Altering Microbiomes with Hydroxyapatite Nanoparticles: A Metagenomic Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
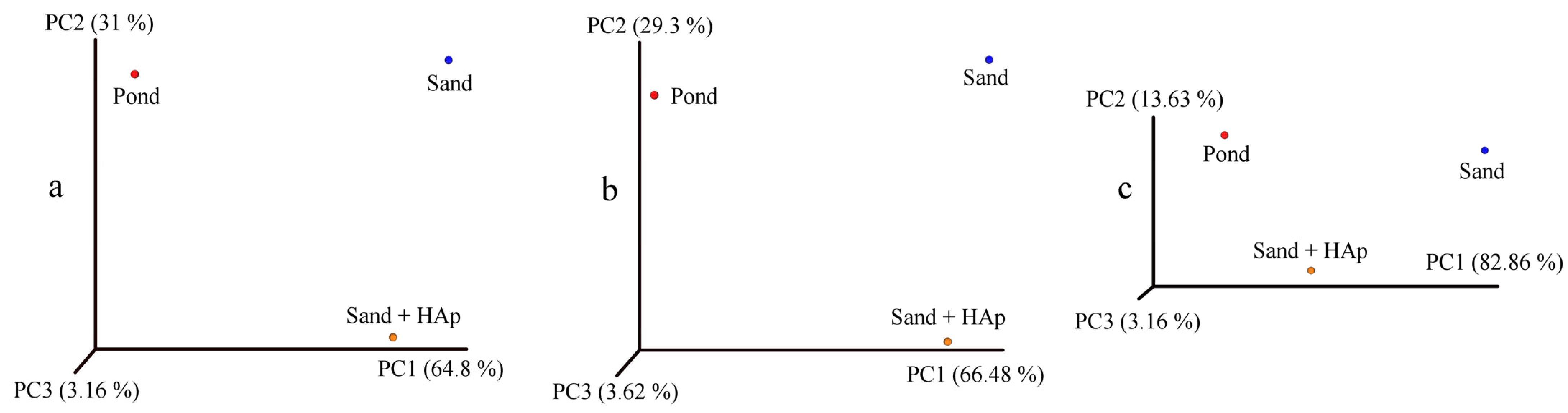
3.1. Study Design
3.2. Analysis of the Global Effects
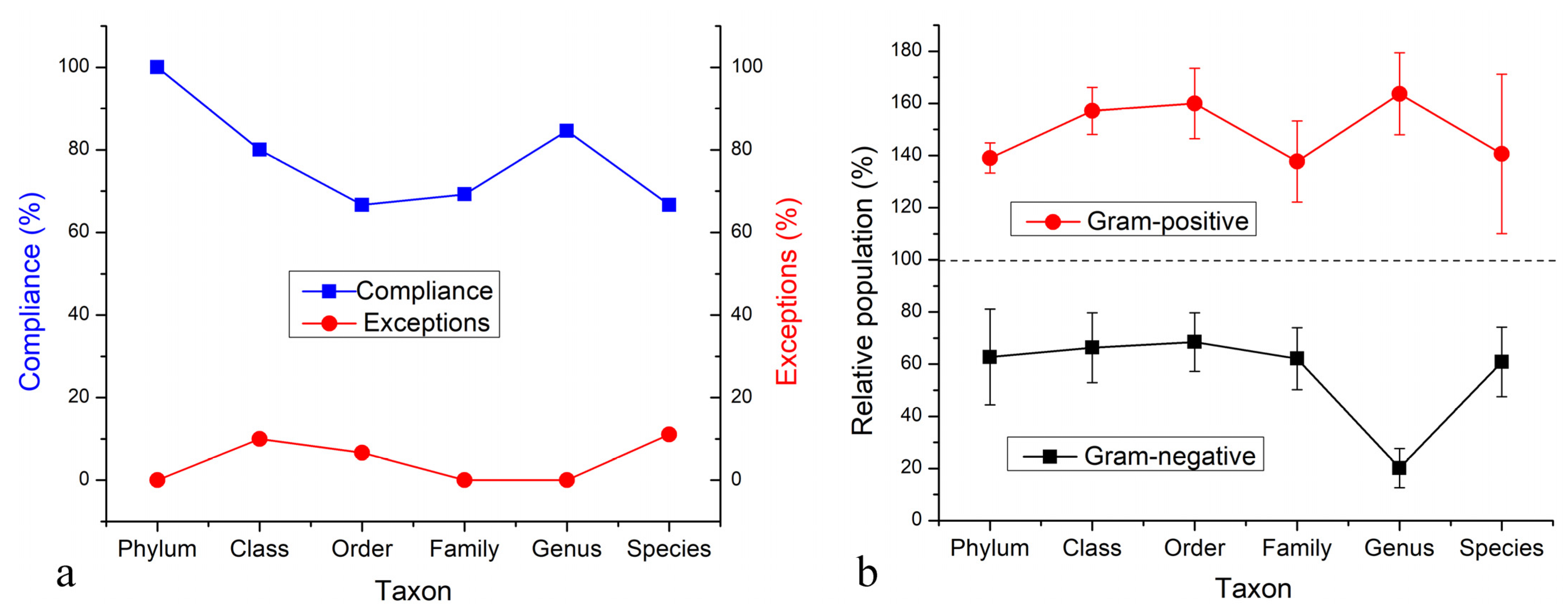
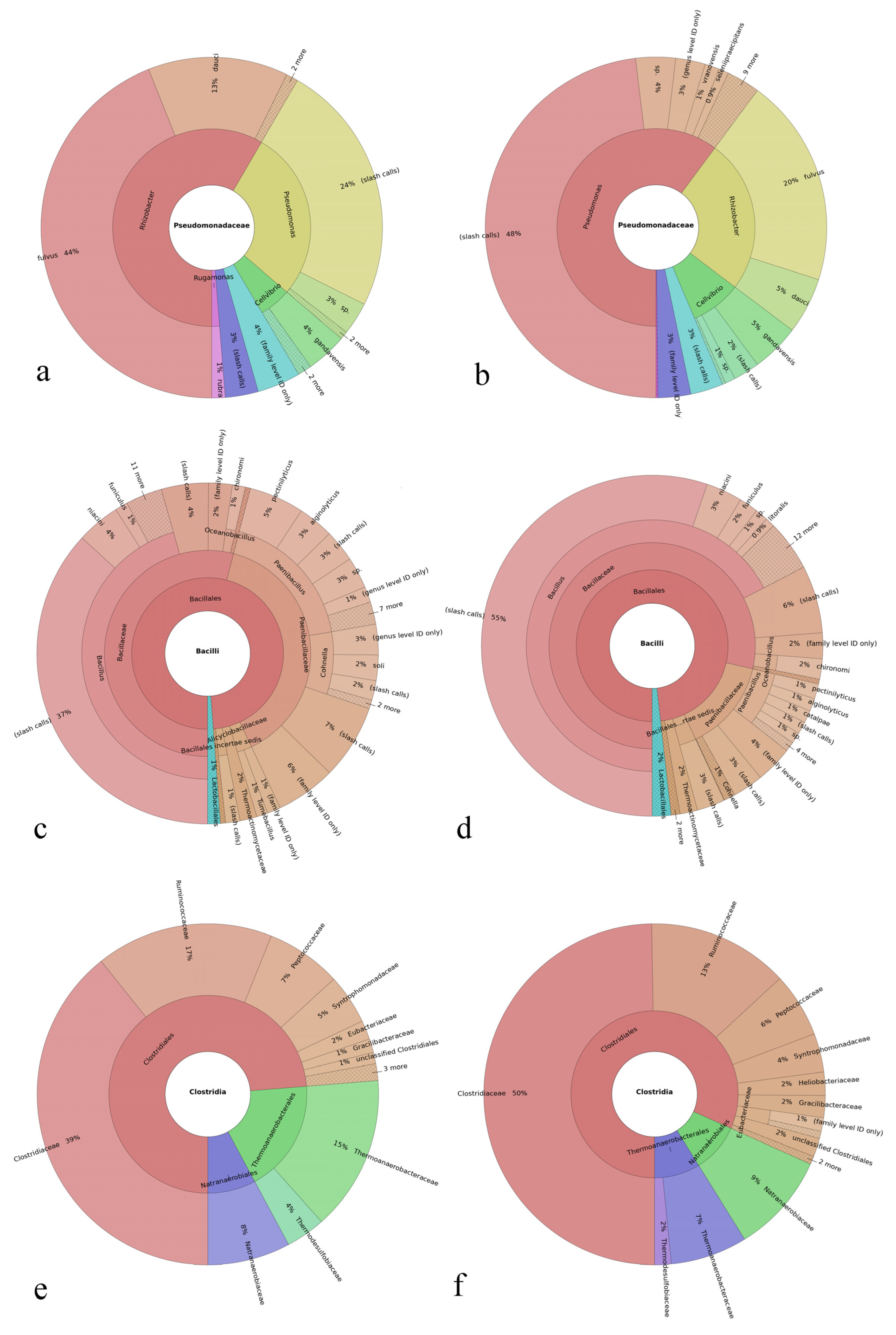
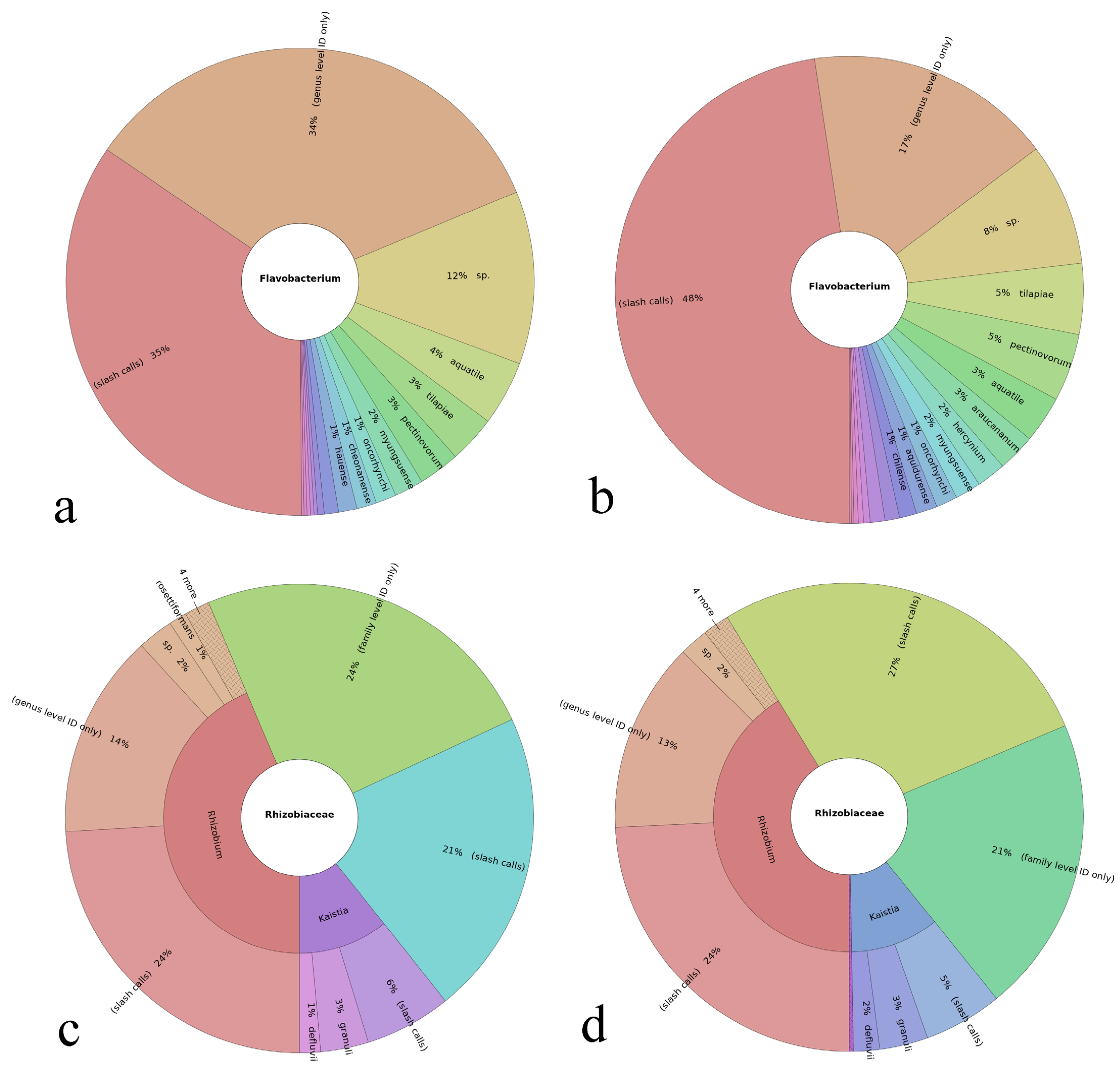
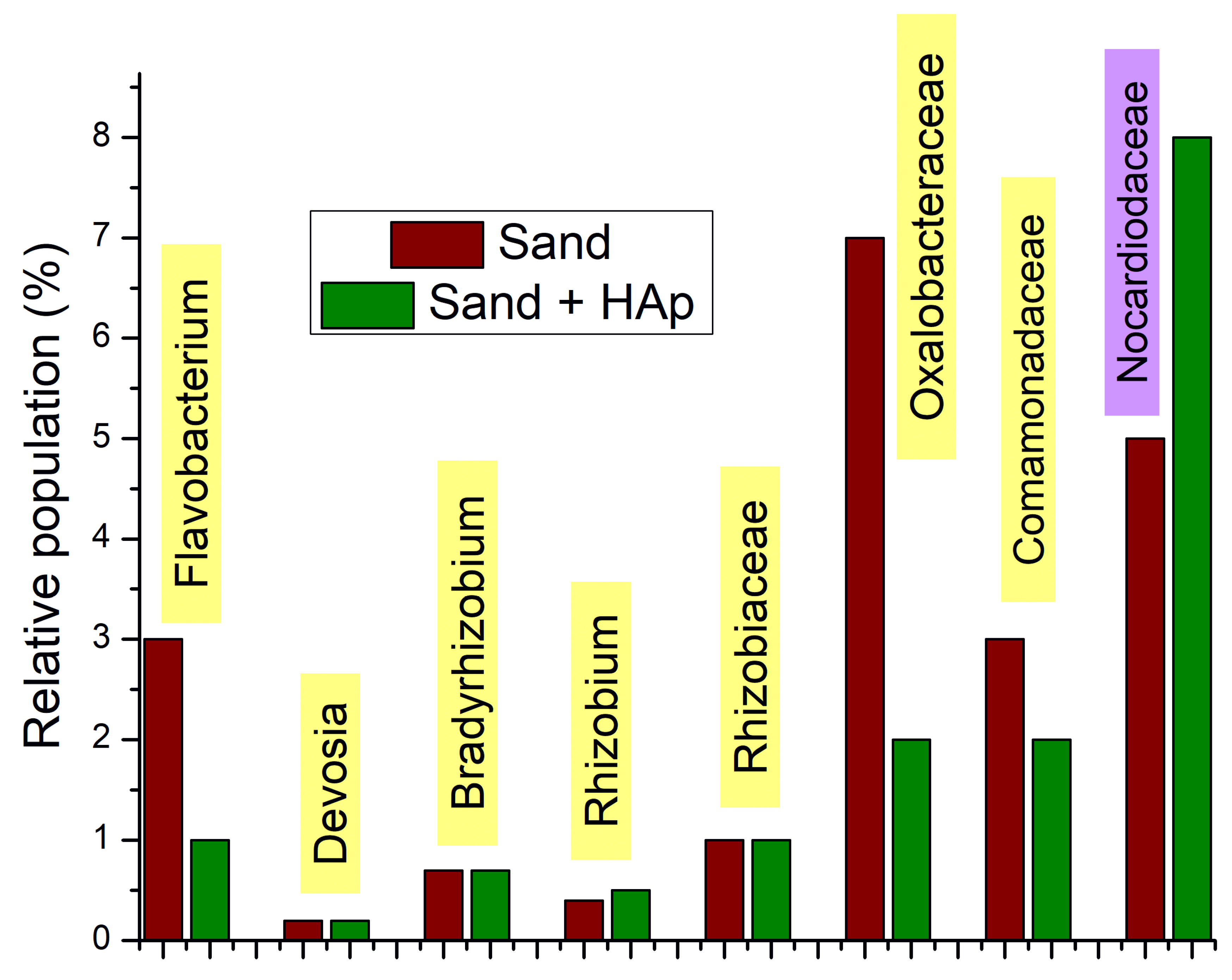
3.3. Results on the Most Abundant Taxa
3.4. Analysis of the Effect on Potential Human Pathogens
3.5. Analysis of the Effect on Positive Bacteria in the Soil
3.6. Mechanism of Gram-Negative vs. Gram-Positive Selectivity
3.7. Mechanism of Selectivity within the Gram-Negative Niche
3.8. Potential Use for HAp as a Microbiome Balancer or Remediation Agent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef] [PubMed]
- Linares, D.; Ross, P.; Stanton, C. Beneficial microbes: The pharmacy in the gut. Bioengineered 2015, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Capra, F. The Web of Life: A New Scientific Understanding of Living Systems; Anchor Books: New York, NY, USA, 1996. [Google Scholar]
- King, N. The unicellular ancestry of animal development. Dev. Cell 2004, 7, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.T.; Thomas, J.G. Oral microbiome: Contributions to local and systemic infections. Curr. Oral. Health Rep. 2016, 3, 45–55. [Google Scholar] [CrossRef]
- George, S.; Aguilera, X.; Gallardo, P.; Farfán, M.; Lucero, Y.; Torres, J.P.; Vidal, R.; O’Ryan, M. Bacterial gut microbiota and infections during early childhood. Front. Microbiol. 2022, 12, 793050. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.J.; Bates, K.A.; King, K.C. Host microbiota can facilitate pathogen infection. PLoS Pathog. 2021, 17, e1009514. [Google Scholar] [CrossRef]
- Mahana, D.; Trent, C.M.; Kurtz, Z.; Bokulich, N.A.; Battaglia, T.; Chung, J.; Muller, C.L.; Li, H.; Bonneau, R.A.; Blaser, M.J. Antibiotic perturbation of the murine gut microbiome enhances the adiposity, insulin resistance, and liver disease associated with high-fat diet. Genome Med. 2016, 8, 48. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Hall, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiat. 2021, 78, 1343–1354. [Google Scholar] [CrossRef]
- Iljazovic, A.; Roy, U.; Gálvez, E.J.C.; Lesker, T.R.; Zhao, B.; Gronow, A.; Amend, L.; Will, S.E.; Hofmann, J.D.; Pils, M.C.; et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2021, 14, 113–124. [Google Scholar] [CrossRef]
- Gilbert, N.M.; O’Brien, V.P.; Lewis, A.L. Transient microbiota exposures activate dormant Escherichia coli infection in the bladder and drive severe outcomes of recurrent disease. PLoS Pathog. 2017, 13, e1006238. [Google Scholar] [CrossRef]
- McDonnell, L.; Gilkes, A.; Ashworth, M.; Rowland, V.; Harries, T.H.; Armstrong, D.; White, P. Association between antibiotics and gut microbiome dysbiosis in children: Systematic review and meta-analysis. Gut Microbes 2021, 13, 1870402. [Google Scholar] [CrossRef] [PubMed]
- Kawalec, A.; Zwolińska, D. Emerging role of microbiome in the prevention of urinary tract infections in children. Int. J. Mol. Sci. 2022, 23, 870. [Google Scholar] [CrossRef] [PubMed]
- Ganda, E.K.; Gaeta, N.; Sipka, A.; Pomeroy, B.; Oikonomou, G.; Schukken, Y.H.; Bicalho, R.C. Normal milk microbiome is reestablished following experimental infection with Escherichia coli independent of intramammary antibiotic treatment with a third-generation cephalosporin in bovines. Microbiome 2017, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Khamash, D.; Voskertchian, A.; Milstone, A. Manipulating the microbiome: Evolution of a strategy to prevent S. aureus disease in children. J. Perinatol. 2018, 38, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.H.; Kiang, J.H.; Correa, V.; Lopez, M.I.; Chen, P.Y.; McKittrick, J.; Meyers, M.A. Armadillo armor: Mechanical testing and micro-structural evaluation. J. Mech. Behav. Biomed. Mater. 2011, 4, 713–722. [Google Scholar] [CrossRef]
- Uskoković, V.; Wu, V.M. When nothing turns itself inside out and becomes something: Coating poly(lactic-co-glycolic acid) spheres with hydroxyapatite nanoparticles vs. the other way around. J. Funct. Biomater. 2022, 13, 102. [Google Scholar] [CrossRef]
- Alanis-Gómez, R.P.; Rivera-Muñoz, E.M.; Luna-Barcenas, G.; Alanis-Gómez, J.R.; Velázquez-Castillo, R. Improving the mechanical resistance of hydroxyapatite/chitosan composite materials made of nanofibers with crystalline preferential orientation. Materials 2022, 15, 4718. [Google Scholar] [CrossRef]
- Wan, W.; Li, Z.; Wang, X.; Tian, F.; Yang, J. Surface-fabrication of fluorescent hydroxyapatite for cancer cell imaging and bio-printing applications. Biosensors 2022, 12, 419. [Google Scholar] [CrossRef]
- Wu, V.M.; Tang, S.; Uskoković, V. Calcium phosphate nanoparticles as intrinsic inorganic antimicrobials: The antibacterial effect. ACS Appl. Mater. Interfaces 2018, 10, 34013–34028. [Google Scholar] [CrossRef]
- Uskoković, V.; Tang, S.; Nikolić, M.G.; Marković, S.; Wu, V.M. Calcium phosphate nanoparticles as intrinsic inorganic antimicrobials: In search of the key particle property. Biointerphases 2019, 14, 031001. [Google Scholar] [CrossRef]
- Wu, V.M.; Huynh, E.; Tang, S.; Uskoković, V. Calcium phosphate nanoparticles as intrinsic inorganic antimicrobials: Mechanism of action. Biomed. Mater. 2021, 16, 015018. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal disease: The good, the bad, and the unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Twetman, S.; Fisher, J.; Marsh, P.D. Understanding dental caries as a non-communicable disease. Br. Dent. J. 2021, 231, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Song, X.; Wang, Y.; Wang, H.; Ge, S. The sorption and short-term immobilization of lead and cadmium by nano-hydroxyapatite/biochar in aqueous solution and soil. Chemosphere 2022, 286, 131810. [Google Scholar] [CrossRef] [PubMed]
- Lemlikchi, W.; Sharrock, P.; Fiallo, M.; Nzihou, A.; Mecherri, M.-O. Hydroxyapatite and alizarin sulfonate ARS modeling interactions for textile dyes removal from wastewaters. Procedia Eng. 2014, 83, 378–385. [Google Scholar] [CrossRef]
- Uskoković, V.; Desai, T.A. Calcium phosphate nanoparticles: A future therapeutic platform for the treatment of osteomyelitis? Ther. Deliv. 2013, 6, 643–645. [Google Scholar]
- Uskoković, V. X-Ray Photoelectron and ion scattering spectroscopic surface analyses of amorphous and crystalline calcium phosphate nanoparticles with different chemical histories. Phys. Chem. Chem. Phys. 2020, 22, 5531–5547. [Google Scholar] [CrossRef]
- Wang, D.; Paradelo, M.; Bradford, S.A.; Peijnenburg, W.J.; Chu, L.; Zhou, D. Facilitated transport of Cu with hydroxyapatite nanoparticles in saturated sand: Effects of solution ionic strength and composition. Water Res. 2011, 45, 5905–5915. [Google Scholar] [CrossRef]
- Bio-Rad. CHT Ceramic Hydroxyapatite and Crystalline Hydroxyapatite Resins. Bio-Rad Webpage. 2022. Available online: https://www.bio-rad.com/en-us/category/cht-ceramic-hydroxyapatite-crystalline-hydroxyapatite-resins (accessed on 13 January 2022).
- Uskoković, V. Ion-doped hydroxyapatite: An impasse or the road to follow? Ceram. Int. 2020, 46, 11443–11465. [Google Scholar] [CrossRef]
- Uskoković, V. Toward functionalization without functional agents: An X-ray photoelectron spectroscopy study. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129676. [Google Scholar] [CrossRef]
- Bystrova, A.V.; Dekhtyar, Y.D.; Popov, A.I.; Coutinho, J.; Bystrov, V.S. Modified hydroxyapatite structure and properties: Modeling and synchrotron data analysis of modified hydroxyapatite structure. Ferroelectrics 2015, 475, 135–147. [Google Scholar] [CrossRef]
- Hübner, W.; Blume, A.; Pushnjakova, R.; Dekhtyar, Y.; Hein, H.J. The influence of X-ray radiation on the mineral/organic matrix interaction of bone tissue: An FT-IR microscopic investigation. Int. J. Artif. Organs. 2005, 1, 66–73. [Google Scholar] [CrossRef]
- Nibali, L.; Sousa, V.; Davrandi, M.; Liu, L.S.; Spratt, D.; Donos, N. Patterns of subgingival microbiota in different periodontal phenotypes. J. Dent. 2022, 117, 103912. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, B.C.; Ayangbenro, A.S.; Babalola, O.O. Comparative study of microbial structure and functional profile of sunflower rhizosphere grown in two fields. BMC Microbiol. 2021, 21, 337. [Google Scholar] [CrossRef] [PubMed]
- Kudinova, A.G.; Petrova, M.A.; Dolgikh, A.V.; Soina, V.S.; Lysak, L.V.; Maslova, O.A. Taxonomic diversity of bacteria and their filterable forms in the soils of eastern antarctica (Larsemann Hills and Bunger Hills). Microbiol. Russ. Fed. 2020, 89, 574–584. [Google Scholar] [CrossRef]
- Urbaniak, C.; Wong, S.; Tighe, S.; Arumugam, A.; Liu, B.; Parker, C.W.; Wood, J.M.; Singh, N.K.; Skorupa, D.J.; Peyton, B.M.; et al. Validating an automated nucleic acid extraction device for omics in space using whole cell microbial reference standards. Front. Microbiol. 2020, 11, 1909. [Google Scholar] [CrossRef]
- Malla, M.A.; Dubey, A.; Kumar, A.; Yadav, S.; Hashem, A.; Abd Allah, E.F. Exploring the human microbiome: The potential future role of next-generation sequencing in disease diagnosis and treatment. Front. Immunol. 2019, 9, 2868. [Google Scholar] [CrossRef]
- Uskoković, V.; Desai, T.A. Phase composition control of calcium phosphate nanoparticles for tunable drug delivery kinetics and treatment of osteomyelitis. I. Preparation and drug release. J. Biomed. Mater. Res. Part A 2013, 101, 1416–1426. [Google Scholar] [CrossRef]
- Khan, M.A.; Wu, V.M.; Ghosh, S.; Uskoković, V. Gene delivery using calcium phosphate nanoparticles: Optimization of the transfection process and the effects of citrate and poly(L-Lysine) as additives. J. Colloid Interface Sci. 2016, 471, 48–58. [Google Scholar] [CrossRef]
- Uskoković, V.; Tang, S.; Wu, V.M. On grounds of the memory effect in amorphous and crystalline apatite: Kinetics of crystallization and biological response. ACS Appl. Mater. Interfaces 2018, 10, 14491–14508. [Google Scholar] [CrossRef]
- Dassi, E.; Ballarini, A.; Covello, G.; Quattrone, A.; Jousson, O.; de Sanctis, V.; Bertorelli, R.; Denti, M.A.; Segata, N. Enhanced microbial diversity in the saliva microbiome induced by short-term probiotic intake revealed by 16S rRNA sequencing on the IonTorrent PGM platform. J. Biotechnol. 2014, 190, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Andralojc, K.M.; Molina, M.A.; Qiu, M.; Spruijtenburg, B.; Rasing, M.; Pater, B.; Huynen, M.A.; Dutilh, B.E.; Ederveen, T.H.A.; Elmelik, D.; et al. Novel high-resolution targeted sequencing of the cervicovaginal microbiome. BMC Biol. 2021, 19, 267. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Verma, N.; Gupta, G. Iron-carbon nanofibers coated with acylated homoserine lactone enhance plant growth and suppress fusarium wilt disease in cicer arietinum by modulating soil microbiome. ACS Agric. Sci. Technol. 2022, 2, 311–322. [Google Scholar] [CrossRef]
- Gupta, S.; Mortensen, M.S.; Schjørring, S.; Trivedi, U.; Vestergaard, G.; Stokholm, J.; Bisgaard, H.; Krogfelt, K.A.; Sørensen, S.J. Amplicon sequencing provides more accurate microbiome information in healthy children compared to culturing. Commun. Biol. 2019, 2, 291. [Google Scholar] [CrossRef]
- Oktarina, H.; Singleton, I. Soil fungal community responses to the silver nanoparticles contamination as assessed by illumina next generation sequencing. J. Rekayasa Kim. Dan Lingkung. 2020, 15, 99–103. [Google Scholar] [CrossRef]
- Meier, M.J.; Dodge, A.E.; Samarajeewa, A.D.; Beaudette, L.A. Soil exposed to silver nanoparticles reveals significant changes in community structure and altered microbial transcriptional profiles. Environ. Pollut. 2020, 258, 113816. [Google Scholar] [CrossRef]
- McGee, C.F.; Storey, S.; Clipson, N.; Doyle, E. Soil microbial community responses to contamination with silver, aluminium oxide and silicon dioxide nanoparticles. Ecotoxicology 2017, 26, 449–458. [Google Scholar] [CrossRef]
- Uskoković, V. Nanostructured platforms for the sustained and local delivery of antibiotics in the treatment of osteomyelitis. Crit. Rev. Ther. Drug Carr. Syst. 2015, 32, 1–59. [Google Scholar] [CrossRef]
- Fu, X.; Zeng, B.; Wang, P.; Wang, L.; Wen, B.; Li, Y.; Liu, H.; Bai, S.; Jia, G. Microbiome of total versus live bacteria in the gut of rex rabbits. Front. Microbiol. 2018, 9, 733. [Google Scholar] [CrossRef]
- Steinberg, J.P.; Burd, E.M. Other gram-negative and gram-variable bacilli. In Mandell, Douglas and Bennett’s Principles and Practice of Infectious Disease, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Saunders: Philadelphia, PA, USA, 2015; Volume 2, pp. 2667–2683. [Google Scholar]
- Wang, M.; Ahrné, S.; Antonsson, M.; Molin, G. T-RFLP combined with principal component analysis and 16S rRNA gene sequencing: An effective strategy for comparison of fecal microbiota in infants of different ages. J. Microbiol. Methods 2004, 59, 53–69. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Z.; Lu, T.; Yu, Y.; Penuelas, J.; Zhu, Y.-G.; Qian, H. Gammaproteobacteria, a core taxon in the guts of soil fauna, are potential responders to environmental concentrations of soil pollutants. Microbiome 2021, 9, 196. [Google Scholar] [CrossRef]
- Steffan, J.J.; Derby, J.A.; Brevik, E.C. Soil pathogens that may potentially cause pandemics, including severe acute respiratory syndrome (SARS) coronaviruses. Curr. Opin. Env. Sci. Health. 2020, 17, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.B.; Quinn, N.M.; Stapp, P. Use of rodenticide bait stations by commensal rodents in southern california. In Proceedings of the Vertebrate Pest Conference, Santa Babara, CA, USA, 25 February–9 March 2020; Available online: https://escholarship.org/uc/item/2qt80491 (accessed on 16 August 2022).
- Sharma, J.; Li, Q.; Mishra, B.B.; Pena, C.; Teale, J.M. Lethal pulmonary infection with Francisella novicida is associated with severe sepsis. J. Leukoc. Biol. 2009, 86, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Falkinham, J.O., III. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 1996, 9, 177–215. [Google Scholar] [CrossRef] [PubMed]
- Hollister, E.B.; Gao, C.; Versalovic, J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology 2014, 146, 1449–1458. [Google Scholar] [CrossRef]
- Patrick, S. A tale of two habitats: Bacteroides fragilis, a lethal pathogen and resident in the human gastrointestinal microbiome. Microbiol. Read. 2022, 168, 001156. [Google Scholar] [CrossRef]
- Aabenhus, R.; Permin, H.; On, S.L.; Andersen, L.P. Prevalence of campylobacter concisus in diarrhoea of immunocompromised patients. Scand. J. Infect. Dis. 2002, 34, 248–252. [Google Scholar] [CrossRef]
- Anzai, Y.; Kim, H.; Park, J.Y.; Wakabayashi, H.; Oyaizu, H. Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. Int. J. Syst. Evol. Microbiol. 2000, 50, 1563–1589. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Pseudomonas aeruginosa in Healthcare Settings. 2019. Available online: https://www.cdc.gov/hai/organisms/pseudomonas.html (accessed on 21 January 2022).
- Coates, R.; Moran, J.; Horsburgh, M.J. Staphylococci: Colonizers and pathogens of human skin. Future Microbiol. 2014, 9, 75–91. [Google Scholar] [CrossRef]
- Uskoković, V.; Huynh, E.; Wu, V.M. Mimicking the transit of nanoparticles through the body: When the path determines properties at the destination. J. Nanoparticle Res. 2020, 22, 184. [Google Scholar] [CrossRef]
- Blackburn, J.K.; McNyset, K.M.; Curtis, A.; Hugh-Jones, M.E. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecological [corrected] niche modeling. Am. J. Trop. Med. Hyg. 2007, 77, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.L.; Wilkins, T.D. Clostridia: Sporeforming anaerobic bacilli. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 18. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8219/ (accessed on 20 January 2022).
- Maczulak, A. Clostridium. In Encyclopedia of Microbiology; Facts on File: New York, NY, USA, 2011; pp. 168–173. [Google Scholar]
- Bradshaw, W.J.; Roberts, A.K.; Shone, C.C.; Acharya, K.R. The structure of the S-layer of Clostridium difficile. J. Cell Commun. Signal 2018, 12, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Smarda, J.; Smajs, D.; Komrska, J.; Krzyzanek, V. S-layers on cell walls of cyanobacteria. Micron 2002, 33, 257–277. [Google Scholar] [CrossRef]
- Calabi, E.; Ward, S.; Wren, B.; Paxton, T.; Panico, M.; Morris, H.; Dell, A.; Dougan, G.; Fairweather, N. Molecular characterization of the surface layer proteins from Clostridium difficile. Mol. Microbiol. 2001, 40, 1187–1199. [Google Scholar] [CrossRef]
- Spigaglia, P.; Galeotti, C.L.; Barbanti, F.; Scarselli, M.; Van Broeck, J.; Mastrantonio, P. The LMW surface-layer proteins of Clostridium difficile PCR ribotypes 027 and 001 share common immunogenic properties. J. Med. Microbiol. 2011, 60, 1168–1173. [Google Scholar] [CrossRef]
- Fellet, G.; Pilotto, L.; Marchiol, L.; Braidot, E. Tools for nano-enabled agriculture: Fertilizers based on calcium phosphate, silicon, and chitosan nanostructures. Agronomy 2021, 11, 1239. [Google Scholar] [CrossRef]
- Wu, V.M.; Uskoković, V. Waiting for Aπαταω: 250 years later. Found. Sci. 2019, 24, 617–640. [Google Scholar] [CrossRef]
- Jiang, S.-T.; Wang, H.-Y.; Zhou, J.-M.; Chen, Z.-M.; Liu, X.-W.; Jia, Y.-S. Effects of phosphorus fertilizer application methods and types on the yield and phosphorus uptake of winter wheat. Chin. J. Appl. Ecol. 2016, 27, 1503–1510. [Google Scholar]
- Köberl, M.; Wagner, P.; Müller, H.; Matzer, R.; Unterfrauner, H.; Cernava, T.; Berg, G. Unraveling the complexity of soil microbiomes in a large-scale study subjected to different agricultural management in Styria. Front. Microbiol. 2020, 11, 01052. [Google Scholar] [CrossRef]
- Edwards, J.; Santos-Medellín, C.; Nguyen, B.; Kilmer, J.; Liechty, Z.; Veliz, E.; Ni, J.; Phillips, G.; Sundaresan, V. Soil domestication by rice cultivation results in plant-soil feedback through shifts in soil microbiota. Genome Biol. 2019, 20, 221. [Google Scholar] [CrossRef]
- Yeoh, Y.; Dennis, P.G.; Paungfoo-Lonhienne, C.; Weber, L.; Brackin, R.; Ragan, M.A.; Schmidt, S.; Hugenholtz, P. Evolutionary conservation of a core root microbiome across plant phyla along a tropical soil chronosequence. Nat. Commun. 2017, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Klironomos, J.N. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 2002, 417, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Zomer, P.; Farmer, S.; Alibek, K. Quantifying crop rhizosphere microbiome ecology: The next frontier in enhancing the commercial utility of agricultural microbes. Ind. Biotechnol. 2018, 14, 116–119. [Google Scholar]
- Smith, C.R.; Blair, P.L.; Boyd, C.; Cody, B.; Hazel, A.; Hedrick, A.; Kathuria, H.; Khurana, P.; Kramer, B.; Muterspaw, K.; et al. Microbial community responses to soil tillage and crop rotation in a corn/soybean agroecosystem. Ecol. Evol. 2016, 6, 8075–8084. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Uskoković, V.; Castiglione, Z.; Cubas, P.; Zhu, L.; Li, W.; Habelitz, S. Zeta-potential and particle size analysis of recombinant human amelogenins. J. Dent. Res. 2010, 89, 149–153. [Google Scholar] [CrossRef]
- Uskoković, V. Dynamic light scattering and microelectrophoresis: Main prospects and limitations. J. Dispers. Sci. Technol. 2012, 33, 1762–1786. [Google Scholar] [CrossRef]
- Pernal, S.P.; Wu, V.M.; Uskoković, V. Hydroxyapatite as a vehicle for the selective effect of superparamagnetic iron oxide nanoparticles against human glioblastoma cells. ACS Appl. Mater. Interfaces 2017, 9, 39283–39302. [Google Scholar] [CrossRef]
- Li, Z.; Ma, J.; Ruan, J.; Zhuang, X. Using positively charged magnetic nanoparticles to capture bacteria at ultralow concentration. Nanoscale Res. Lett. 2019, 14, 195. [Google Scholar] [CrossRef]
- Cremin, K.; Jones, B.A.; Teahan, J.; Meloni, G.N.; Perry, D.; Zerfass, C.; Asally, M.; Soyer, O.S.; Unwin, P.R. Scanning ion conductance microscopy reveals differences in the ionic environments of gram-positive and negative bacteria. Anal. Chem. 2020, 92, 16024–16032. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Furevi, A.; Perepelov, A.V.; Guo, X.; Cao, H.; Wang, Q.; Reeves, P.R.; Knirel, Y.A.; Wang, L.; Widmalm, G. Structure and genetics of Escherichia coli O antigens. FEMS Microbiol. Rev. 2020, 44, 655–683. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.C.; Lindhout, T.; Beveridge, T.J.; Dutcher, J.R.; Lam, J.S. Differential lipopolysaccharide core capping leads to quantitative and correlated modifications of mechanical and structural properties in Pseudomonas aeruginosa biofilms. J. Bacteriol. 2009, 191, 6618–6631. [Google Scholar] [CrossRef] [PubMed]
- Cochet, F.; Peri, F. The role of carbohydrates in the lipopolysaccharide (LPS)/Toll-like receptor 4 (TLR4) signaling. Int. J. Mol. Sci. 2017, 18, 2318. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, G.I.; Hopkins, J.A.; Blaser, M.J. Lipopolysaccharide structures in Enteribacteriaceae, Pseudomonas aeruginosa, and Vibrio cholera are immunologically related to Campylobacter spp. Infect. Immun. 1986, 51, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.A.F.; Carnell, O.T.; Lafage, L.; Gray, J.; Biboy, J.; Gibson, J.F.; Pollitt, J.G.; Tazoll, S.C.; Turnbull, W.; Hajdamoxicz, N.H.; et al. Staphylococcus aureus cell wall structure and dynamics during host-pathogen interaction. PLoS Pathog. 2021, 17, e1009468. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, V.; Varela, C.; Javid, A.; Singh, A.; Besra, G.S.; Bhatt, A. Mycolic acids: Deciphering and targeting the Achilles’ heel of the tubercle bacillus. Mol. Microbiol. 2015, 98, 7–16. [Google Scholar] [CrossRef]
- Alderwick, L.J.; Harrison, J.; Lloyd, G.S.; Birch, H.L. The mycobacterial cell wall—Peptidoglycan and arabinogalactan. Cold Spring Harb. Perspect. Med. 2015, 5, a021113. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta (BBA)—Biomembr. 2016, 1858, 936–946. [Google Scholar] [CrossRef]
- Christie, W.W.; Lipid A and Bacterial Lipopolysaccharides. The Lipid Web. 2022. Available online: https://www.lipidmaps.org/resources/lipidweb/lipidweb_html/lipids/simple/lipidA/index.htm (accessed on 24 January 2022).
- Navarre, W.W.; Schneewind, O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 1999, 63, 174–229. [Google Scholar] [CrossRef]
- Kim, S.J.; Chang, J.; Singh, M. Peptidoglycan architecture of Gram-positive bacteria by solid-state NMR. Biochim. Biophys. Acta (BBA)—Biomembr. 2015, 1848, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Silipo, A.; Erbs, G.; Shinya, T.; Dow, J.M.; Parrilli, M.; Lanzetta, R.; Shibuya, N.; Newman, M.-A.; Molinaro, A. Glyco-conjugates as elicitors or suppressors of plant innate immunity. Glycobiology 2010, 20, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.E.S.; Pol-Fachin, L.; Lins, R.D.; Soares, T.A. Polymyxin binding to the bacterial outer membrane reveals cation displacement and increasing membrane curvature in susceptible but not in resistant lipopolysaccharide chemotypes. J. Chem. Inf. Model. 2017, 57, 2181–2193. [Google Scholar] [CrossRef] [PubMed]
- Lerouge, I.; Laeremans, T.; Verreth, C.; Vanderleyden, J.; Van Soom, C.; Tobin, A.; Carlson, R.W. Identification of an ATP-binding cassette transporter for export of the O-antigen across the inner membrane in Rhizobium etli based on the genetic, functional, and structural analysis of an lps mutant deficient in O-antigen. J. Biol. Chem. 2001, 276, 17190–17198. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, T.; Uskoković, E.; Wu, V.; Uskoković, V. Calcium phosphate and senescence of orange jubilees in the summertime. ACS Appl. Bio Mater. 2020, 3, 3770–3784. [Google Scholar] [CrossRef] [PubMed]
- Dzink-Fox, J.L.; Leadbetter, E.R.; Godchaux, W. Acetate acts as a protonophore and differentially affects bead movement and cell migration of the gliding bacterium Cytophaga johnsonae (Flavobacterium johnsoniae). Microbiology 1997, 143, 3693–3701. [Google Scholar] [CrossRef][Green Version]
- Uskoković, V.; Lee, K.; Lee, P.P.; Fischer, K.E.; Desai, T.A. Shape effect in the design of nanowire-coated microparticles as epithelial drug delivery devices. ACS Nano 2012, 6, 7832–7841. [Google Scholar] [CrossRef]
- Lerouge, I.; Vanderleyden, J. O-antigen structural variation: Mechanisms and possible roles in animal/plant–microbe interactions. FEMS Microbiol. Rev. 2002, 26, 17–47. [Google Scholar] [CrossRef]
- Steimle, A.; Autenrieth, I.B.; Frick, J.-S. Structure and function: Lipid A modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef]
- Lam, J.; Taylor, V.; Islam, S.; Hao, Y.; Kocíncová, D. Genetic and functional diversity of pseudomonas aeruginosa lipopolysaccharide. Front. Microbiol. 2011, 2, 00118. [Google Scholar] [CrossRef]
- Eberspächer, J.; Lingens, F. The genus phenylobacterium. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 250–256. [Google Scholar]
- Irschik, H.; Reichenbach, H. Intracellular location of flexirubins in Flexibacter elegans (Cytophagales). Biochim. Biophys. Acta 1978, 510, 1–10. [Google Scholar] [CrossRef]
- Gomi, K.; Kawasaki, K.; Kawai, Y.; Shiozaki, M.; Nishijima, M. Toll-like receptor 4-md-2 complex mediates the signal transduction induced by flavolipin, an amino acid-containing lipid unique to Flavobacterium meningosepticum. J. Immunol. 2002, 168, 2939–2943. [Google Scholar] [CrossRef] [PubMed]
- Silipo, A.; Molinaro, A.; Jiang, C.-L.; Jiang, Y.; Xu, P.; Xu, L.-H.; Lanzetta, R.; Parrilli, M. The O-chain structure from the LPS of the bacterium Naxibacter alkalitolerans YIM 31775T. Carbohydr. Res. 2007, 342, 757–761. [Google Scholar] [CrossRef]
- MacLean, L.L.; Vinogradov, E.; Crump, E.M.; Perry, M.B.; Kay, W.W. The structure of the lipopolysaccharide O-antigen produced by Flavobacterium psychrophilum (259-93). Eur. J. Biochem. 2001, 268, 2710–2716. [Google Scholar] [CrossRef]
- Cisar, J.O.; Bush, C.A.; Wiens, G.D. Comparative structural and antigenic characterization of genetically distinct flavobacterium psychrophilum O-polysaccharides. Front. Microbiol. 2019, 10, 1041. [Google Scholar] [CrossRef]
- Knirel, Y.A.; Vinogradov, E.V.; Shashkov, A.S.; Wilkinson, S.G.; Tahara, Y.; Dmitriev, B.A.; Kochetkov, N.K.; Stanislavsky, E.S.; Mashilova, G.M. Somatic antigens of Pseudomonas aeruginosa. The structure of O-specific polysaccharide chains of the lipopolysaccharides from P. aeruginosa O1 (Lányi), O3 (Habs), O13 and O14 (Wokatsch), and the serologically related strain NCTC 8505. Eur. J. Biochem. 1986, 155, 659–669. [Google Scholar] [CrossRef]
- Lindberg, A.A.; Weintraub, A.; Zähringer, U.; Rietschel, E.T. Structure-activity relationships in lipopolysaccharides of Bacteroides fragilis. Rev. Infect. Dis. 1990, 12, S133–S141. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, L.S.; Bhat, U.R.; Carlson, R.W. Structural characterization of the O-antigenic polysaccharide of the lipopolysaccharide from Rhizobium etli strain CE3. A unique O-acetylated glycan of discrete size, containing 3-O-methyl-6-deoxy-L-talose and 2,3,4-tri-O-,methyl-l fucose. J. Biol. Chem. 2000, 275, 18851–18863. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Wang, X.; Xu, C.; Han, T.; Guo, X. Genetic characterization of the O-antigen and development of a molecular serotyping scheme for enterobacter cloacae. Front. Microbiol. 2020, 11, 00727. [Google Scholar]
- Nazarenko, E.L.; Crawford, R.J.; Ivanova, E.P. The structural diversity of carbohydrate antigens of selected gram-negative marine bacteria. Mar. Drugs 2011, 9, 1914–1954. [Google Scholar] [CrossRef]
- Sandaa, R.; Torsvik, V.; Enger, V.; Daae, F.L.; Castberg, T.; Hahn, D. Analysis of bacterial communities in heavy metal-contaminated soils at different levels of resolution. FEMS Microbiol. Ecol. 1999, 30, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Chodak, M.; Gołebiewski, M.; Justyna, M.P.; Katarzyna, K.; Maria, N. Diversity of microorganisms from forest soils differently polluted with heavy metals. Appl. Soil. Ecol. 2013, 64, 7–14. [Google Scholar] [CrossRef]
- Hemmat-Jou, M.H.; Safari-Sinegani, A.A.; Mirzaie-Asl, A.; Tahmeourespour, A. Analysis of microbial communities in heavy metals-contaminated soils using the metagenomic approach. Ecotoxicology 2018, 27, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Hua, X.; Müller, K.; Wang, H.; Yang, T.; Wang, Q.; Peng, X.; Wang, M.; Pang, Y.; et al. Glyphosate application increased catabolic activity of gram-negative bacteria but impaired soil fungal community. Environ. Sci. Pollut. Res. Int. 2018, 25, 14762–14772. [Google Scholar] [CrossRef]
- Pan, X.; Richardson, M.D.; Deng, S.; Kremer, R.J.; English, J.T.; Mihail, J.D.; Sams, C.E.; Scharf, P.C.; Veum, K.S.; Xiong, X. Effect of organic amendment and cultural practice on large patch occurrence and soil microbial community. Crop Sci. 2017, 57, 2263–2272. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, X.; Huang, G.; Li, W.; Wei, Q.; Zhang, J.; Han, F. Oil degradation and variation of microbial communities in contaminated soils induced by different bacterivorous nematodes species. Ecotoxicol. Environ. Saf. 2022, 229, 113079. [Google Scholar] [CrossRef]
- Galitskaya, P.; Biktasheva, L.; Blagodatsky, S.; Selivanovskaya, S. Response of bacterial and fungal communities to high petroleum pollution in different soils. Sci. Rep. 2021, 11, 164. [Google Scholar] [CrossRef]
- Börjesson, G.; Menichetti, L.; Kirchmann, H.; Kätterer, T. Soil microbial community structure affected by 53 years of nitrogen fertilisation and different organic amendments. Biol. Fertil. Soils. 2012, 48, 245–257. [Google Scholar] [CrossRef]
- Chodak, M.; Golebiewski, M.; Morawska-Ploskonska, J.; Kuduk, K.; Niklinska, M. Soil chemical properties affect the reaction of forest soil bacteria to drought and rewetting stress. Ann. Microbiol. 2015, 65, 1627–1637. [Google Scholar] [CrossRef]
- Pouralibaba, F.; Balaei, E.; Kashefimehr, A. Evaluation of gram negative bacterial contamination in dental unit water supplies in a university clinic in Tabriz, Iran. J. Dent. Res. Dent. Clin. Dent. Prospects. 2011, 5, 94–97. [Google Scholar]
- Cristina, M.L.; Spagnolo, A.M.; Casini, B.; Baggiani, A.; Del Giudice, P.; Brusaferro, S.; Poscia, A.; Moscato, U.; Perdelli, F.; Orlando, P. The impact of aerators on water contamination by emerging gram-negative opportunists in at-risk hospital departments. Infect. Control. Hosp. Epidemiol. 2014, 35, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Penna, V.T.C.; Martins, S.A.M.; Mazzola, P.G. Identification of bacteria in drinking and purified water during the monitoring of a typical water purification system. BMC Public Health 2002, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Torkar, K.G.; Dražetić, M. The microbial contamination and the presence of β-lactamase producing Gram-negative bacteria in the water and on the surfaces of public recreation water facilities. Int. J. Environ. Health Res. 2017, 27, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Mazzulli, T.; Skulnick, M.; Small, G.; Marshall, W.; Hoban, D.J.; Zhanel, G.G.; Finn, S.; Low, D.E. Susceptibility of community Gram-negative urinary tract isolates to mecillinam and other oral agents. Can. J. Infect. Dis. 2001, 12, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, K.; Carey, R.M.; Lin, X.; Seckar, T.D.; Wei, Z.; Chorath, K.; Newman, J.G.; O’Malley, B.W.; Weinstein, G.S.; Feldman, M.D.; et al. The microbiome of HPV-positive tonsil squamous cell carcinoma and neck metastasis. Oral Oncol. 2021, 117, 105305. [Google Scholar] [PubMed]
- Salguero, M.V.; Al-Obaide, M.A.I.; Singh, R.; Siepmann, T.; Vasylyeva, T.L. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp. Ther. Med. 2019, 18, 3461–3469. [Google Scholar] [CrossRef]
- Matsue, M.; Mori, Y.; Nagase, S.; Sugiyama, Y.; Hirano, R.; Ogai, K.; Ogura, K.; Kurihara, S.; Okamoto, S. Measuring the antimicrobial activity of lauric acid against various bacteria in human gut microbiota using a new method. Cell Transpl. 2019, 28, 1528–1541. [Google Scholar] [CrossRef]
- Nath, S.G.; Raveendran, R. Microbial dysbiosis in periodontitis. J. Indian Soc. Periodontol. 2013, 17, 543–545. [Google Scholar] [CrossRef]







| Gram Stain | Species | Lab Strain | MDR Strain |
|---|---|---|---|
| IC50 (HAp) (mg/mL) | IC50 (HAp) (mg/mL) | ||
| Gram-negative | E. coli | 29 | / |
| P. aeruginosa | 59 | 10 | |
| S. aureus | >100 | 98 | |
| Gram-positive | S. epidermis | >100 | / |
| E. faecalis | 93 | / |
| Taxon | Motile | Shape | Catalase-Active | Oxidase-Active | Urea Hydrolysis | Esculin Hydrolysis | Citrate Utilization | Casein Hydrolysis |
|---|---|---|---|---|---|---|---|---|
| Flavobacterii | +(glide) | Filamentous | + | + | + | + | + | + |
| Oxalobacteraceae | +/− | Rods | + | + | +/− | +/− | +/− | +/− |
| Phenylobacterium | – | Rods | + | + | – | – | + | – |
| Adhaeribacter | – | Rods | + | + | + | + | – | + |
| Taxon | Motile | Shape | Catalase-Active | Oxidase-Active | Urea Hydrolysis | Esculin Hydrolysis | Citrate Utilization | Casein Hydrolysis |
|---|---|---|---|---|---|---|---|---|
| Bacteroidaceae | +(flagella) | Rods | – | + | – | – | + | + |
| Enterobacter | +(flagella) | Rods | +/− | – | + | + | + | + |
| Rhizobium | +(flagella) | Rods | + | + | – | + | – | + |
| Amaricoccus macauensis | – | Coccus | + | + | + | – | – | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uskoković, V.; Wu, V.M. Altering Microbiomes with Hydroxyapatite Nanoparticles: A Metagenomic Analysis. Materials 2022, 15, 5824. https://doi.org/10.3390/ma15175824
Uskoković V, Wu VM. Altering Microbiomes with Hydroxyapatite Nanoparticles: A Metagenomic Analysis. Materials. 2022; 15(17):5824. https://doi.org/10.3390/ma15175824
Chicago/Turabian StyleUskoković, Vuk, and Victoria M. Wu. 2022. "Altering Microbiomes with Hydroxyapatite Nanoparticles: A Metagenomic Analysis" Materials 15, no. 17: 5824. https://doi.org/10.3390/ma15175824
APA StyleUskoković, V., & Wu, V. M. (2022). Altering Microbiomes with Hydroxyapatite Nanoparticles: A Metagenomic Analysis. Materials, 15(17), 5824. https://doi.org/10.3390/ma15175824






