Preparation, Antimicrobial Properties under Different Light Sources, Mechanisms and Applications of TiO2: A Review
Abstract
:1. Introduction
2. TiO2 and Its Composites Materials
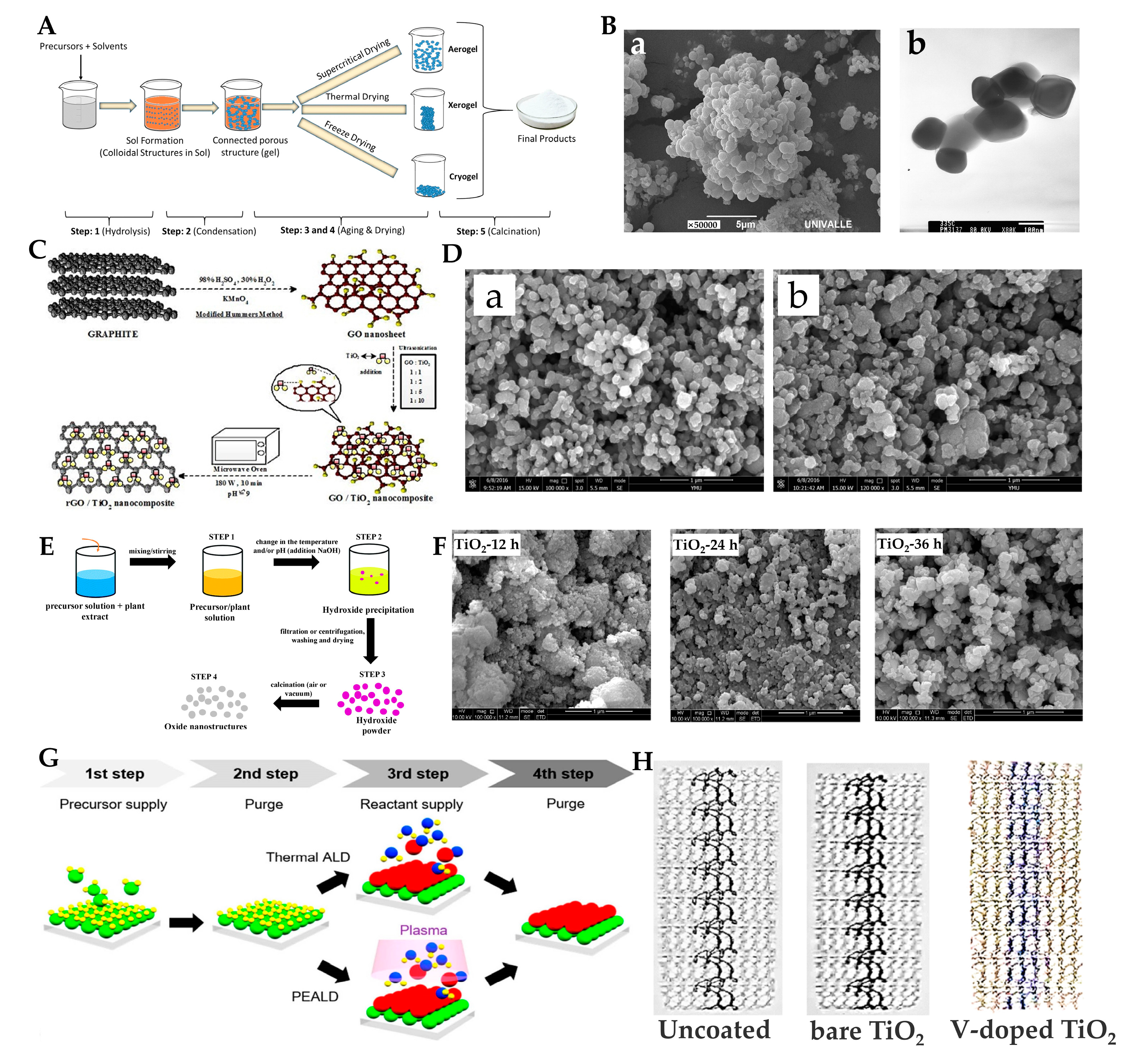
2.1. Synthesis Methods
2.1.1. Sol–Gel Method
2.1.2. Microwave-Assisted Method
2.1.3. Green Synthesis Method
2.1.4. Hydrothermal Method
2.1.5. Atomic Layer Deposition and Magnetron Sputtering Methods
2.2. Antimicrobial Activity under UV Light
| Material | Grain Size: nm | Microorganism | Light Source | Antimicrobial Activity | Reference |
|---|---|---|---|---|---|
| A-TiO2, M-TiO2, R-TiO2 | Approximately 300 nm | Streptococcus sanguinis, Actinomyces naeslundii, and Fusobacterium nucleatum | UVA 2 × 15 W (λ = 350 nm and intensity = 1.62 mW/cm2) | Approximately 99.9% for M-TiO2 and 99% for A-TiO2, R-TiO2 had no antimicrobial activity | [48] |
| Ag-TiO2 | 1–3 (Ag) | E. coli, the influenza A virus (H1N1), and enterovirus | UVA (20 W/cm2) | >99.9% (1 wt% Ag/TiO2) | [49] |
| TiO2(NS1.0, NS1.2, NS1.5, NS1.8, and NS2.0 with different F/Ti ratios) | 200–600 (length), 6–20 (thickness) | S. mutans | 365 nm light (2.5 mW/cm2) | Approximately 90% (NS1.0) | [51] |
| Cu/TiO2 nonwoven fabric (NWF) | 20 (TiO2), 1–5 (Cu) | HuNoV genogroup II genotype 4 (HuNoV GII.4) | 373-nm UVA-LED source | The optimum treatment conditions for inactivating the HuNoV GII.4 droplets were as follows: Cu:TiO2 ratio of 1:7.7 and the use of a 373 nm UVA-LED source for 48.08 min | [52] |
| Polyurethane-acrylate-Ag/TiO2 | TiO2: 30(length), 3–4(diameter), Ag: 6(diameter) | E. coli | 36 W mercury lamp (wavelength >245 nm) | Approximately 97% | [53] |
| Fe-TiO2 | 12–15 | E. coli | Black light blue lamp with the major fraction of irradiation occurring at 365 nm | Almost 100% (0.5% Fe-doped, 10 min) | [54] |
| SiO2@ TiO2 | 20.49 | E. coli | UVA (30 W) | 99.3% | [50] |
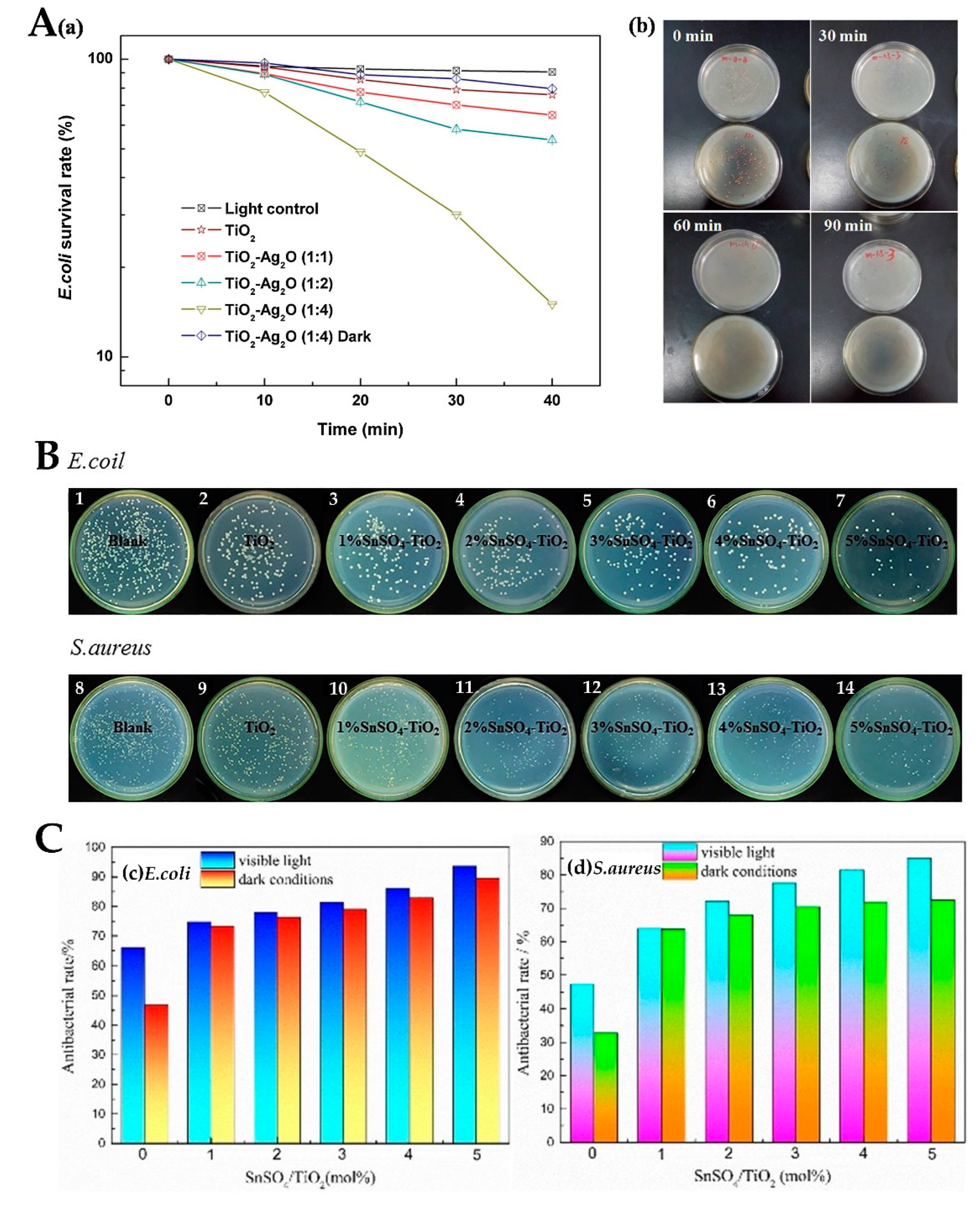
2.3. Antimicrobial Activity under Visible Light
2.3.1. TiO2-Metal Composites
2.3.2. Nonmetal Doping
2.3.3. Polymer Doping, Oxides and Others
| Material | Grain Size: nm | Microorganism | Light Source | Antimicrobial Effect | Reference |
|---|---|---|---|---|---|
| Au/Pt-TiO2 | Au (1 min of coating): 9.36 ± 1.88 (diameter), 8.77 ± 1.90 (height) Pt (2 min of coating): 20.72 ± 5.21 (diameter), 30.42 ± 6.01 (height) | S. aureus | Visible light (470 nm) | Au TiO2 NTs: colony forming unit decreased from approximately 235 to 60 (×104 CFU/mL) Pt TiO2 NTs: colony forming unit decreased from approximately 290 to 75 (×104 CFU/mL) | [59] |
| Ag and Au/TiO2 | Ag:6 Au:14 | E. coli | Visible light (>420 nm) | Ag TiO2 NTs: almost 100% after 1 h Au TiO2 NTs: increased 4 times compared to that under UV irradiation (77.5%) | [89] |
| Cr-TiO2 | 5.8 | E. coli | Visible light (360–740 nm) | >95% (100μg/mL) | [62] |
| Cu, Pt and Ag-TiO2 | 12–15 (catalyst) | E. coli | Visible light (>450 nm) | >99% | [58] |
| Fe-TiO2 | 19.24–22.24 | E. coli | Visible light (400–700 nm) | 97.57% (0.1 at% of Fe-doped TiO2 films) | [61] |
| TiO2(B) | 117–484 (thickness) | E. coli, A. baumannii, S. aureus and S. pyogenes | Visible light (60 W, incandescent lamp) | Approximately 50% | [66] |
| B-TiO2-CNT | 12–18 | E. coli | Visible light (>400 nm) | Almost 100% (3B-TiO2-CNT nanocomposites) | [69] |
| B/Ce-TiO2 | 23 | E. coli and S. aureus | Visible light | Almost 99% (S. aureus) Almost 100% (E. coli) | [71] |
| Ag2O-TiO2 | 5–30 | E. coli | Visible light (>400 nm) | Almost 100% | [83] |
| Cu2O/TiO2 | 8 | E. coli and C. albicans | Visible light (>420 nm) | >99% | [87] |
| CuO/TiO2 | 23.6–24.2(anatase), 42.8–44.5(rutile) | S. aureus | Visible light | Almost 100% | [90] |
| Polyaniline-kapok fiber/TiO2 | 6.06 | E. coli | Visible light | 30% less than that in the dark | [91] |
| Au/ZnO-TiO2 | 10 (Au), 50 (ZnO) | E. coli | - | 98.2% | [92] |
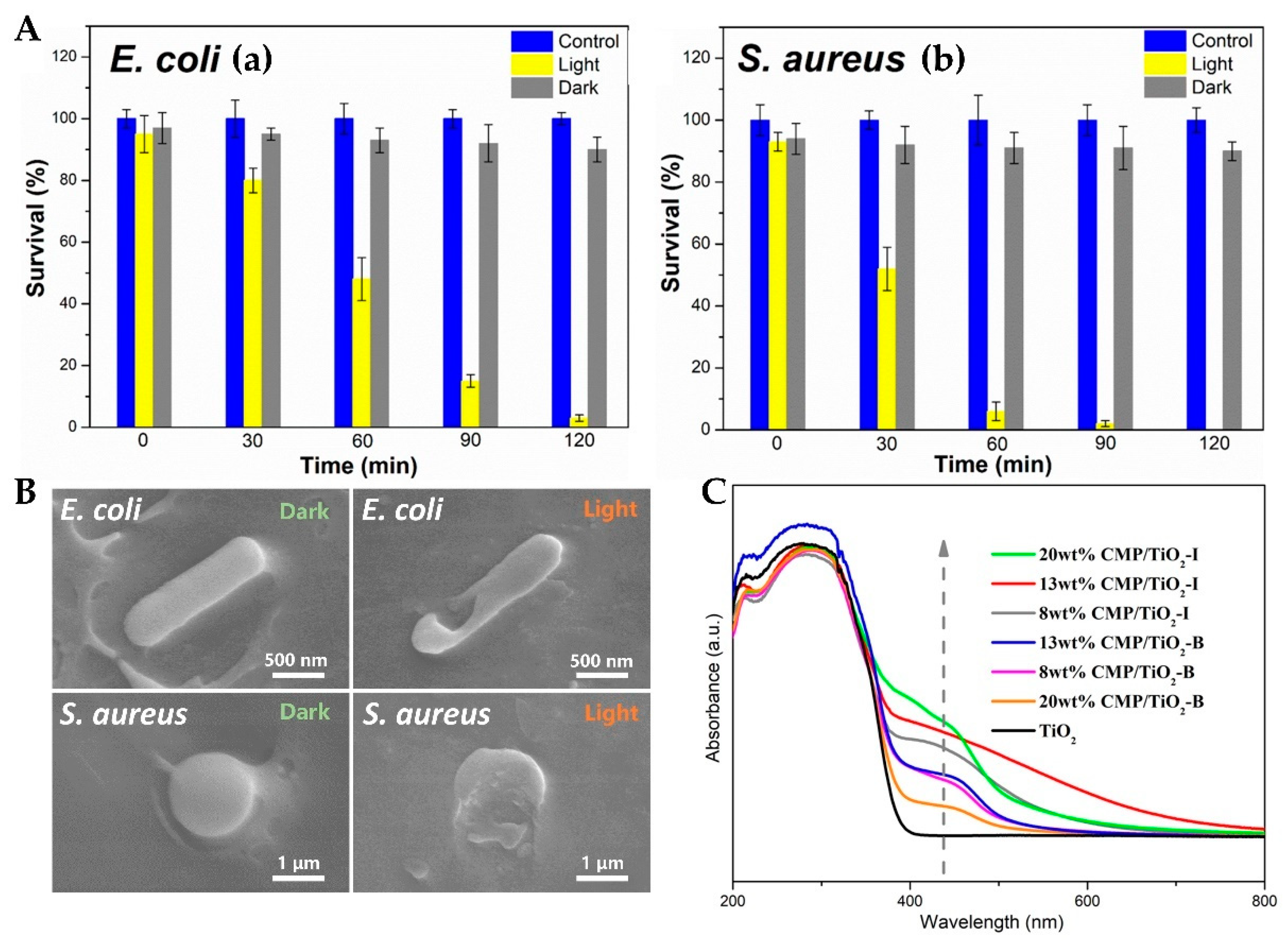
| CMP/TiO2 | 650–950 | E. coli and S. aureus | Visible light (LED) | 98.14% (E. coli), nearly100% (S. aureus) | [75] |
3. Antimicrobial Mechanism
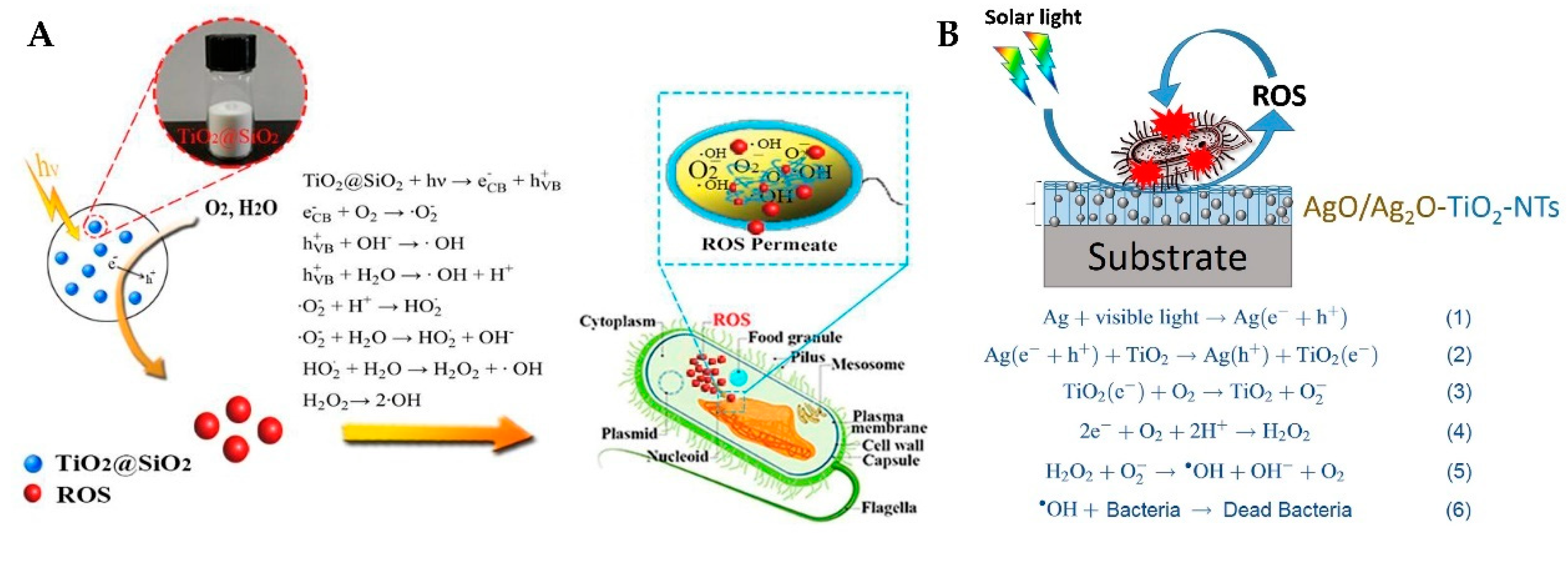
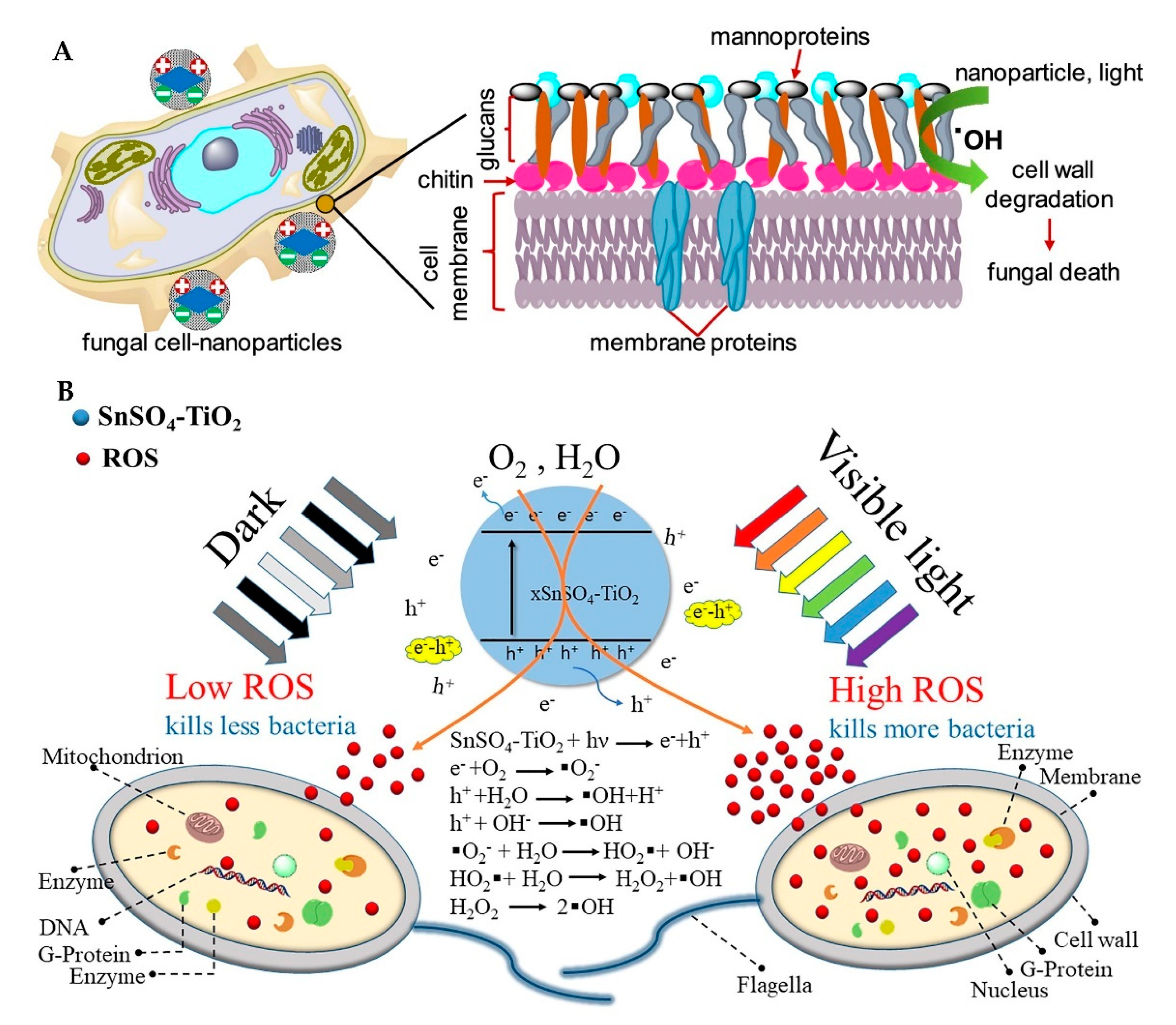
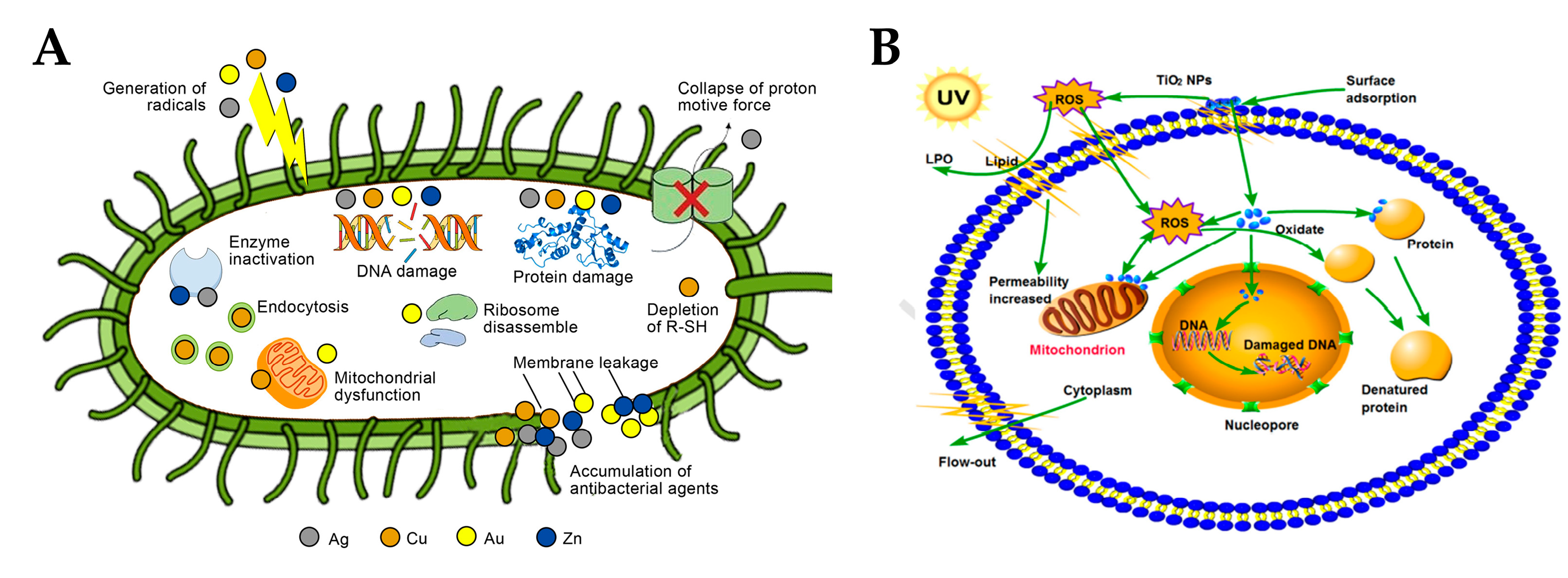
3.1. Production and Function of Reactive Oxygen Species
3.1.1. Production of ROS
3.1.2. Function of ROS
3.2. Release and Function of Metal Ions
4. Applications
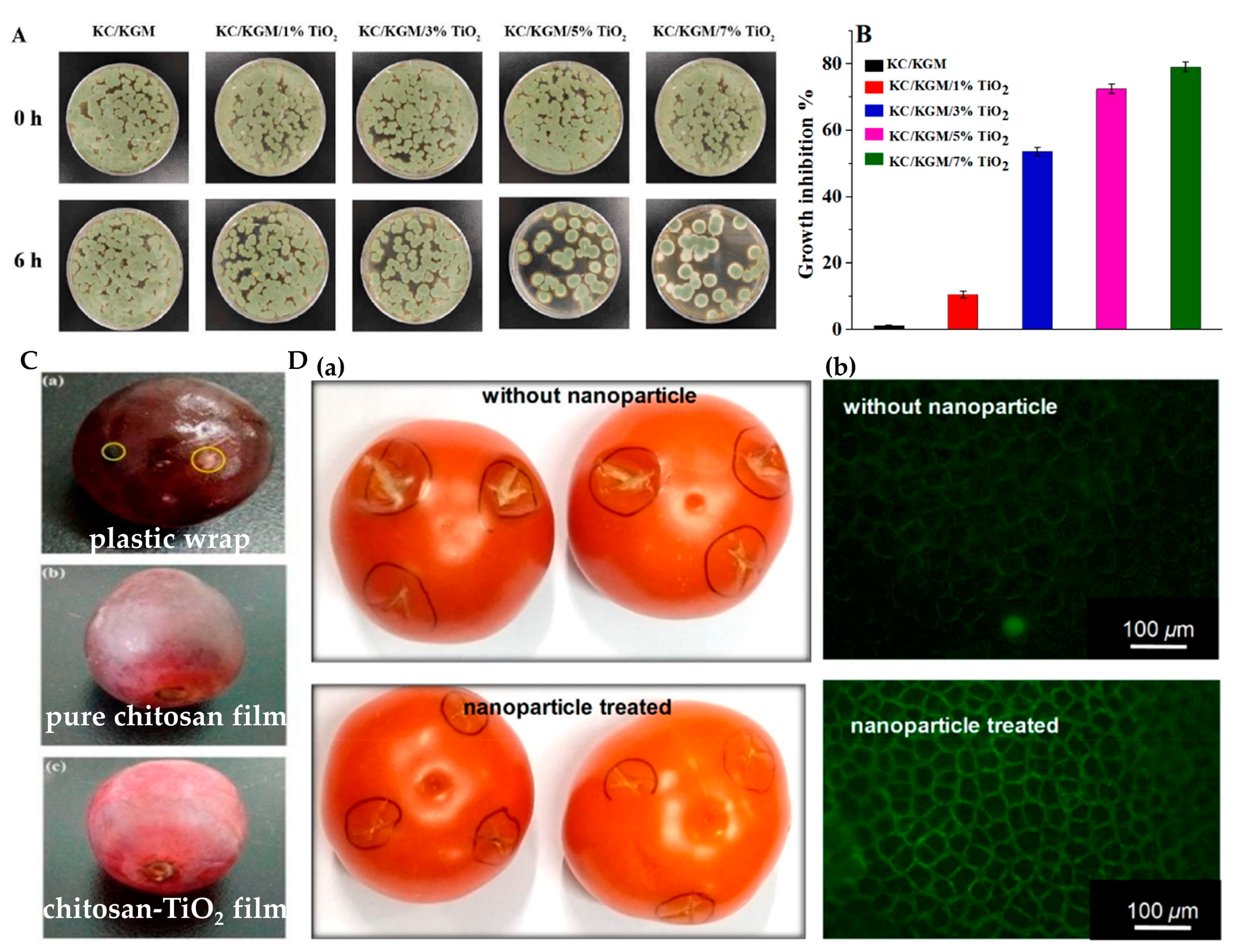
4.1. Food Packaging and Preservation
4.2. Self-Cleaning Fabric
4.3. Others
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Eichelberger, K.R.; Cassat, J.E. Metabolic Adaptations During Staphylococcus aureus and Candida albicans Co-Infection. Front. Immunol. 2021, 12, 797550. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Shen, Y.; Yang, M.; Chi, K.M.; Guo, N. Hazard of Staphylococcal Enterotoxins in Food and Promising Strategies for Natural Products against Virulence. J. Agric. Food Chem. 2022, 70, 2450–2465. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L. Bacterial diarrhoea. Curr. Opin. Pediatr. 2022, 34, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Hadidi, M.; Forough, M.; Nafchi, A.M.; Khaneghah, A.M. The control of fungi and mycotoxins by food active packaging: A review. Crit. Rev. Food Sci. Nutr. 2022, in press. [Google Scholar] [CrossRef]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial textile: Recent developments and functional perspective. Polym. Bull. 2022, 79, 5747–5771. [Google Scholar] [CrossRef]
- Sayinli, B.; Dong, Y.J.; Park, Y.; Bhatnagar, A.; Sillanpaa, M. Recent progress and challenges facing ballast water treatment-A review. Chemosphere 2022, 291, 132776. [Google Scholar] [CrossRef]
- Stanaszek-Tomal, E. Environmental Factors Causing the Development of Microorganisms on the Surfaces of National Cultural Monuments Made of Mineral Building Materials-Review. Coatings 2020, 10, 1203. [Google Scholar] [CrossRef]
- Li, C.; Lin, Q.F.; Dong, F.L.; Li, Y.H.; Luo, F.; Zhang, K.J. Formation of iodinated trihalomethanes during chlorination of amino acid in waters. Chemosphere 2019, 217, 355–363. [Google Scholar] [CrossRef]
- Mustapha, S.; Jimoh, T.; Ndamitso, M.; Abdulkareem, S.A.; Taye, S.D.; Mohammed, A.K.; Amigun, A.T. The Occurrence of N-nitrosodimethylamine (NDMA) in Swimming Pools: An Overview. Environ. Health Insights 2021, 15, 117863022110365. [Google Scholar] [CrossRef]
- Rosenman, K.D.; Reilly, M.J.; Wang, L. Calls to a State Poison Center Concerning Cleaners and Disinfectants From the Onset of the COVID-19 Pandemic through April 2020. Public Health Rep. 2021, 136, 27–31. [Google Scholar] [CrossRef]
- Bonin, L.; Vitry, V.; Olivier, M.G.; Bertolucci-Coelho, L. COVID-19: Effect of disinfection on corrosion of surfaces. Corros. Eng. Sci. Technol. 2020, 55, 693–695. [Google Scholar] [CrossRef]
- Chomcheon, S.; Lenbury, Y.; Sarika, W. Stability, Hopf bifurcation and effects of impulsive antibiotic treatments in a model of drug resistance with conversion delay. Adv. Differ. Equ. 2019, 2019, 274. [Google Scholar] [CrossRef]
- Mohanty, H.; Pachpute, S.; Yadav, R.P. Mechanism of drug resistance in bacteria: Efflux pump modulation for designing of new antibiotic enhancers. Folia Microbiol. 2021, 66, 727–739. [Google Scholar] [CrossRef]
- Goodeve, C.F.; Kitchener, J.A. Photosensitisation by titanium dioxide. Trans. Faraday Soc. 1938, 34, 570–579. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.-S.; Pawar, S.H. Developments in photocatalytic antibacterial activity of nano TiO2: A review. Korean J. Chem. Eng. 2016, 33, 1989–1998. [Google Scholar] [CrossRef]
- Muruganandham, M.; Zhang, Y.F.; Suri, R.; Lee, G.J.; Chen, P.K.; Hsieh, S.H.; Sillanpaa, M.; Wu, J.J. Environmental Applications of ZnO Materials. J. Nanosci. Nanotechnol. 2015, 15, 6900–6913. [Google Scholar] [CrossRef]
- Verbic, A.; Gorjanc, M.; Simoncic, B. Zinc Oxide for Functional Textile Coatings: Recent Advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol-gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Faustini, M.; Grosso, D.; Boissiere, C.; Backov, R.; Sanchez, C. “Integrative sol-gel chemistry”: A nanofoundry for materials science. J. Sol-Gel Sci. Technol. 2014, 70, 216–226. [Google Scholar] [CrossRef]
- Faustini, M. Sol-gel engineering to tune structural colours. J. Sol-Gel Sci. Technol. 2020, 95, 504–516. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Recent Advances in TiO2 Films Prepared by Sol-Gel Methods for Photocatalytic Degradation of Organic Pollutants and Antibacterial Activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review on the synthesis of TiO2 nanoparticles by solution route. Cent. Eur. J. Chem. 2012, 10, 279–294. [Google Scholar] [CrossRef]
- Vargas, M.A.; Rodriguez-Paez, J.E. Amorphous TiO2 nanoparticles: Synthesis and antibacterial capacity. J. Non-Cryst. Solids 2017, 459, 192–205. [Google Scholar] [CrossRef]
- Tahmasebizad, N.; Hamedani, M.T.; Shaban Ghazani, M.; Pazhuhanfar, Y. Photocatalytic activity and antibacterial behavior of TiO2 coatings co-doped with copper and nitrogen via sol-gel method. J. Sol-Gel Sci. Technol. 2020, 93, 570–578. [Google Scholar] [CrossRef]
- Karthik, K.; Vijayalakshmi, S.; Phuruangrat, A.; Revathi, V.; Verma, U. Multifunctional Applications of Microwave-Assisted Biogenic TiO2 Nanoparticles. J. Clust. Sci. 2019, 30, 965–972. [Google Scholar] [CrossRef]
- Stanciu, I.; Predoana, L.; Cusu, J.P.; Preda, S.; Anastasescu, M.; Vojisavljevic, K.; Malic, B.; Zaharescu, M. Thermal behaviour of the TiO2-based gels obtained by microwave-assisted sol-gel method. J. Therm. Anal. Calorim. 2017, 130, 639–651. [Google Scholar] [CrossRef]
- Liu, W.; Ge, M.; Zhu, Y.; Gao, Q. Photocatalytic Dye Degradation by TiO2 Nanocups Synthesized via Microwave-Assisted Solvothermal Method. Nanosci. Nanotechnol. Lett. 2017, 9, 1579–1583. [Google Scholar] [CrossRef]
- Ates, M.; Bayrak, Y.; Yoruk, O.; Caliskan, S. Reduced graphene oxide/Titanium oxide nanocomposite synthesis via microwave-assisted method and supercapacitor behaviors. J. Alloys Compd. 2017, 728, 541–551. [Google Scholar] [CrossRef]
- Lu, Y.J.; Wang, X.; Bi, X.J. Study on the preparation of Ag doped TiO2 catalyst by microwave hydrothermal method and its photocatalytic activity. In Proceedings of the 7th International Conference on Education, Management, Computer and Society (EMCS), Shenyang, China, 17–19 March 2017; pp. 839–845. [Google Scholar]
- Lu, Y.J.; Wang, X.; Bi, X.J. Study on the Preparation of Sm Doped TiO2 Catalyst by Microwave Hydrothermal Method and its Photocatalytic Activity. In Proceedings of the 7th International Conference on Education, Management, Computer and Society (EMCS), Shenyang, China, 17–19 March 2017; pp. 110–117. [Google Scholar]
- Irshad, M.A.; Nawaz, R.; Rehman, M.Z.U.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef] [PubMed]
- Nabi, G.; Qurat ul, A.; Khalid, N.R.; Tahir, M.B.; Rafique, M.; Rizwan, M.; Hussain, S.; Iqbal, T.; Majid, A. A Review on Novel Eco-Friendly Green Approach to Synthesis TiO2 Nanoparticles Using Different Extracts. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1552–1564. [Google Scholar] [CrossRef]
- Goncalves, R.A.; Toledo, R.P.; Joshi, N.; Berengue, O.M. Green Synthesis and Applications of ZnO and TiO2 Nanostructures. Molecules 2021, 26, 2236. [Google Scholar] [CrossRef]
- Sagadevan, S.; Lett, J.A.; Vennila, S.; Prasath, P.V.; Kaliaraj, G.S.; Fatimah, I.; Leonard, E.; Mohammad, F.; Al-Lohedan, H.A.; Alshahateet, S.F.; et al. Photocatalytic activity and antibacterial efficacy of titanium dioxide nanoparticles mediated by Myristica fragrans seed extract. Chem. Phys. Lett. 2021, 771, 138527. [Google Scholar] [CrossRef]
- Marfur, N.A.; Jaafar, N.F.; Khairuddean, M.; Nordin, N. A Review on Recent Progression of Modifications on Titania Morphology and its Photocatalytic Performance. Acta Chim. Slov. 2020, 67, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qian, J.; Zhao, Q.R.; Yang, S.S.; Yang, Y.; Zhao, Q. Hydrothermal synthesis of rutile TiO2 nanotubes film on Ti foil for photocatalytic degradation. Dig. J. Nanomater. Biostruct. 2021, 16, 579–584. [Google Scholar]
- Tang, M.; Xia, Y.; Yang, D.; Liu, J.; Zhu, X.; Tang, R. Effects of Hydrothermal Time on Structure and Photocatalytic Property of Titanium Dioxide for Degradation of Rhodamine B and Tetracycline Hydrochloride. Materials 2021, 14, 5674. [Google Scholar] [CrossRef]
- Chiappim, W.; Testoni, G.; Miranda, F.; Fraga, M.; Furlan, H.; Saravia, D.A.; Sobrinho, A.D.; Petraconi, G.; Maciel, H.; Pessoa, R. Effect of Plasma-Enhanced Atomic Layer Deposition on Oxygen Overabundance and Its Influence on the Morphological, Optical, Structural, and Mechanical Properties of Al-Doped TiO2 Coating. Micromachines 2021, 12, 588. [Google Scholar] [CrossRef]
- Al-Maliki, F.J.; Al-Rubaiy, M.A. Synthesis and study the concentration effect on the photocatalytic activity of titania nanoparticles as anti-bactria using reactive magnetron sputtering technique. Opt. Quantum Electron. 2022, 54, 1–11. [Google Scholar] [CrossRef]
- Lou, B.S.; Chen, W.T.; Diyatmika, W.; Lu, J.H.; Chang, C.T.; Chen, P.W.; Lee, J.W. High power impulse magnetron sputtering (HiPIMS) for the fabrication of antimicrobial and transparent TiO2 thin films. Curr. Opin. Chem. Eng. 2022, 36, 100782. [Google Scholar] [CrossRef]
- Pessoa, R.S.; dos Santos, V.P.; Cardoso, S.B.; Doria, A.; Figueira, F.R.; Rodrigues, B.V.M.; Testoni, G.E.; Fraga, M.A.; Marciano, F.R.; Lobo, A.O.; et al. TiO2 coatings via atomic layer deposition on polyurethane and polydimethylsiloxane substrates: Properties and effects on C. albicans growth and inactivation process. Appl. Surf. Sci. 2017, 422, 73–84. [Google Scholar] [CrossRef]
- Abdulagatov, I.M.; Ragimov, R.M.; Khamidov, M.A.; Maksumova, A.M.; Abdullaeva, N.M. ALD coated polypropylene hernia meshes for prevention of mesh-related post-surgery complications: An experimental study in animals. Biomed. Mater. 2022, 17, 015006. [Google Scholar] [CrossRef]
- Qi, L.; Guo, B.H.; Lu, Q.; Gong, H.H.; Wang, M.; He, J.L.; Jia, B.; Ren, J.; Zheng, S.C.; Lu, Y.F. Preparation and Photocatalytic and Antibacterial Activities of Micro/Nanostructured TiO2-Based Photocatalysts for Application in Orthopedic Implants. Front. Mater. 2022, 9, 914905. [Google Scholar] [CrossRef]
- Smietana, M.; Drazewski, T.; Firek, P.; Mikulic, P.; Bock, W.J. Comparison of Al2O3 nano-overlays deposited with Magnetron Sputtering and Atomic Layer Deposition on optical fibers for sensing purposes. In Proceedings of the Conference on Micro/Nano Materials, Devices, and Systems, Melbourne, Australia, 9–11 December 2013. [Google Scholar]
- Sibanda, D.; Oyinbo, S.T.; Jen, T.C. A review of atomic layer deposition modelling and simulation methodologies: Density functional theory and molecular dynamics. Nanotechnol. Rev. 2022, 11, 1332–1363. [Google Scholar] [CrossRef]
- Pantaroto, H.N.; Ricomini-Filho, A.P.; Bertolini, M.M.; Dias da Silva, J.H.; Azevedo Neto, N.F.; Sukotjo, C.; Rangel, E.C.; Barao, V.A.R. Antibacterial photocatalytic activity of different crystalline TiO2 phases in oral multispecies biofilm. Dent. Mater. 2018, 34, E182–E195. [Google Scholar] [CrossRef]
- Moongraksathum, B.; Chien, M.-Y.; Chen, Y.-W. Antiviral and Antibacterial Effects of Silver-Doped TiO2 Prepared by the Peroxo Sol-Gel Method. J. Nanosci. Nanotechnol. 2019, 19, 7356–7362. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, X.; Gao, X.; Zhang, B.; Luo, Y.; Yao, X. Antimicrobial property and photocatalytic antibacterial mechanism of the TiO2-doped SiO2 hybrid materials under ultraviolet-light irradiation and visible-light irradiation. Ceram. Int. 2019, 45, 15505–15513. [Google Scholar] [CrossRef]
- Hayashi, K.; Nozaki, K.; Tan, Z.; Fujita, K.; Nemoto, R.; Yamashita, K.; Miura, H.; Itaka, K.; Ohara, S. Enhanced Antibacterial Property of Facet-Engineered TiO2 Nanosheet in Presence and Absence of Ultraviolet Irradiation. Materials 2020, 13, 78. [Google Scholar] [CrossRef]
- Moon, E.W.; Lee, H.-W.; Rok, J.H.; Ha, J.-H. Photocatalytic inactivation of viral particles of human norovirus by Cu-doped TiO2 non-woven fabric under UVA-LED wavelengths. Sci. Total Environ. 2020, 749, 141574. [Google Scholar] [CrossRef]
- Li, X.Y.; Xie, J.G.; Liao, L.Y.; Jiang, X.; Fu, H.Q. UV-curable polyurethane acrylate-Ag/TiO2 nanocomposites with superior UV light antibacterial activity. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 835–843. [Google Scholar] [CrossRef]
- Stoyanova, A.M.; Hitkova, H.Y.; Ivanova, N.K.; Bachvarova-Nedelcheva, A.D.; Iordanova, R.S.; Sredkova, M.P. Photocatalytic and antibacterial activity of Fe-doped TiO2 nanoparticles prepared by nonhydrolytic sol-gel method. Bulg. Chem. Commun. 2013, 45, 497–504. [Google Scholar]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C-Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.e.K.; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef] [PubMed]
- Wysocka, I.; Kowalska, E.; Ryl, J.; Nowaczyk, G.; Zielinska-Jurek, A. Morphology, Photocatalytic and Antimicrobial Properties of TiO2 Modified with Mono- and Bimetallic Copper, Platinum and Silver Nanoparticles. Nanomaterials 2019, 9, 1129. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.-S.; Choi, E.-J.; Bae, J.-M.; Park, Y.-B.; Oh, S. Visible Light-Enhanced Antibacterial and Osteogenic Functionality of Au and Pt Nanoparticles Deposited on TiO2 Nanotubes. Materials 2020, 13, 3721. [Google Scholar] [CrossRef]
- Lee, H.; Jang, H.S.; Kim, N.Y.; Joo, J.B. Cu-doped TiO2 hollow nanostructures for the enhanced photocatalysis under visible light conditions. J. Ind. Eng. Chem. 2021, 99, 352–363. [Google Scholar] [CrossRef]
- Meng, D.; Liu, X.; Xie, Y.; Du, Y.; Yang, Y.; Xiao, C. Antibacterial Activity of Visible Light-Activated TiO2 Thin Films with Low Level of Fe Doping. Adv. Mater. Sci. Eng. 2019, 2019, 5819805. [Google Scholar] [CrossRef]
- Gomez-Polo, C.; Larumbe, S.; Gil, A.; Munoz, D.; Fernandez, L.R.; Barquin, L.F.; Garcia-Prieto, A.; Fdez-Gubieda, M.L.; Muela, A. Improved photocatalytic and antibacterial performance of Cr doped TiO2 nanoparticles. Surf. Interfaces 2021, 22, 100867. [Google Scholar] [CrossRef]
- Yoo, S.M.; Rawal, S.B.; Lee, J.E.; Kim, J.; Ryu, H.-Y.; Park, D.-W.; Lee, W.I. Size-dependence of plasmonic Au nanoparticles in photocatalytic behavior of Au/TiO2 and Au@SiO2/TiO2. Appl. Catal. A-Gen. 2015, 499, 47–54. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, X.; Liu, J.; Tian, Z.; Dai, L.; He, B.; Han, C.; Wu, Y.; Zeng, Z.; Hu, Z. On the role of localized surface plasmon resonance in UV-Vis light irradiated Au/TiO2 photocatalysis systems: Pros and cons. Nanoscale 2015, 7, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
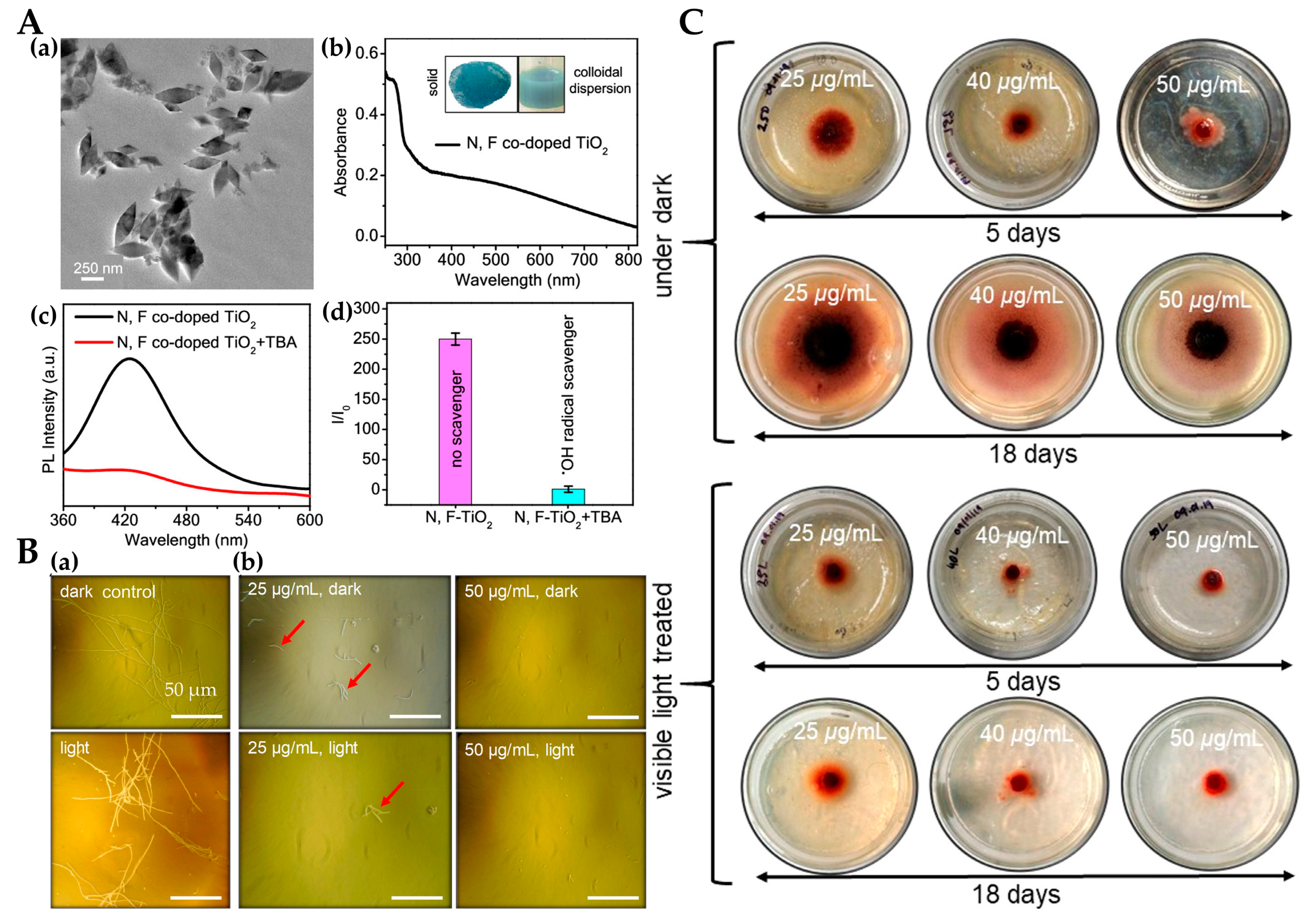
- Mukherjee, K.; Acharya, K.; Biswas, A.; Jana, N.R. TiO2 Nanoparticles Co-doped with Nitrogen and Fluorine as Visible-Light-Activated Antifungal Agents. ACS Appl. Nano Mater. 2020, 3, 2016–2025. [Google Scholar] [CrossRef]
- Wong, M.-S.; Sun, M.-T.; Sun, D.-S.; Chang, H.-H. Visible-Light-Responsive Antibacterial Property of Boron-Doped Titania Films. Catalysts 2020, 10, 1349. [Google Scholar] [CrossRef]
- Sun, D.-S.; Kau, J.-H.; Huang, H.-H.; Tseng, Y.-H.; Wu, W.-S.; Chang, H.-H. Antibacterial Properties of Visible-Light-Responsive Carbon-Containing Titanium Dioxide Photocatalytic Nanoparticles against Anthrax. Nanomaterials 2016, 6, 237. [Google Scholar] [CrossRef]
- Islam, S.Z.; Nagpure, S.; Kim, D.Y.; Rankin, S.E. Synthesis and Catalytic Applications of Non-Metal Doped Mesoporous Titania. Inorganics 2017, 5, 15. [Google Scholar] [CrossRef]
- Koli, V.B.; Ke, S.-C.; Dodamani, A.G.; Deshmukh, S.P.; Kim, J.-S. Boron-Doped TiO2-CNT Nanocomposites with Improved Photocatalytic Efficiency toward Photodegradation of Toluene Gas and Photo-Inactivation of Escherichia coli. Catalysts 2020, 10, 632. [Google Scholar] [CrossRef]
- Kilic, A.; Beyazsakal, L.; Findik, B.T.; Incebay, H. Synthesis and electrochemical investigation of chiral amine bis(phenolate)- boron complexes: In vitro antibacterial activity screening of boron compounds. Inorg. Chim. Acta 2020, 510, 119777. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Wu, Y.S.; Yang, H.; Wang, M.; Shi, X.G.; Wang, C.; Zhang, S.W. Effect of calcination temperature on the microstructure and antimicrobial activity of boron and cerium co-doped titania nanomaterials. Mater. Technol. 2018, 33, 48–56. [Google Scholar] [CrossRef]
- Raja, A.; Selvakumar, K.; Rajasekaran, P.; Arunpandian, M.; Ashokkumar, S.; Kaviyarasu, K.; Bahadur, S.A.; Swaminathan, M. Visible active reduced graphene oxide loaded titania for photodecomposition of ciprofloxacin and its antibacterial activity. Colloids Surf. A-Physicochem. Eng. Asp. 2019, 564, 23–30. [Google Scholar] [CrossRef]
- Nasr, M.; Balme, S.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Enhanced Visible-Light Photocatalytic Performance of Electrospun rGO/TiO2 Composite Nanofibers. J. Phys. Chem. C 2017, 121, 261–269. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Gao, X.; Li, T.; Zhang, M.; Niu, X.; Zhang, D.; Wang, Y.; Fan, H.; Wang, K. Preparation and Performance Study of Antibacterial Materials Based on GO-TiO2. Chemistryselect 2021, 6, 7880–7886. [Google Scholar] [CrossRef]
- Wu, Y.; Zang, Y.; Xu, L.; Wang, J.; Jia, H.; Miao, F. Synthesis of high-performance conjugated microporous polymer/TiO2 photocatalytic antibacterial nanocomposites. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 126, 112121. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Kubacka, M.; Gabala, E.; Dobrowolska, A.; Synoradzki, K.; Siwinska-Ciesielczyk, K.; Czaczyk, K.; Jesionowski, T. Hydrothermally Assisted Fabrication of TiO2-Fe3O4 Composite Materials and Their Antibacterial Activity. Materials 2020, 13, 4715. [Google Scholar] [CrossRef] [PubMed]
- Tekin, D.; Birhan, D.; Kiziltas, H. Thermal, photocatalytic, and antibacterial properties of calcinated nano-TiO2/polymer composites. Mater. Chem. Phys. 2020, 251, 123067. [Google Scholar] [CrossRef]
- Yuan, C.; Hung, C.-H.; Yuan, C.-S.; Li, H.-W. Preparation and Application of Immobilized Surfactant-Modified PANi-CNT/TiO2 under Visible-Light Irradiation. Materials 2017, 10, 877. [Google Scholar] [CrossRef]
- Saravanan, R.; Aviles, J.; Gracia, F.; Mosquera, E.; Gupta, V.K. Crystallinity and lowering band gap induced visible light photocatalytic activity of TiO2/CS (Chitosan) nanocomposites. Int. J. Biol. Macromol. 2018, 109, 1239–1245. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, S.; Li, S.; Zhou, X.; Yin, J.; Liu, P.; Shi, W.; Wu, M.; Shen, L. Electrospinning visible light response Bi2MoO6/Ag3PO4 composite photocatalytic nanofibers with enhanced photocatalytic and antibacterial activity. Appl. Surf. Sci. 2021, 569, 150955. [Google Scholar] [CrossRef]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent developments in the use of metal oxides for photocatalytic degradation of pharmaceutical pollutants in water—A review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Yan, Y.; Li, C.Q.; Wu, Y.H.; Gao, J.K.; Zhang, Q.C. From isolated Ti-oxo clusters to infinite Ti-oxo chains and sheets: Recent advances in photoactive Ti-based MOFs. J. Mater. Chem. A 2020, 8, 15245–15270. [Google Scholar] [CrossRef]
- Du, Y.; Tang, D.; Zhang, G.; Wu, X. Facile synthesis of Ag2O-TiO2/sepiolite composites with enhanced visible-light photocatalytic properties. Chin. J. Catal. 2015, 36, 2219–2228. [Google Scholar] [CrossRef]
- Ton, N.Q.T.; Le, T.N.T.; Kim, S.; Dao, V.A.; Yi, J.; Vu, T.H.T. High-Efficiency Photo-Generated Charges of ZnO/TiO2 Heterojunction Thin Films for Photocatalytic and Antibacterial Performance. J. Nanosci. Nanotechnol. 2020, 20, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Mu, L.; Han, B.; Zhang, J.; Shi, H. Fabrication of TiO2/Ag2O heterostructure with enhanced photocatalytic and antibacterial activities under visible light irradiation. Appl. Surf. Sci. 2017, 396, 1596–1603. [Google Scholar] [CrossRef]
- Kiwi, J.; Rtimi, S. Mechanisms of the Antibacterial Effects of TiO2-FeOx under Solar or Visible Light: Schottky Barriers versus Surface Plasmon Resonance. Coatings 2018, 8, 391. [Google Scholar] [CrossRef]
- Janczarek, M.; Endo, M.; Zhang, D.; Wang, K.; Kowalska, E. Enhanced Photocatalytic and Antimicrobial Performance of Cuprous Oxide/Titania: The Effect of Titania Matrix. Materials 2018, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.Y.; Zhang, B.; Cui, S.; Yang, S.; Tang, X.N. Fabrication of SnSO4-modified TiO2 for enhance degradation performance of methyl orange (MO) and antibacterial activity. Appl. Surf. Sci. 2021, 551, 149419. [Google Scholar] [CrossRef]
- Endo, M.; Wei, Z.; Wang, K.; Karabiyik, B.; Yoshiiri, K.; Rokicka, P.; Ohtani, B.; Markowska-Szczupak, A.; Kowalska, E. Noble metal-modified titania with visible-light activity for the decomposition of microorganisms. Beilstein J. Nanotechnol. 2018, 9, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, P.; Eslami, A.; Bahrami, A.; Hosseini-Abari, A.; Saber, F.Y.; Mohammadi, R.; Mehr, M.Y. Surface modification of TiO2 nanoparticles with CuO for visible-light antibacterial applications and photocatalytic degradation of antibiotics. Ceram. Int. 2021, 47, 33875–33885. [Google Scholar] [CrossRef]
- Gapusan, R.B.; Balela, M.D.L. Visible light-induced photocatalytic and antibacterial activity of TiO2/polyaniline-kapok fiber nanocomposite. Polym. Bull. 2021, 47, 33875–33885. [Google Scholar] [CrossRef]
- Liang, Y.; Li, W.; Wang, X.; Zhou, R.; Ding, H. TiO2-ZnO/Au ternary heterojunction nanocomposite: Excellent antibacterial property and visible-light photocatalytic hydrogen production efficiency. Ceram. Int. 2022, 48, 2826–2832. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef]
- Selvam, S.; Sundrarajan, M. Functionalization of cotton fabric with PVP/ZnO nanoparticles for improved reactive dyeability and antibacterial activity. Carbohydr. Polym. 2012, 87, 1419–1424. [Google Scholar] [CrossRef]
- Guo, M.Y.; Ng, A.M.C.; Liu, F.Z.; Djurisic, A.B.; Chan, W.K. Photocatalytic activity of metal oxides-The role of holes and OH center dot radicals. Appl. Catal. B -Environ. 2011, 107, 150–157. [Google Scholar] [CrossRef]
- Guo, M.Y.; Ng, A.M.C.; Liu, F.Z.; Djurisic, A.B.; Chan, W.K.; Su, H.M.; Wong, K.S. Effect of Native Defects on Photocatalytic Properties of ZnO. J. Phys. Chem. C 2011, 115, 11095–11101. [Google Scholar] [CrossRef]
- Yu, J.L.; Tahmasebi, A.; Han, Y.; Yin, F.; Li, X. A review on water in low rank coals: The existence, interaction with coal structure and effects on coal utilization. Fuel Processing Technol. 2013, 106, 9–20. [Google Scholar] [CrossRef]
- Yougbare, S.; Mutalik, C.; Okoro, G.; Lin, I.H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.C.; Kuo, T.R. Emerging Trends in Nanomaterials for Antibacterial Applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef]
- Aruoja, V.; Dubourguier, H.-C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef]
- Li, K.; Chen, Y.; Zhang, W.; Pu, Z.; Jiang, L.; Chen, Y. Surface Interactions Affect the Toxicity of Engineered Metal Oxide Nanoparticles toward Paramecium. Chem. Res. Toxicol. 2012, 25, 1675–1681. [Google Scholar] [CrossRef]
- Agmon, E.; Solon, J.; Bassereau, P.; Stockwell, B.R. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci. Rep. 2018, 8, 5515. [Google Scholar] [CrossRef]
- Hossein, M.M.; Potash, S.A.; Takikawa, M.; Shubhra, R.D.; Saha, T.; Islam, Z.; Hossein, S.; Hasan, M.A.; Takeoka, S.; Sarker, S.R. Investigation of the Antibacterial Activity and in vivo Cytotoxicity of Biogenic Silver Nanoparticles as Potent Therapeutics. Front. Bioeng. Biotechnol. 2019, 7, 239. [Google Scholar] [CrossRef]
- Niloy, M.S.; Hossain, M.M.; Takikawa, M.; Shakil, M.S.; Polash, S.A.; Mahmud, K.M.; Uddin, M.F.; Alam, M.; Shubhra, R.D.; Shawan, M.; et al. Synthesis of Biogenic Silver Nanoparticles Using Caesalpinia digyna and Investigation of Their Antimicrobial Activity and In Vivo Biocompatibility. ACS Appl. Bio Mater. 2020, 3, 7722–7733. [Google Scholar] [CrossRef]
- Yan, B.; Ai, Y.W.; Sun, Q.; Ma, Y.; Cao, Y.; Wang, J.W.; Zhang, Z.Y.; Wang, X.D. Membrane Damage during Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1. Mol. Cell 2021, 81, 355–369.e10. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-E.; Li, Z.-H.; Zheng, W.; Zhao, Y.-F.; Jin, Y.-F.; Tang, Z.-X. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: A review. Food Addit. Contam. Part A -Chem. Anal. Control Expo. Risk Assess. 2014, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yi, Z.; Chen, D.; Duan, X.; Zhou, Z.; Fan, X.; Jiang, M. Evaluation of photocatalytic production of active oxygen and decomposition of phenol in ZnO suspensions. Rare Met. 2011, 30, 188–191. [Google Scholar] [CrossRef]
- Yang, C.; Li, Q.; Tang, L.; Xin, K.; Bai, A.; Yu, Y. Synthesis, photocatalytic activity, and photogenerated hydroxyl radicals of monodisperse colloidal ZnO nanospheres. Appl. Surf. Sci. 2015, 357, 1928–1938. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Wang, L.; Hao, Y.; Yang, L.; He, P.; Xue, J. Preparation and Characterization of Sulfated TiO2/zeolite Composite Catalysts with Enhanced Photocatalytic Activity. Nano 2018, 13, 49–59. [Google Scholar] [CrossRef]
- Gao, X.D.; Fei, G.T.; Xu, S.H.; Zhong, B.N.; Ouyang, H.M.; Li, X.H.; Zhang, L.D. Porous Ag/TiO2-Schottky-diode based plasmonic hot-electron photodetector with high detectivity and fast response. Nanophotonics 2019, 8, 1247–1254. [Google Scholar] [CrossRef]
- Mandal, S.S.; Bhattacharyya, A.J. Electrochemical sensing and photocatalysis using Ag-TiO2 microwires. J. Chem. Sci. 2012, 124, 969–978. [Google Scholar] [CrossRef]
- Hajjaji, A.; Elabidi, M.; Trabelsi, K.; Assadi, A.A.; Bessais, B.; Rtimi, S. Bacterial adhesion and inactivation on Ag decorated TiO2-nanotubes under visible light: Effect of the nanotubes geometry on the photocatalytic activity. Colloids Surf. B -Biointerfaces 2018, 170, 92–98. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Ma, J.; Peng, Y.; Wang, A. A review on bidirectional analogies between the photocatalysis and antibacterial properties of ZnO. J. Alloys Compd. 2019, 783, 898–918. [Google Scholar] [CrossRef]
- Cadet, J.; Teoule, R. Comparative study of oxidation of nucleic acid components by hydroxyl radicals, singlet oxygen and superoxide anion radicals. Photochem. Photobiol. 1978, 28, 661–667. [Google Scholar] [CrossRef]
- Chen, B.B.; Pan, N.L.; Liao, J.X.; Huang, M.Y.; Jiang, D.C.; Wang, J.J.; Qiu, H.J.; Chen, J.X.; Li, L.; Sun, J. Cyclometalated iridium(III) complexes as mitochondria-targeted anticancer and antibacterial agents to induce both autophagy and apoptosis. J. Inorg. Biochem. 2021, 219, 111450. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Mani, A. Photocatalytic, antibacterial and anticancer activity of silver-doped zinc oxide nanoparticles. J. Saudi Chem. Soc. 2020, 24, 1010–1024. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.Z.; Binh, C.T.T.; Kelly, J.J.; Gaillard, J.F.; Gray, K.A. Cytotoxicity of commercial nano-TiO2 to Escherichia coli assessed by high-throughput screening: Effects of environmental factors. Water Res. 2013, 47, 2352–2362. [Google Scholar] [CrossRef]
- Li, H.Y.; Gao, Y.C.; Li, C.X.; Ma, G.; Shang, Y.L.; Sun, Y. A comparative study of the antibacterial mechanisms of silver ion and silver nanoparticles by Fourier transform infrared spectroscopy. Vib. Spectrosc. 2016, 85, 112–121. [Google Scholar] [CrossRef]
- Shaligram, S.; Campbell, A. Toxicity of copper salts is dependent on solubility profile and cell type tested. Toxicol. Vitr. 2013, 27, 844–851. [Google Scholar] [CrossRef]
- Chang, L.; Duan, W.J.; Chen, A.G.; Li, J.J.; Huang, S.Q.; Tang, H.J.; Pan, G.; Deng, Y.; Zhao, L.N.; Li, D.F.; et al. Preparation of polyacrylonitrile-based fibres with chelated Ag ions for antibacterial applications. R. Soc. Open Sci. 2020, 7, 200324. [Google Scholar] [CrossRef]
- Saidin, S.; Jumat, M.A.; Amin, N.; Al-Hammadi, A.S.S. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mat. Sci. Eng. C-Mater. 2021, 118, 111382. [Google Scholar] [CrossRef]
- Shi, A.Q.; Zhu, C.S.; Fu, S.; Wang, R.X.; Qin, G.W.; Chen, D.F.; Zhang, E.L. What controls the antibacterial activity of Ti-Ag alloy, Ag ion or Ti2Ag particles? Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 109, 110548. [Google Scholar] [CrossRef]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 2010, 34, 389–412. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. Fems Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced Antibacterial Activity of Nanocrystalline ZnO Due to Increased ROS-Mediated Cell Injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Wahab, R.; Siddiqui, M.A.; Saquib, Q.; Dwivedi, S.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Shin, H.-S. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. B -Biointerfaces 2014, 117, 267–276. [Google Scholar] [CrossRef]
- Engineering, F. Antimicrobial materials, coatings and biomimetic surfaces with modified microtography to control microbial fouling of product contact surfaces within food processing equipment: Legislation, requirements, effectiveness and challenges. J. Hyg. Eng. Des. 2014, 7, 8–29. [Google Scholar]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-Light Active Titanium Dioxide Nanomaterials with Bactericidal Properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef]
- Yuan, X.; Wei, Q.; Ke, H.; Huang, Z.; Chen, D. Structural color and photocatalytic property of polyester fabrics coated with Ag/ZnO composite films. Int. J. Cloth. Sci. Technol. 2019, 31, 487–494. [Google Scholar] [CrossRef]
- Akyildiz, H.I.; Diler, S.; Islam, S. Evaluation of TiO2 and ZnO atomic layer deposition coated polyamide 66 fabrics for photocatalytic activity and antibacterial applications. J. Vac. Sci. Technol. A 2021, 39, 022405. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.L.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Lim, E.S.; Kim, J.J.; Sul, W.J.; Kim, J.S.; Kim, B.; Kim, H.; Koo, O.K. Metagenomic Analysis of Microbial Composition Revealed Cross-Contamination Pathway of Bacteria at a Foodservice Facility. Front. Microbiol. 2021, 12, 636329. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Nonthermal Processes for Shelf-Life Extension of Seafoods: A Revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Forghani, F.; Daliri, E.B.-M.; Li, J.; Liao, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Microbial response to some nonthermal physical technologies. Trends Food Sci. Technol. 2020, 95, 107–117. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Cheng, J.-H.; Sun, D.-W. Effects of nonthermal food processing technologies on food allergens: A review of recent research advances. Trends Food Sci. Technol. 2018, 74, 12–25. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef]
- Duan, N.; Li, Q.; Meng, X.; Wang, Z.; Wu, S. Preparation and characterization of k-carrageenan/konjac glucomannan/TiO2 nanocomposite film with efficient anti-fungal activity and its application in strawberry preservation. Food Chem. 2021, 364, 130441. [Google Scholar] [CrossRef]
- Roman, L.E.; Huachani, J.; Uribe, C.; Solis, J.L.; Gomez, M.M.; Costa, S.; Costa, S. Blocking erythemally weighted UV radiation using cotton fabrics functionalized with ZnO nanoparticles in situ. Appl. Surf. Sci. 2019, 469, 204–212. [Google Scholar] [CrossRef]
- Tian, H.; Zhai, Y.; Xu, C.; Liang, J. Durable Antibacterial Cotton Fabrics Containing Stable Acyclic N-Halamine Groups. Ind. Eng. Chem. Res. 2017, 56, 7902–7909. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, S.X.; Peng, L.; Wang, Y.; Shang, S.; Miao, D.; Guo, R. AgNps-PVA-coated woven cotton fabric: Preparation, water repellency, shielding properties and antibacterial activity. J. Ind. Text. 2019, 48, 1545–1565. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M. Production of cotton fabrics with durable antibacterial property by using gum tragacanth and silver. Int. J. Biol. Macromol. 2018, 109, 476–482. [Google Scholar] [CrossRef]
- Zhu, Y.; Mao, K.L.; Rong, J.; Zheng, Y.H.; Yang, D.Y.; Zhang, T.; Qiu, F.X. Fabrication of titanium incorporation into zeolitic imidazolate framework-L on fabric carrier for visible-light driven photocatalytic antibacterial activity. Opt. Mater. 2022, 125, 112085. [Google Scholar] [CrossRef]
- Biswas, A.; Jana, N.R. Cotton Modified with Silica Nanoparticles, N,F Codoped TiO2 Nanoparticles, and Octadecyltrimethoxysilane for Textiles with Self-Cleaning and Visible Light-Based Cleaning Properties. ACS Appl. Nano Mater. 2021, 4, 877–885. [Google Scholar] [CrossRef]
- Yuzer, B.; Aydin, M.I.; Con, A.H.; Inan, H.; Can, S.; Selcuk, H.; Kadmi, Y. Photocatalytic, self-cleaning and antibacterial properties of Cu(II) doped TiO2. J. Environ. Manag. 2022, 302, 114023. [Google Scholar] [CrossRef] [PubMed]
- Verdier, T.; Bertron, A.; Erable, B.; Roques, C. Bacterial Biofilm Characterization and Microscopic Evaluation of the Antibacterial Properties of a Photocatalytic Coating Protecting Building Material. Coatings 2018, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Janus, M.; Kusiak-Nejman, E.; Rokicka-Konieczna, P.; Markowska-Szczupak, A.; Zajac, K.; Morawski, A.W. Bacterial Inactivation on Concrete Plates Loaded with Modified TiO2 Photocatalysts under Visible Light Irradiation. Molecules 2019, 24, 3026. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cheng, J.; Lo, I.M.C. Green photocatalytic disinfection of real sewage: Efficiency evaluation and toxicity assessment of eco-friendly TiO2-based magnetic photocatalyst under solar light. Water Res. 2021, 190, 116705. [Google Scholar] [CrossRef]
- Tshangana, C.; Chabalala, M.; Muleja, A.; Nxumalo, E.; Mamba, B. Shape-dependant photocatalytic and antimicrobial activity of ZnO nanostructures when conjugated to graphene quantum dots. J. Environ. Chem. Eng. 2020, 8, 103930. [Google Scholar] [CrossRef]
- Sen Chang, J.; Chong, M.N.; Poh, P.E.; Ocon, J.D.; Zoqratt, M.Z.H.M.; Lee, S.M. Impacts of morphological-controlled ZnO nanoarchitectures on aerobic microbial communities during real wastewater treatment in an aerobic-photocatalytic system. Environ. Pollut. 2020, 259, 113867. [Google Scholar] [CrossRef]
- Melinte, V.; Buruiana, T.; Rosca, I.; Chibac, A.L. TiO2-Based Photopolymerized Hybrid Catalysts with Visible Light Catalytic Activity Induced by In Situ Generated Ag/Au NPs. Chemistryselect 2019, 4, 5138–5149. [Google Scholar] [CrossRef]
- Si, Y.; Liu, H.; Yu, H.; Jiang, X.; Sun, D. A heterogeneous TiO2/SrTiO3 coating on titanium alloy with excellent photocatalytic antibacterial, osteogenesis and tribocorrosion properties. Surf. Coat. Technol. 2022, 431, 128008. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, C.; Bu, J.; Song, C. Preparation, Antimicrobial Properties under Different Light Sources, Mechanisms and Applications of TiO2: A Review. Materials 2022, 15, 5820. https://doi.org/10.3390/ma15175820
Shang C, Bu J, Song C. Preparation, Antimicrobial Properties under Different Light Sources, Mechanisms and Applications of TiO2: A Review. Materials. 2022; 15(17):5820. https://doi.org/10.3390/ma15175820
Chicago/Turabian StyleShang, Changyu, Junyu Bu, and Cui Song. 2022. "Preparation, Antimicrobial Properties under Different Light Sources, Mechanisms and Applications of TiO2: A Review" Materials 15, no. 17: 5820. https://doi.org/10.3390/ma15175820
APA StyleShang, C., Bu, J., & Song, C. (2022). Preparation, Antimicrobial Properties under Different Light Sources, Mechanisms and Applications of TiO2: A Review. Materials, 15(17), 5820. https://doi.org/10.3390/ma15175820







