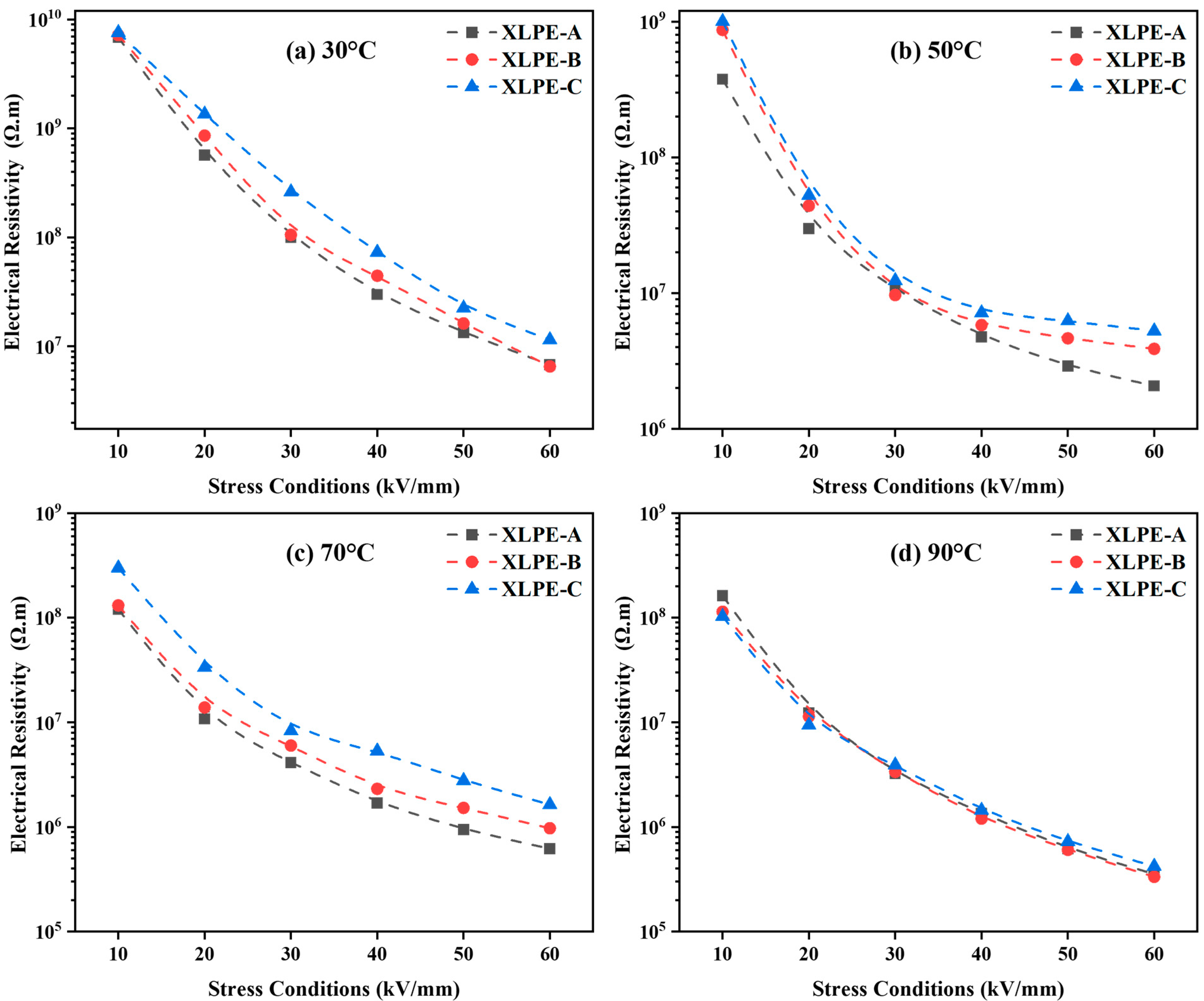
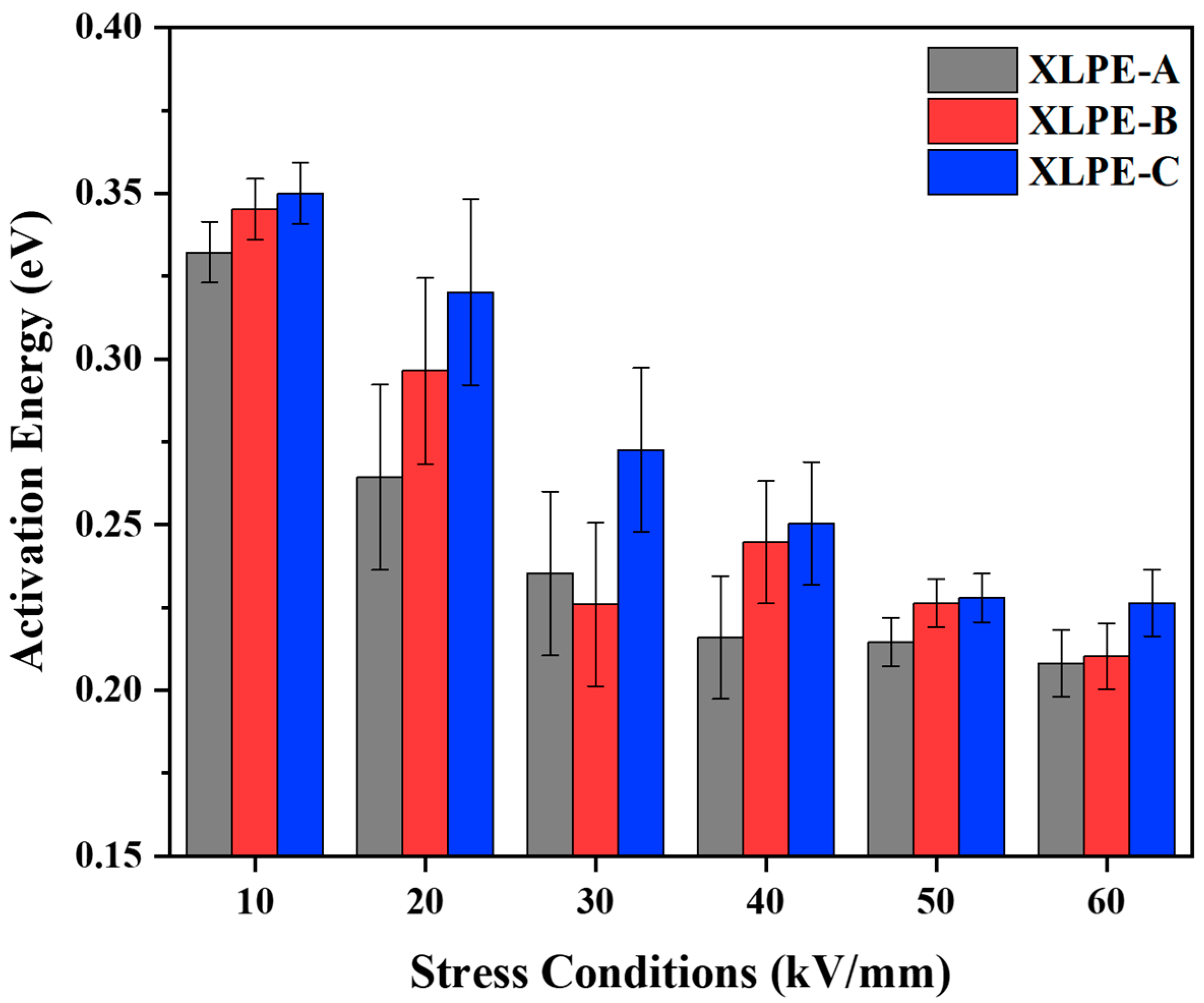
3.5. Electrical Resistivity and Activation Energy Analysis
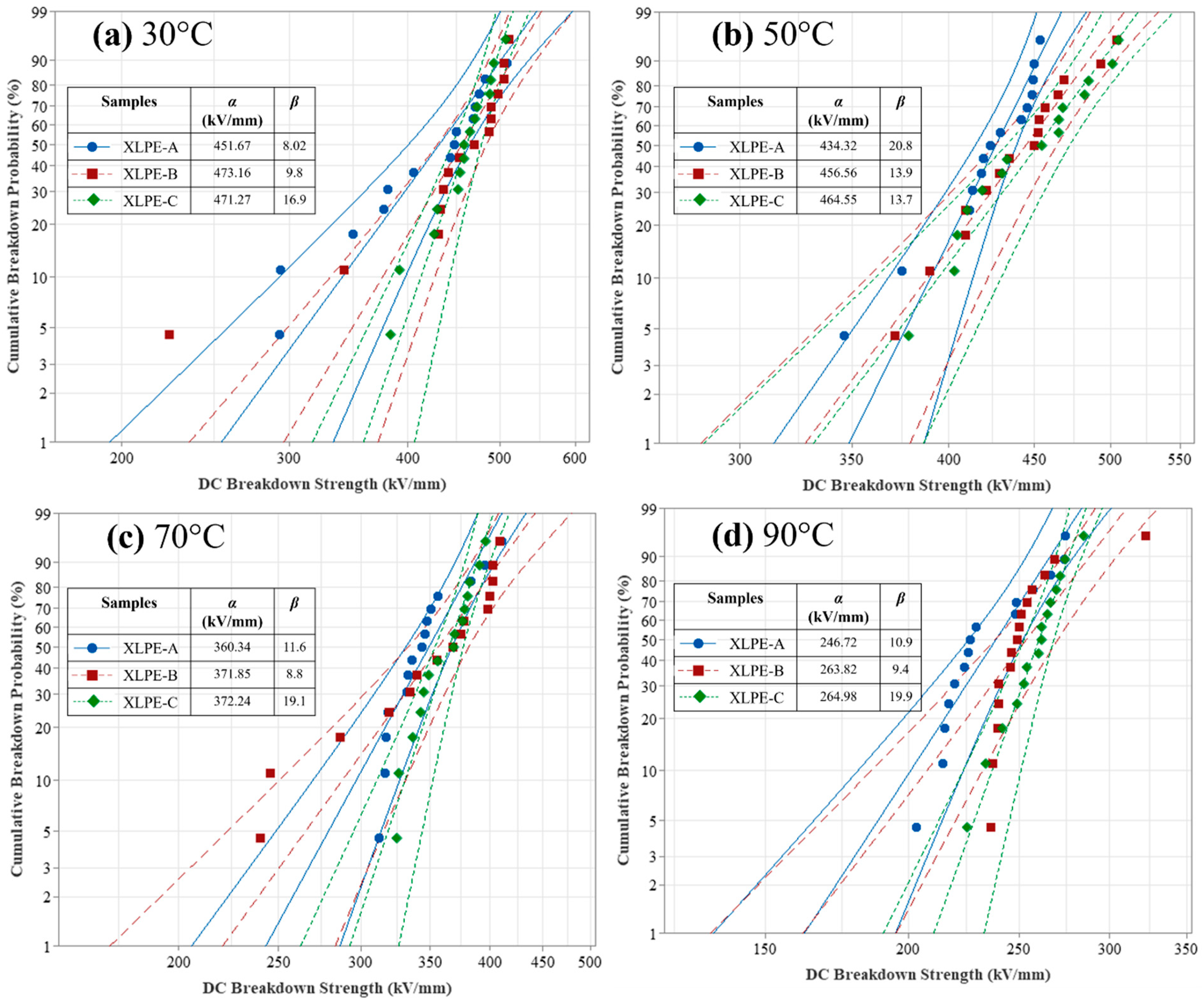
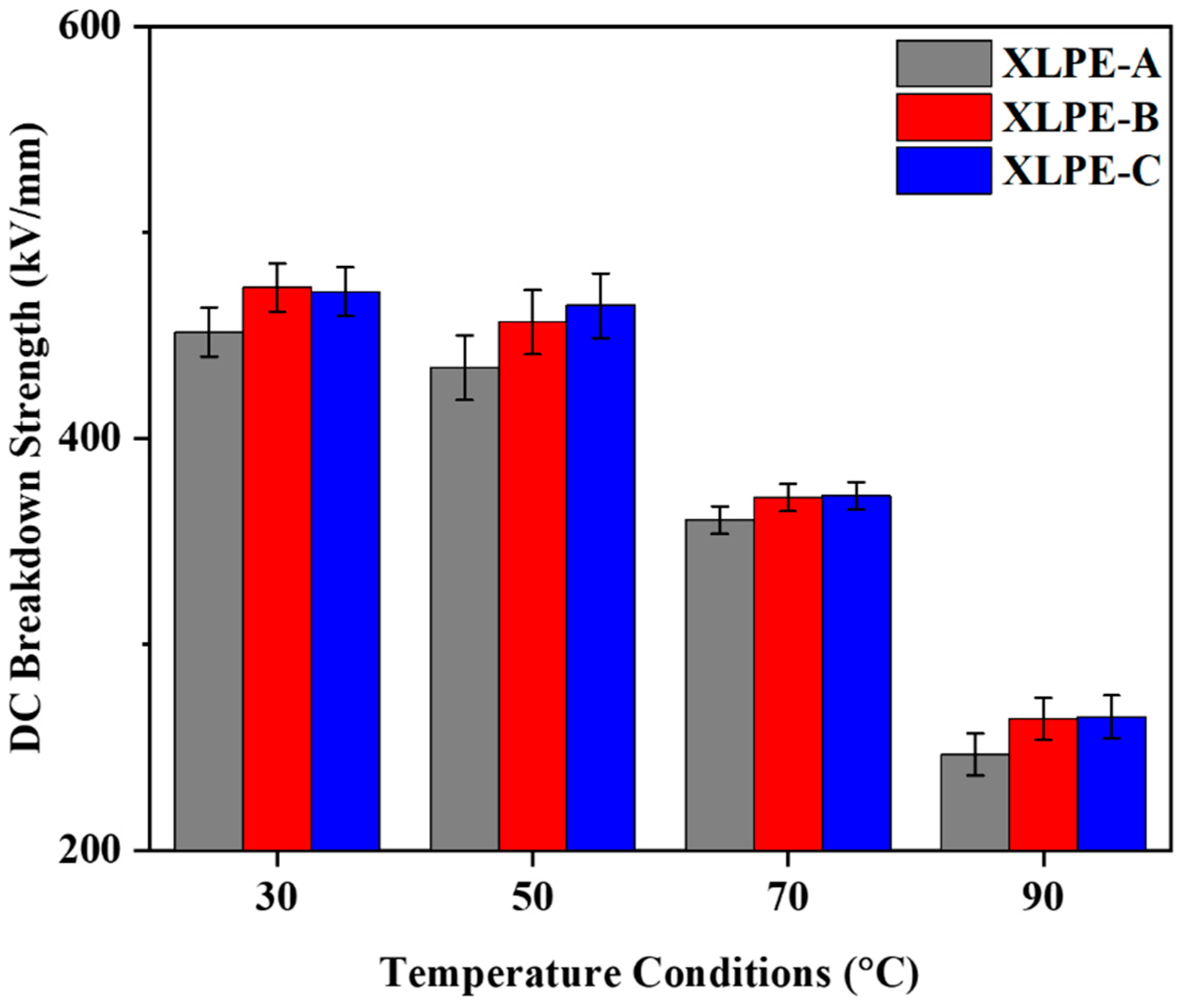
3.6. DC Breakdown Analysis
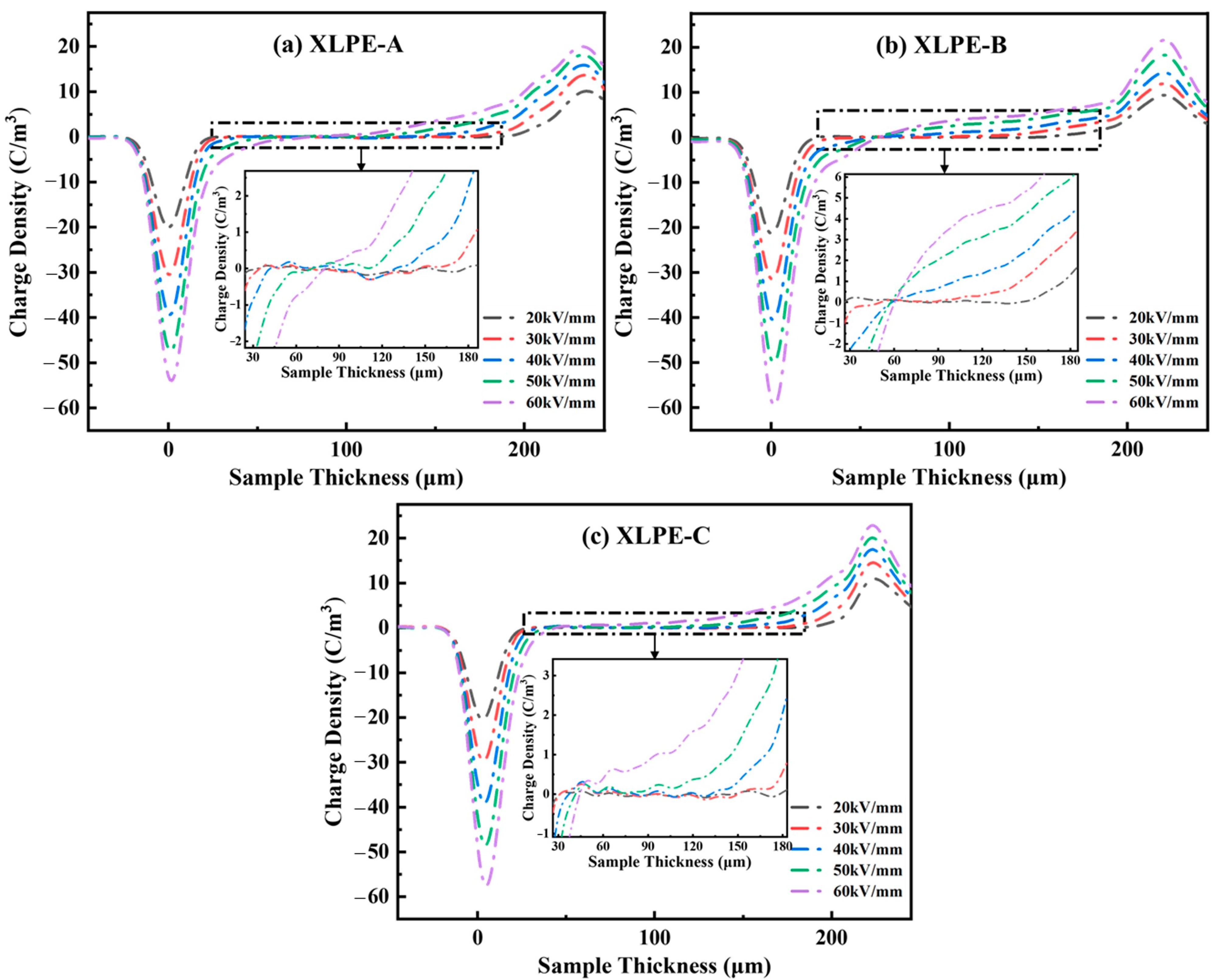
3.7. Space Charge Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, W.; Nilsson, S. HVDC Transmission: Yesterday and Today. IEEE Power Energy Mag. 2007, 5, 22–31. [Google Scholar] [CrossRef]
- Jeroense, M. HVDC, the next Generation of Transmission Highlights with Focus on Extruded Cable Systems. In Proceedings of the 2008 International Symposium on Electrical Insulating Materials (ISEIM 2008), Yokkaichi, Japan, 7–11 September 2008; pp. 10–15. [Google Scholar] [CrossRef]
- Chen, G.; Hao, M.; Xu, Z.; Vaughan, A.; Cao, J.; Wang, H. Review of High Voltage Direct Current Cables. CSEE J. Power Energy Syst. 2015, 1, 9–21. [Google Scholar] [CrossRef]
- Hanley, T.L.; Burford, R.P.; Fleming, R.J.; Barber, K.W. A General Review of Polymeric Insulation for Use in HVDC Cables. IEEE Electr. Insul. Mag. 2003, 19, 13–24. [Google Scholar] [CrossRef]
- Mostofi Sarkari, N.; Mohseni, M.; Ebrahimi, M. Examining Impact of Vapor-Induced Crosslinking Duration on Dynamic Mechanical and Static Mechanical Characteristics of Silane-Water Crosslinked Polyethylene Compound. Polym. Test. 2021, 93, 106933. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Song, Y.; Zheng, Q. Influence of Crosslinking on Physical Properties of Low Density Polyethylene. Chin. J. Polym. Sci. 2012, 30, 837–844. [Google Scholar] [CrossRef]
- Hampton, R.N. Feature Article—Some of the Considerations for Materials Operating under High-Voltage, Direct-Current Stresses. IEEE Electr. Insul. Mag. 2008, 24, 5–13. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Q.; Liao, R.; Xing, L.; Wu, N.; Jiang, Q. The Impact of Cross-Linking Effect on the Space Charge Characteristics of Cross-Linked Polyethylene with Different Degrees of Cross-Linking under Strong Direct Current Electric Field. Polymers 2019, 11, 1149. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yang, K.; Zhang, Y.; Wang, S.; Li, J. Crosslinking Dependence of Trap Distribution and Breakdown Performance of Crosslinked Polyethylene. J. Mater. Sci. Mater. Electron. 2019, 30, 20605–20613. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, L.; Cao, L.; Li, W.; Gao, J.; Chen, G. Temperature Stability of DC Conductivity and Breakdown of Crosslinked LLDPE. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 2064–2070. [Google Scholar] [CrossRef]
- Nilsson, S.; Hjertberg, T.; Smedberg, A. Structural Effects on Thermal Properties and Morphology in XLPE. Eur. Polym. J. 2010, 46, 1759–1769. [Google Scholar] [CrossRef]
- Meng, F.-B.; Chen, X.; Xu, X.; Dai, C.; Paramane, A.; Tanaka, Y. Effect of Degassing Treatment Durations on Physico-Chemical and Electrical Properties of 500 kV Extra HVDC XLPE Cable Insulation. Polym. Degrad. Stab. 2021, 188, 109566. [Google Scholar] [CrossRef]
- Lei, Z.; Fabiani, D.; Palmieri, F.; Li, C.; Han, T.; Selleri, G.; Grolli, F.; Speranza, M.; Suraci, S.V. Space Charge Characteristics of XLPE and Semiconductive Layer Coated with Graphene. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 128–131. [Google Scholar] [CrossRef]
- Ouyang, B.; Li, H.; Zhang, X.; Wang, S.; Li, J. The Role of Micro-Structure Changes on Space Charge Distribution of XLPE during Thermo-Oxidative Ageing. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3849–3859. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, X.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Effect of Temperature, Crystallinity and Molecular Chain Orientation on the Thermal Conductivity of Polymers: A Case Study of PLLA. J. Mater. Sci. 2018, 53, 10543–10553. [Google Scholar] [CrossRef]
- Cao, L.; Zhong, L.; Li, Y.; Gao, J.; Chen, G. Crosslinking Dependence of Direct Current Breakdown Performance for XLPE-PS Composites at Different Temperatures. Polymers 2021, 13, 219. [Google Scholar] [CrossRef]
- Akbarian, D.; Hamedi, H.; Damirchi, B.; Yilmaz, D.E.; Penrod, K.; Woodward, W.H.H.; Moore, J.; Lanagan, M.T.; van Duin, A.C.T. Atomistic-Scale Insights into the Crosslinking of Polyethylene Induced by Peroxides. Polymer 2019, 183, 121901. [Google Scholar] [CrossRef]
- Sahyoun, J.; Crepet, A.; Gouanve, F.; Keromnes, L.; Espuche, E. Diffusion Mechanism of Byproducts Resulting from the Peroxide Crosslinking of Polyethylene. J. Appl. Polym. Sci. 2017, 134, 44525. [Google Scholar] [CrossRef]
- Andersson, L.H.U.; Hjertberg, T. The Effect of Different Structure Parameters on the Crosslinking Behaviour and Network Performance of LDPE. Polymer 2006, 47, 200–210. [Google Scholar] [CrossRef]
- Goshowaki, M.; Endoh, I.; Noguchi, K.; Kawabe, U.; Sekii, Y. Influence of Antioxidants on Electrical Conduction in LDPE and XLPE. J. Electrost. 2007, 65, 551–554. [Google Scholar] [CrossRef]
- Habibi, H.; Alam, A.S.; Prasetyawan, O.; Supriyadi, G. FTIR and SEM Analysis of Breakdown XLPE Cable Insulation. In Proceedings of the 2021 3rd International Conference on High Voltage Engineering and Power Systems (ICHVEPS), Bandung, Indonesia, 5–6 October 2021; pp. 314–317. [Google Scholar] [CrossRef]
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the Carbonyl Index of Polyethylene and Polypropylene Using Specified Area under Band Methodology with ATR-FTIR Spectroscopy. e-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Ren, H.; Zhong, L.; Zhao, W.; Liy, M.; Yang, X.; Li, Y.; Yu, Q.; Cao, L.; Zhou, Z. Influence of Crosslinking Byproducts on DC Conductivity of HVDC XLPE Cable Insulation. In Proceedings of the 2018 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Cancun, Mexico, 21–24 October 2018; pp. 90–93. [Google Scholar] [CrossRef]
- Ahmed, M.; Lisheng, Z.; Li, F.; Xu, N.; Ren, H. DC Conductivity Fluctuation Due to Temperature Dependence and Cross-Linking Byproducts of XLPE Insulation Material in HVDC Cables. In Proceedings of the 2021 IEEE Electrical Insulation Conference (EIC), Denver, CO, USA, 7–28 June 2021; pp. 173–176. [Google Scholar] [CrossRef]
- Kanai, T.; Fujitomi, T.; Miyake, H.; Tanaka, Y. Reduction Effect of Cross-Linking by-Products on Dielectric Strength in Polyethylene under DC Stress. In Proceedings of the 2017 International Symposium on Electrical Insulating Materials (ISEIM), Toyohashi, Japan, 11–15 September 2017; Volume 2, pp. 489–492. [Google Scholar] [CrossRef]
- Kumara, S.; Xu, X.; Hammarström, T.; Ouyang, Y.; Pourrahimi, A.M.; Müller, C.; Serdyuk, Y.V. Electrical Characterization of a New Crosslinked Copolymer Blend for DC Cable Insulation. Energies 2020, 13, 1434. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Q.; Liu, M.; Li, Y.; Zhong, L.; Fu, M.; Hou, S. DC Electrical Breakdown Dependence on the Radial Position of Specimens within HVDc XLPE Cable Insulation. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1476–1484. [Google Scholar] [CrossRef]
- Kim, C.; Jiang, P.; Liu, F.; Hyon, S.; Ri, M.; Yu, Y.; Ho, M. Investigation on Dielectric Breakdown Behavior of Thermally Aged Cross-Linked Polyethylene Cable Insulation. Polym. Test. 2019, 80, 106045. [Google Scholar] [CrossRef]

| Designation | LDPE Content/phr | DCP Content/phr | Antioxidant 300/phr |
|---|---|---|---|
| XLPE-A | 100 | 1.4 | 0.3 |
| XLPE-B | 100 | 1.7 | 0.3 |
| XLPE-C | 100 | 2.0 | 0.3 |
| Samples | ΔHm (J/g) | L (nm) | |||
|---|---|---|---|---|---|
| XLPE-A | 105.85 | 106.5 | 93.3 | 7.63 | 36.84 |
| XLPE-B | 103.97 | 105.2 | 91.7 | 7.36 | 36.19 |
| XLPE-C | 102.12 | 103.9 | 91.0 | 7.11 | 35.54 |
| Peaks Correspond to the Wavenumber (cm−1) | XLPE-A | XLPE-B | XLPE-C |
|---|---|---|---|
| 467.04 (Aryl disulfides) | 0.111 | 0.101 | 0.115 |
| 724.03 (Rocking vibration peak) | 3.690 | 3.911 | 4.510 |
| 890.21 (Vinylidene out blend) | 0.189 | 0.173 | 0.203 |
| 1080.08 (Alkyl substituted ether) | 0.359 | 0.322 | 0.340 |
| 1264.91 (Aromatic ethers) | 0.483 | 0.439 | 0.527 |
| 1303.07 (Vinylidene in blend) | 0.579 | 0.554 | 0.586 |
| 1373.34 (CH3 symmetrical peak) | 1.032 | 0.985 | 1.094 |
| 1451.05 (CH3 asymmetrical peak) | 2.826 | 2.764 | 3.190 |
| 1720.37 (Carbonyl compounds) 1750.48 (C=O stretch) | 0.232 0.112 | 0.220 0.110 | 0.234 0.124 |
| 1896.26 (Aromatic combination) 2020.03 (Skeleton vibration peak) 2640.21 (Thiols S-H stretch) | 0.145 0.193 0.744 | 0.138 0.185 0.706 | 0.146 0.194 0.740 |
| 2855.02 (CH2 symmetrical peak) | 6.064 | 6.120 | 6.184 |
| 2923.12 (CH2 asymmetrical peak) | 5.526 | 5.635 | 6.000 |
| 3604.03 (Non-bonded hydroxy) | 0.264 | 0.249 | 0.261 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Zhong, L.; Li, F.; Xu, N.; Gao, J. Improving the DC Dielectric Properties of XLPE with Appropriate Content of Dicumyl Peroxide for HVDC Cables Insulation. Materials 2022, 15, 5857. https://doi.org/10.3390/ma15175857
Ahmed M, Zhong L, Li F, Xu N, Gao J. Improving the DC Dielectric Properties of XLPE with Appropriate Content of Dicumyl Peroxide for HVDC Cables Insulation. Materials. 2022; 15(17):5857. https://doi.org/10.3390/ma15175857
Chicago/Turabian StyleAhmed, Muneeb, Lisheng Zhong, Fei Li, Nuo Xu, and Jinghui Gao. 2022. "Improving the DC Dielectric Properties of XLPE with Appropriate Content of Dicumyl Peroxide for HVDC Cables Insulation" Materials 15, no. 17: 5857. https://doi.org/10.3390/ma15175857
APA StyleAhmed, M., Zhong, L., Li, F., Xu, N., & Gao, J. (2022). Improving the DC Dielectric Properties of XLPE with Appropriate Content of Dicumyl Peroxide for HVDC Cables Insulation. Materials, 15(17), 5857. https://doi.org/10.3390/ma15175857








