Abstract
Materials based on the NiAl-Cr-Mo system with zirconium oxide or aluminum-magnesium spinel nanoparticle small additions were obtained by spark plasma sintering. Thermodynamic modeling was carried out to predict the phase formation in the NiAl-Cr-Mo system and its change depending on temperature, considering the presence of a small amount of carbon in the system. The phase composition and microstructure of materials were studied. NiAl (B2) and CrMo phases were found in the sintered samples. Bending strength measurements at different temperatures shows that nanoparticles of insoluble additives lead to an increase in bending strength, especially at high temperatures. A fractographic analysis of the sample’s fractures shows their hybrid nature and intercrystalline fracture, which is confirmed by the clearly visible matrix grains similar to cleavage. The maximum strength at 700 °C (475 MPa) was found for material with the addition of 0.1 wt.% zirconium oxide nanoparticles. In the study of internal friction, typical peaks of a nickel-aluminum alloy were found in the temperature ranges of 150–200 °C and 350–400 °C.
1. Introduction
Nickel-aluminum materials are promising for different parts of the manufacture of power plants and engines operating at high temperatures and in aggressive media. Compared to nickel alloys, the NiAl intermetallic compound has a high melting point (1911 K) and low density, a high heat resistance up to 1273 K, and good thermal conductivity (70–80 W/(m·K) at temperature range 25–1127 K) [1,2,3,4]. The NiAl compound has a density of 5.7–6.3 g/cm3 and its operating temperature can reach 1000–1100 °C. In addition, the material has good high-temperature strength, corrosion resistance, and creep resistance [5,6,7,8,9]. However, nickel-aluminum is non-plastic and brittle at room temperature, which reduces the possibility of its application [10,11,12,13]. There are several ways to improve the characteristics of this material using additives of transition, rare-earth, and noble metals [14,15,16,17,18,19,20]. The development of so-called eutectic alloys based on NiAl containing Cr, Mo and other elements improves its functional properties [12]. Eutectic materials in the NiAl-Cr-Mo system have high thermodynamic stability [14,15,16,17,21].
Intermetallic compounds have a high potential for use in composites with high heat resistance and high-temperature strength for rocket and space technology, power engineering and the aircraft industry [22,23]. In addition to its attractiveness as an engineering material, NiAl has such important properties as a simple crystalline structure, a high-ordered crystalline lattice, stability over a wide range of concentrations, a reversible shape memory effect, and anisotropic elastic and plastic behavior. Casting, rapid crystallization, powder metallurgy and mechanical synthesis are used to obtain materials based on NiAl. Limited plasticity and impact strength at room temperature make them difficult to manufacture using traditional processing methods. Meanwhile, due to the high melting point of NiAl and the requirements for vacuum facilities, casting methods have significant production costs [24]. The application of the powder metallurgy method is complicated by the presence of a stable surface oxide layer, and therefore, most aluminides are difficult to sinter to maximum density without pressure use [25]. Powder metallurgy makes it possible to economically create new materials from mixtures of mechanically activated metals and intermetallic compounds. In particular, the use of spark plasma sintering facilitates their rapid production with high density. According to [26], the chemical elements added to nickel alloys affect its structure and properties in different ways; for example, Al, Cr, and Mo increase strength and corrosion resistance.
One of the reasons for the use of intermetallic materials, including NiAl, is that the chemical bonds in them consist of combinations of partially ionic or covalent bonds, which have a high strength compared to weak metallic bonds. CrMo alloys consisting of a solid solution are characterized by high heat resistance and corrosion resistance, which makes them promising as additions to NiAl-based materials. Moreover, the addition of small amounts of insoluble nanoparticles to a metal or alloy matrix leads to an increase in the strength characteristics and creep resistance, as well as to an improvement in a number of functional properties [27,28,29]. This paper describes the study of materials based on the system (at.%) NiAl-13Cr-13Mo (marked as “M”) with small additions of zirconium oxide (0.01–0.1 wt.%) or aluminum magnesium spinel (0.01–0.2 wt.%) nanoparticles, formed by spark plasma sintering of mechanically activated powders. The effect of small additions of nanoparticles on mechanical properties has not yet been fully explained. In a number of previous works, it has been found that in the case of a soft matrix, for example, aluminum [29,30], small additions of high-modulus insoluble in the matrix nanoparticles provide not only an increase in strength, but also in elastic modulus. Moreover, for a number of cases, such dependencies of properties on the concentration of additives of nanoparticles have an extreme character. This behavior phenomenon is explained by the formation of interfacial hardening zones around nanoparticles, which, according to the Obraztsov-Lurie-Belov theory, have a gradient change in the elastic modulus or strength from the boundary with the nanoparticle to the matrix [31]. In this work, we investigated the effect of small amounts of ZrO2 and MgAl2O4 nanoparticles, which have Young’s modulus of 577 GPa [32] and 275 GPa [33], respectively, on mechanical properties of the NiAl-CrMo matrix. Thermodynamic modeling was carried out in the Terra® software environment [34] to predict the phase formation in the NiAl-Cr-Mo system, as well as with a small amount of carbon that enters the matrix from graphite molds during spark plasma sintering [35,36], considering the formation of a regular Cr-Mo solid solution. A fractographic analysis of the obtained samples was carried out, as well as studies of the features of the temperature dependence of internal friction.
2. Materials and Methods
The technology of nickel-aluminum matrix composites manufacturing included the preparation and mixing of initial powders NiAl (99.5%, 45 μm, PN70Yu30), JSC “Polema” (Tula, Russia), Cr (98.5%, 150 μm, PH1S, JSC “Polema” (Tula, Russia)), Mo (99.5%, 5 μm, MPCh, LLC “Atomspecsplav”, Saint Petersburg, Russia), with small amounts of MgAl2O4 nanoparticles (specific surface area—120 m2/g, average diameter—15 nm) or ZrO2-8Y2O3 nanoparticles (specific surface area—25 m2/g, average diameter—40 nm).
The sieving of the matrix powder was carried out in a special vibratory sieve to remove contaminants and large particles aggregates (with size more than 20 μm). Mechanical activation was carried out in the planetary mill «Activator—2SL». Mixing was carried out in steel cups in argon with steel balls. The ratio of powder to balls was 1:5, and the mixing time was 20 min. The disk rotation frequency was 33 Hz. After mixing in a planetary mill, the powders were extracted into the air. Then, isopropyl alcohol was added to them in the amount 1:5 of powder to liquid. Mixing was carried out with an overhead stirrer at a speed of 300 to 450 rpm to prevent foaming formation. During 5 min of stirring, the jar with the powder was in an ultrasonic bath at a frequency of 20 kHz. While stirring, the addition of zirconium oxide or aluminum-magnesium spinel nanoparticles pre-dispersed in isopropyl alcohol was carried out in various amounts (in the range of 0.01–0.2 wt.%). After finish of mixing, the jar was left to air dry for 24 h under a hood. Then the powder was extracted and pressed into graphite molds with a diameter of 30 mm. Sintering was carried out by the spark plasma sintering (SPS) using FCT System GmbH equipment at a pressure of 40 MPa and a temperature of 1300 °C in argon for 30 min with 130°/min heating rate to sintering temperature. There was no additional heat treatment of the sintered samples.
The study of the matrix “M” powder sample was carried out by thermal analysis using differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA) by NETZSCH© STA449F 1 Jupiter thermal analyzer in alumina crucible in the temperature range from 25 °C to 1500 °C with heating rate 10°/min in an inert atmosphere (argon).
The morphology and composition of the materials were studied by scanning electron microscope (SEM) Quanta 600 with TRIDENT XM4 energy-dispersive X-ray spectroscopy (EDX) equipment. X-ray phase analysis (XRD) was performed in β-filtered cobalt radiation by a horizontal 2θ-θ X-ray diffractometer HZG-4. The phases were identified using the ICDD PDF-2 database. The ultimate bending strength was determined by the three-point method on a Test Systems-VacEto universal mechanical testing machine. Young’s modulus and internal friction were measured by the ultrasonic method on the MUZA® device.
Thermodynamic modeling was carried out using the Terra® software v. 6.2 by Bauman Moscow State Technical University, Moscow, Russia [34,37], which helps to study the chemistry of transformations in a wide range of compositions, temperatures and pressures. Equilibrium compositions depending on temperature, total enthalpy, entropy and equilibrium heat capacity of the system were found. The correct calculations of the system equilibrium imply the availability of data on the existing phases, their transformations, and thermodynamic properties. Terra® software allows modeling of partially non-equilibrium processes using the following methods [38]:
- exclusion from the calculation of phases that do not have enough time to form. For example, during SPS, rapid heating and rapid quenching of the material occur, and the formation of a number of intermetallic compounds or solid solutions can be excluded;
- ‘freezing’ the absolute content of some reagents, for example, with high phase stability, as well as low reactivity or short process times.
In addition, a number of assumptions was made during the calculations: the systems are closed and isolated in a state of internal thermodynamic equilibrium, surface interfaces at the phase boundaries are not considered, substances in different phases are considered different components.
The results of thermodynamic modeling were compared with experimental data from [39,40,41,42,43,44].
3. Results
3.1. Study of the Matrix Powder and Nanoparticles
Figure 1 shows the general view of the used nanoparticles to modify the intermetallic matrix. The zirconium oxide and spinel nanoparticles previously has spherical or near-spherical form (Figure 1a,b).
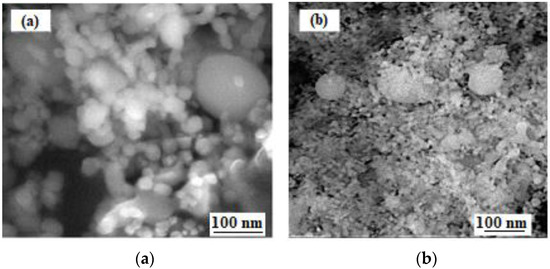
Figure 1.
SEM images in the secondary electrons of zirconium oxide (a) and aluminum-magnesium spinel (b) nanopowders.
Figure 2 shows the microstructure of the matrix powders NiAl, Cr, Mo (without the addition of nanoparticles) after mixing the initial powders in a ball mill for 2 days. Matrix particles have a shape close to spherical, with a diameter from 5 to 40 μm. The average diameter of mixed powder particles before milling was 40 μm, and after milling—25 μm.
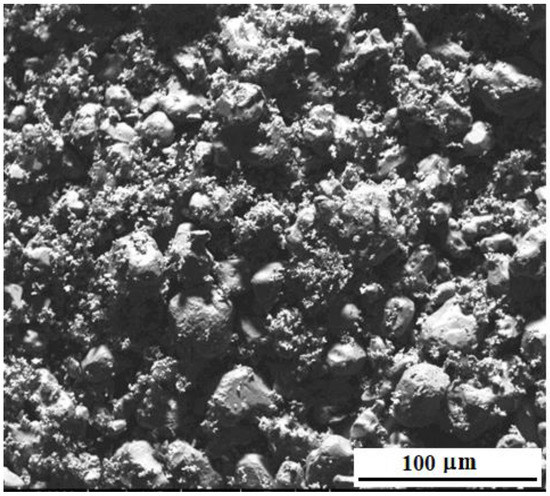
Figure 2.
Microstructure of “M” powders after mixing in a ball mill.
Thermal analysis of the sample shows that it is stable up to 1290 °C because there are not any peaks on the DSC curve. In the temperature range from 1290 °C to about 1350 °C, the DSC curve shows a double overlapping endothermic process with peaks at temperatures of 1300 °C and 1335 °C (Figure 3a). Further, at a temperature of 1450 °C, the beginning of another endothermic process is observed. In this case, there is practically no change in mass. A slight mass increase of less than 0.4% approximately corresponds to the observational error. The endothermic peak is associated with the onset of early melting. XRD analysis after DSC shows formation of Al5Mo intermetallic compound (Figure 3b). In addition, this could be due to the dissolution of the CrMo phase in the NiAl matrix.
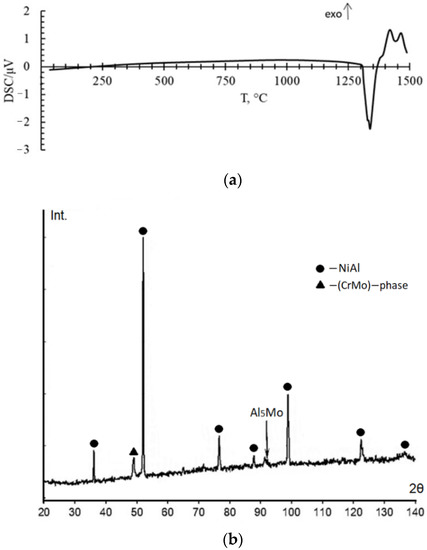
Figure 3.
Results of DSC analysis of the matrix powders (a) and XRD spectrum of matrix samples after DSC (b).
Figure 4 shows the results of thermogravimetric analysis of the matrix powder. Weight gain starts at 700 °C and reaches 0.36% at 1500 °C due to slight oxidation in the presence of impurity oxygen.
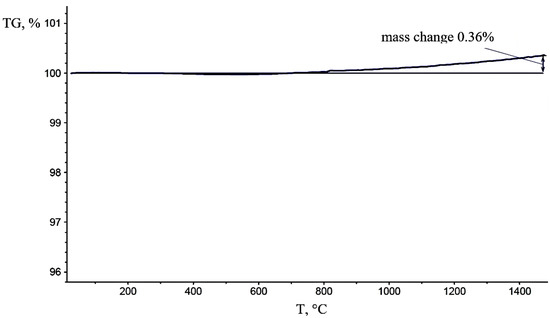
Figure 4.
Change in the mass of the “M” composites powder.
3.2. Results of Thermodynamic Modeling
Thermodynamic calculations with varying initial parameters help to reduce the number of experiments to find the optimal composition and technological parameters of new materials formation process. This is especially important for non-equilibrium or quasi-equilibrium processes occurring under conditions of rapid temperature change. Data on the intermetallic compounds and various phases formation are taken from a number of verifiable sources [39,45,46,47,48,49,50,51,52,53,54,55,56], as well as form the IVTANTERMO® database, the US National Bureau of Standards [57,58]. Due to the lack of research and the values of changes in thermodynamic properties, the standard entropy, standard enthalpy, density, and coefficients for the heat capacity polynomial are calculated according to the well-known methods [59,60,61,62,63]. In the database of thermodynamic substances used in the Terra® software, properties are expressed in different ways. In thermodynamic modeling the concept of total enthalpy I is also used [64,65]. It is necessary to know the formula of the compound, as well as the values of the enthalpy of formation , standard enthalpy and entropy . All necessary initial data for calculations are taken from [66,67,68]. The results of thermodynamic modeling are shown in Figure 5.
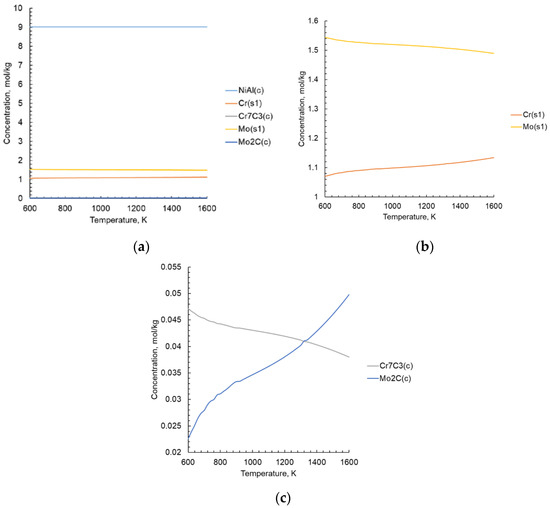
Figure 5.
Results of calculating the equilibrium phase composition in the NiAl-Cr-Mo-C system: (a) all phases, (b) solid solution (s1) components, (c) carbide phases.
3.3. XRD Analysis
The diffraction patterns of the matrix sintered samples («M», NiAl-13Cr-13Mo, at.%) with MgAl2O4 or ZrO2 nanoparticles additions are shown in Figure 6. NiAl B2, CrMo phases were found in the sintered samples.
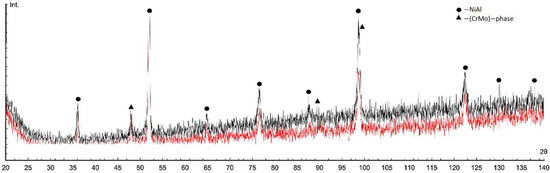
Figure 6.
X-ray diffraction patterns of sintered matrix samples with 0.01 wt.% MgAl2O4 (black) or 0.01 wt.% ZrO2 (red) nanoparticles additions (Int.—intensity in arbitrary units).
Due to the closeness and high intensity of the main phases peaks and the peaks of intermetallic phases that could be formed during sintering (for example, related to the Al-Mo system) they could not be identified.
3.4. Elemental Composition
The map of the elements distribution in the M-0.01 MgAl2O4 composite structure is shown in Figure 7. The light areas in the figures represent clusters of molybdenum particles, which also contain chromium and nickel. Such regions were observed throughout the material. The formation of these areas is associated with the complexity of the distribution of molybdenum small particles among large particles of the nickel-aluminum matrix during stirring in a ball mill. However, since these areas are distributed throughout the material, they contribute to an increase in mechanical characteristics and prevent crack growth, according to [69,70,71]. The presence of chromium is noted both in molybdenum-enriched areas and evenly in the nickel-aluminum matrix, most likely in the form of a solid solution.
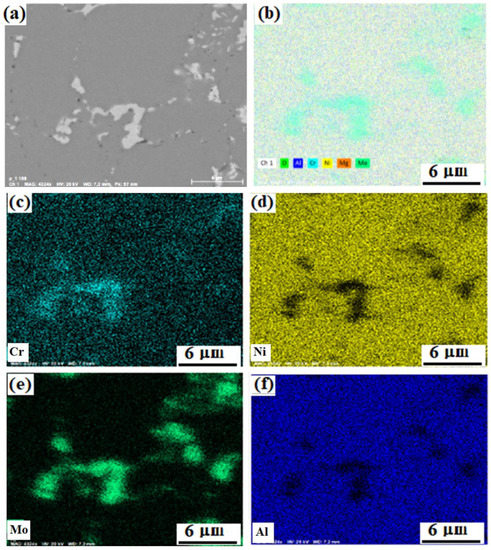
Figure 7.
Distribution of elements in the structure of the composite M-0.01 wt.% of MgAl2O4: SEM backscattered electron image of the study region (a), summary map of the elements distribution (b), distribution maps of Cr (c), Ni (d), Mo (e), Al (f).
The distribution of all elements over their phase-space looks uniform. Nevertheless, according to the distribution maps of chromium and molybdenum in the NiAl matrix, it can be concluded that it contains much more chromium than molybdenum. Chromium is distributed between two phases, which is consistent with the results obtained in [72,73].
Figure 8 shows the distribution of elements in a composite sample reinforced with zirconium oxide nanoparticles. The phase regions of NiAl and CrMo are clearly observed. The CrMo phase is also present in the form of finely dispersed irregularly shaped inclusions along the grain boundaries.
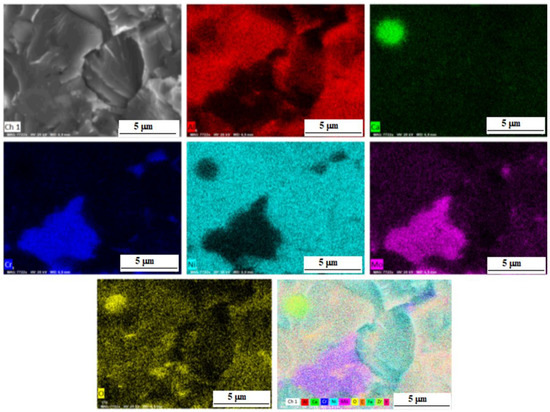
Figure 8.
SEM secondary electron image, maps of the elements distribution in the sample M-0.05 wt.% ZrO2 and a summary map.
The presence of calcium oxide is explained that the NiAl powder (PN70Yu30) and chromium powder (PH1S) may content ~0.1 wt.% Ca according to the manufacturer.
3.5. Bending Strength
Figure 9 shows the results of measuring the bending strength at 25 and 700 °C of composite samples modified with zirconium oxide and spinel nanoparticles obtained in this work compared to date for NiAl-Mo materials from other researchers [74,75]. The maximum strength of the studied in this work materials (475 MPa) at 700 °C corresponds to the sample reinforced by zirconium oxide nanoparticles in the amount of 0.1 wt.%.
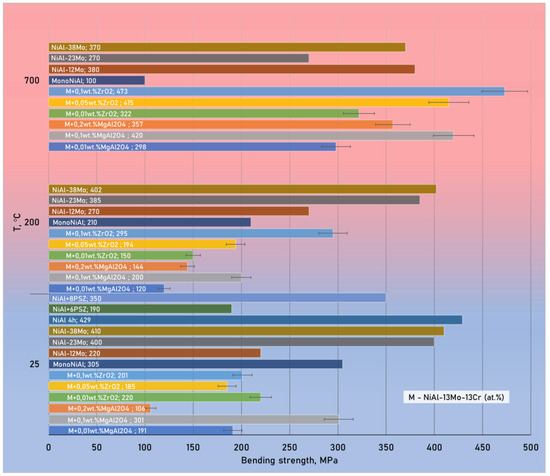
Figure 9.
Change in bending strength of various composites versus test temperature (values for materials with the letter “M” were obtained in this investigation; other data are taken from [74,75]).
A clear dynamic of the growth in bending strength was observed for samples with 0.05 and 0.1 wt.% of ZrO2 nanoparticles and 0.2 wt.% of MgAl2O4 nanoparticles with an increase in their concentration and test temperature. Samples with 0.01 wt.% of ZrO2, 0.01 wt.% and 0.1 wt.% of MgAl2O4 nanoparticles have an extreme behavior of the strength change on temperature increase. An interesting fact is that the addition of zirconium oxide or spinel nanoparticles significantly increases the high-temperature strength of the material. At higher additions, the tendency to aggregation is inherent for nanoparticles, and it is more difficult to distribute them uniformly, so it may decrease the matrix mechanical properties. It can show up in character of the properties-nanoparticles percent dependence curve as the presence of several extremums.
Young’s modulus was determined by the ultrasonic method (Figure 10). The average Young’s modulus of M was 205 GPa. The porosity of composites was in the range of 2.9–4.4%.
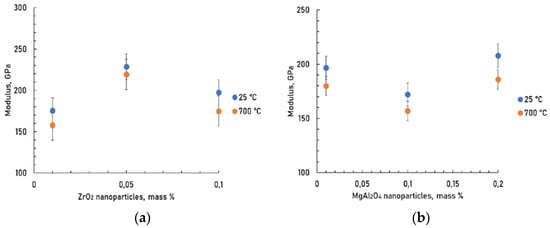
Figure 10.
Young’s modulus of composites at test temperatures of 25 and 700 °C depending on the concentration of ZrO2 (a) and MgAl2O4 (b) nanoparticles.
It seems to show that a change in the Young’s modulus-nanoparticles concentration curve of composites reinforced with spinel and zirconium oxide nanoparticles has extremums. The values of Young’s modulus are somewhat higher for the composite strengthened with 0.05 wt.% ZrO2 and 0.2 wt.% MgAl2O4 nanoparticles additions.
3.6. Microstructure of Material Fractures
Figure 11 shows the fracture microstructure of sintered samples of the M system. The typical pure M structure is shown in Figure 11a. The matrix grains size ranges from 5 to 25 μm. Chromium-molybdenum phase particles are located in chains along the boundaries of the matrix, that can be seen in Figure 11a,b. The size of the CrMo phase areas ranges from a few to tens of μm. The matrix fractured surface particulate qualities can be found in Figure 11c,d with cracks and grooves.
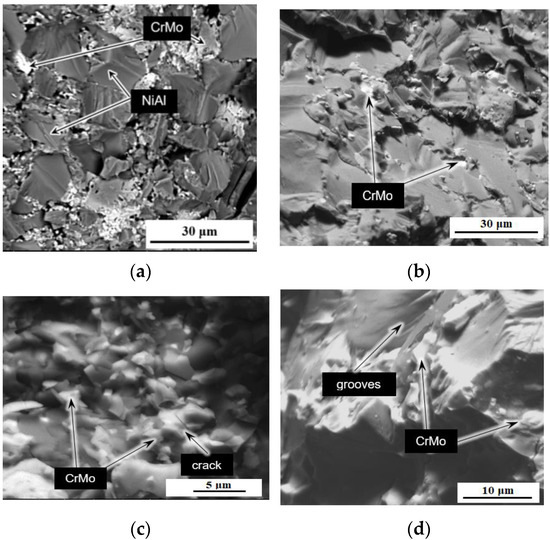
Figure 11.
Microstructure of the sintered sample M without the addition of nanoparticles, SEM images in backscattered electrons: (a,b)—general views of the matrix microstructure, view of fractured matrix grains (c,d).
Figure 12 shows the cluster or clusters (aggregates) of aluminum-magnesium spinel nanoparticles and the resulting crack in the sample M-0.01 wt.% of MgAl2O4 after bending tests. As was shown in [75], the reasons for the strengthening of materials based on NiAl couldn’t be explained by any single effect. In relation to the materials obtained in this work, such reasons include: dispersion strengthening by microparticles of the CrMo phase, dispersion strengthening due to the addition of small amounts of nanoparticles of refractory compounds (ZrO2, MgAl2O4) [75]. In addition, the cause of strengthening is crack bending due to the creation of obstacles by the second phase, as can be seen in Figure 12. At the same time, it is important to remember that the difference between the elastic moduli of molybdenum (324 GPa) [76], chromium (279 GPa) and nickel-aluminum (188 GPa) [77] leads to the residual stresses increase around fine particles of the eutectic phase due to external impact [78]. This effect helps to limit the growth of cracks.
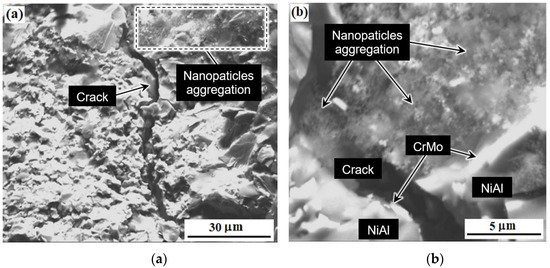
Figure 12.
The microstructure of the material M-0.01 wt.% of MgAl2O4 after bending tests: microstructure of the formed crack area (a), clusters of aluminum-magnesium spinel nanoparticles (b).
Figure 13 shows fracture images of composites with ZrO2 nanoparticles after bending tests at different temperatures. A hybrid nature of fractures is observed. There is intercrystalline fracture, which is confirmed by clearly visible crystal grains of the matrix, similar to chips.
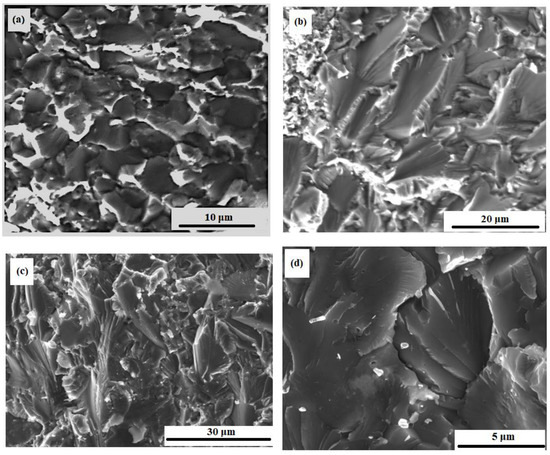
Figure 13.
Fracture microstructure of the M-0.05 wt.% of ZrO2 sample after bending tests at: 20 °C (a), 200 °C (b), and 700 °C (c,d).
The crack is spreading on the surface of the grain faces and along the interphase surface (between the matrix and the eutectic phase). The destruction is also brittle that wavy microrelief indicates, i.e., the presence of chip facets with steps in the form of a wavy pattern. A crack usually grows along a crystallographic plane with small indices [79]. The chip planes have different directions, which is associated with the corresponding orientation of individual crystal grains. This is due to the characteristic type of fracture in the fractographic analysis of the microstructure of the material (Figure 13c) [79,80,81]. Steps of wavy fracture are typically the result of cleavage along second-order chip planes. They combine and spread in the direction of the crack to minimize energy. This can also be explained as capturing cracks by regions with higher fracture toughness, in this case it is CrMo [80] as in Figure 12. It is possible to determine the trajectory of crack propagation along the direction of wavy patterns [81]. Intercrystalline failure is observed in the areas of molybdenum particles clusters. The nature of the fractures does not change with an increase in the test temperature to 700 °C. Micropores are visible in many grains.
3.7. Results of Internal Friction Investigation
Figure 14 shows curves of internal friction depending on temperature (25–700 °C). Peaks are visible in the temperature ranges of 150–200 °C and 350–400 °C. The presence of these peaks is typical for a nickel-aluminum alloy, they are clearly visible in almost all diagrams. Relaxation occurs at 420–450 °C, and the peaks at these temperatures are most likely associated with grain boundary sliding (Ke peaks), as well as with dislocation motion. The decline after the peak at about 400 °C may be associated with the deceleration of this motion by inclusions in the composites.
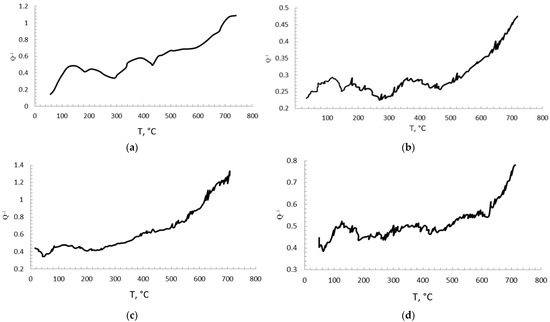
Figure 14.
Damping curves of composites: M + 0.01 MgAl2O4 (a), M + 0.2 MgAl2O4 (b), M + 0.01 ZrO2 (c), M + 0.1 ZrO2 (d).
In general, the curves of internal friction show a high phase stability of materials with an M matrix with the addition of nanoparticles in the considered test temperature range.
4. Discussion
According to [39], phase decomposition is observed at 1173 K for the Cr-Mo alloy with 44.2 at.% Mo. Continuous solutions in the liquid and solid state and the absence of any intermediate phases are typical for this system. A miscibility gap occurs below 880 °C in the solid state [39]. The oxidation resistance of Cr-Mo alloys rapidly decreases with increasing Mo content at the temperature about of 1000 °C [40,41], so its amount should not be too large. Based on the results of work [42], EDS and XRD methods show well the content of various phases formed in the studied alloys. The authors note that the NiAl-40Cr alloy consists mainly of two BCC phases, NiAl (B2) and Cr (A2). The addition of Mo leads to the formation of two intermetallic phases (AlMo3 and MoNi). AlMo3 seems to disappear at high Mo additions (over 20% of Mo), while MoNi is retained at even higher Mo concentrations. The NiAl-40Mo alloy practically contains the Mo and NiAl phases [42].
In [43], as a result of sintering the NiAl-28Cr-5.5Mo-0.5Zr powder by spark plasma method, the phase composition of the obtained material included NiAl phases and Cr (Mo) phases. The alloy obtained by electric arc melting of NiAl-20Cr-20Mo alloy contained BCC-B2 NiAl, BCC-Cr, AlMo3, NiMo (orthorombic), MexOy phases [42].
In this work, the main phases of the material are NiAl and CrMo after spark plasma sintering of the NiAl-13Cr-13Mo powder mixture at 1300 °C. These phase formations are also predicted by thermodynamic modeling in the Terra® software. Free carbon from molds and graphite paper, which diffusion is due to electrotransfer, for example, can lead to the formation of chromium and molybdenum carbides in the sintered material.
A strengthening effect with increasing test temperature for materials with nanoparticles additions (Figure 9) can be associated both with homogenization (same as solution heat treatment), and additional dissolution of chromium or molybdenum in NiAl and partial ordering of the BCC phase, and with the resistance of strengthening phases to the dislocations motion. The homogenization of materials may occur due to slow heating via bending testing (10°/min in 60 min). Moreover, it is well known that NiAl has a brittle to ductile transition at 300–400 °C [1,82]. In addition, it is known for a number of brittle materials [83,84], that with an increase in the temperature of bending tests, an increase in the strength of the test sample occurs as a result of plastic effects. This is because the deformation leads to the elimination of residual pores and the healing of the formed cracks, which increases the bending strength of the material.
5. Conclusions
The calculations of the equilibrium phase composition of the NiAl-Cr-Mo-C system is carried out at a temperature range of 25–1327 °C and a pressure of 0.1 MPa. The main components presented in the system are NiAl and solid solution of CrMo. Via thermodynamic modelling, the existence of carbides Cr7C3 and Mo2C are also found. There is a change in the composition of the Cr-Mo solid solution with an increase in temperature, with an increase in chromium and a decrease in molybdenum amount, as thermochemistry calculations have shown.
Samples of new composites containing of MgAl2O4 or ZrO2 nanoparticles were obtained by spark plasma sintering of mechanically activated matrix powder. The main phases of the materials are NiAl and CrMo.
The ultimate bending strength of the obtained materials versus the test temperature relation is studied. The maximum strength at 700 °C (475 MPa) is found for material with zirconium oxide nanoparticles addition in the amount of 0.1 wt.%.
The fractures of materials have a hybrid nature: there is intercrystalline failure, which is confirmed by the fact that grains of the matrix, similar to chips, are clearly visible. The destruction also has a fragile nature, which is determined by the wavy microrelief.
In the study of internal friction, typical peaks of a nickel-aluminum alloy are found in the temperature ranges of 150–200 °C and 350–400 °C. Relaxation occurs at 420–450 °C, and the peaks at these temperatures are most likely associated with grain boundary sliding (Ke peaks), as well as with dislocations motion. The decrease in the peak after 400 °C may be associated with the inhibition of this movement by inclusions in the composites. The decline in the peak after 400 °C may be associated with the deceleration of the motion due to inclusions in the composites.
Author Contributions
Conceptualization, L.A. and V.K.; methodology, S.S.; software, L.A.; validation, S.S.; formal analysis, M.L.; investigation, L.A. and S.S.; resources, Z.E.; data curation, Z.E.; writing—original draft preparation, L.A.; writing—review and editing, S.S.; project administration, V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation in the framework of the “Goszadanie”, grant number FSFF-2020–0014.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge the support received by the employees of the Department of Nanotechnology of JSC “Keldysh Research Center” for assistance in the research and valuable advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miracle, D.B. The physical and mechanical properties of NiAl. Acta Metall. 1993, 41, 649–684. [Google Scholar] [CrossRef]
- Noebe, R.D.; Bowman, R.R.; Nathal, M.V. Physical and mechanical properties of the B2 compound NiAl. Int. Mater. Rev. 1993, 38, 193–232. [Google Scholar] [CrossRef]
- George, E.P.; Liu, C.T. Brittle fracture and grain boundary chemistry of microalloyed NiAl. J. Mater. Res. 1990, 5, 754–762. [Google Scholar] [CrossRef]
- Barrett, C.A. Effect of 0.1 at.% Zirconium on the cyclic oxidation resistance of β-NiAl. Oxid. Met. 1988, 30, 361–390. [Google Scholar] [CrossRef]
- Geist, D.; Gammer, C.; Rentenberger, C.; Karnthaler, H.P. Sessile dislocations by reactions in NiAl severely deformed at room temperature. J. Alloys Compd. 2015, 621, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Bao, H. Decompose the electron and phonon thermal transport of intermetallic compounds NiAl and Ni3Al by first-principles calculations. Int. J. Heat Mass Transfer. 2018, 117, 972–977. [Google Scholar] [CrossRef]
- Wang, L.; Shen, J.; Zhang, G.; Zhang, Y.; Guo, L.; Ge, Y.; Gao, L.; Fu, H. Stability of lamellar structure of directionally solidified NiAl-28Cr-6Mo eutectic alloy at different withdrawal rates and temperatures. Intermetallics 2018, 94, 83–91. [Google Scholar] [CrossRef]
- Fan, G.H.; Wang, Q.W.; Du, Y.; Geng, L.; Hu, W.; Zhang, X.; Huang, Y.D. Producing laminated NiAl with bimodal distribution of grain size by solid–liquid reaction treatment. Mater. Sci. Eng. A 2014, 590, 318–322. [Google Scholar] [CrossRef]
- Wei, N.; Cui, H.; Ma, L.; Song, X.; Liu, W.; Hou, N. Porous TiC–TiB2–NiAl composites and effect of NiAl contents on pore structure and microstructure. Powder Metall. 2015, 58, 273–280. [Google Scholar] [CrossRef]
- Liu, E.; Jia, J.; Bai, Y.; Wang, W.; Gao, Y. Study on preparation and mechanical property of nanocrystalline NiAl intermetallic. Mater. Des. 2014, 53, 596–601. [Google Scholar] [CrossRef]
- Andasmas, M.; Chauveau, T.; Fagnon, N.; Vrel, D. Synthesis of NiAl intermetallics from cold-extruded samples. Intermetallics 2013, 32, 137–144. [Google Scholar] [CrossRef]
- Wang, B.; Wang, D.; Ning, H.; Liu, G. Study of NiAl-based alloy parts produced by metal injection moulding. Powder Metall. 2022, 65, 52–60. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Shrivastava, S. Brittle-to-ductile transition in NiAl single crystal. Acta Mater. 1998, 46, 1493–1502. [Google Scholar] [CrossRef]
- Walter, J.L.; Cline, H.E. The effect of solidification rate on structure and high-temperature strength of the eutectic NiAl-Cr. Metall. Mater. Trans. 1970, 1, 1221–1229. [Google Scholar] [CrossRef]
- Ferrandini, P.; Batista, W.W.; Caram, R. Influence of growth rate on the microstructure and mechanical behaviour of a NiAl-Mo eutectic alloy. J. Alloys Compd. 2004, 381, 91–98. [Google Scholar] [CrossRef]
- Bei, H.; George, E.P. Microstructures and mechanical properties of a directionally solidified NiAl-Mo eutectic alloy. Acta Mater. 2005, 53, 69–77. [Google Scholar] [CrossRef]
- Zhang, J.F.; Shen, J.; Shang, Z.; Feng, Z.R.; Wang, L.S.; Fu, H.Z. Microstructure and room temperature fracture toughness of directionally solidified NiAl-Mo eutectic in situ composites. Intermetallics 2012, 21, 18–25. [Google Scholar] [CrossRef]
- Bogner, S.; Hu, L.; Hollad, S.; Hu, W.; Gottstein, G.; Bührig-Polaczek, A. Microstructure of a eutectic NiAl-Mo alloy directionally solidified using an industrial scale and a laboratory scale bridgman furnace. Int. J. Mat. Res. 2012, 103, 17–23. [Google Scholar] [CrossRef]
- Hu, L.; Hu, W.; Gottstein, G.; Bogner, S.; Hollad, S.; Bührig-Polaczek, A. Investigation into microstructure and mechanical properties of NiAl-Mo composites produced by directional solidification. Mater. Sci. Eng. A 2012, 539, 211–222. [Google Scholar] [CrossRef]
- Chen, X.F.; Johnson, D.R.; Noebe, R.D.; Oliver, B.F. Deformation and fracture of a directionally solidified NiAl-28Cr-6Mo eutectic alloy. J. Mater. Res. 1995, 10, 1159–1170. [Google Scholar] [CrossRef] [Green Version]
- Dudová, M.; Kuchařová, K.; Barták, T.; Bei, H.; George, E.P.; Somsen, C.; Dlouhý, A. Creep in directionally solidified NiAl-Mo eutectics. Scr. Mater. 2011, 65, 699–702. [Google Scholar] [CrossRef]
- Ivchenko, M.V.; Pushin, V.G.; Wanderka, N. Vysokoentropiynyye ekviatomnyye splavy AlCrFeCoNiCu: Gipotezy i eksperimental’nyye fakty. Zhurnal Tekhnicheskoy Fiziki 2014, 84, 57–69. (In Russian) [Google Scholar]
- Darolia, R. NiAl alloys for high-temperature structural applications. JOM 1991, 43, 44–49. [Google Scholar] [CrossRef]
- Shekari, M.; Adeli, M.; Khobzi, A.; Kobashi, M.; Kanetake, N. Induction-activated self-propagating, high-temperature synthesis of nickel aluminide. Adv. Powder Technol. 2017, 28, 2974–2979. [Google Scholar] [CrossRef]
- Dong, S.; Hou, P.; Cheng, H.; Yang, H.; Zou, G. Fabrication of intermetallic NiAl by self-propagating high-temperature synthesis reaction using aluminium nanopowder under high pressure. J. Phys. Cond. Matt. 2002, 14, 11023–11030. [Google Scholar] [CrossRef]
- Donachie, M.J.; Stephen, J.D. Superalloys: A Technical Guide, 2nd ed.; ASM International: Materials Park, MA, USA, 2002; 439p. [Google Scholar]
- Agureev, L.E.; Ivanov, B.S.; Savushkina, S.V.; Laptev, I.N.; Ashmarin, A.A.; Ivanov, A.V.; Sivtsova, G.V.; Kostikov, V.I.; Eremeeva, Z.V. Study of the structure and properties of cermets based on the NiAl–Al2O3 system. Russ. J. Non-Ferr. Met. 2021, 62, 763–770. [Google Scholar] [CrossRef]
- Agureev, L.E.; Laptev, I.N.; Kanushkin, A.I.; Ivanov, A.V.; Ashmarin, A.A.; Vysotina, E.A.; Ivanov, B.S.; Kostikov, V.I.; Eremeeva, Z.V. Preparation and study of Ni–Al–O system cermet composites with a small addition of MgAl2O4 nanoparticles. Russ. J. Non-Ferr. Met. 2020, 61, 375–381. [Google Scholar] [CrossRef]
- Agureev, L.E.; Laptev, I.N.; Ivanov, B.S.; Kanushkin, A.I.; Rizakhanov, R.N.; Ashmarin, A.A.; Ivanov, A.V.; Vysotina, E.A.; Panasova, G.V.; Kostikov, V.I.; et al. Development of heat resistant aluminum composite with minor addition of alumina nanofibers (nafen™). Inorg. Mat. Appl. Res. 2020, 11, 1045–1050. [Google Scholar] [CrossRef]
- Mironov, V.V.; Agureev, L.; Eremeeva, Z.V.; Kostikov, V.I. Effect of Small Additions of Alumina Nanoparticles on the Strength Characteristics of an Aluminum Material. Dokl. Phys. Chem. 2018, 481, 110–113. [Google Scholar] [CrossRef]
- Lurie, S.; Volkov-Bogorodskiy, D.; Solyaev, Y.; Rizakhanov, R.; Agureev, L. Multiscale modelling of aluminium-based metal–matrix composites with oxide nanoinclusions. Comp. Mater. Sci. 2016, 116, 62–73. [Google Scholar] [CrossRef]
- Dauda, I.; Mokhtar, M.; Mokhtar, A.; Othman, M. Modelling the elastic constants of cubic zirconia using molecular dynamics simulations. Adv. Mat. Res. 2014, 845, 387–391. [Google Scholar]
- Fedotov, A.F. Analysis of the adequacy and selection of phenomenological models of the elastic properties of porous powder materials. J. Mater. Sci. 2017, 52, 2964–2973. [Google Scholar] [CrossRef]
- Trusov, B.G. TERRA software system for modeling phase and chemical equilibria in plasma-chemical systems. In Proceedings of the 3rd International Symposium on Theoretical and Applied Plasma Chemistry, Collection of Materials, Ples, Russia, 16–21 September 2002; pp. 217–220. (In Russian). [Google Scholar]
- Bokhonov, B.B.; Ukhina, A.V.; Dudina, D.V.; Anisimov, A.G.; Malic, V.I.; Batraev, I.S. Carbon uptake during Spark Plasma Sintering: Investigation through the analysis of the carbide “footprint” in a Ni–W alloy. RSC Adv. 2015, 5, 80228–80237. [Google Scholar] [CrossRef]
- Agureev, L.; Kostikov, V.; Eremeeva, Z.; Savushkina, S.; Ivanov, B.; Khmelenin, D.; Belov, G.; Solyaev, Y. Influence of Alumina Nanofibers Sintered by the Spark Plasma Method on Nickel Mechanical Properties. Metals 2021, 11, 548. [Google Scholar] [CrossRef]
- Simonyan, L.M.; Alpatova, A.A.; Demidova, N.V. The EAF dust chemical and phase composition research techniques. J. Mater. Res. Technol. 2019, 8, 1601–1607. [Google Scholar] [CrossRef]
- Ilinykh, N.; Krivorogova, A. Thermodynamic modelling of fluxing alloys of Ni-C-Cr-Si-B. Eur. Phys. J. Spec. Top. 2017, 226, 1115–1121. [Google Scholar] [CrossRef]
- Jindal, V.; Sarma, B.N.; Lele, S. A thermodynamic assessment of the Cr–Mo system using CE-CVM. Calphad 2013, 43, 80–85. [Google Scholar] [CrossRef]
- Venkatraman, M.; Neumann, J.P. The Cr-Mo (Chromium-Molybdenum) System. Bull. Alloy. Phase Diagr. 1987, 8, 216–220. [Google Scholar] [CrossRef]
- Ning, H.; Wang, D.; Wang, B.; Liu, G. Investigations on the NiAl–Cr(Mo) eutectic alloy with optimized microstructure and improved room-temperature compressive properties. Mater. Sci. Eng. A 2021, 813, 141138. [Google Scholar] [CrossRef]
- Mathiou, C.; Giorspyros, K.; Georgatis, E.; Poulia, A.; Karantzalis, A.E. NiAl-Cr-Mo Medium Entropy Alloys: Microstructural Verification, Solidification Considerations, and Sliding Wear Response. Materials 2020, 13, 3445. [Google Scholar] [CrossRef]
- Liu, Z.; Ning, H.; Lin, Z.; Wang, D. Influence of Spark Plasma Sintering Parameters on the Microstructure and Room-Temperature Mechanical Properties of NiAl-28Cr-5.5Mo-0.5Zr Alloy. Acta Metall. Sin. 2021, 57, 1579–1587. [Google Scholar]
- Mathiou, C.; Kyrtsidou, K.; Georgatis, E.; Karantzalis, A.E. Primary Nano-Indentation Assessment of Low Entropy NiAl-Cr Alloys. SOJ Mater. Sci. Eng. 2022, 10, 1–15. [Google Scholar]
- Begunov, A.I.; Kuz’min, M.P. Thermodynamic Stability of Intermetallic Compounds in Technical Aluminum. J. Sib. Fed. Univ. Eng. Technol. 2014, 2, 132–137. [Google Scholar]
- Onderka, B.; Sypień, A.; Wierzbicka-Miernik, A.; Czeppe, T.; Zabdyr, L.A. Heat capacities of some binary intermetallic compounds in Al-Fe-Ni-Ti system. Arch. Metall. Mater. 2010, 55, 435–439. [Google Scholar]
- Yao, Z.-H.; Ruan, Y.-T.; Dong, J.-X.; Yu, Q.-Y.; Zhang, S.-Q.; Chen, X. Study on Gamma Prime and Carbides of Alloy A286 by Traditional Thermodynamic Calculation. High Temp. Mater. Proc. 2018, 37, 495–507. [Google Scholar] [CrossRef]
- Geramifard, G. Thermodynamic Investigation of Oxidation of Nial-(Cr, Mo) Alloys Used for Manufacturing Metal Matrix Composites by Directional Solidification; Karlsruher Institut für Technologie: Karlsruhe, Germany, 2021. [Google Scholar]
- Zhang, Z.; Li, M.; Flores, K.; Mishra, R. Machine learning formation enthalpies of intermetallics. J. Appl. Phys. 2020, 128, 105103. [Google Scholar] [CrossRef]
- Akande, T.; Matthew-Ojelabi, F.; Agunbiade, G.; Faweya, E. Thermodynamic properties of V, Cr, Mo, and Fe metals and their binary alloys. Turk. J. Phys. 2019, 43, 606–617. [Google Scholar] [CrossRef]
- Turchi, P.E.A.; Kaufman, L.; Liu, Z.-K. Modeling of Ni–Cr–Mo based alloys: Part I—Phase stability. Calphad 2006, 30, 70–87. [Google Scholar] [CrossRef]
- Cutler, R.W. The 1200 °C Isothermal Sections of the Ni-Al-Cr and the Ni-Al-Mo Ternary Phase. Master’s Thesis, The Ohio State University, Columbus, OH, USA, 2011. [Google Scholar]
- Peng, J. Experimental Investigation and Thermodynamic Modeling of the Al-Cr-Mo-Ni System and Its Subsystems. Ph.D. Thesis, Karlsruher Institut für Technologie, Karlsruhe, Germany, 2016. [Google Scholar]
- Sigli, C.; Kosugi, M.; Sanchez, J.M. Calculation of Thermodynamic Properties and Phase Diagrams of Binary Transition-Metal Alloys. Phys. Rev. Lett. 1986, 57, 253–256. [Google Scholar] [CrossRef]
- Berdnikov, V.I.; Gudim, Y.A. Termodinamicheskiye svoystva binarnykh metallichskikh sistem, soderzhashchikh intermetallidnyye soyedineniya. Izv. Vyssh. Uch. Zaved. Chern. Met. 2013, 5, 37–41. (In Russian) [Google Scholar]
- Chen, Q.; Huang, Z.; Zhao, Z.; Hu, C. First-principles study on the structural, elastic, and thermodynamics properties of Ni3X (X: Al, Mo, Ti, Pt, Si, Nb, V, and Zr) intermetallic compounds. Appl. Phys. A 2014, 116, 1161–1172. [Google Scholar] [CrossRef]
- Bale, C.W.; Belisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; Pelton, A.D.; et al. FactSage Thermochemical Software and Databases—Recent developments. Calphad 2009, 33, 295–311. [Google Scholar] [CrossRef]
- Roine, A. Outokumpu HSC Chemistry for Windows. Chemical Reactions and Equilibrium Software with Extensive Thermochemical Database; Outokumpu Research OY: Pori, Finland, 2002; 268p. [Google Scholar]
- Andreyev, O.L.; Bushkova, O.V.; Batalov, N.N. Raschot termodinamicheskikh svoystv oksidov kobalta (III,IV) I kobaltita litiya. Electrokhim. Ener. 2006, 6, 187–191. (In Russian) [Google Scholar]
- Morachevskiy, A.G.; Sladkov, I.B. Termodinamicheskiye Raschoty v Metallurgii; Metallurgiya: Moscow, Russia, 1985; 136p. (In Russian) [Google Scholar]
- Vasilyev, V.P.; Taldrick, A.F.; Ilinykh, N.I. New correlative method of thermodynamic analysis of the inorganic compounds. In Proceedings of the MATEC Web of Conferences XXXIX JEEP—39th Edition of the Joint European Days on Equilibrium between Phases, Nancy, France, 19–21 March 2013; EDP Sciences: Paris, France, 2013. 8p. [Google Scholar]
- Kuzmin, M.P.; Begunov, A.I. Priblizhonnyye raschoty termodinamicheskikh kharakteristik intermetallicheskih soedinenij na osnove alyuminiya. Vestn. IrGTU 2013, 72, 98–101. (In Russian) [Google Scholar]
- Moiseyev, G.K.; Vatolin, N.A.; Marshuk, L.A.; Il’inykh, N.I. Temperaturnyye Zavisimosti Privedennoy Energii Gibbsa Nekotorykh Neorganicheskikh Veshchestv (Al’ternativnyy Bank Dannykh ACTPA.OWN); UrO RAN: Yekaterinburg, Russia, 1997; 231p. (In Russian) [Google Scholar]
- Khina, B.B. Thermodynamic Models of Condensed Phases and Their Application in Materials Science. Adv. Mater. Technol. 2018, 1, 41–47. [Google Scholar] [CrossRef]
- Kubashevskij, O.; Olkokk, S.B. Metallurgicheskaja Termohimija [Metallurgical Thermochemistry]; Metallurgija: Moscow, Russia, 1982; 392p. (In Russian) [Google Scholar]
- Moiseyev, G.K. Otsenka termokhimicheskikh svoystv i termodinamicheskikh funktsiy nekotorykh letuchikh i kondensirovannykh klasterov shchelochnykh metallov (SHCHM). RASPL 2003, 4, 59–84. (In Russian) [Google Scholar]
- Gurvich, L.V.; Bergman, G.A.; Veitz, I.V.; Medvedev, V.A.; Khachkuruzov, G.A.; Yungman, V.S. Thermodynamic Properties of Individual Substances; Glushko, V.P., Ed.; High Temperature Institute: Moscow, Russia, 1982; Volume 1–4. [Google Scholar]
- Bystrov, P.N.; Kagan, D.N.; Krechetova, G.A.; Shpilrain, E.E. Liquid Metal Heat Carriers of Heat Pipes and Power Plants; Moscow Nauka: Moscow, Russia, 1988; 264p. (In Russian) [Google Scholar]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Kostikov, V.I.; Agureev, L.E.; Eremeeva, Z.V. Development of nanoparticle-hardened aluminum composites for rocket and space technology. Russ. J. Non-Ferr. Met. 2015, 56, 325–328. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, H.; Yee, J.K.; Mo, X.; Topping, T.; Lavernia, E.J.; Schoenung, J.M. Toughening of aluminum matrix nanocomposites via spatial arrays of boron carbide spherical nanoparticles. Act. Mater. 2016, 103, 128–140. [Google Scholar] [CrossRef]
- Gombola, C.; Kaumann, A.; Geramifard, G.; Blankenburg, M.; Heilmaier, M. Microstructural Investigations of Novel High Temperature Alloys Based on NiAl-(Cr, Mo). Metals 2020, 10, 961. [Google Scholar] [CrossRef]
- Misra, A.; Gibala, R.; Noebe, R.D. Optimization of toughness and strength in multiphase intermetallics. Intermetallics 2001, 9, 971–978. [Google Scholar] [CrossRef]
- Subramanian, P.R.; Mendiratta, M.G.; Miracle, D.B. Influence of non-reactive particles on the microstructure of NiAl and NiAl–ZrO2 processed by thermal explosion Intermetallics. Metall. Mater. Trans. A 2008, 16, 732–737. [Google Scholar]
- Subramanian, P.R.; Mendiratta, M.G.; Miracle, D.B. Microstructures and Mechanical Behavior of NiAl-Mo and NiAl-Mo-Ti Two-Phase Alloys. Metall. Mater. Trans. A 1994, 25A, 2770. [Google Scholar] [CrossRef]
- Boyer, H.E.; Gall, T.L. (Eds.) Metals Handbook, Desk Edition, 2nd ed.; ASM International: Metals Park, MA, USA, 1985; 1521p. [Google Scholar]
- Rusovic, N.; Warlimont, H. Young’s modulus of β2-NiAl alloys. Phys. Stat. Sol. A 1979, 53, 283–288. [Google Scholar] [CrossRef]
- Davidge, R.W.; Green, T.J. The strength of two-phase ceramic/glass materials. J. Mater. Sci. 1968, 3, 629–634. [Google Scholar] [CrossRef]
- Klevtsov, G.V.; Botvina, L.R.; Klevtsova, N.A.; Limar, L.V. Fractodiagnostics of Destruction of Metallic Materials and Structures; MISiS: Moscow, Russia, 2007; 264p. (In Russian) [Google Scholar]
- Bochenek, K.; Węglewski, W.; Morgiel, J.; Maj, M.; Basist, M. Enhancement of fracture toughness of hot-pressed NiAl-Re material by aluminum oxide addition. Mater. Sci. Eng. A 2020, 790, 139670. [Google Scholar] [CrossRef]
- Fellows, J. (Ed.) Fractography and Atlas of Fractograms. Handbook; Metallurgy: Moscow, Russia, 1982; 489p. (In Russian) [Google Scholar]
- Margevicius, R.W.; Cotton, J.D. Study of the brittle-to-ductile transition in NiA1 by texture analysis. Acta Metall. Mater. 1995, 43, 645–655. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Jang, S.H.; Nishimura, T.; Choi, S.-Y. Microstructure and high temperature strength of silicon carbide with 2000 ppm yttria. J. Eur. Ceram. Soc. 2017, 37, 4449–4455. [Google Scholar] [CrossRef]
- Izhevskyi, V.A.; Bressiani, A.H.A.; Bressiani, J.C. Effect of liquid phase sintering on microstructure and mechanical properties of Yb2O3-AlN containing SiC-based ceramics. J. Am. Ceram. Soc. 2005, 88, 1115–1121. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).