Variously Prepared Zeolite Y as a Modifier of ANFO
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterization Methods
3. Results and Discussion
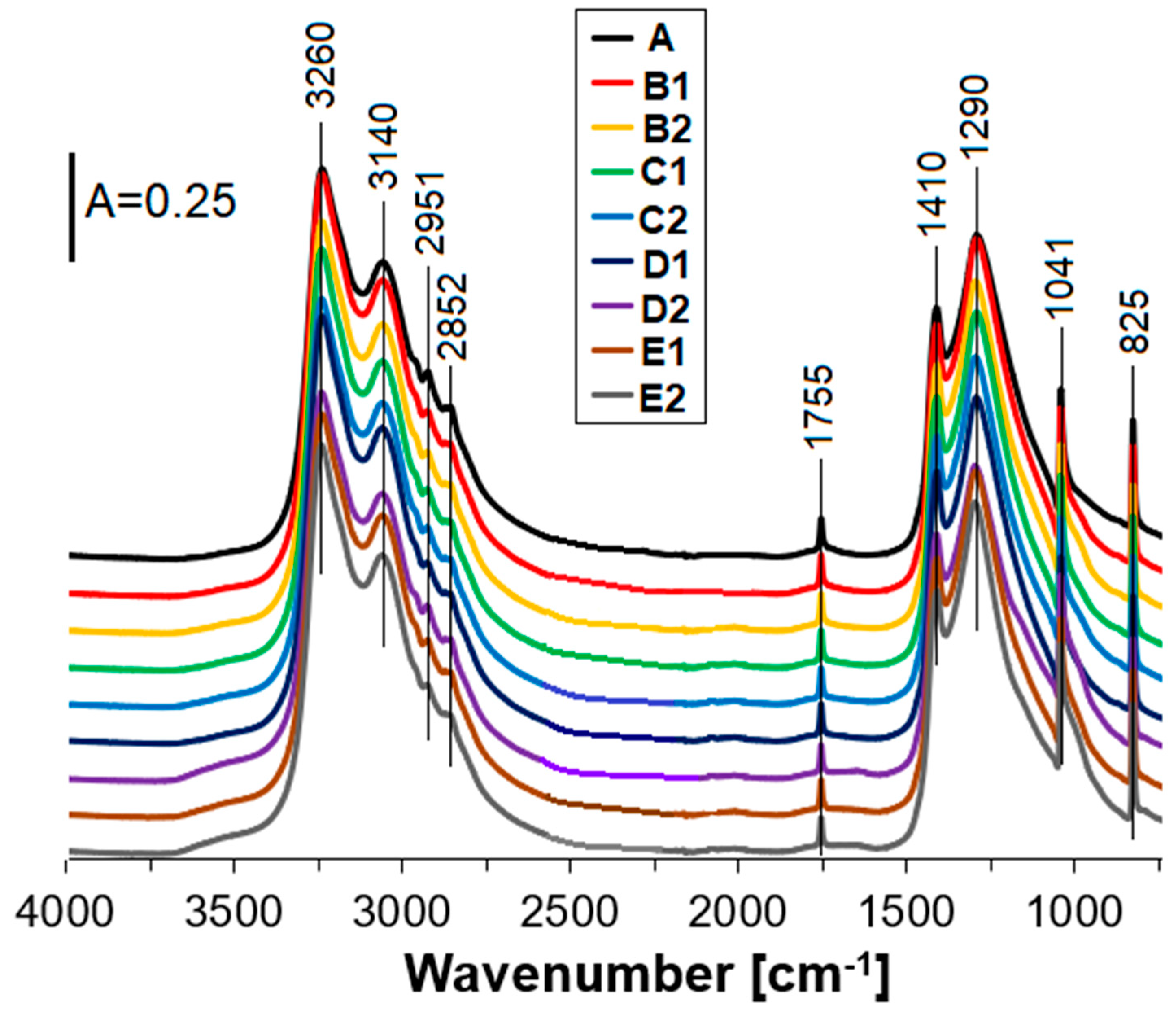
3.1. Structure
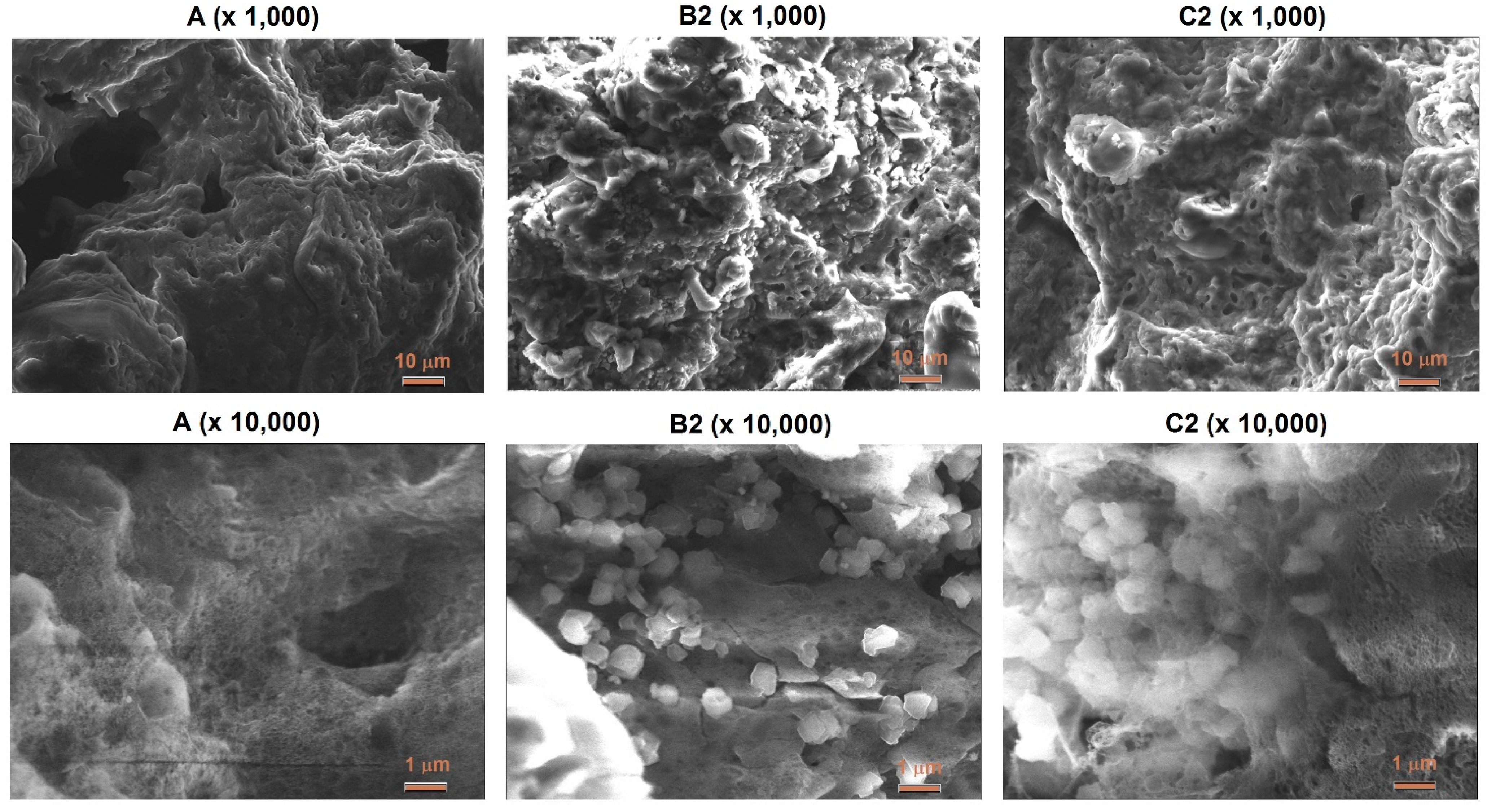
3.2. Morphology and Surface
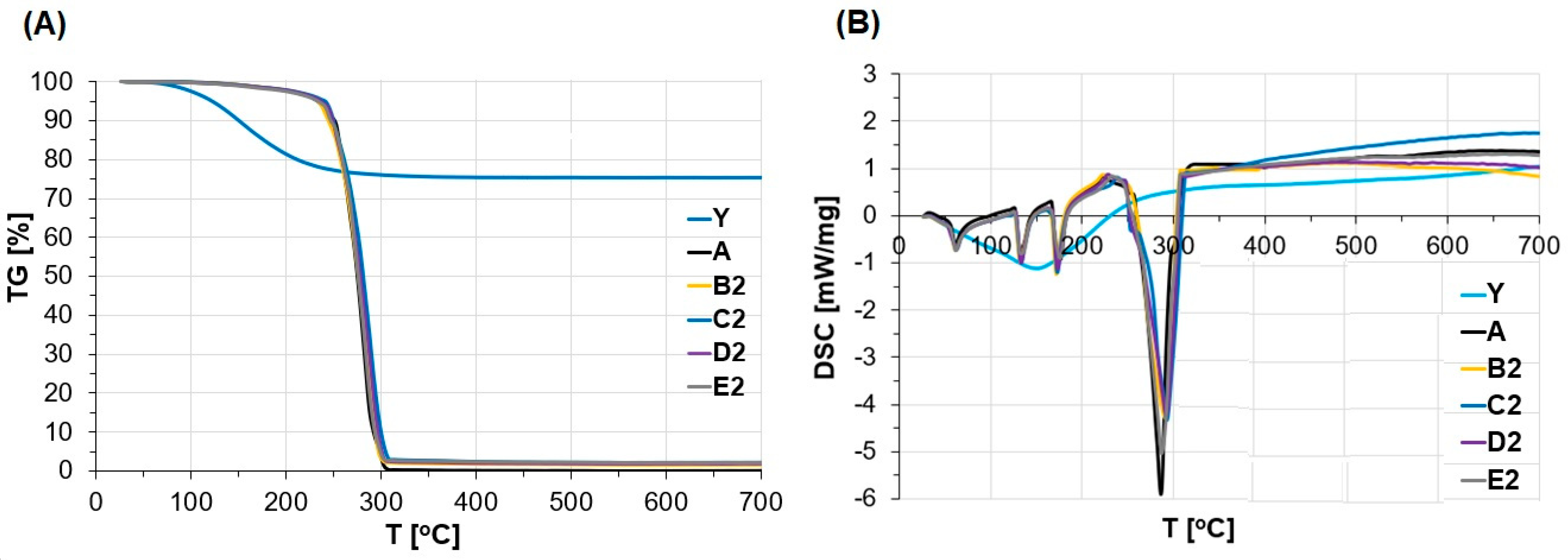
3.3. Thermal Properties
3.4. Detonation Properties
4. Conclusions
- In the present work, we presented the assessment of the blasting properties of Ammonium Nitrate Fuel Oil (ANFO) with the addition of variously modified zeolite Y.
- The presence of zeolite Y in ANFO did not change the structure, but altered ANFO’s surface, morphology, and influenced slightly thermal properties of such synthesized ANFO.
- The addition of zeolite Y to ANFO led to the growth of the detonation pressure, temperature, and heat of the explosion.
- We can control the VOD of ANFO by the choice of the way of the modification of zeolite Y additive. For bare zeolite Y and Mg-Y prepared via the impregnation method, the velocity of detonation (VOD) rose. The opposite effect was observed for ANFO modified with Mg-Y obtained from the deposition of Mg over zeolite Y via the ultrasonic-assisted procedure.
- The utilization of variously modified zeolite Y as an ANFO modifier generally reduced the volume of (COx + NOx) post-blast fumes, which is desired from an ecological point of view.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, M.A. The Science of Industrial Explosives; IRECO Chemicals: Salt Lake City, UT, USA, 1974. [Google Scholar]
- Mathiak, H. Pothole Blasting for Wildlife; Wisconsin Conservation Department: Madison, WI, USA, 1965. [Google Scholar]
- Biessikirski, A.; Kuterasinski, Ł. Research on Morphology and Topology of ANFO Based on Various Types of Oxygen Component; AGH: Kraków, Poland, 2018. [Google Scholar]
- Zygmunt, B.; Buczkowski, D. Influence of Ammonium Nitrate Prills’ Properties on DetonationVelocity of ANFO. Propellants Explos. Pyrotech. 2007, 32, 411–414. [Google Scholar] [CrossRef]
- Zygmunt, B. Detonation parameters of mixtures containing ammonium nitrate and aluminum. Cent. Eur. J. Energ. Mater. 2009, 6, 57–66. [Google Scholar]
- Miyake, A.; Takahara, K.; Ogawa, T.; Ogata, Y.; Wada, Y.; Arai, H. Influence of physical properties of ammonium nitrate on the detonation behavior of ANFO. J. Loss Prev. Process Ind. 2001, 14, 533–538. [Google Scholar] [CrossRef]
- Cummock, N.R.; Mares, J.O.; Gunduz, I.E.; Son, S.F. Relating a small-scale shock sensitivity experiment to large-scale failure diameter in an aluminized ammonium nitrate non-ideal explosive. Combust. Flame 2018, 194, 271–277. [Google Scholar] [CrossRef]
- Davis, W.; Fauquignon, C. Classical theory of detonation. J. Phys. IV Colloq. 1995, 4, 3–21. [Google Scholar] [CrossRef][Green Version]
- Smirnov, E.B.; Kostitsin, O.; Koval, A.V.; Akhlyustin, I.A. Model of non-ideal detonation of condensed high explosives. In Journal of Physics: Conference Series, Proceedings of the XXXI International Conference on Equations of State for Matter, Elbrus, Russia, 1–6 March 2016; IOP Publishing: Bristol, UK, 2016; Volume 774, p. 012076. [Google Scholar]
- Paszula, J.; Kowalewski, E. Study of the detonation development of non-ideal explosive. High Energy Mater. 2015, 7, 95–105. [Google Scholar]
- Clark, G.B. Principles of Fragmentation; Wiley-Interscience: New York, NY, USA, 1987. [Google Scholar]
- Kwok, Q.S.M.; Jones, D.E.G. Thermodesorption studies of ammonium nitrate prills by high-resolution thermogravimetry. J. Therm. Anal. Calorim. 2003, 74, 57–63. [Google Scholar] [CrossRef]
- Lenspiece, E.; Petr, V. The characterization of ammonium nitrate mini-prills. In Dynamic Behavior of Materials; Song, B., Casem, D., Kimberley, J., Eds.; Conference Proceedings of the Society for Experimental Mechanics Series; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Biessikirski, A.; Kuterasiński, Ł.; Dworzak, M.; Pyra, J.; Twardosz, M. Comparison of structure, morphology, and topography of fertilizer-based explosives applied in the mining industry. Microchem. J. 2019, 144, 39–44. [Google Scholar] [CrossRef]
- Oxley, J.C.; Smith, J.L.; Rogers, E.; Yu, M. Ammonium nitrate: Thermal stability and explosivity modifiers. Thermochim. Acta 2002, 384, 23–45. [Google Scholar] [CrossRef]
- Oxley, J.C.; Smith, J.L.; Wang, W. Compatibility of Ammonium Nitrate with Monomolecular Explosives 2. Nitroarenes12. J. Phys. Chem. 1994, 98, 3901. [Google Scholar] [CrossRef]
- Brower, K.R.; Oxley, J.C.; Tewari, M. Evidence for Homolytic Decomposition of Ammonium Nitrate at High Temperature. J. Phys. Chem. 1989, 93, 4029. [Google Scholar] [CrossRef]
- Atlagic, S.G.; Biessikirski, A.; Kuterasiński, Ł.; Dworzak, M.; Twardosz, M.; Sorogas, N.; Arvanitidis, J. On the Investigation of Microstructured Charcoal as an ANFO Blasting Enhancer. Energies 2020, 13, 4681. [Google Scholar] [CrossRef]
- Sinditskii, V.P.; Egorshev, V.Y.; Levshenkov, A.I.; Serushkin, V.V. Ammonium nitrate: Combustion mechanism and the role of additives. Propellants Explos. Pyrotech. 2005, 30, 269–280. [Google Scholar] [CrossRef]
- Maranda, A.; Gałęzowski, D.; Papliński, A. Investigation on Detonation and Thermochemical Parameters of Aluminized ANFO. J. Energ. Mater. 2003, 21, 1–3. [Google Scholar] [CrossRef]
- Maranda, A.; Paszula, J.; Zawadzka-Małota, I.; Kuczyńska, B.; Witkowski, W.; Nikolczuk, K.; Wilk, Z. Aluminum powder influence on ANFO detonation parameters. Cent. Eur. J. Energ. Mater. 2011, 8, 279–293. [Google Scholar]
- Izato, Y.; Miyake, A.; Date, S. Combustion characteristics of ammonium nitrate and carbon mixtures based on a thermal decomposition mechanism. Propellants Explos. Pyrotech. 2013, 38, 129–135. [Google Scholar] [CrossRef]
- Hussain, G.; Rees, G. Combustion of NH4NO3 and carbon-based mixtures. Fuel 1993, 72, 1475–1479. [Google Scholar] [CrossRef]
- Lurie, B.A.; Lianshen, C. Kinetics and mechanism of thermal decomposition of ammonium nitrate powder under the action of carbon black. Combust. Explos. Shock Waves 2000, 36, 607–617. [Google Scholar] [CrossRef]
- Miyake, A.; Kobayashi, H.; Echigoya, H.; Ogawa, T. Combustion and ignition properties of ammonium nitrate and activated carbon mixtures. Int. J. Energ. Mater. Chem. Propuls. 2009, 8, 411–419. [Google Scholar] [CrossRef]
- Wang, L.; Ago, M.; Borghei, M.; Ishaq, A.; Papageorgiou, A.C.; Lundahl, M.; Rojas, O.J. Conductive Carbon Microfibers Derived from Wet-Spun Lignin/Nanocellulose Hydrogels. ACS Sustain. Chem. Eng. 2019, 7, 6013–6022. [Google Scholar] [CrossRef]
- Nazarian, A.; Presser, C. Forensic methodology for the thermochemical characterization of ANNM and ANFO homemade explosives. Thermochim. Acta 2015, 608, 65–75. [Google Scholar] [CrossRef]
- Gunawan, R.; Zhang, D. Thermal stability and kinetics of decomposition of ammonium nitrate in the presence of pyrite. J. Hazard. Mater. 2009, 165, 751–758. [Google Scholar] [CrossRef]
- Gunawan, R.; Freij, S.; Zhang, D.; Beach, F.; Littlefair, M. A mechanistic study into the reactions of ammonium nitrate with pyrite. Chem. Eng. Sci. 2006, 61, 5781–5790. [Google Scholar] [CrossRef]
- Kramarczyk, B.; Pytlik, M.; Mertuszka, P. Effect of aluminum additives on selected detonation parameters of a bulk emulsion explosive. High-Energy Mater. 2020, 12, 99–113. [Google Scholar]
- Biessikirski, A.; Ziąbka, M.; Dworzak, M.; Kuterasiński, Ł.; Twardosz, M. Effect of metal addition on ANFO’s morphology and energy detonation. Przem. Chem. 2019, 98, 928–931. [Google Scholar]
- Gómez-Rico, M.F.; Marty´ın-Gullón, I.; Fullana, A.; Conesa, J.A.; Font, R. Pyrolysis and combustion kinetics and emissions of waste lube oils. J. Anal. Appl. Pyrolysis 2003, 68–69, 527–546. [Google Scholar] [CrossRef]
- Hakki Metecan, I.; Ozkan, A.R.; Isler, R.; Yanik, J.; Saglam, M.; Yuksel, M. Naphtha derived from polyolefins. Fuel 2005, 84, 619. [Google Scholar] [CrossRef]
- Sriningsih, W.; Garby Saerodji, M.; Trisunaryanti, W.; Triyono; Armunanto, R.; Falah, I.I. Fuel production from LDPE plastic waste over natural zeolite supported Ni, Ni-Mo, Co and Co-Mo metals. Procedia Environ. Sci. 2014, 20, 215–224. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Chong, C.T.; Lam, W.H.; Nyuk, T.N.S.T.A.; Moh, L.M.; Ibrahim, D.; Lama, S.S. Microwave co-pyrolysis of waste polyolefins and waste cooking oil: Influence of N2 atmosphere versus vacuum environment. Energy Convers. Manag. 2018, 171, 1292–1301. [Google Scholar] [CrossRef]
- Owusu, P.A.; Banadda, N.E.; Zziwa, A.; Seay, J.; Kiggundu, N. Reverse engineering of plastic waste into useful fuel products. J. Anal. Appl. Pyrolysis 2018, 130, 285–293. [Google Scholar] [CrossRef]
- Kaniewski, M.; Homann, K.; Homann, J. Influence of selected potassium salts on thermal stability of ammonium nitrate. Thermochim. Acta 2019, 678, 178313. [Google Scholar] [CrossRef]
- Maranda, A. Influence of aluminum chemical activity on detonation parameters of composite explosive containing aluminum powders (CX-Al). Wiad. Chem. 2001, 55, 353–375. [Google Scholar]
- Barański, K. Analysis of the Possibility of Using “Green Pyrotechnics” in the Detonators MW. Ph.D. Thesis, Faculty of Mining and Geoengineering, AGH University of Science and Technology in Cracow, Cracow, Poland, 27 September 2019. [Google Scholar]
- Biessikirski, A.; Barański, K.; Pytlik, M.; Kuterasiński, Ł.; Biegańska, J.; Słowiński, K. Application of Silicon Dioxide as the Inert Component or Oxide Component Enhancer in ANFO. Energies 2021, 14, 2152. [Google Scholar] [CrossRef]
- Biessikirski, A.; Wądrzyk, M.; Janus, R.; Biegańska, J.; Jodłowski, G.; Kuterasiński, Ł. Study on fuel oils used in ammonium nitrate-based explosives. Przem. Chem. 2018, 97, 457–462. [Google Scholar]
- EN 13631–14:2003; Explosives for Civil Uses. High Explosives. Part 14: Determination of Velocity of Detonation, European Committee for Standardization: Brussels, Belgium, 2003.
- EN 13631-16:2004; Explosives for Civil Uses. High Explosives. Part 16: Detection and Measurement of Toxic Gases, European Committee for Standardization:: Brussels, Belgium, 2004.
- European Union. Council directive 93/15/EEC of 5 April 1993 on the harmonization of the provisions relating to the placing on the market and supervision of explosives for civil uses. Off. J. Eur. Union 1993, 12, 20–36. [Google Scholar]
- Biessikirski, A.; Pytlik, M.; Kuterasiński, Ł.; Dworzak, M.; Twardosz, M.; Napruszewska, B.D. Influence of the Ammonium(V) Nitrate Porous Prill Assortments and Absorption Index on Ammonium Nitrate Fuel Oil Blasting Properties. Energies 2020, 13, 3763. [Google Scholar] [CrossRef]
- Biessikirski, A.; Atlagic, S.G.; Pytlik, M.; Kuterasinski, Ł.; Dworzak, M.; Twardosz, M.; Nowak-Senderowska, D.; Napruszewska, B.D. The Influence of Microstructured Charcoal Additive on ANFO’s Properties. Energies 2021, 14, 4354. [Google Scholar] [CrossRef]
- Suceska, M. Calculation of Detonation Parameters by EXPLO5 Computer Program. Mater. Sci. Forum 2004, 465–466, 325–330. [Google Scholar] [CrossRef]
- Hendricks, S.B.; Posnjak, E.; Kracek, F.C. Molecular rotation in the solid state. The variation of the crystal structure of ammonium nitrate with temperature. J. Am. Chem. Soc. 1932, 54, 2766–2786. [Google Scholar] [CrossRef]
- Vargeese, A.A.; Joshi, S.S.; Krishnamurthy, V.N. Effect of the method of crystallization on the IV–III and IV–II polymorphic transitions of ammonium nitrate. J. Hazard. Mater. 2009, 161, 373–379. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Fu, X.-Q.; Wang, Q. Phase stability of ammonium nitrate with organic potassium salts. Cent. Eur. J. Energ. Mater. 2016, 13, 736–754. [Google Scholar] [CrossRef]
- Ferg, E.; Masalova, I. Using PXRD to Investigate the Crystallization of Highly Concentrated Emulsions of NH4NO3. S. Afr. J. Chem. 2011, 64, 7–16. [Google Scholar]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- National Institute of Standards and Technology: “Ammonium Nitrate”. Available online: https://webbook.nist.gov/cgi/cbook.cgi?Name=ammonium+nitrate&Units=SI&cIR=on#Refs (accessed on 1 January 2022).
- Wu, B.H.; Chan, M.N.; Chan, C.K. FTIR characterization of polymorphic transformation of ammonium nitrate. Aerosol Sci. Technol. 2007, 41, 581–588. [Google Scholar] [CrossRef]
- Steele, B.A.; Oleynik, I.I. New crystal phase ammonium nitrate: First-principles prediction and characterization. In Proceedings of the AIP Conference Proceedings, Tampa Bay, FL, USA, 14–19 June 2015; Volume 1793, p. 130008. [Google Scholar]
- Pretsch, E.; Buhlmann, P.; Badertscher, M. Structure Determination of Organic Compounds. Tables of Spectral Data; Springer: Berlin, Germany, 2009. [Google Scholar]
- Aksay, S. Effects of Al dopant on XRD, FT-IR and UV–vis properties of MgO films. Phys. B Condens. Matter. 2019, 570, 280–284. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asifa, M.; Shamsuzzaman. Microwave-assisted MgO NP catalyzed one-pot multicomponent synthesis of polysubstituted steroidal pyridines. New J. Chem. 2018, 42, 184–197. [Google Scholar] [CrossRef]
- Chen, N.Y. Hydrophobic properties of zeolites. J. Phys. Chem. 1976, 80, 60–64. [Google Scholar] [CrossRef]
- Theoret, A.; Sandorey, C. Infrared Spectra and Crystalline Phase Transitions of Ammonium Nitrate. Can. J. Chem. 1964, 42, 57. [Google Scholar] [CrossRef]
- Biessikirski, A.; Kuterasiński, Ł.; Dworzak, M.; Twardosz, M.; Tatko, M.; Napruszewska, B.D. On the Influence of the Ammonium Nitrate(V) Provenance on Its Usefulness for the Manufacture of ANFO Type Explosives. Energies 2020, 13, 4942. [Google Scholar] [CrossRef]
- Fedoro, B.T.; Aaronson, H.A.; Sheeld, O.E.; Reese, E.F.; Clift, G.D.; Dunkle, C.G.; Walter, H.; McLean, D.C. Encyclopedia of Explosives and Related Items; US Department of the Army, Picatinny Arsenal: Dover, NJ, USA, 1960; Volume 1. [Google Scholar]
- Muzyk, R.; Topolnicka, T. Direct determination of oxygen content in coals by the use of the high-temperature pyrolysis method. Prz. Gor. 2013, 69, 100–104. [Google Scholar]
- Bhattacharyya, M.M.; Singh, P.K.; Singh, R.R. Laboratory methodology for assessment of toxic fumes in post-detonation gases from explosives. Min. Res. Eng. 2001, 10, 171–183. [Google Scholar] [CrossRef]
- Sapko, M.; Rowland, J.; Mainiero, R.; Zlochower, I. Chemical and physical factors that influence NOx production during blasting–exploratory study. In Proceedings of the 28th Conference on ‘Explosives and Blasting Technique’, Las Vegas, NV, USA, 10 February 2002; pp. 317–330. [Google Scholar]
- Mainiero, R.; Harris, M.; Rowland, J. Dangers of toxic fumes from blasting. In Proceedings of the 33rd Conference on ‘Explosives and Blasting Technique’, Nashville, TN, USA, 28–31 January 2007; Volume 1, pp. 1–6. [Google Scholar]
- Onederra, I.; Bailey, V.; Cavanough, G.; Torrance, A. Understanding main causes of nitrogen oxide fumes in surface blasting. Min. Technol. 2012, 121, 151–159. [Google Scholar] [CrossRef]
- Chaiken, R.F.; Cook, E.B.; Ruhe, T.C. Toxic Fumes from Explosives: Ammonium Nitrate-Fuel Oil Mixtures; BuMines RI 7867; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1974. [Google Scholar]
- Rowland, J.H., III; Mainiero, R. Factors Affecting ANFO Fumes Production. In Proceedings of the 26th Annual Conference on Explosives and Blasting Technique, Cleveland, OH, USA, 13–16 February 2000; International Society of Explosives Engineers: Warrensville Heights, OH, USA, 2000; pp. 163–174. [Google Scholar]
- Biessikirski, A.; Czerwonka, D.; Biegańska, J.; Kuterasiński, Ł.; Ziąbka, M.; Dworzak, M.; Twardosz, M. Research on the Possible Application of Polyolefin Waste-Derived Pyrolysis Oils for ANFO Manufacturing. Energies 2021, 14, 172. [Google Scholar] [CrossRef]
- Salzano, E.; Basco, A. Comparison of the explosion thermodynamics of TNT and black powder using Le Chatelier diagrams. Propellants Explos. Pyrotech. 2012, 37, 724–731. [Google Scholar] [CrossRef]
- Zawadzka-Małota, I. Testing of mining explosives with regard to the content of carbon oxides and nitrogen oxides in their detonation products. J. Sustain. Min. 2015, 14, 173–178. [Google Scholar] [CrossRef]



| Sample | Chemical Composition [% wt.] | Description | ||
|---|---|---|---|---|
| Ammonium Nitrate | Fuel Oil | Zeolite Y | ||
| A | 94.00 | 6.00 | 0.00 | Commercial ANFO, reference sample (5.00 g) |
| B1 | 93.06 | 5.94 | 1.00 | ANFO (4.95 g) + zeolite Y (0.05 g) |
| B2 | 92.12 | 5.88 | 2.00 | ANFO (4.90 g) + zeolite Y (0.10 g) |
| C1 | 93.06 | 5.94 | 1.00 | ANFO (4.95 g) + Mg-Y (0.05 g). Zeolite Y containing Mg introduced via the impregnation method. |
| C2 | 92.12 | 5.88 | 2.00 | ANFO (4.90 g) + Mg-Y (0.10 g). Zeolite Y containing Mg introduced via the impregnation method. |
| D1 | 93.06 | 5.94 | 1.00 | ANFO (4.95 g) + Mg-Y (0.05 g). Zeolite Y containing Mg introduced via the ion-exchange method. |
| D2 | 92.12 | 5.88 | 2.00 | ANFO (4.90 g) + Mg-Y (0.10 g). Zeolite Y containing Mg introduced via the ion-exchange method. |
| E1 | 93.06 | 5.94 | 1.00 | ANFO (4.95 g) + Mg-Y (0.05 g). Zeolite Y containing Mg introduced via ultrasonic-assisted impregnation method. |
| E2 | 92.12 | 5.88 | 2.00 | ANFO (4.90 g) + Mg-Y (0.10 g). Zeolite Y containing Mg introduced via ultrasonic-assisted impregnation method. |
| Sample | CO2 [dm3/kg] | CO [dm3/kg] | NOx [dm3/kg] | COx and NOx Post-Blast Volume [dm3/kg] | VOD [m/s] | Density [kg/m3] |
|---|---|---|---|---|---|---|
| A | 121.7 | 4.01 | 7.64 | 133.4 | 2024 | 695 |
| B1 | 119.5 | 4.41 | 6.83 | 130.7 | 2129 | 683 |
| B2 | 114.1 | 4.69 | 7.24 | 126.0 | 2176 | 672 |
| C1 | 117.0 | 4.17 | 7.96 | 129.1 | 2096 | 676 |
| C2 | 116.0 | 4.85 | 9.00 | 129.8 | 2122 | 671 |
| D1 | 116.4 | 4.18 | 8.73 | 129.3 | 1947 | 684 |
| D2 | 117.9 | 3.80 | 9.94 | 131.6 | 2078 | 676 |
| E1 | 118.4 | 3.81 | 8.73 | 130.9 | 1945 | 686 |
| E2 | 119.5 | 4.48 | 9.83 | 133.8 | 1981 | 686 |
| Sample | Detonation Pressure [MPa] | Detonation Temperature [K] | Heat of Explosion [kJ/kg] | Compression Energy [kJ/kg] | Oxygen Balance [%] |
|---|---|---|---|---|---|
| A | 3838 | 2970 | 3913 | 795 | −0.99 |
| B1 | 3871 | 3204 | 4352 | 819 | −0.98 |
| B2 | 3954 | 3428 | 4785 | 873 | −0.97 |
| C1 | 3844 | 3220 | 4381 | 835 | −0.98 |
| C2 | 3961 | 3443 | 4811 | 877 | −0.97 |
| D1 | 3966 | 3224 | 4379 | 856 | −0.98 |
| D2 | 4008 | 3441 | 4813 | 878 | −0.97 |
| E1 | 3900 | 3211 | 4384 | 821 | −0.98 |
| E2 | 4126 | 3441 | 4816 | 889 | −0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuterasiński, Ł.; Wojtkiewicz, A.M.; Sadowska, M.; Żeliszewska, P.; Napruszewska, B.D.; Zimowska, M.; Pytlik, M.; Biessikirski, A. Variously Prepared Zeolite Y as a Modifier of ANFO. Materials 2022, 15, 5855. https://doi.org/10.3390/ma15175855
Kuterasiński Ł, Wojtkiewicz AM, Sadowska M, Żeliszewska P, Napruszewska BD, Zimowska M, Pytlik M, Biessikirski A. Variously Prepared Zeolite Y as a Modifier of ANFO. Materials. 2022; 15(17):5855. https://doi.org/10.3390/ma15175855
Chicago/Turabian StyleKuterasiński, Łukasz, Agnieszka M. Wojtkiewicz, Marta Sadowska, Paulina Żeliszewska, Bogna D. Napruszewska, Małgorzata Zimowska, Mateusz Pytlik, and Andrzej Biessikirski. 2022. "Variously Prepared Zeolite Y as a Modifier of ANFO" Materials 15, no. 17: 5855. https://doi.org/10.3390/ma15175855
APA StyleKuterasiński, Ł., Wojtkiewicz, A. M., Sadowska, M., Żeliszewska, P., Napruszewska, B. D., Zimowska, M., Pytlik, M., & Biessikirski, A. (2022). Variously Prepared Zeolite Y as a Modifier of ANFO. Materials, 15(17), 5855. https://doi.org/10.3390/ma15175855








