Upcycling Glass Waste into Porous Microspheres for Wastewater Treatment Applications: Efficacy of Dye Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Microspheres
2.2. Characterisation Methods for RG and PRGMs
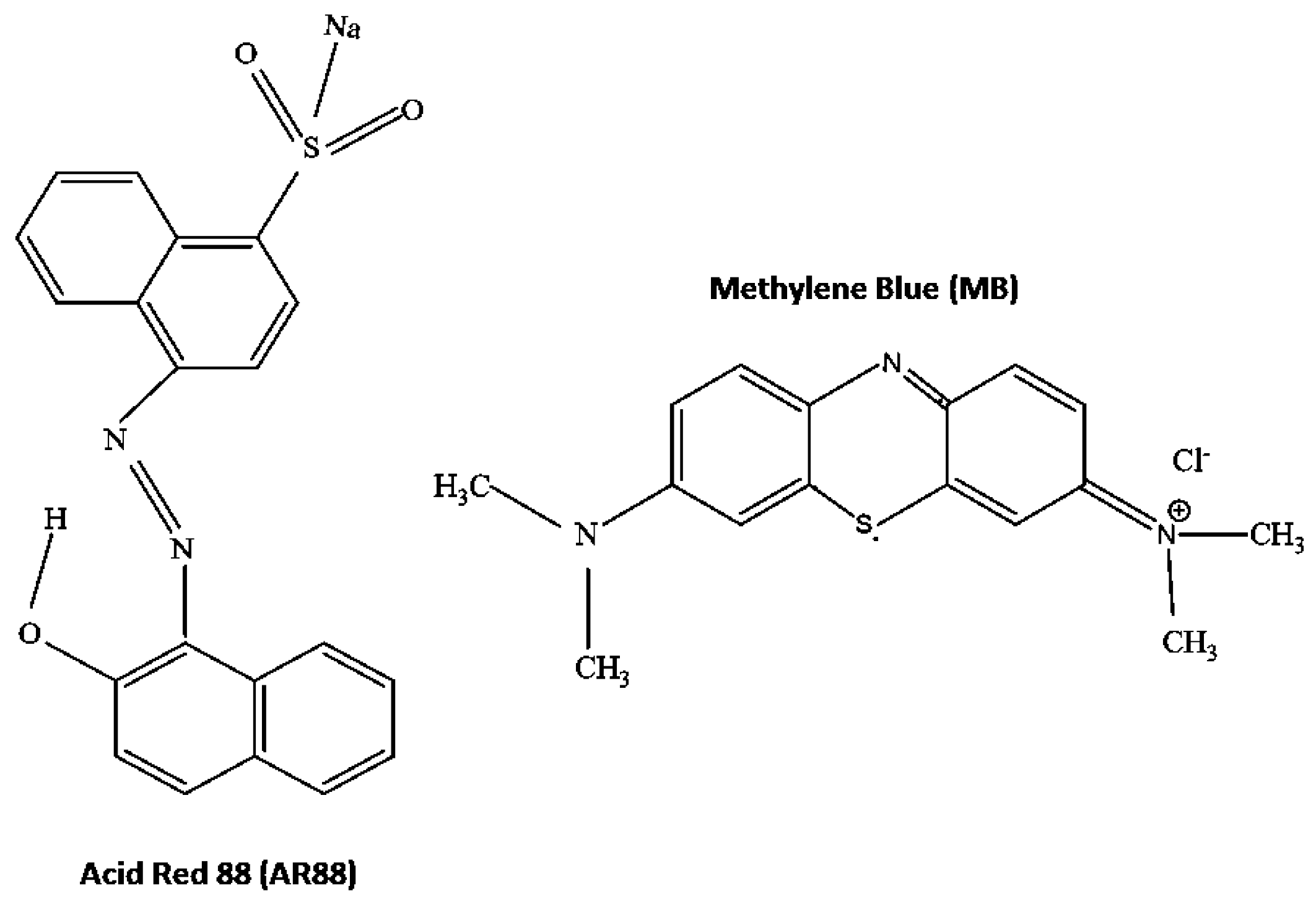
2.3. Dye Removal Studies
2.3.1. Batch Adsorption Experiments
2.3.2. Coagulation Study
2.3.3. Column Adsorption Experiment
2.3.4. Statistical Analysis
2.3.5. Recyclability Study of PRGMs
2.4. Dye Removal Mechanism Investigation via Structural Analysis
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.4.2. X-ray Photoelectron Spectroscopy (XPS) Analysis
3. Results
3.1. Characterisation of RG and PRGMs
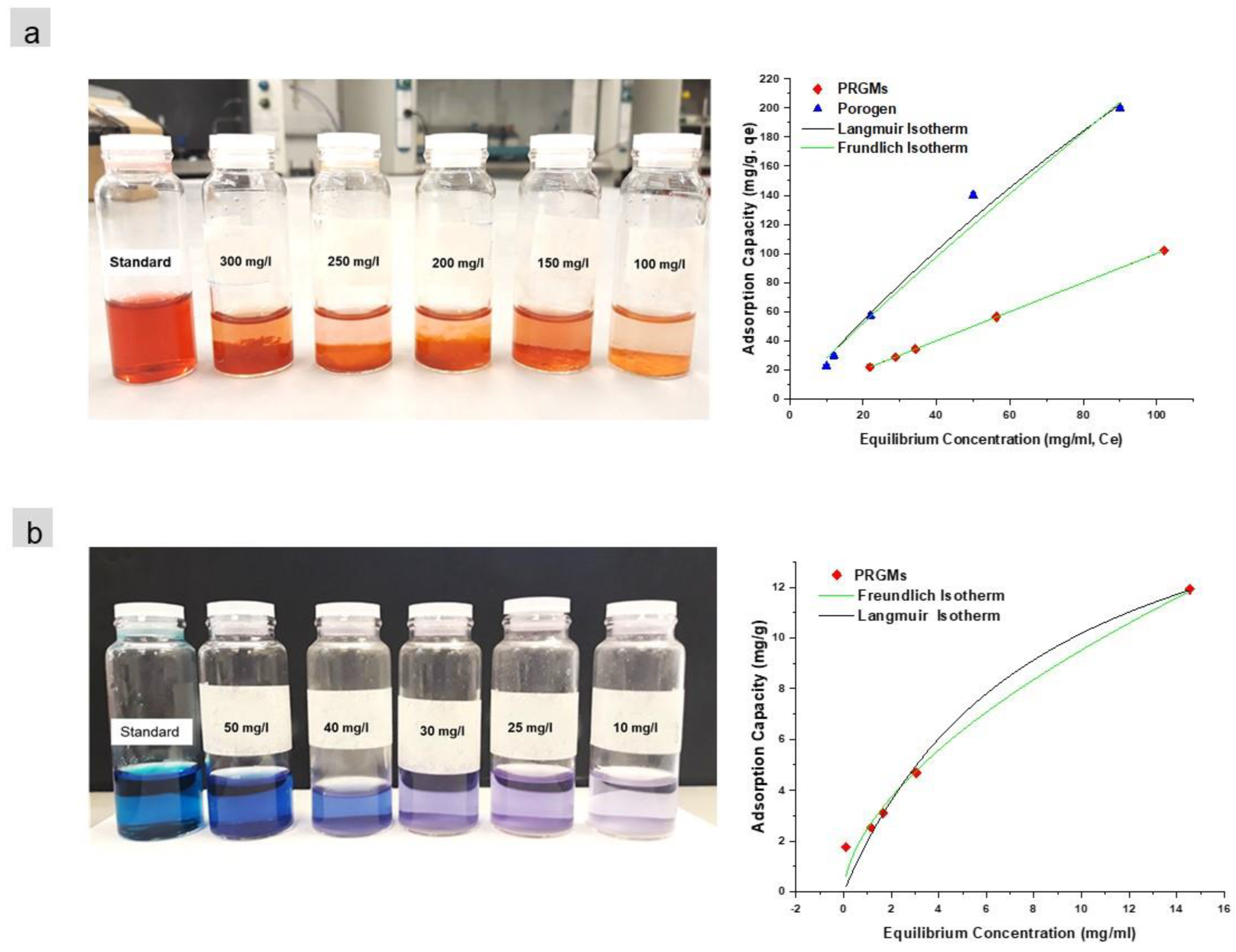
3.2. Dye Adsorption Studies
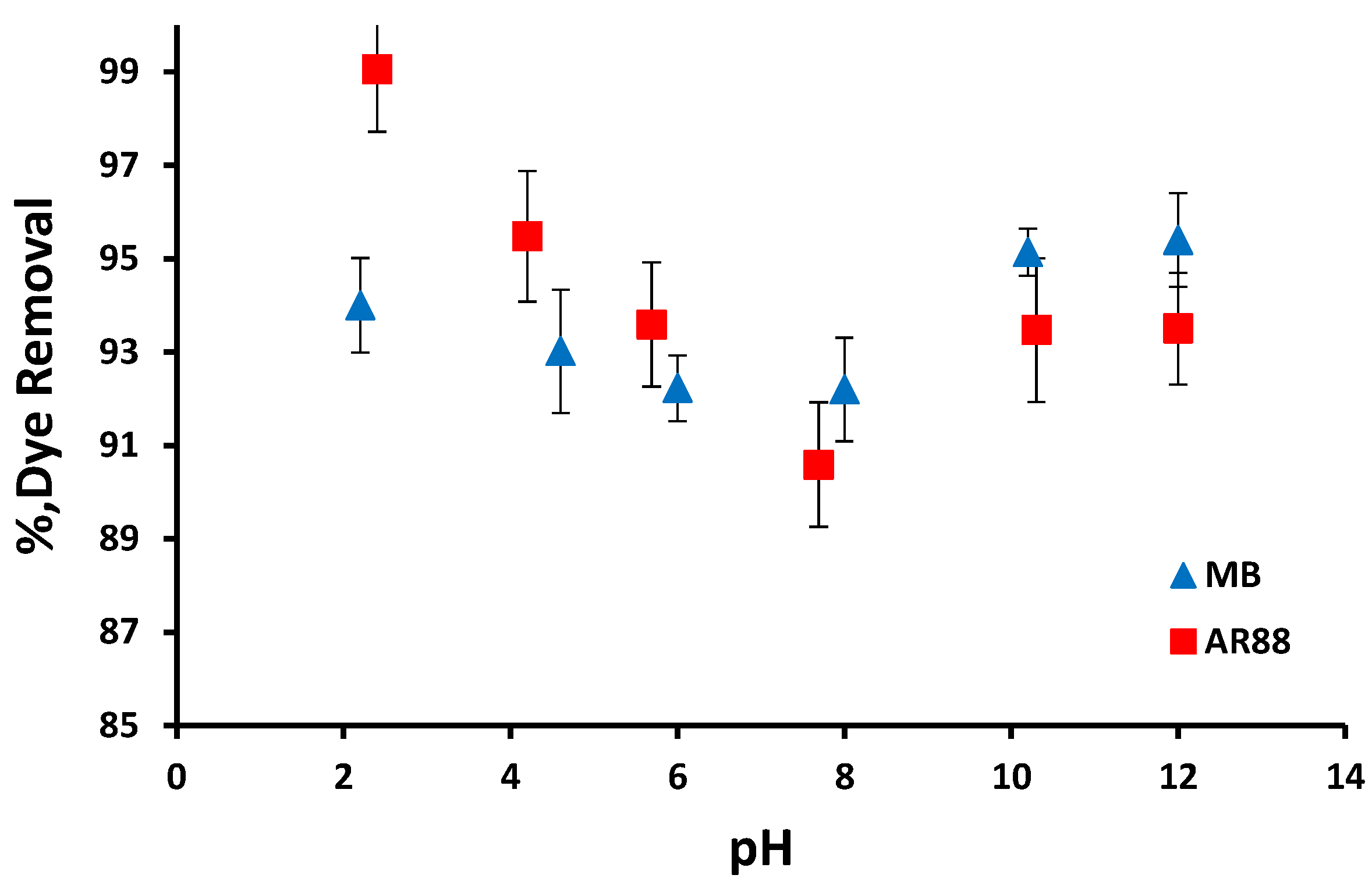
3.2.1. Effect of Initial pH
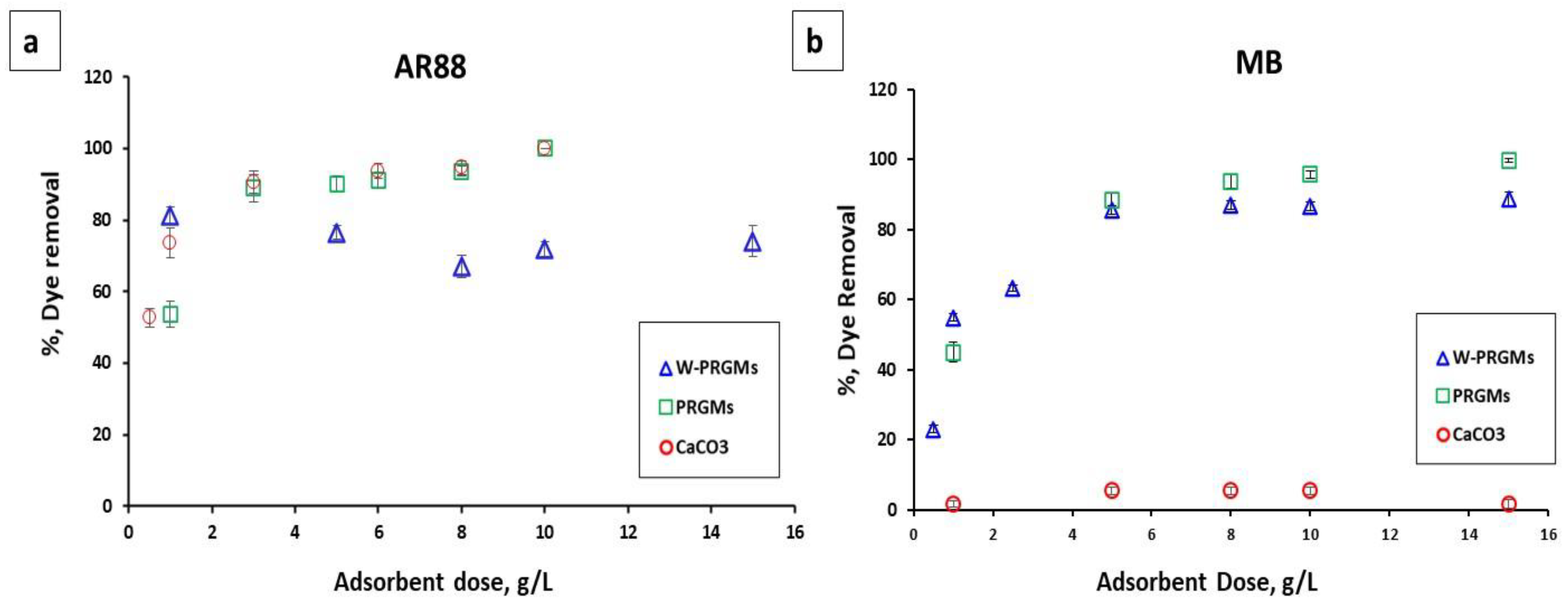
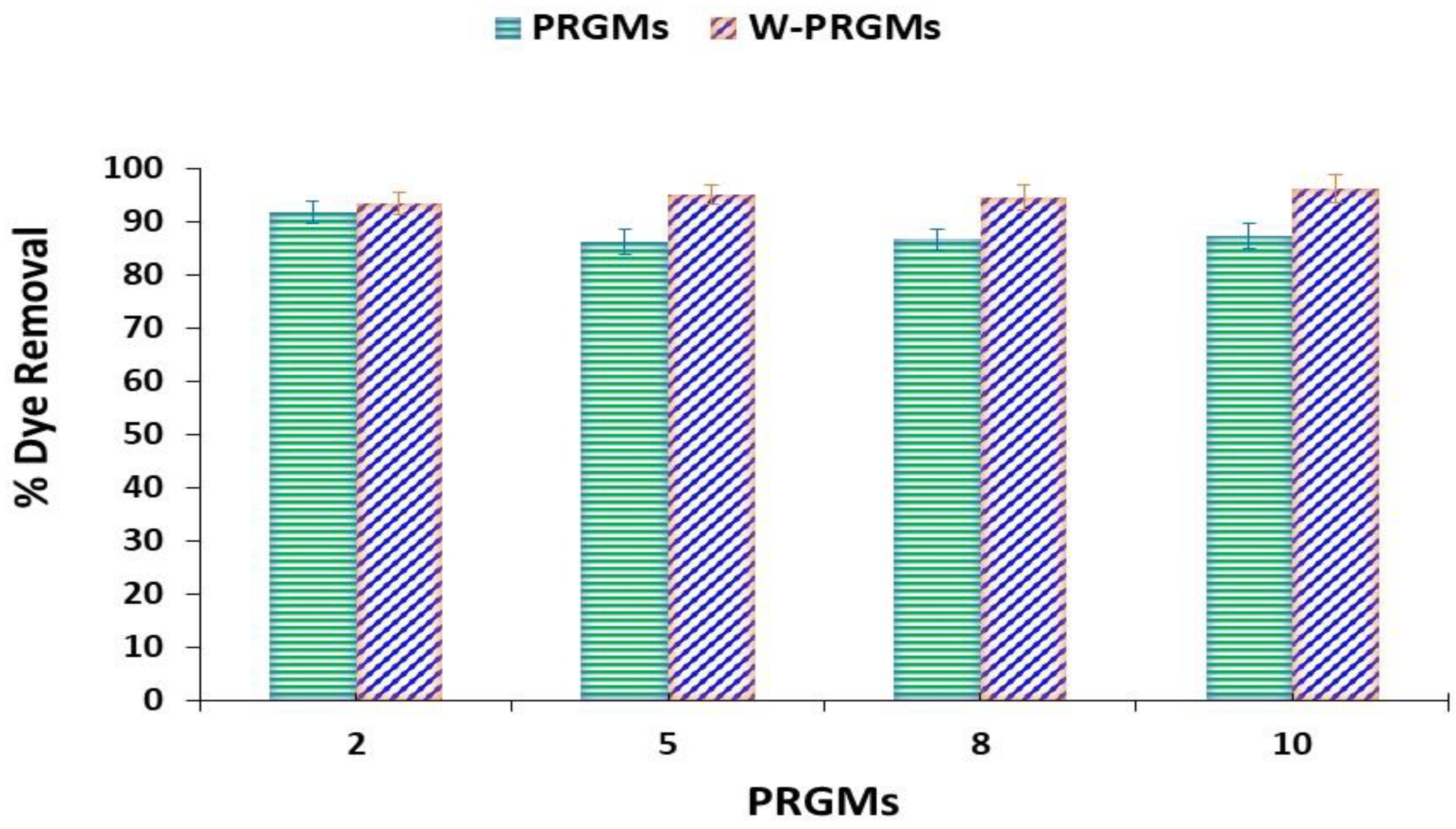
3.2.2. Effect of Adsorbent Dose
3.2.3. Adsorption Isotherms
3.3. Coagulation Study
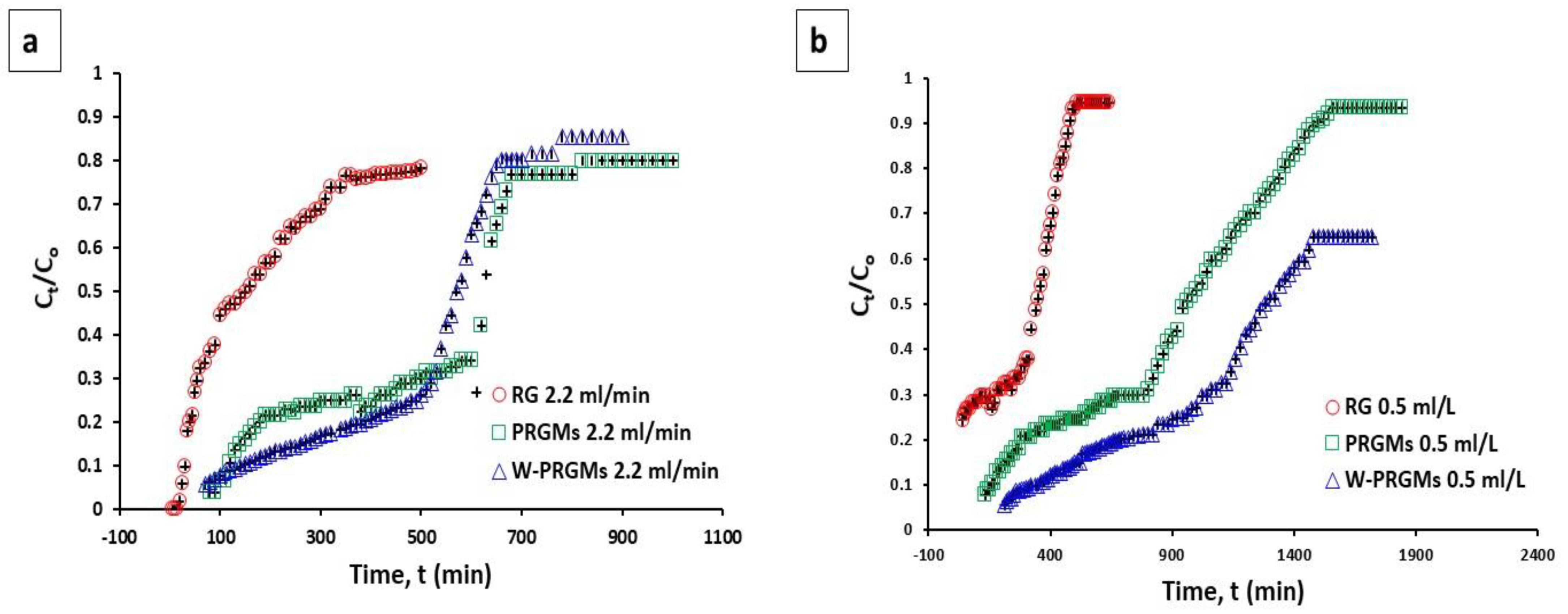
3.4. Column Adsorption Studies
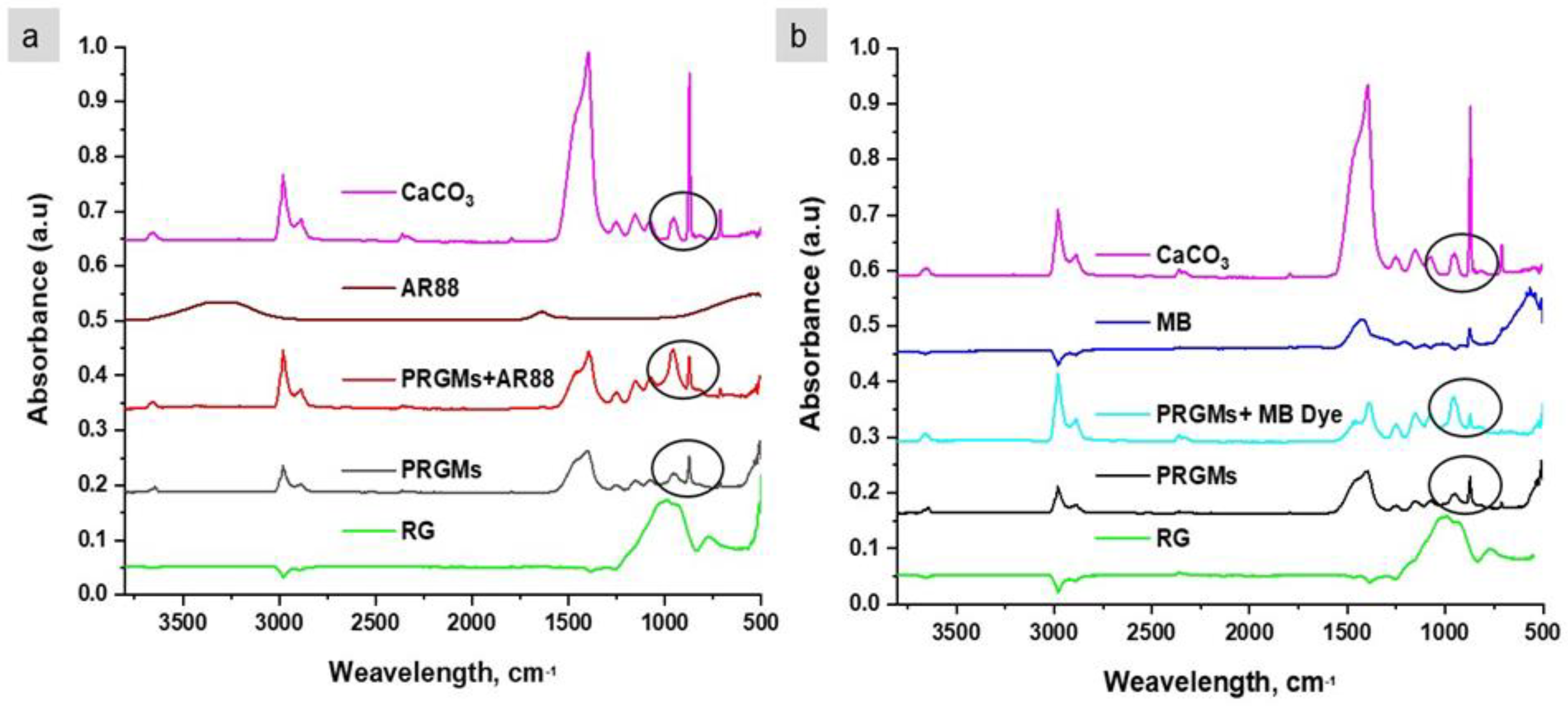
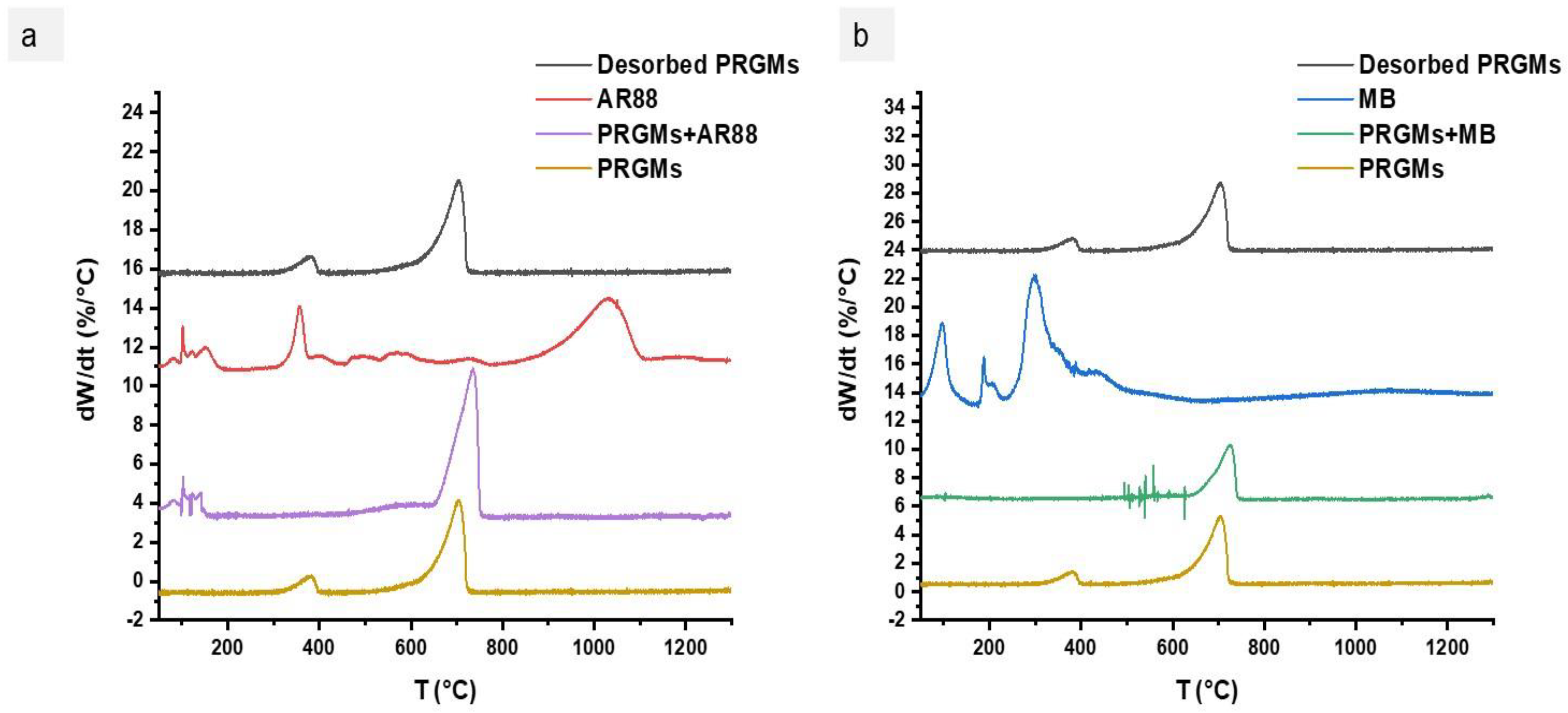
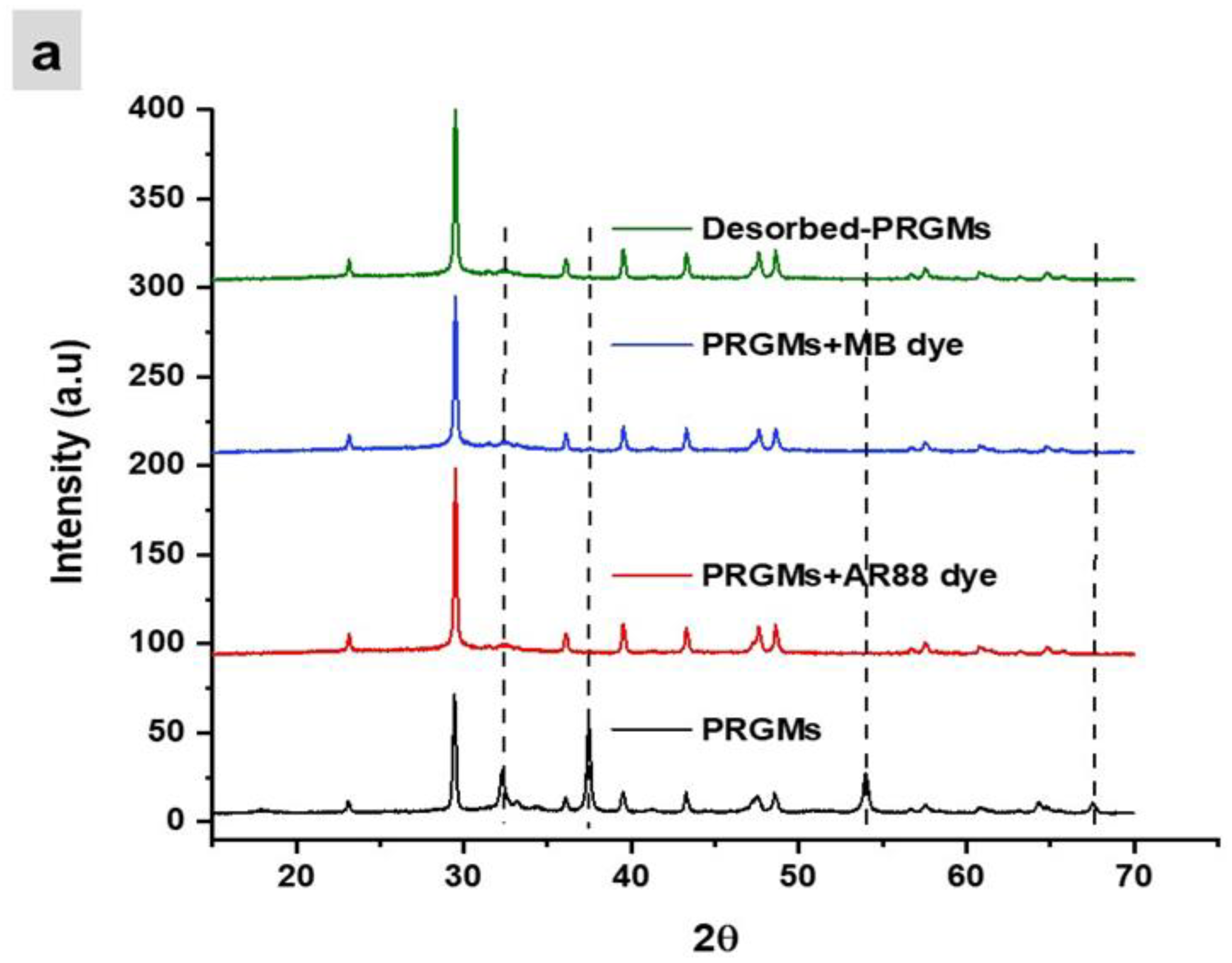
3.5. Interaction of Dye with PRGMs
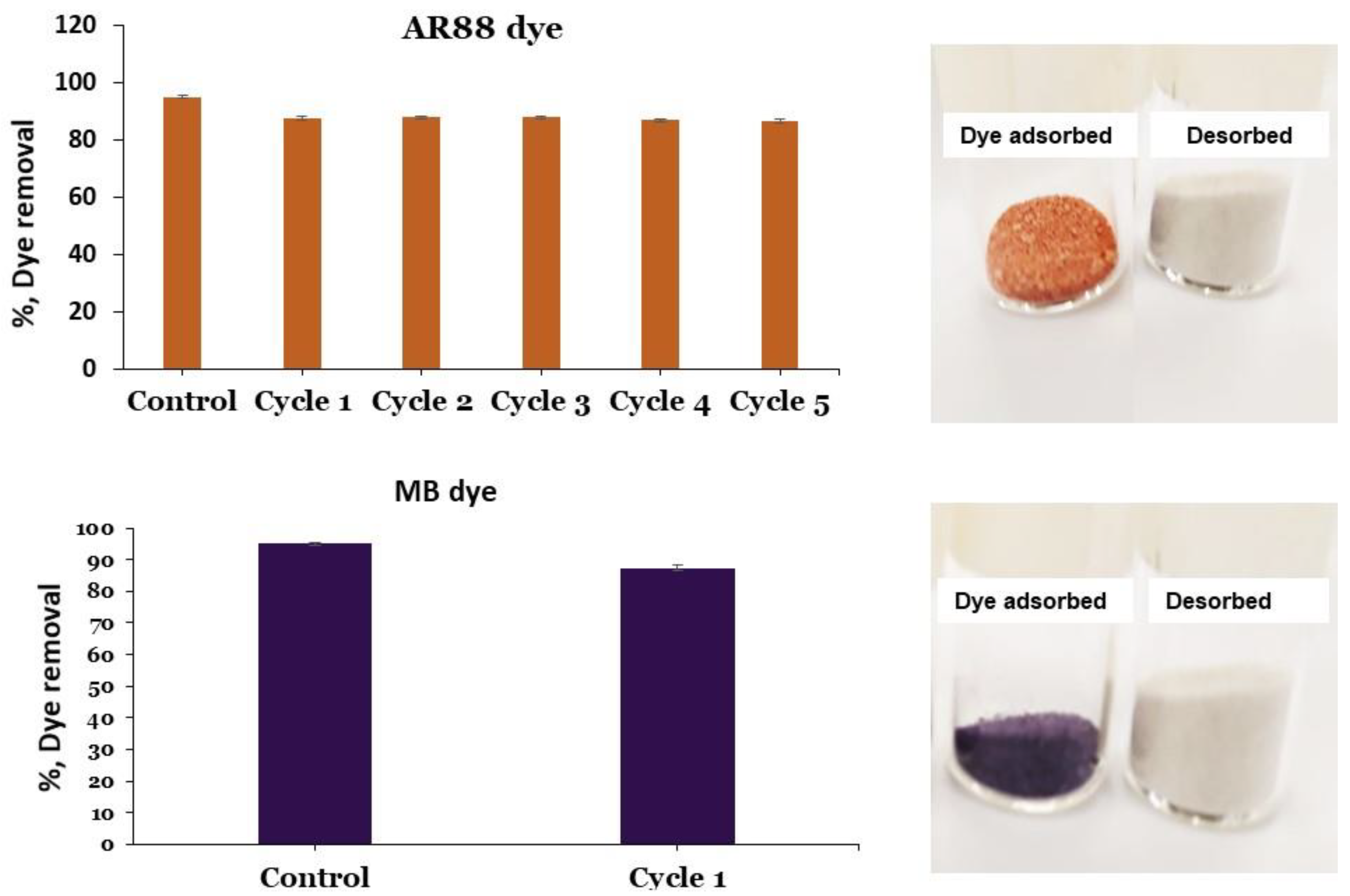
3.6. Reusability of PRGMs
4. Discussion
4.1. Processing of RG Particles into Porous Microspheres
4.2. Factors Influencing Dye Removal by PRGMs
- For the AR88 sorption in acidic medium
- PRGMs + H+ → PRGMs–H+ (Protonation of PRGMs)
- PRGMs–H+ + AR88− → PRGMs–H−AR88
- For the MB sorption in basic medium
- PRGMs + OH−→ PRGMs–OH− (de-protonation of PRGMs)
- PRGMs–OH−+ MB+ → PRGMs–OH−MB
4.3. Dye Removal Mechanism and Desorption Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, J.; Remmen, A. Enabling circular economy through product stewardship. Procedia Manuf. 2017, 8, 377–384. [Google Scholar] [CrossRef]
- Lieder, M.; Rashid, A. Towards circular economy implementation: A comprehensive review in context of manufacturing industry. J. Clean. Prod. 2016, 115, 36–51. [Google Scholar] [CrossRef]
- Fisher, O.J.; Watson, N.; Escrig, J.; Gomes, R.L. Intelligent Resource Use to Deliver Waste Valorisation and Process Resilience in Manufacturing Environments: Moving towards sustainable process manufacturing. Johns. Matthey Technol. Rev. 2020, 64, 93–99. [Google Scholar] [CrossRef]
- Addis, B. Building with Reclaimed Components and Materials: A Design Handbook for Reuse and Recycling; Taylor & Francis Group: Abingdon, UK, 2012. [Google Scholar]
- Garetti, M.; Taisch, M. Sustainable manufacturing: Trends and research challenges. Prod. Plan. Control. 2012, 23, 83–104. [Google Scholar] [CrossRef]
- Facts and Figures about Materials, Waste and Recycling. Environmental Protection Agency. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling (accessed on 1 January 2020).
- Miezah, K.; Obiri-Danso, K.; Kádár, Z.; Fei-Baffoe, B.; Mensah, M.Y. Municipal solid waste characterization and quantification as a measure towards effective waste management in Ghana. Waste Manag. 2015, 46, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Imteaz, M.A.; Ali, M.Y.; Arulrajah, A. Possible environmental impacts of recycled glass used as a pavement base material. Waste Manag. Res. 2012, 30, 917–921. [Google Scholar] [CrossRef]
- Bernardo, E.; Esposito, L.; Rambaldi, E.; Tucci, A.; Hreglich, S. Recycle of waste glass into “glass–ceramic stoneware”. J. Am. Ceram. Soc. 2008, 91, 2156–2162. [Google Scholar] [CrossRef]
- Nodehi, M.; Taghvaee, V.M. Sustainable concrete for circular economy: A review on use of waste glass. Glas. Struct. Eng. 2021, 7, 3–22. [Google Scholar] [CrossRef]
- Silva, R.V.; De Brito, J.; Lye, C.Q.; Dhir, R.K. The role of glass waste in the production of ceramic-based products and other applications: A review. J. Clean. Prod. 2017, 167, 346–364. [Google Scholar] [CrossRef]
- Mattson, J.S.; Mark, H.B. Activated Carbon: Surface Chemistry and Adsorption from Solution; M. Dekker: New York, NY, USA, 1971. [Google Scholar]
- Vanderborght, B.M.; Van Grieken, R.E. Enrichment of trace metals in water by adsorption on activated carbon. Anal. Chem. 1977, 49, 311–316. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dye. Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Arena, N.; Lee, J.; Clift, R. Life Cycle Assessment of activated carbon production from coconut shells. J. Clean. Prod. 2016, 125, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Ragan, S.; Megonnell, N. Activated carbon from renewable resources—Lignin. Cellul. Chem. Technol. 2011, 45, 527–531. [Google Scholar]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Soyer, E.; Akgiray, O.; Eldem, N.; Saatçı, A.M. Crushed recycled glass as a filter medium and comparison with silica sand. CLEAN–Soil Air Water 2010, 38, 927–935. [Google Scholar] [CrossRef]
- Cescon, A.; Jiang, J.-Q. Filtration process and alternative filter media material in water treatment. Water 2020, 12, 3377. [Google Scholar] [CrossRef]
- Bové, J.; Arbat, G.; Duran-Ros, M.; Pujol, T.; Velayos, J.; De Cartagena, F.R.; Puig-Bargués, J. Pressure drop across sand and recycled glass media used in micro irrigation filters. Biosyst. Eng. 2015, 137, 55–63. [Google Scholar] [CrossRef]
- Fernández, A.; Lassaletta, G.; Jiménez, V.M.; Justo, A.; González-Elipe, A.R.; Herrmann, J.-M.; Tahiri, H.; Ait-Ichou, Y. Preparation and characterization of TiO2 photocatalysts supported on various rigid supports (glass, quartz and stainless steel). Comparative studies of photocatalytic activity in water purification. Appl. Catal. B Environ. 1995, 7, 49–63. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, X. Effect of surface treatment on the photocatalytic activity and hydrophilic property of the sol-gel derived TiO2 thin films. Mater. Res. Bull. 2001, 36, 97–107. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, M.; Vaish, R. Transparent ferroelectric glass–ceramics for wastewater treatment by piezocatalysis. Commun. Mater. 2020, 1, 100. [Google Scholar] [CrossRef]
- Nishida, T.; Morimoto, A.; Yamamoto, Y.; Kubuki, S. Waste water purification using new porous ceramics prepared by recycling waste glass and bamboo charcoal. Appl. Water Sci. 2017, 7, 4281–4286. [Google Scholar] [CrossRef]
- Hussain, Z.; Sultan, N.; Ali, M.; Naz, M.Y.; AbdEl-Salam, N.M.; Ibrahim, K.A. Thermochemical conversion of waste glass and mollusk shells into an absorbent material for separation of direct blue 15 azo dye from industrial wastewater. ACS Omega 2020, 5, 18114–18122. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Stucky, G.D. Synthesis of highly ordered mesoporous silica materials using sodium silicate and amphiphilic block copolymers. Chem. Commun. 2000, 13, 1159–1160. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, Y.; Tang, F.; Liu, S. Mesoporous/microporous silica materials: Preparation from natural sands and highly efficient fixed-bed adsorption of methylene blue in wastewater. Microporous Mesoporous Mater. 2018, 257, 9–18. [Google Scholar] [CrossRef]
- Dahane, S.; Galera, M.M.; Marchionni, M.E.; Viciana, M.M.S.; Derdour, A.; García, M.D.G. Mesoporous silica based MCM-41 as solid-phase extraction sorbent combined with micro-liquid chromatography–quadrupole-mass spectrometry for the analysis of pharmaceuticals in waters. Talanta 2016, 152, 378–391. [Google Scholar] [CrossRef]
- MacArthur, E. Towards a circular economy: Business rationale for an accelerated transition. Greener Manag. Int. 2015, 20. [Google Scholar]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Bui, T.H.; Hong, S.P.; Yoon, J. Development of nanoscale zirconium molybdate embedded anion exchange resin for selective removal of phosphate. Water Res. 2018, 134, 22–31. [Google Scholar] [CrossRef]
- Maheshwari, U.; Gupta, S. Removal of Cr (VI) from wastewater using activated neem bark in a fixed-bed column: Interference of other ions and kinetic modelling studies. Desalination Water Treat. 2016, 57, 8514–8525. [Google Scholar] [CrossRef]
- Xu, Z.; Cai, J.-G.; Pan, B.-C. Mathematically modeling fixed-bed adsorption in aqueous systems. J. Zhejiang Univ. Sci. A 2013, 14, 155–176. [Google Scholar] [CrossRef] [Green Version]
- Markandeya, S.; Kisku, G. Linear and nonlinear kinetic modeling for adsorption of disperse dye in batch process. Res. J. Environ. Toxicol. 2015, 9, 320–331. [Google Scholar]
- Knight, A.W.; Tigges, A.B.; Ilgen, A.G. Adsorption of copper (II) on mesoporous silica: The effect of nano-scale confinement. Geochem. Trans. 2018, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Dada, A. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.d.; León, J.J.L.; de Matos, T.N. Elucidation of mechanism involved in adsorption of Pb (II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Han, R.; Wang, Y.; Yu, W.; Zou, W.; Shi, J.; Liu, H. Biosorption of methylene blue from aqueous solution by rice husk in a fixed-bed column. J. Hazard. Mater. 2007, 141, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Ruj, B.; Sadhukhan, A.; Gupta, P. A TG-FTIR investigation on the co-pyrolysis of the waste HDPE, PP, PS and PET under high heating conditions. J. Energy Inst. 2020, 93, 1020–1035. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Usher, C.R.; Grassian, V.H. Reactions of sulfur dioxide on calcium carbonate single crystal and particle surfaces at the adsorbed water carbonate interface. Phys. Chem. Chem. Phys. 2007, 9, 3011–3024. [Google Scholar] [CrossRef]
- Das, S.K.; Dickinson, C.; Lafir, F.; Brougham, D.F.; Marsili, E. Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with Rhizopus oryzae protein extract. Green Chem. 2012, 14, 1322–1334. [Google Scholar] [CrossRef]
- Sharma, A.; Jain, H.; Miller, A. Surface modification of a silicate glass during XPS experiments. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Film. 2001, 31, 369–374. [Google Scholar] [CrossRef]
- Sordello, F.; Zeb, G.; Hu, K.; Calza, P.; Minero, C.; Szkopek, T.; Cerruti, M. Tuning TiO2 nanoparticle morphology in graphene–TiO2 hybrids by graphene surface modification. Nanoscale 2014, 6, 6710–6719. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Zhao, S.; Ma, X.; Liu, S.; Shen, Q. A fast π-π stacking self-assembly of cobalt terephthalate dihydrate and the twelve-electron lithiation-delithiation of anhydrous cobalt terephthalate. J. Power Sources 2019, 426, 23–32. [Google Scholar] [CrossRef]
- Nesbitt, H.; Bancroft, G.; Henderson, G.; Ho, R.; Dalby, K.; Huang, Y.; Yan, Z. Bridging, non-bridging and free (O2–) oxygen in Na2O-SiO2 glasses: An. X-ray Photoelectron Spectroscopic (XPS) and Nuclear Magnetic Resonance (NMR) study. J. Non-Cryst. Solids 2011, 357, 170–180. [Google Scholar] [CrossRef]
- Serra, J.; González, P.; Liste, S.; Serra, C.; Chiussi, S.; León, B.; Pérez-Amor, M.; Ylänen, H.; Hupa, M. FTIR and XPS studies of bioactive silica based glasses. J. Non-Cryst. Solids 2003, 332, 20–27. [Google Scholar] [CrossRef]
- Larionau, P.; Hujova, M.; Michalková, M.; Mahmoud, M.; Švančárková, A.; Galusková, D.; Parchoviansky, M.; Bernardo, E.; Galusek, D.; Kraxner, J. Low-alkali borosilicate glass microspheres from waste cullet prepared by flame synthesis. Int. J. Appl. Glass Sci. 2021, 12, 562–569. [Google Scholar] [CrossRef]
- Levine, A.D.; Tchobanoglous, G.; Asano, T. Characterization of the size distribution of contaminants in wastewater: Treatment and reuse implications. Water Pollut. Control. Fed. 1985, 57, 805–816. [Google Scholar]
- Madaeni, S. The application of membrane technology for water disinfection. Water Res. 1999, 33, 301–308. [Google Scholar] [CrossRef]
- Zhang, Y.; Diehl, A.; Lewandowski, A.; Gopalakrishnan, K.; Baker, T. Removal efficiency of micro-and nanoplastics (180 nm–125 μm) during drinking water treatment. Sci. Total Environ. 2020, 720, 137383. [Google Scholar] [CrossRef]
- Kumar, P.S.; Prot, T.; Korving, L.; Keesman, K.J.; Dugulan, I.; van Loosdrecht, M.C.; Witkamp, G.-J. Effect of pore size distribution on iron oxide coated granular activated carbons for phosphate adsorption–Importance of mesopores. Chem. Eng. J. 2017, 326, 231–239. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.H. Membrane bioreactor (MBR) technology for wastewater treatment and reclamation: Membrane fouling. Membranes 2016, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.R.; Santiano, B.; Kim, H.; Kan, E. Heterogeneous oxidation of methylene blue with surface-modified iron-amended activated carbon. Am. J. Anal. Chem. 2013, 4, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Lum, P.T.; Lim, K.Y.; Zakaria, N.A.; Foo, K.Y. A novel preparation of visible light driven Durio zibethinus shell ash supported CuO nanocomposite for the photocatalytic degradation of acid dye. J. Mater. Res. Technol. 2020, 9, 168–179. [Google Scholar] [CrossRef]
- Konicki, W.; Sibera, D.; Narkiewicz, U. Adsorption of Acid Red 88 Anionic Dye from Aqueous Solution onto ZnO/ZnMn2O4 Nanocomposite: Equilibrium, Kinetics, and Thermodynamics. Pol. J. Environ. Stud. 2017, 26, 2585–2593. [Google Scholar] [CrossRef]
- Vaghetti, J.C.; Lima, E.C.; Royer, B.; Brasil, J.L.; da Cunha, B.M.; Simon, N.M. Application of Brazilian-pine fruit coat as a biosorbent to removal of Cr (VI) from aqueous solution—Kinetics and equilibrium study. Biochem. Eng. J. 2008, 42, 67–76. [Google Scholar] [CrossRef]
- Boughzala, K.; Kooli, F.; Meksi, N.; Bechrifa, A.; Bouzouita, K. Waste products from the phosphate industry as efficient removal of Acid Red 88 dye from aqueous solution: Their regeneration uses and batch design adsorber. Desalination Water Treat 2020, 202, 410–419. [Google Scholar] [CrossRef]
- Zhao, D.; Sheng, G.; Hu, J.; Chen, C.; Wang, X. The adsorption of Pb (II) on Mg2Al layered double hydroxide. Chem. Eng. J. 2011, 171, 167–174. [Google Scholar] [CrossRef]
- Yan, H.; Li, H.; Yang, H.; Li, A.; Cheng, R. Removal of various cationic dyes from aqueous solutions using a kind of fully biodegradable magnetic composite microsphere. Chem. Eng. J. 2013, 223, 402–411. [Google Scholar] [CrossRef]
- Wang, W.; Cai, K.; Wu, X.; Shao, X.; Yang, X. A novel poly (m-phenylenediamine)/reduced graphene oxide/nickel ferrite magnetic adsorbent with excellent removal ability of dyes and Cr (VI). J. Alloy. Compd. 2017, 722, 532–543. [Google Scholar] [CrossRef]
- Padmesh, T.; Vijayaraghavan, K.; Sekaran, G.; Velan, M. Batch and column studies on biosorption of acid dyes on fresh water macro alga Azolla filiculoides. J. Hazard. Mater. 2005, 125, 121–129. [Google Scholar] [CrossRef]
- Balarak, D.; Mahdavi, Y. Experimental and Kinetic Studies on Acid Red 88 Dye (AR88) Adsorption by Azolla Filiculoides. Biochem. Physiol. 2016, 5, 1–5. [Google Scholar] [CrossRef]
- Chen, S.; Yue, Q.; Gao, B.; Li, Q.; Xu, X.; Fu, K. Adsorption of hexavalent chromium from aqueous solution by modified corn stalk: A fixed-bed column study. Bioresour. Technol. 2012, 113, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Abdolali, A.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Zhang, J.; Liang, S.; Chang, S.W.; Nguyen, D.D.; Liu, Y. Application of a breakthrough biosorbent for removing heavy metals from synthetic and real wastewaters in a lab-scale continuous fixed-bed column. Bioresour. Technol. 2017, 229, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazari, G.; Abolghasemi, H.; Esmaieli, M.; Pouya, E.S. Aqueous phase adsorption of cephalexin by walnut shell-based activated carbon: A fixed-bed column study. Appl. Surf. Sci. 2016, 375, 144–153. [Google Scholar] [CrossRef]
- Bohart, G.S.; Adams, E.Q. Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc. 1920, 42, 523–544. [Google Scholar] [CrossRef] [Green Version]
- Thomas, H.C. Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Sun, X.; Imai, T.; Sekine, M.; Higuchi, T.; Yamamoto, K.; Kanno, A.; Nakazono, S. Adsorption of phosphate using calcined Mg3–Fe layered double hydroxides in a fixed-bed column study. J. Ind. Eng. Chem. 2014, 20, 3623–3630. [Google Scholar] [CrossRef]
- Ahmed, M.; Hameed, B. Removal of emerging pharmaceutical contaminants by adsorption in a fixed-bed column: A review. Ecotoxicol. Environ. Saf. 2018, 149, 257–266. [Google Scholar] [CrossRef]
- Benjedim, S.; Romero-Cano, L.A.; Pérez-Cadenas, A.F.; Bautista-Toledo, M.I.; Lotfi, E.M.; Carrasco-Marín, F. Removal of emerging pollutants present in water using an E-coli biofilm supported onto activated carbons prepared from argan wastes: Adsorption studies in batch and fixed bed. Sci. Total Environ. 2020, 720, 137491. [Google Scholar] [CrossRef]
- Egbosiuba, T.C.; Abdulkareem, A.S.; Kovo, A.S.; Afolabi, E.A.; Tijani, J.O.; Bankole, M.T.; Bo, S.; Roos, W.D. Adsorption of Cr (VI), Ni (II), Fe (II) and Cd (II) ions by KIAgNPs decorated MWCNTs in a batch and fixed bed process. Sci. Rep. 2021, 11, 1–20. [Google Scholar] [CrossRef]
- Esfandian, H.; Samadi-Maybodi, A.; Khoshandam, B.; Parvini, M. Experimental and CFD modeling of diazinon pesticide removal using fixed bed column with Cu-modified zeolite nanoparticle. J. Taiwan Inst. Chem. Eng. 2017, 75, 164–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, F.; Shen, Z.; Wang, F.; Lynch, R.; Al-Tabbaa, A. Adsorption of methyl tert-butyl ether (MTBE) onto ZSM-5 zeolite: Fixed-bed column tests, breakthrough curve modelling and regeneration. Chemosphere 2019, 220, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Garg, A. Adsorption and oxidation of ciprofloxacin in a fixed bed column using activated sludge derived activated carbon. J. Environ. Manag. 2019, 250, 109474. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Shariff, A.; Lau, K.; Bustam, M. Factors affecting CO2 absorption efficiency in packed column: A review. J. Ind. Eng. Chem. 2012, 18, 1874–1883. [Google Scholar] [CrossRef]
- Yang, Z.; Asoh, T.-A.; Uyama, H. Removal of cationic or anionic dyes from water using ion exchange cellulose monoliths as adsorbents. Bull. Chem. Soc. Jpn. 2019, 92, 1453–1461. [Google Scholar] [CrossRef]
- Jahangiri-rad, M.; Jamshidi, A.; Rafiee, M.; Nabizadeh, R. Adsorption performance of packed bed column for nitrate removal using PAN-oxime-nano Fe2O3. J. Environ. Health Sci. Eng. 2014, 12, 1–5. [Google Scholar]
- Miralles, N.; Valderrama, C.; Casas, I.; Martínez, M.; Florido, A. Cadmium and lead removal from aqueous solution by grape stalk wastes: Modeling of a fixed-bed column. J. Chem. Eng. Data 2010, 55, 3548–3554. [Google Scholar] [CrossRef] [Green Version]
- Kafshgari, F.; Keshtkar, A.R.; Mousavian, M.A. Study of Mo. (VI) removal from aqueous solution: Application of different mathematical models to continuous biosorption data. Iran. J. Environ. Health Sci. Eng. 2013, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Aroonwilas, A.; Veawab, A. Characterization and comparison of the CO2 absorption performance into single and blended alkanolamines in a packed column. Ind. Eng. Chem. Res. 2004, 43, 2228–2237. [Google Scholar] [CrossRef]
- Yu, H.; Morgan, S.; Allport, A.; Cottrell, A.; Do, T.; Wardhaugh, J.M.G.L.; Feron, P. Results from trialling aqueous NH3 based post-combustion capture in a pilot plant at Munmorah power station: Absorption. Chem. Eng. Res. Des. 2011, 89, 1204–1215. [Google Scholar] [CrossRef]
- Askari, M.; Salehi, E.; Ebrahimi, M.; Barati, A. Application of breakthrough curve analysis and response surface methodology for optimization of a hybrid separation system consisting of fixed-bed column adsorption and dead-end depth filtration. Chem. Eng. Processing-Process Intensif. 2019, 143, 107594. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, V.C.; Mall, I.D. Fixed-bed study for adsorptive removal of furfural by activated carbon. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 50–56. [Google Scholar] [CrossRef]
- Otaru, A.; Kennedy, A.R. The permeability of virtual macroporous structures generated by sphere packing models: Comparison with analytical models. Scr. Mater. 2016, 124, 30–33. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Lee, J.-W.; Yang, S.-Y.; Lee, H.; Koh, Y.-H.; Kim, H.-E. Dual-scale porous biphasic calcium phosphate gyroid scaffolds using ceramic suspensions containing polymer microsphere porogen for digital light processing. Ceram. Int. 2020, 47, 11285–11293. [Google Scholar] [CrossRef]
- Netpradit, S.; Thiravetyan, P.; Towprayoon, S. Evaluation of metal hydroxide sludge for reactive dye adsorption in a fixed-bed column system. Water Res. 2004, 38, 71–78. [Google Scholar] [CrossRef]
- Qi, C.; Zhao, L.; Lin, Y.; Wu, D. Graphene oxide/chitosan sponge as a novel filtering material for the removal of dye from water. J. Colloid Interface Sci. 2018, 517, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, S. Use of granular slag columns for lead removal. Water Res. 2002, 36, 4001–4008. [Google Scholar] [CrossRef]
- Xie, W.; Liang, D.; Li, L.; Qu, S.; Tao, W. Surface chemical properties and pore structure of the activated coke and their effects on the denitrification activity of selective catalytic reduction. Int. J. Coal Sci. Technol. 2019, 6, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Tang, Q.; Jiang, T.; Cheng, Y. Adsorption performance of hydrophobic/hydrophilic silica aerogel for low concentration organic pollutant in aqueous solution. Nanotechnol. Rev. 2019, 8, 266–274. [Google Scholar] [CrossRef]
- Suzuki, M.; Maruyama, S.; Umesaki, N.; Tanaka, T. Hydroxyl-Group Identification Using O K-Edge XAFS in Porous Glass Fabricated by Hydrothermal Reaction and Low-Temperature Foaming. Molecules 2019, 24, 3488. [Google Scholar] [CrossRef] [Green Version]
- Samad, S.A.; Arafat, A.; Ferrari, R.; Gomes, R.L.; Lester, E.; Ahmed, I. Adsorption studies and effect of heat treatment on porous glass microspheres. Int. J. Appl. Glass Sci. 2022, 13, 63–81. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Liu, J.; Liu, X.; Chen, C.; Li, G.; Meng, Y. Graphene oxides cross-linked with hyperbranched polyethylenimines: Preparation, characterization and their potential as recyclable and highly efficient adsorption materials for lead (II) ions. Chem. Eng. J. 2016, 285, 698–708. [Google Scholar] [CrossRef] [Green Version]
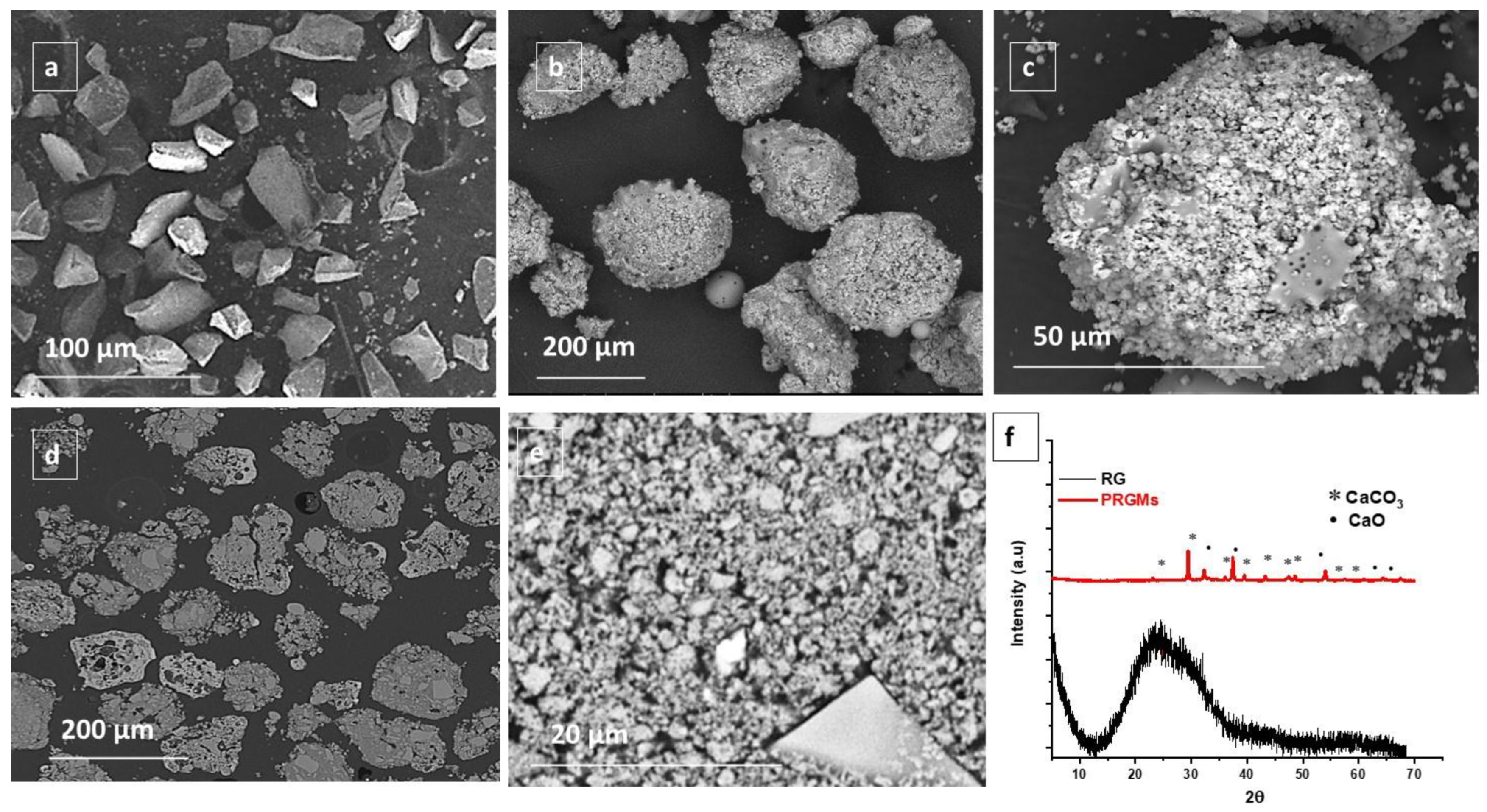


| Sample | Surface Area (m2/g) | Amicro (m2/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Vtotal (cm3/g) |
|---|---|---|---|---|---|
| RG | 0.03 | 0.006 | 0 | 0 | 0 |
| PRGMs | 8 | 1.5 | 0.002 | 0.03 | 0.06 |
| Langmuir Isotherm | Freundlich Isotherm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AR88 dye | Sample | qm (mg/g) | R2 | X2 | qe (mg/g) | Kf (mL/g) | 1/n | R2 | X2 |
| PRGMs | 78 ± 4 | 0.99 | 2.1 | 99 | 4 ± 0.01 | 0.71 ± 0.1 | 0.99 | 1.2 | |
| RG | -- | -- | -- | --- | -- | -- | -- | -- | |
| Porogen | 227 ± 5 | 0.99 | 3.07 | 200 | 2 ± 0.001 | 1 ± 0.2 | 0.99 | 5.3 | |
| MB dye | PRGMs | 20 ± 3 | 0.98 | 0.37 | 14 | 3 ± 0.003 | 0.58 | 0.98 | 1.2 |
| RG | -- | -- | -- | -- | -- | -- | -- | -- | |
| Porogen | -- | -- | -- | -- | -- | -- | -- | -- | |
| Column Study | Batch Study | ||||
|---|---|---|---|---|---|
| Sample | Flow Rate, (mL\min) | EBCT, (min) | qtot (mg) (±3) | qeq (mg\g) (±3) | qe (mg\g) (±4) |
| RG | 2.2 | 1 | 235 | 84 | -- |
| RG | 0.5 | 5 | 89 | 42 | |
| PRGMs | 2.2 | 1 | 763 | 381 | 98 |
| PRGMs | 0.5 | 5 | 425 | 202 | |
| W-PRGMs | 2.2 | 1 | 611 | 305 | -- |
| W-PRGMs | 0.5 | 5 | 282 | 157 | |
| Thomas Model | Adams-Bohart Model | Yoon-Nelson Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Flow Rate, (mL/min) | Qe exp, mg/g | qth, mg/g | Kth (mL/min/mg) | R2 | N0 (mg/L) | KAB (mL/min/mg) | R2 | τcal (min) | τexp (min) | R2 |
| RG | 2.2 | 84 | 12 | 0.001 | 0.64 | 39 | 4 × 10−5 | 0.92 | 175 | 204 | 0.94 |
| 0.5 | 42 | 33 | 0.001 | 0.94 | 25 | 9 × 10−5 | 0.4 | 245 | 255 | 0.9 | |
| PRGMs | 2.2 | 381 | 250 | 6.8 × 10−5 | 0.88 | 193 | 5.4 × 10−5 | 0.77 | 420 | 622 | 0.88 |
| 0.5 | 202 | 231 | 3.1 × 10−5 | 0.80 | 135 | 2.2 × 10−5 | 0.61 | 800 | 914 | 0.86 | |
| W-PRGMs | 2.2 | 305 | 244 | 9.5 × 10−5 | 0.83 | 222 | 5.4 × 10−5 | 0.7 | 400 | 549 | 0.83 |
| 0.5 | 157 | 168 | 4.5 × 10−5 | 0.86 | 95 | 2.5 × 10−5 | 0.61 | 750 | 1285 | 0.80 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samad, S.A.; Arafat, A.; Lester, E.; Ahmed, I. Upcycling Glass Waste into Porous Microspheres for Wastewater Treatment Applications: Efficacy of Dye Removal. Materials 2022, 15, 5809. https://doi.org/10.3390/ma15175809
Samad SA, Arafat A, Lester E, Ahmed I. Upcycling Glass Waste into Porous Microspheres for Wastewater Treatment Applications: Efficacy of Dye Removal. Materials. 2022; 15(17):5809. https://doi.org/10.3390/ma15175809
Chicago/Turabian StyleSamad, Sabrin A., Abul Arafat, Edward Lester, and Ifty Ahmed. 2022. "Upcycling Glass Waste into Porous Microspheres for Wastewater Treatment Applications: Efficacy of Dye Removal" Materials 15, no. 17: 5809. https://doi.org/10.3390/ma15175809
APA StyleSamad, S. A., Arafat, A., Lester, E., & Ahmed, I. (2022). Upcycling Glass Waste into Porous Microspheres for Wastewater Treatment Applications: Efficacy of Dye Removal. Materials, 15(17), 5809. https://doi.org/10.3390/ma15175809