Studies of Monoamine Neurotransmitters at Nanomolar Levels Using Carbon Material Electrodes: A Review
Abstract
1. Introduction
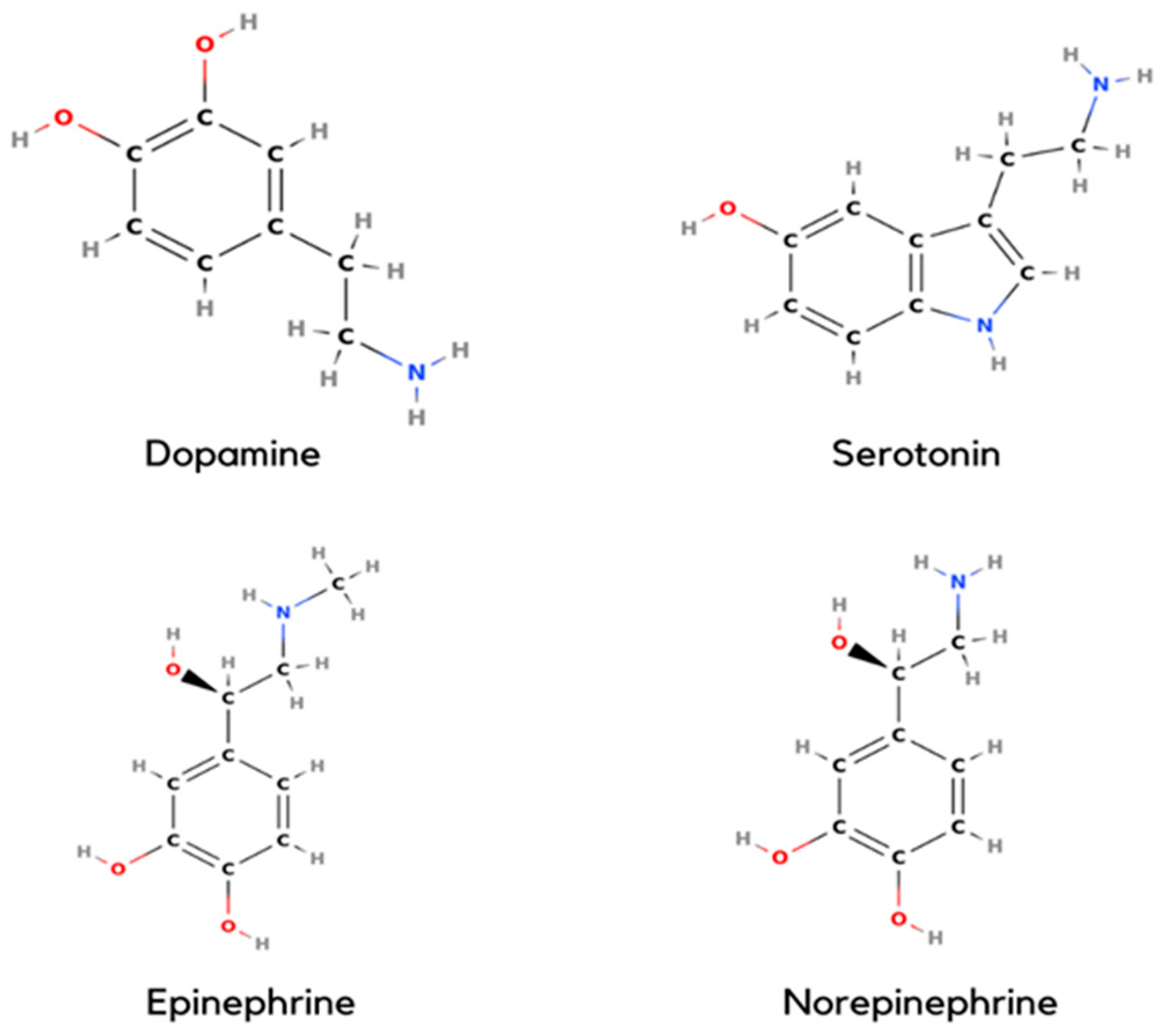
1.1. Dopamine
1.2. Serotonin
1.3. Epinephrine
1.4. Norepinephrine
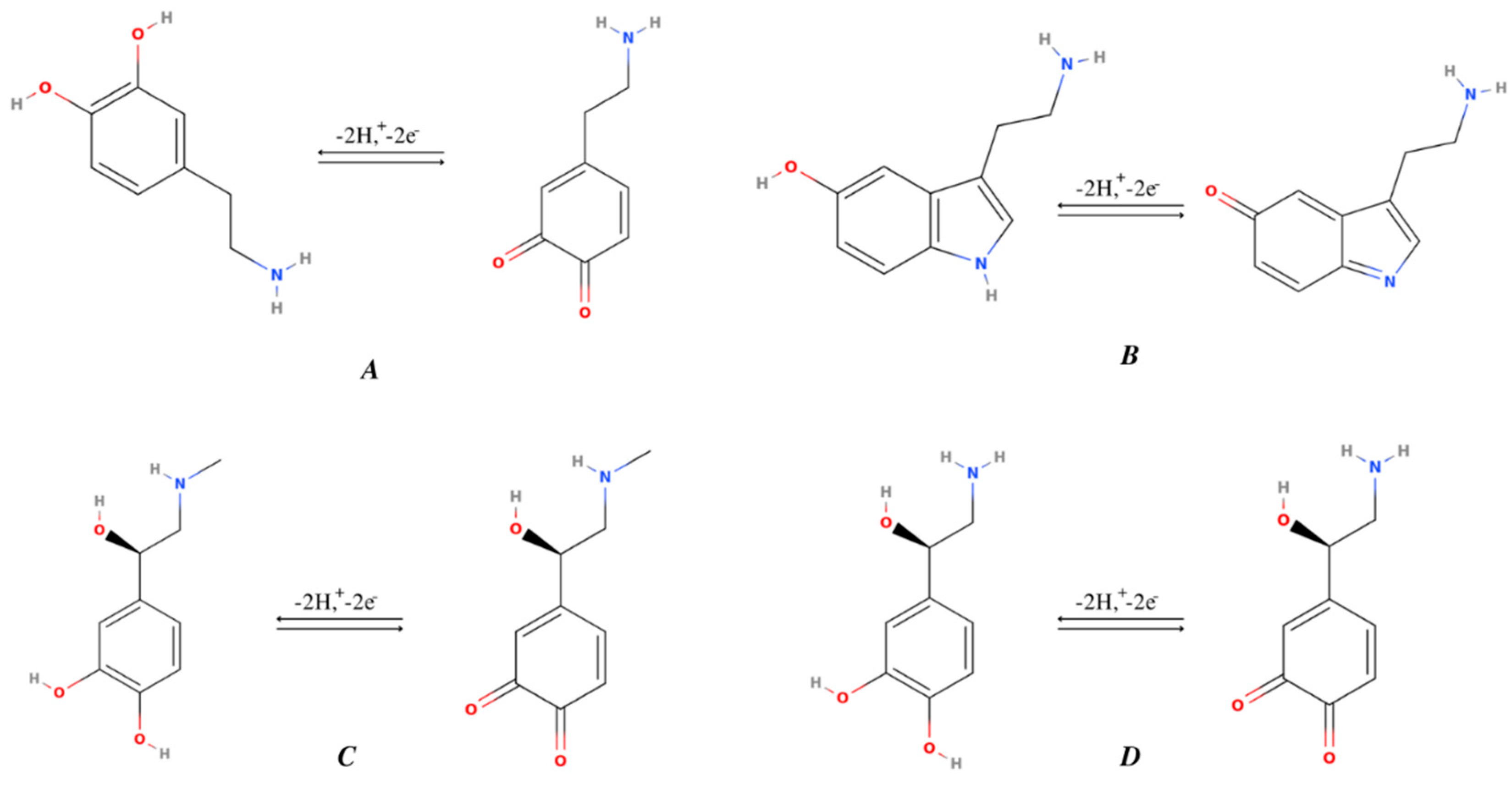
2. Comparison of Structures and Mechanism of Monoamine NTs Based on the Position of the Hydroxyl Group
3. Neurotransmitter Biosensing
4. Neurosensing via Electrochemical Studies
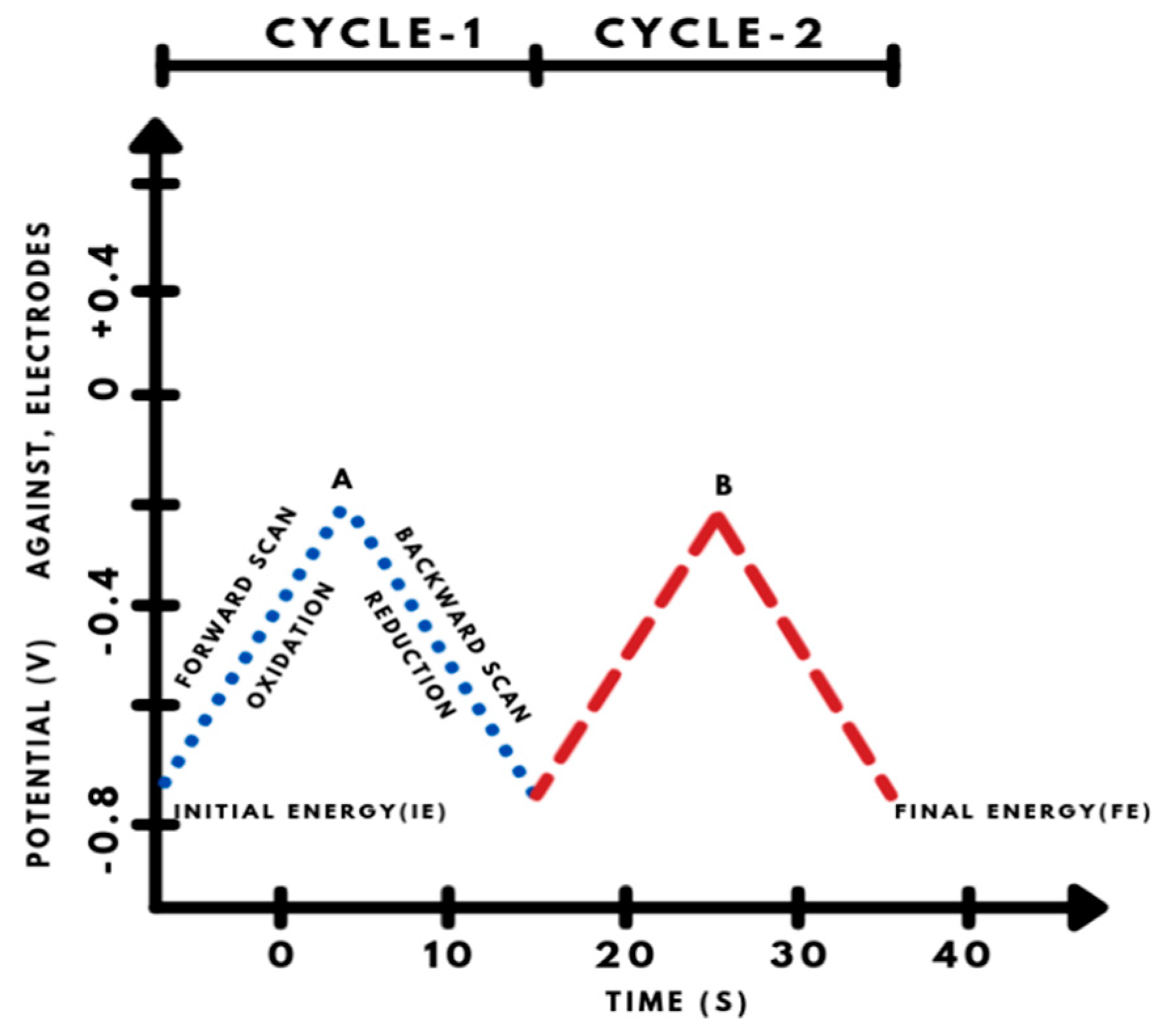
4.1. Cyclic Voltammetry (CV)
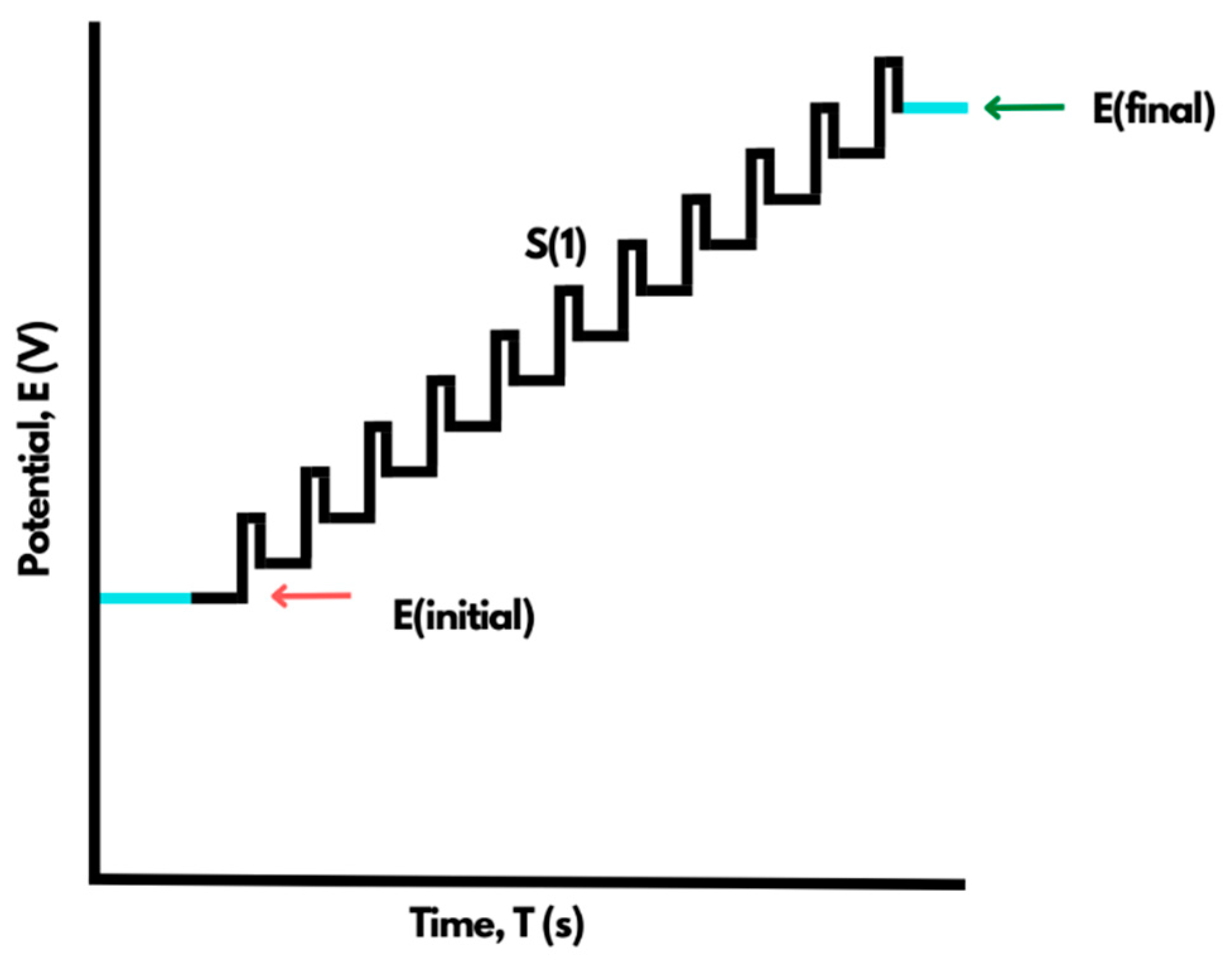
4.2. Differential Pulse Voltammetry (DPV)
5. Sensing of Monoamine NTs Using Surface-Modified Electrodes
5.1. Dopamine
5.1.1. Carbon Paste Electrode (CPE) Modified with Activated Carbon
5.1.2. CPE Modified with Polyalizarin Yellow R (PAYR)
5.1.3. CPE Modified with Sulfated β-Cyclodextrin (S-CD)
5.1.4. Pencil Graphite Electrode (PGE) Modified with Uric Acid and Ascorbic Acid at Poly(Xylenol Orange)
5.1.5. PGE Modified with Cu/CuxO Nanoparticles
5.1.6. CPE Modified with Poly(Reactive Blue) and Lysine
5.2. Serotonin
5.2.1. GCE Modified with Conducting Polyelectrolytes and Polymers
5.2.2. GCE Modified with Carbon Nanomaterials
5.2.3. Metal or Metal Oxides Nanoparticles Modified GCE
5.2.4. Graphite and Carbon Fiber Electrode and Surface Modifications
5.2.5. t-ZrO2 NPs Modified CPE
5.2.6. Diamond, Screen Printed, and Metal or Metal Oxide-Based Electrodes with Surface Modification
5.3. Epinephrine
5.3.1. CPE Modified with 3,4-Dihydroxybenzaldehyde-2,4-dinitrophenylhydrazone (DDP) and Carbon Nanotubes (CNTs)
5.3.2. Gold Electrode Modified with Polyaniline (PANI), MWCNT, RuO2, TiO2, MWCNT-PANI-TiO2, and MWCNT-PANI-RuO2 Suspensions
5.3.3. Gold Disk Electrode Using Biomimic Hemin Modified Molecular Imprinted Polymers (MIP)
5.3.4. GCE Modified with L-Glutamic Acid-Functionalized Graphene
5.4. Norepinephrine
5.4.1. GCE Modified with CNTs and Cobalt Ferrite Magnetic Nanoparticles
5.4.2. GCE Modified with Graphene
5.4.3. GCE Modified with MoO3 Nanowires (NWs)
MoO3.H2O → MoO3 + H2O
5.4.4. GCE Modified with Cetyltrimethylammonium Bromide Assisted SnO2 Nanoparticles for Concurrent Sensing of Epinephrine and Norepinephrine
5.4.5. Bare Boron-Doped Diamond Electrode
5.4.6. CPE Modified with Graphene-QDs/ILs for Simultaneous Detection of Acetylcholine and Norepinephrine
6. Conclusions and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tank, A.W.; Wong, D.L. Peripheral and Central Effects of Circulating Catecholamines. Compr. Physiol. 2014, 5, 1–15. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K. Chemical control of nanotube electronics. Nanotechnology 2000, 11, 57–60. [Google Scholar] [CrossRef]
- Wu, K.; Fei, J.; Hu, S. Simultaneous determination of dopamine and serotonin on a glassy carbon electrode coated with a film of carbon nanotubes. Anal. Biochem. 2003, 318, 100–106. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, N.; Tomar, V.; Chandra, R. A review on electrochemical detection of serotonin based on surface modified electrodes. Biosens. Bioelectron. 2018, 107, 76–93. [Google Scholar] [CrossRef]
- Albert, P.R.; Vahid-Ansari, F.; Luckhart, C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: Pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front. Behav. Neurosci. 2014, 8, 199. [Google Scholar] [CrossRef]
- Jones, B.J.; Blackburn, T.P. The medical benefit of 5-HT research. Pharmacol. Biochem. Behav. 2002, 71, 555–568. [Google Scholar] [CrossRef]
- McGuinness, O.P.; Shau, V.; Benson, E.M.; Lewis, M.; Snowden, R.T.; Greene, J.E.; Neal, D.W.; Cherrington, A.D. Role of epinephrine and norepinephrine in the metabolic response to stress hormone infusion in the conscious dog. Am. J. Physiol. Metab. 1997, 273, E674–E681. [Google Scholar] [CrossRef]
- Lowe, C.R. Biosensors. Trends Biotechnol. 1984, 2, 59–65. [Google Scholar] [CrossRef]
- Ardakani, T.; Hosu, O.; Cristea, C.; Ardakani, M.; Marrazza, G. Latest Trends in Electrochemical Sensors for Neurotransmitters: A Review. Sensors 2019, 19, 2037. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal, M.K.; Mansha, M.; Alsharaa, A.; Jillani, S.M.S.; Basheer, C. Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: A review. TrAC Trends Anal. Chem. 2015, 76, 15–29. [Google Scholar] [CrossRef]
- Silverberg, A.B.; Shah, S.D.; Haymond, M.W.; Cryer, P.E. Norepinephrine: Hormone and neurotransmitter in man. Am. J. Physiol. Metab. 1978, 234, E252–E256. [Google Scholar] [CrossRef]
- Peters, A.; Palay, S.L. The morphology of synapses. J. Neurocytol. 1996, 25, 687–700. [Google Scholar] [CrossRef]
- Di Chiara, G.; Morelli, M.; Consolo, S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994, 17, 228–233. [Google Scholar] [CrossRef]
- Moukhles, H.; Bosler, O.; Bolam, J.P.; Vallée, A.; Umbriaco, D.; Geffard, M.; Doucet, G. Quantitative and morphometric data indicate precise cellular interactions between serotonin terminals and postsynaptic targets in rat substantia nigra. Neuroscience 1997, 76, 1159–1171. [Google Scholar] [CrossRef]
- Banerjee, S.; McCracken, S.; Hossain, F.; Slaughter, G. Electrochemical Detection of Neurotransmitters. Biosensors 2020, 10, 101. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J. A simple and low-cost poly (dl-phenylalanine) modified carbon sensor for the improved electrochemical analysis of Riboflavin. J. Sci. Adv. Mater. Devices 2020, 5, 502–511. [Google Scholar] [CrossRef]
- Raril, C.; Manjunatha, J.G. Fabrication of novel polymer-modified graphene-based electrochemical sensor for the determination of mercury and lead ions in water and biological samples. J. Anal. Sci. Technol. 2020, 11, 3. [Google Scholar] [CrossRef]
- Charithra, M.M.; Manjunatha, J.G.G.; Raril, C. Surfactant Modified Graphite Paste Electrode as an Electrochemical Sensor for the Enhanced Voltammetric Detection of Estriol with Dopamine and Uric acid. Adv. Pharm. Bull. 2020, 10, 247–253. [Google Scholar] [CrossRef]
- Westbroek, P. Electrochemical methods. Anal. Electrochem. Text. 2005, 37–69. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.; McCarthy, B.D.; Rountree, E.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Kissinger, P.T.; Heineman, W.R. Cyclic voltammetry. J. Chem. Educ. 1983, 60, 702. [Google Scholar] [CrossRef]
- Osteryoung, J.G.; Schreiner, M.M. Recent Advances in Pulse Voltammetry. Crit. Rev. Anal. Chem. 1988, 19, S1–S27. [Google Scholar] [CrossRef]
- Farahi, A.; Hammani, H.; Kajai, A.; Lahrich, S.; Bakasse, M.; El Mhammedi, M. Electro-catalytic detection of dopamine at carbon paste electrode modified with activated carbon: Analytical application in blood samples. Int. J. Environ. Anal. Chem. 2020, 100, 295–310. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, W.; Wang, J.; Zhang, G.; Chai, S.; Zhang, L.; Liu, T. Selective determination of dopamine and uric acid using electrochemical sensor based on poly (alizarin yellow R) film-modified electrode. Anal. Methods 2014, 6, 3474–3481. [Google Scholar] [CrossRef]
- Gnahore, G.T.; Velasco-Torrijos, T.; Colleran, J. The Selective Electrochemical Detection of Dopamine Using a Sulfated β-Cyclodextrin Carbon Paste Electrode. Electrocatalysis 2017, 8, 459–471. [Google Scholar] [CrossRef]
- Chandra, U.; Swamy, B.E.K.; Gilbert, O.; Sherigara, B.S. Voltammetric Detection of Dopamine in Presence of Ascorbic Acid and Uric Acid at Poly (Xylenol Orange) Film-Coated Graphite Pencil Electrode. Int. J. Electrochem. 2011, 2011, 512692. [Google Scholar] [CrossRef][Green Version]
- Bahrami, E.; Amini, R.; Vardak, S. Electrochemical detection of dopamine via pencil graphite electrodes modified by Cu/CuxO nanoparticles. J. Alloys Compd. 2020, 855, 157292. [Google Scholar] [CrossRef]
- Shashikumara, J.; Swamy, B.K. Electrochemical investigation of dopamine in presence of Uric acid and ascorbic acid at poly (Reactive Blue) modified carbon paste electrode: A voltammetric study. Sens. Int. 2020, 1, 100008. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Swamy, B.E.K.; Ramírez, H.N.G.; Ekanthappa, M.T.; Flores-Moreno, R. Quantum chemical and electrochemical studies of lysine modified carbon paste electrode surfaces for sensing dopamine. N. J. Chem. 2018, 42, 4501–4506. [Google Scholar] [CrossRef]
- Kumar, M.; Fu, Y.; Wang, M.; Swamy, B.K.; Jayaprakash, G.K.; Zhao, W. Influence of cationic surfactant cetyltrimethylammonium bromide for electrochemical detection of guanine, uric acid and dopamine. J. Mol. Liq. 2021, 321, 114893. [Google Scholar] [CrossRef]
- Fooladsaz, K. Dopamine determination with a biosensor based on catalase and modified carbon paste electrode with zinc oxide nanoparticles. Int. J. Electrochem. 2012, 7, 9892–9908. [Google Scholar]
- Mazloum-Ardakani, M.; Khoshroo, A. Electrocatalytic properties of functionalized carbon nanotubes with titanium dioxide and benzofuran derivative/ionic liquid for simultaneous determination of isoproterenol and serotonin. Electrochim. Acta 2014, 130, 634–641. [Google Scholar] [CrossRef]
- Reddy, S.; Swamy, B.K.; Jayadevappa, H. CuO nanoparticle sensor for the electrochemical determination of dopamine. Electrochim. Acta 2012, 61, 78–86. [Google Scholar] [CrossRef]
- Lai, G.-S.; Zhang, H.-L.; Han, D.-Y. Electrocatalytic Response of Dopamine at an Iron Nanoparticles–Nafion–Modified Carbon Paste Electrode. Anal. Lett. 2008, 41, 3088–3099. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Bong, S.; Kang, Y.-J.; Yang, Y.; Mahajan, R.K.; Kim, J.S.; Kim, H. Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens. Bioelectron. 2010, 25, 2366–2369. [Google Scholar] [CrossRef]
- Plowman, B.J.; Mahajan, M.; O’Mullane, A.P.; Bhargava, S.K. Electrochemical detection of dopamine and cytochrome c at a nanostructured gold electrode. Electrochim. Acta 2010, 55, 8953–8959. [Google Scholar] [CrossRef]
- Baloach, Q.-U.; Nafady, A.; Tahira, A.; Sirajuddin; Sherazi, S.T.H.; Shaikh, T.; Arain, M.; Willander, M.; Ibupoto, Z.H. An amperometric sensitive dopamine biosensor based on novel copper oxide nanostructures. Microsyst. Technol. 2016, 23, 1229–1235. [Google Scholar] [CrossRef]
- Li, B. Highly selective and sensitive determination of dopamine by the novel molecularly imprinted poly (nicotinamide)/CuO nanoparticles modified electrode. Biosens. Bioelectron. 2015, 67, 121–128. [Google Scholar] [CrossRef]
- Zheng, Z.; Qiu, H.; Zheng, M.; Weng, S.; Huang, Z.; Xian, R.; Lin, X. Selective electrochemical determination of dopamine in serum in the presence of ascorbic acid and uric acid by using a CuO nanoleaf electrode. Anal. Methods 2014, 6, 7923–7927. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Sudha, V.; Kumar, S.M.; Thangamuthu, R. Simultaneous determination of dopamine and uric acid using copper oxide nano-rice modified electrode. J. Alloys Compd. 2018, 748, 338–347. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Lu, K.; Ye, B. A simple but highly sensitive and selective calixarene-based voltammetric sensor for serotonin. Electrochim. Acta 2013, 87, 756–762. [Google Scholar] [CrossRef]
- Zen, J.-M.; Chen, I.-L.; Shih, Y. Voltammetric determination of serotonin in human blood using a chemically modified electrode. Anal. Chim. Acta 1998, 369, 103–108. [Google Scholar] [CrossRef]
- Jin, G.-P.; Lin, X.-Q.; Gong, J.-M. Novel choline and acetylcholine modified glassy carbon electrodes for simultaneous determination of dopamine, serotonin and ascorbic acid. J. Electroanal. Chem. 2004, 569, 135–142. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Chen, Y.; Wang, L.; Lin, X. Simultaneous determination of dopamine and serotonin by use of covalent modification of 5-hydroxytryptophan on glassy carbon electrode. Microchim. Acta 2009, 164, 107–112. [Google Scholar] [CrossRef]
- Gong, J.-M.; Lin, X.-Q. Electrochemical Determination of Serotonin and the Competitive Adsorption with Dopamine at 5,5-Ditetradecyl-2-(2-trimethylammonioethyl)-1,3-dioxane Bromide Lipid Film Modified by Glassy Carbon Electrode. Anal. Sci. 2004, 20, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, T.; Ramaraj, R. Simultaneous determination of ascorbic acid, dopamine and serotonin at poly(phenosafranine) modified electrode. Electrochem. Commun. 2003, 5, 667–672. [Google Scholar] [CrossRef]
- Yao, H.; Li, S.; Tang, Y.; Chen, Y.; Chen, Y.; Lin, X. Selective oxidation of serotonin and norepinephrine over eriochrome cyanine R film modified glassy carbon electrode. Electrochim. Acta 2009, 54, 4607–4612. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J.G. Surfactant and polymer layered carbon composite electrochemical sensor for the analysis of estriol with ciprofloxacin. Mater. Res. Innov. 2020, 24, 349–362. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J.G.; Raril, C.; Tigari, G. Sensitive and Selective Electrochemical Resolution of Tyrosine with Ascorbic Acid through the Development of Electropolymerized Alizarin Sodium Sulfonate Modified Carbon Nanotube Paste Electrodes. ChemistrySelect 2019, 4, 4559–4567. [Google Scholar] [CrossRef]
- Sun, Y.; Fei, J.; Hou, J.; Zhang, Q.; Liu, Y.; Hu, B. Simultaneous determination of dopamine and serotonin using a carbon nanotubes-ionic liquid gel modified glassy carbon electrode. Microchim. Acta 2009, 165, 373–379. [Google Scholar] [CrossRef]
- Satyanarayana, M.; Reddy, K.K.; Gobi, K.V. Nanobiocomposite Based Electrochemical Sensor for Sensitive Determination of Serotonin in Presence of Dopamine, Ascorbic Acid and Uric Acid In Vitro. Electroanalysis 2014, 26, 2365–2372. [Google Scholar] [CrossRef]
- Ran, G.; Chen, C.; Gu, C. Serotonin sensor based on a glassy carbon electrode modified with multiwalled carbon nanotubes, chitosan and poly(p-aminobenzenesulfonate). Microchim. Acta 2015, 182, 1323–1328. [Google Scholar] [CrossRef]
- Babaei, A.; Taheri, A.R. Nafion/Ni(OH)2 nanoparticles-carbon nanotube composite modified glassy carbon electrode as a sensor for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. Sens. Actuators B Chem. 2013, 176, 543–551. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, D.; Jeon, S. Electrochemical determination of serotonin on glassy carbon electrode modified with various graphene nanomaterials. Sens. Actuators B Chem. 2012, 174, 285–291. [Google Scholar] [CrossRef]
- Han, H.S.; You, J.-M.; Jeong, H.; Jeon, S. Synthesis of graphene oxide grafted poly(lactic acid) with palladium nanoparticles and its application to serotonin sensing. Appl. Surf. Sci. 2013, 284, 438–445. [Google Scholar] [CrossRef]
- Goyal, R.N.; Oyama, M.; Gupta, V.K.; Singh, S.P.; Sharma, R.A. Sensors for 5-hydroxytryptamine and 5-hydroxyindole acetic acid based on nanomaterial modified electrodes. Sens. Actuators B Chem. 2008, 134, 816–821. [Google Scholar] [CrossRef]
- Li, J.; Lin, X. Simultaneous determination of dopamine and serotonin on gold nanocluster/overoxidized-polypyrrole composite modified glassy carbon electrode. Sens. Actuators B Chem. 2007, 124, 486–493. [Google Scholar] [CrossRef]
- Wei, X.; Wang, F.; Yin, Y.; Liu, Q.; Zou, L.; Ye, B. Selective detection of neurotransmitter serotonin by a gold nanoparticle-modified glassy carbon electrode. Analyst 2010, 135, 2286–2290. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Wang, X.; Zhu, W.; Han, Q.; Zhu, C.; Hong, J.; Zhou, X.; Jiang, H. Electrochemical serotonin sensing interface based on double-layered membrane of reduced graphene oxide/polyaniline nanocomposites and molecularly imprinted polymers embedded with gold nanoparticles. Sens. Actuators B Chem. 2014, 196, 57–63. [Google Scholar] [CrossRef]
- Sadanandhan, N.K.; Cheriyathuchenaaramvalli, M.; Devaki, S.J.; Menon, A.R. PEDOT-reduced graphene oxide-silver hybrid nanocomposite modified transducer for the detection of serotonin. J. Electroanal. Chem. 2017, 794, 244–253. [Google Scholar] [CrossRef]
- Anithaa, A.; Asokan, K.; Sekar, C. Highly sensitive and selective serotonin sensor based on gamma ray irradiated tungsten trioxide nanoparticles. Sens. Actuators B Chem. 2017, 238, 667–675. [Google Scholar] [CrossRef]
- Dinesh, B.; Veeramani, V.; Chen, S.-M.; Saraswathi, R. In situ electrochemical synthesis of reduced graphene oxide-cobalt oxide nanocomposite modified electrode for selective sensing of depression biomarker in the presence of ascorbic acid and dopamine. J. Electroanal. Chem. 2017, 786, 169–176. [Google Scholar] [CrossRef]
- Ran, G.; Chen, X.; Xia, Y. Electrochemical detection of serotonin based on a poly(bromocresol green) film and Fe3O4 nanoparticles in a chitosan matrix. RSC Adv. 2017, 7, 1847–1851. [Google Scholar] [CrossRef]
- Miyazaki, K.; Matsumoto, G.; Yamada, M.; Yasui, S.; Kaneko, H. Simultaneous voltammetric measurement of nitrite ion, dopamine, serotonin with ascorbic acid on the GRC electrode. Electrochim. Acta 1999, 44, 3809–3820. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Liang, Q.-L.; Wang, Y.-M.; Luo, G.-A. Carbon nanotube-intercalated graphite electrodes for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. J. Electroanal. Chem. 2003, 540, 129–134. [Google Scholar] [CrossRef]
- Kachoosangi, R.T.; Compton, R.G. A simple electroanalytical methodology for the simultaneous determination of dopamine, serotonin and ascorbic acid using an unmodified edge plane pyrolytic graphite electrode. Anal. Bioanal. Chem. 2007, 387, 2793–2800. [Google Scholar] [CrossRef]
- Gupta, P.; Goyal, R.N. Polymelamine modified edge plane pyrolytic graphite sensor for the electrochemical assay of serotonin. Talanta 2 0143, 120, 17–22. [Google Scholar] [CrossRef]
- Lu, L.; Wang, S.; Lin, X. Attachment of DNA to the Carbon Fiber Microelectrode via Gold Nanoparticles for Simultaneous Determination of Dopamine and Serotonin. Anal. Sci. 2004, 20, 1131–1135. [Google Scholar] [CrossRef]
- Özcan, A.; Ilkbaş, S. Poly(pyrrole-3-carboxylic acid)-modified pencil graphite electrode for the determination of serotonin in biological samples by adsorptive stripping voltammetry. Sens. Actuators B Chem. 2015, 215, 518–524. [Google Scholar] [CrossRef]
- Jin, G.-P.; Chen, Q.-Z.; Ding, Y.-F.; He, J.-B. Electrochemistry behavior of adrenalin, serotonin and ascorbic acid at novel poly rutin modified paraffin-impregnated graphite electrode. Electrochim. Acta 2007, 52, 2535–2541. [Google Scholar] [CrossRef]
- Oni, J.; Nyokong, T. Simultaneous voltammetric determination of dopamine and serotonin on carbon paste electrodes modified with iron(II) phthalocyanine complexes. Anal. Chim. Acta 2001, 434, 9–21. [Google Scholar] [CrossRef]
- Wei, J.; He, J.-B.; Cao, S.-Q.; Zhu, Y.-W.; Wang, Y.; Hang, G.-P. Enhanced sensing of ascorbic acid, dopamine and serotonin at solid carbon paste electrode with a nonionic polymer film. Talanta 2010, 83, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Babazadeh, M. A Selective Simultaneous Determination of Levodopa and Serotonin Using a Glassy Carbon Electrode Modified with Multiwalled Carbon Nanotube/Chitosan Composite. Electroanalysis 2011, 23, 1726–1735. [Google Scholar] [CrossRef]
- Rivot, J.-P.; Cespuglio, R.; Puig, S.; Jouvet, M.; Besson, J.-M. In Vivo Electrochemical Monitoring of Serotonin in Spinal Dorsal Horn with Nafion-Coated Multi-Carbon Fiber Electrodes. J. Neurochem. 2002, 65, 1257–1263. [Google Scholar] [CrossRef]
- Jackson, B.P.; Dietz, S.M.; Wightman, R.M. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal. Chem. 1995, 67, 1115–1120. [Google Scholar] [CrossRef]
- de Irazu, S.; Unceta, N.; Sampedro, M.C.; Goicolea, M.A.; Barrio, R.J. Multimembrane carbon fiber microelectrodes for amperometric determination of serotonin in human urine. Analyst 2001, 126, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Swamy, B.E.K.; Venton, B.J. Carbon nanotube-modified microelectrodes for simultaneous detection of dopamine and serotonin in vivo. Analyst 2007, 132, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Njagi, J.; Ball, M.; Best, M.; Wallace, K.N.; Andreescu, S. Electrochemical Quantification of Serotonin in the Live Embryonic Zebrafish Intestine. Anal. Chem. 2010, 82, 1822–1830. [Google Scholar] [CrossRef]
- Jiang, X.; Lin, X. Overoxidized polypyrrole film directed DNA immobilization for construction of electrochemical micro-biosensors and simultaneous determination of serotonin and dopamine. Anal. Chim. Acta 2005, 537, 145–151. [Google Scholar] [CrossRef]
- Matt, S.B.; Shivanna, M.; Manjunath, S.; Siddalinganahalli, M.; Siddalingappa, D.M. Electrochemical Detection of Serotonin Using t-ZrO2 Nanoparticles Modified Carbon Paste Electrode. J. Electrochem. Soc. 2020, 167, 155512. [Google Scholar] [CrossRef]
- Sarada, B.V.; Rao, T.N.; Tryk, D.A.; Fujishima, A. Electrochemical Oxidation of Histamine and Serotonin at Highly Boron-Doped Diamond Electrodes. Anal. Chem. 2000, 72, 1632–1638. [Google Scholar] [CrossRef]
- Liu, M.; Xiang, J.; Zhou, J.; Ding, H. A disposable amperometric sensor for rapid detection of serotonin in the blood and brain of the depressed mice based on Nafion membrane-coated colloidal gold screen-printed electrode. J. Electroanal. Chem. 2010, 640, 1–7. [Google Scholar] [CrossRef]
- Gomez, F.J.; Martín, A.; Silva, M.F.; Escarpa, A. Screen-printed electrodes modified with carbon nanotubes or graphene for simultaneous determination of melatonin and serotonin. Microchim. Acta 2015, 182, 1925–1931. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Tao, L.; Min, Q.; Xiang, J.; Wang, Q.; Xie, J.; Yue, Y.; Wu, S.; Li, X.; et al. A disposable electrochemical sensor for simultaneous determination of norepinephrine and serotonin in rat cerebrospinal fluid based on MWNTs-ZnO/chitosan composites modified screen-printed electrode. Biosens. Bioelectron. 2015, 65, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Tertis, M.; Cernat, A.; Lacatiș, D.; Florea, A.; Bogdan, D.; Suciu, M.; Săndulescu, R.; Cristea, C. Highly selective electrochemical detection of serotonin on polypyrrole and gold nanoparticles-based 3D architecture. Electrochem. Commun. 2017, 75, 43–47. [Google Scholar] [CrossRef]
- Goyal, R.N.; Gupta, V.K.; Oyama, M.; Bachheti, N. Gold nanoparticles modified indium tin oxide electrode for the simultaneous determination of dopamine and serotonin: Application in pharmaceutical formulations and biological fluids. Talanta 2007, 72, 976–983. [Google Scholar] [CrossRef]
- Goyal, R.N.; Agrawal, B. Ag ion irradiated based sensor for the electrochemical determination of epinephrine and 5-hydroxytryptamine in human biological fluids. Anal. Chim. Acta 2012, 743, 33–40. [Google Scholar] [CrossRef]
- Lin, S.-H.; Lee, L.-T.; Yang, Y.K. Serotonin and Mental Disorders: A Concise Review on Molecular Neuroimaging Evidence. Clin. Psychopharmacol. Neurosci. 2014, 12, 196–202. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J.; Van Hien, H.; Kim, N.H.; Lee, J.H. A novel sensitive sensor for serotonin based on high-quality of AuAg nanoalloy encapsulated graphene electrocatalyst. Biosens. Bioelectron. 2017, 96, 186–193. [Google Scholar] [CrossRef]
- Li, Y.-T.; Tang, L.-N.; Ning, Y.; Shu, Q.; Liang, F.-X.; Wang, H.; Zhang, G.-J. In vivo Monitoring of Serotonin by Nanomaterial Functionalized Acupuncture Needle. Sci. Rep. 2016, 6, 28018. [Google Scholar] [CrossRef]
- Cesarino, I.; Galesco, H.V.; Machado, S.A. Determination of serotonin on platinum electrode modified with carbon nanotubes/polypyrrole/silver nanoparticles nanohybrid. Mater. Sci. Eng. C 2014, 40, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, S.A.; Chang, T.; Park, S.-M. Self-assembled monolayer as a pre-concentrating receptor for selective serotonin sensing. Biosens. Bioelectron. 2010, 26, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Mazloum-Ardakani, M.; Beitollahi, H.; Mirjalili, B.B.; Akbari, A. Determination of epinephrine in the presence of uric acid and folic acid using nanostructure-based electrochemical sensor. J. Nanostruct. 2011, 1, 181–190. [Google Scholar]
- Tsele, T.P.; Adekunle, A.S.; Fayemi, O.E.; Ebenso, E.E. Electrochemical detection of Epinephrine using Polyaniline nanocomposite films doped with TiO2 and RuO2 Nanoparticles on Multi-walled Carbon Nanotube. Electrochim. Acta 2017, 243, 331–348. [Google Scholar] [CrossRef]
- Tadi, K.K.; Motghare, R.V.; Ganesh, V. Electrochemical detection of epinephrine using a biomimic made up of hemin modified molecularly imprinted microspheres. RSC Adv. 2015, 5, 99115–99124. [Google Scholar] [CrossRef]
- Cui, F.; Zhang, X. Electrochemical sensor for epinephrine based on a glassy carbon electrode modified with graphene/gold nanocomposites. J. Electroanal. Chem. 2012, 669, 35–41. [Google Scholar] [CrossRef]
- Kang, H.; Jin, Y.; Han, Q. Electrochemical Detection of Epinephrine Using an L-Glutamic Acid Functionalized Graphene Modified Electrode. Anal. Lett. 2014, 47, 1552–1563. [Google Scholar] [CrossRef]
- Hu, W.; Sun, D.; Ma, W. Simultaneous electrochemical determination of dopamine and epinephrine with a silver-doped Poly(L-glutamic acid) modified electrode. Chem. Anal. 2008, 53, 703–716. [Google Scholar]
- Joseph, T.; Thomas, N. A facile electrochemical sensor based on titanium oxide (TiO2)/reduced graphene oxide (RGO) nano composite modified carbon paste electrode for sensitive detection of epinephrine (EP) from ternary mixture. Mater. Today Proc. 2021, 41, 606–609. [Google Scholar] [CrossRef]
- De Queiroz, D.F.; Dadamos, T.R.D.L.; Machado, S.A.S.; Martines, M.A.U. Electrochemical Determination of Norepinephrine by Means of Modified Glassy Carbon Electrodes with Carbon Nanotubes and Magnetic Nanoparticles of Cobalt Ferrite. Sensors 2018, 18, 1223. [Google Scholar] [CrossRef]
- Samdani, K.J.; Joh, D.W.; Rath, M.K.; Lee, K.T. Electrochemical mediatorless detection of norepinephrine based on MoO3 nanowires. Electrochim. Acta 2017, 252, 268–274. [Google Scholar] [CrossRef]
- Lavanya, N.; Sekar, C. Electrochemical sensor for simultaneous determination of epinephrine and norepinephrine based on cetyltrimethylammonium bromide assisted SnO2 nanoparticles. J. Electroanal. Chem. 2017, 801, 503–510. [Google Scholar] [CrossRef]
- Yadav, S.K.; Agrawal, B.; Oyama, M.; Goyal, R.N. Graphene modified palladium sensor for electrochemical analysis of norepinephrine in pharmaceuticals and biological fluids. Electrochim. Acta 2014, 125, 622–629. [Google Scholar]
- Łuczak, T. Determination of norepinephrine alone and in the presence of ascorbic and uric acids using a gold electrode modified with gold nanoparticles and self- assembled layers of meso-2,3-dimercaptosuccinic acid. Electroanalysis 2014, 26, 1461. [Google Scholar] [CrossRef]
- Goyal, R.N.; Bishnoi, S. Simultaneous determination of epinephrine and norepinephrine in human blood plasma and urine samples using nanotubes modified edge plane pyrolytic graphite electrode. Talanta 2011, 84, 78. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, M.; Li, X.; Purushothaman, A.; Li, F. Electrochemical Detection of Norepinephrine in the Presence of Epinephrine, Uric Acid and Ascorbic Acid Using a Graphene-modified Electrode. Int. J. Electrochem. Sci. 2012, 7, 991–1000. Available online: http://www.electrochemsci.org (accessed on 29 October 2021).
- Keskin, E.; Yardim, Y.; Levent, A.; Şentürk, Z. Electrochemical determination of norepinephrine by adsorptive stripping voltammetry using a bare boron-doped diamond electrode. Rev. Roum. Chim. 2020, 64, 1063–1072. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S. Voltametric Behavior of Noradrenaline at 2-Mercaptoethanol Self-Assembled Monolayer Modified Gold Electrode and its Analytical Application. Sensors 2003, 3, 61–68. [Google Scholar] [CrossRef]
- Kalimuthu, P.; John, A. Selective determination of norepinephrine in the presence of ascorbic and uric acids using an ultrathin polymer film modified electrode. Electrochim. Acta 2011, 56, 2428–2432. [Google Scholar] [CrossRef]
- Huang, S.-H.; Liao, H.-H.; Chen, D.-H. Simultaneous determination of norepinephrine, uric acid, and ascorbic acid at a screen printed carbon electrode modified with polyacrylic acid-coated multi-wall carbon nanotubes. Biosens. Bioelectron. 2010, 25, 2351–2355. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Liu, Y.; Lai, G.-S.; Yu, A.-M.; Huang, Y.-M.; Jin, C.-M. Calix[4]arene crown-4 ether modified glassy carbon electrode for electrochemical determination of norepinephrine. Analyst 2009, 134, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, Y.; Yuan, Z. Electrochemical Behavior of Norepinephrine at Poly(2,4,6-trimethylpyridine) Modified Glassy Carbon Electrode. Electroanalysis 2002, 14, 445–448. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X.; Wang, X.; Ma, X.; Li, X.; Cao, K. Voltammetric determination of dopamine and norepinphrine on a glassy carbon electrode modified with poly (L-aspartic acid). J. Chem. Sci. 2012, 124, 537–544. [Google Scholar] [CrossRef]
- Beitollahi, H.; Mohadesi, A.; Mahani, S.K.; Karimi-Maleh, H.; Akbari, A. New voltammetric strategy for simultaneous determination of norepinephrine, acetaminophen, and folic acid using a 5-amino-3′,4′-dimethoxy-biphenyl-2-ol/carbon nanotube paste electrode. Ionics 2012, 18, 703–710. [Google Scholar] [CrossRef]
- Jahani, P.M. Graphene quantum dots/ionic liquid-Modified Carbon Paste Electrode-Based Sensor for Simultaneous voltammetric determination of norepinephrine and acetylcholine. Int. J. Electrochem. Sci. 2020, 15, 947–958. [Google Scholar] [CrossRef]
- Fajardo, A.; Tapia, D.; Pizarro, J.; Segura, R.; Jara, P. Determination of norepinephrine using a glassy carbon electrode modified with graphene quantum dots and gold nanoparticles by square wave stripping voltammetry. J. Appl. Electrochem. 2019, 49, 423–432. [Google Scholar] [CrossRef]
- Chen, J.; Huang, H.; Zeng, Y.; Tang, H.; Li, L. A novel composite of molecularly imprinted polymer-coated PdNPs for electrochemical sensing norepinephrine. Biosens. Bioelectron. 2015, 65, 366–374. [Google Scholar] [CrossRef]


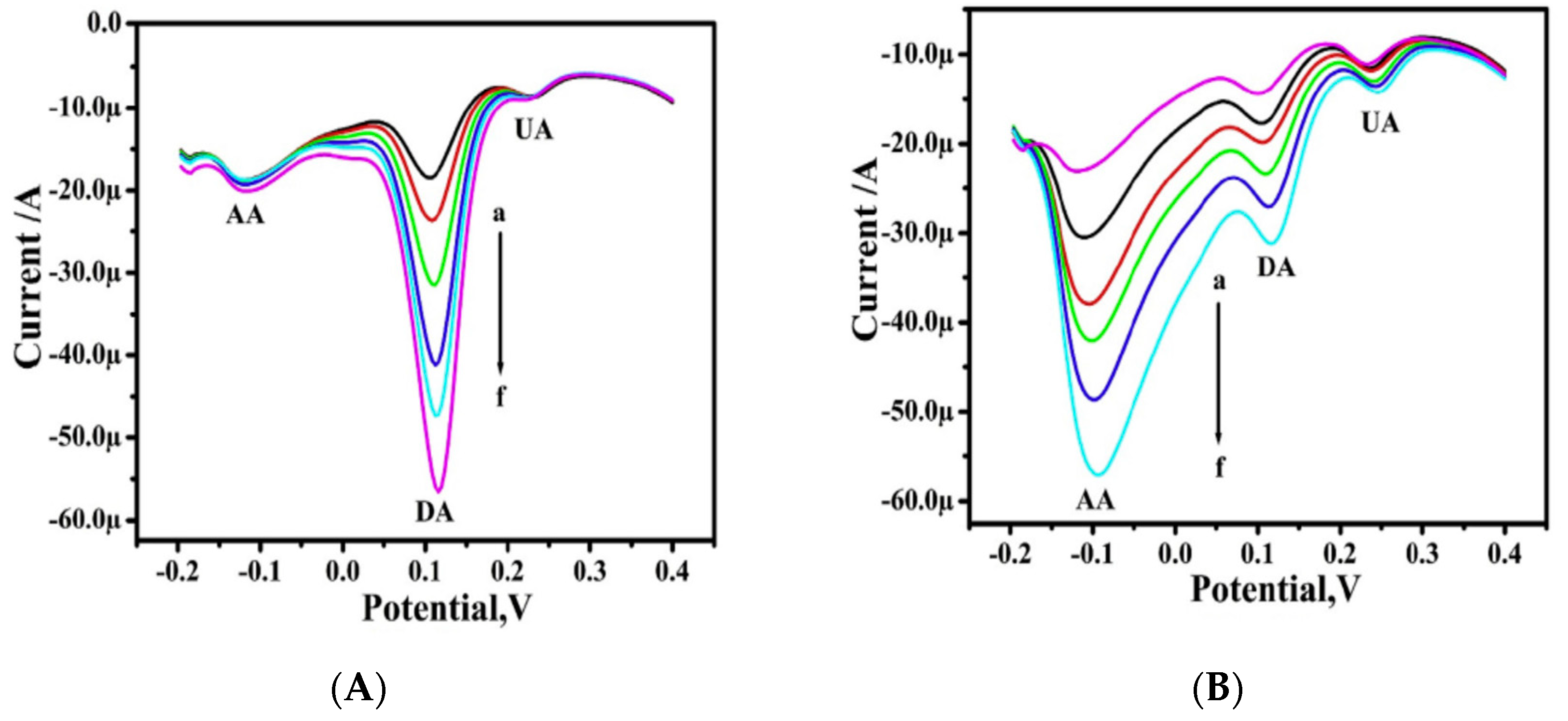
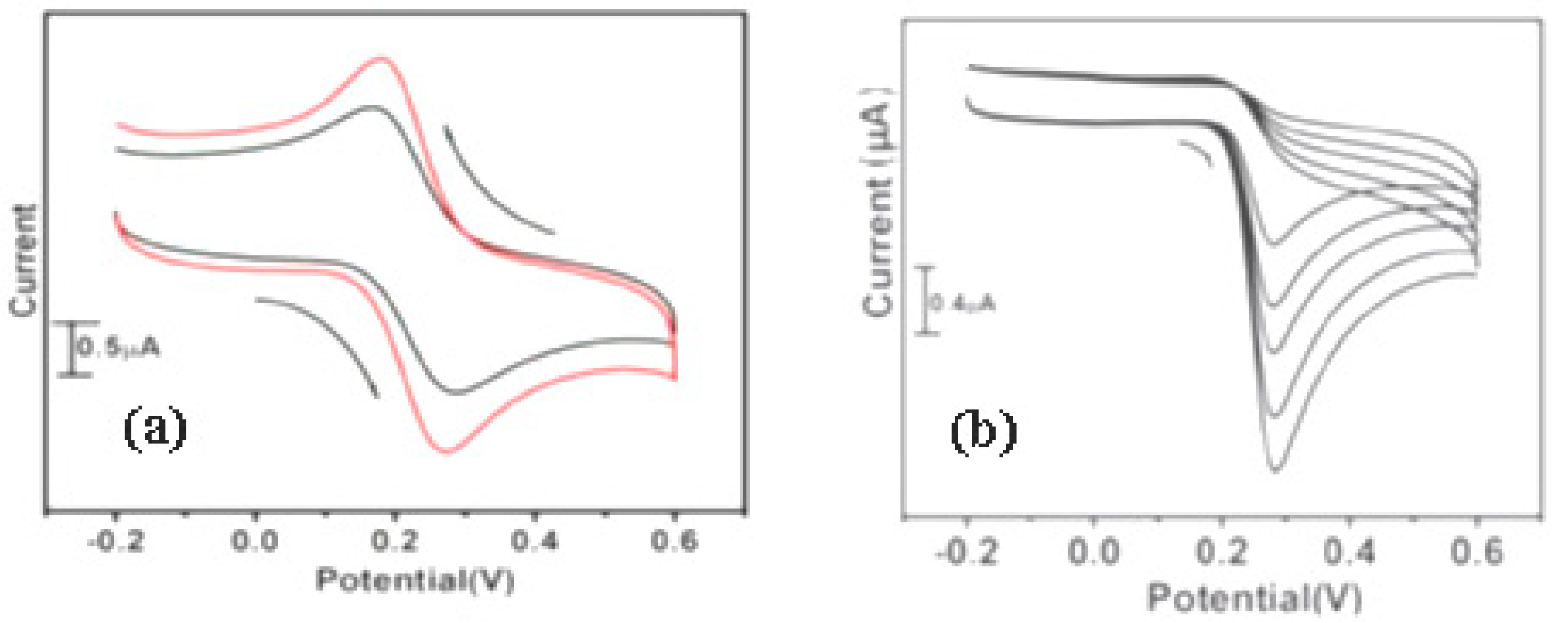

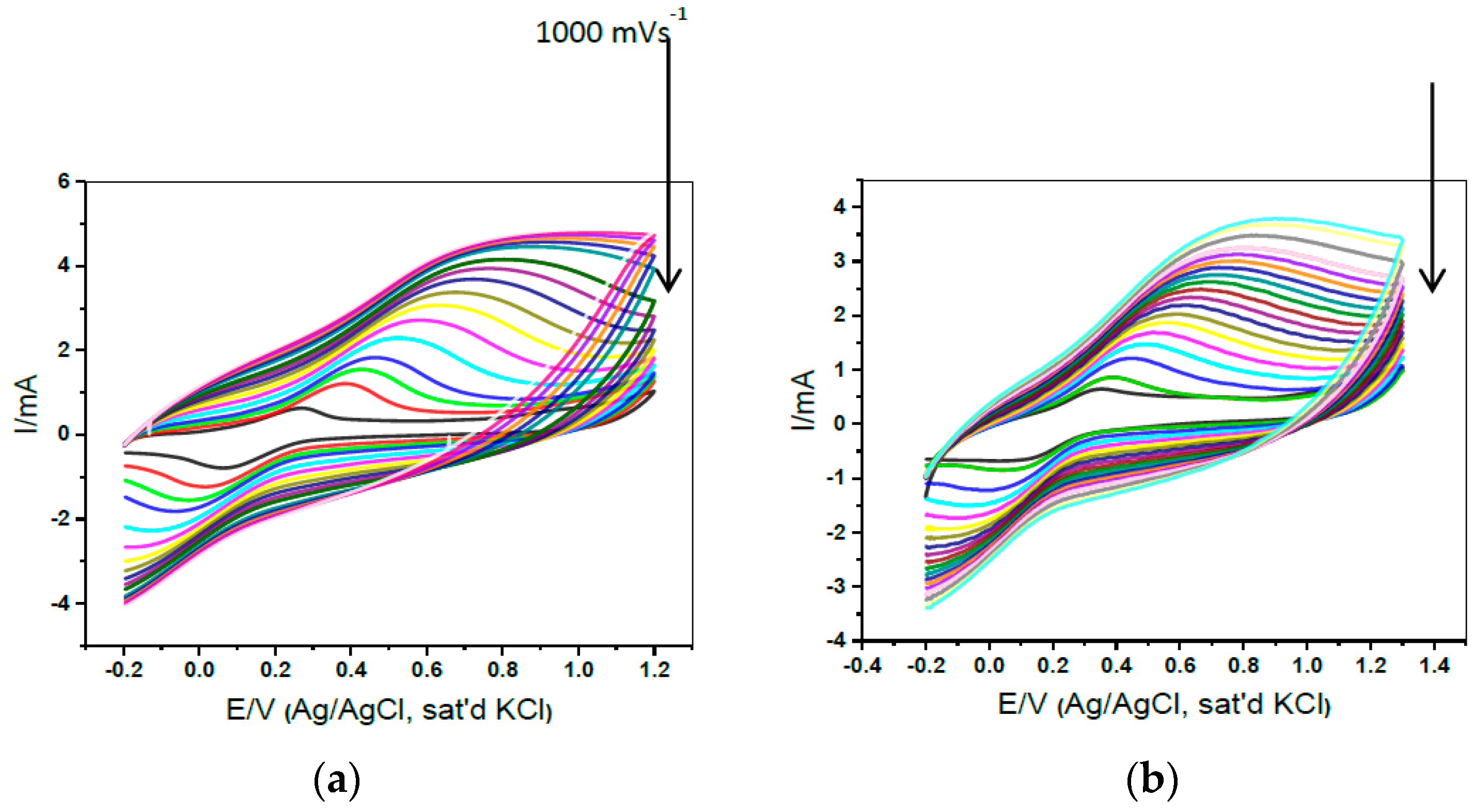

| Name | Localization | General Detection Method | Functions |
|---|---|---|---|
| Dopamine | Central and peripheral nervous system | ELISA and HPLC | Regulation of trennel, hormonal, and CNS, neuromodulatory |
| Serotonin | Central and peripheral nervous system | ~ELISA | Embryo-genesis and morphogenesis |
| Epinephrine | Sympathetic nerves and adrenal medulla | Gas-liquid chromatography | Heart, muscle contraction, dilation of airways, glycogenolysis |
| Norepinephrine | Sympathetic nerves and adrenal medulla | Gas-liquid chromatography | Same as above |
| S. No. | Electrode | Method | pH | Range (nM) | LOD (nM) | Sensitivity (µAµM−1) | Reference |
|---|---|---|---|---|---|---|---|
| 1. | CAT/ZnONPs/CPE | CV | 7 | 5000–41,000 | 3000 | 0.42 | [31] |
| 2. | TiO2 NP/CPE | SWV | 7 | 1000–6000 | 840 | 0.042, 0.043 | [32] |
| 3. | Rod-shaped CuO NP/MCPE | DPV | 6 | 300–1400 | 180 | 0.29 | [33] |
| 4. | INP-Nafion-modified CPE | DPV | 7 | 10,000–110,000 | 3300 | 0.16 | [34] |
| 5. | GS/DMF/GCE | DPV | 7.4 | 4000–100,000 | 2640 | 0.07 | [35] |
| 6. | Nanostructured Gold | DPV | 7 | 10,000–100,000 | 5000 | 0.14 | [36] |
| 7. | CuO/GCE | CA | 7.4 | 5000–40,000 | 100 | 0 | [37] |
| 8. | MIPs/CuO/GCE | CV | 7.5 | 20–25,000 | 10 | 0.27 | [38] |
| 9. | CuO Nanoleaf | DPV | 8 | 1000–1750 | 500 | 0.0229, 0.664 | [39] |
| 10. | S-β-CDCPE | CV DPV | 7 | 500–50,000 | 13,300 | [25] | |
| 11. | CuO nano rice/GCE | DPV | 7 | 1000–150,000 | 420 | 0.04 | [40] |
| 12z. | PAYR/CPE | CV DPV | 7 | 490–70,100 | 160 | [24] | |
| 13. | Cu/CuxO NPs/PGE | DPV | 5.8 | 300–53,000 | 1070 | 0.51 | [27] |
| 14. | poly(XO)/GPE | CV DPV | 7 | - | 9100 | [26] | |
| 15. | MCPE-poly (Reactive blue) | CV DPV | 4 | 5000–30,000 | 4600 | - | [28] |
| 16. | CTAB/CPE | CV | 7 | 1000–70,000 | 200 | - | [30] |
| S. No. | Coating | Technique Used | LOD (nM) | Range (nM) | Interference | Sample | Reference |
|---|---|---|---|---|---|---|---|
| 1. | C-undecyl Calix resorcinarene | DPV CV CC | 30 | 100–10,000 | DA AA FA EP | Human serum | [41] |
| 2. | Nafion/RuO2 pyrochlore | SWV | 2 | 10–500 2000–20,000 | UA AA glucose oxalate | Human blood Plasma | [42] |
| 3. | Ch or Ach | CV DPV | 500 | 1000–30,000 | DA AA | Human plasma | [43] |
| 4. | 5-HTP | DPV | 1700 | 5000–35,000 | DA | Human serum | [44] |
| 5. | DTDB | CV DPV | 50 | 200–10,000 | DA AA KCl NaCl UA Ca Glucose | Serum | [45] |
| 6. | Poly(phenosafranine) | DPV | 5 | - | AA DA | Serum | [46] |
| 7. | Eriochrome Cyanine R | CV DPV | 50 | 50–5000 | NE AA UA | Serum | [47] |
| 8. | Poly(safranine O) | CV DPV | 5 | 30–10,000 | DA AA UA bilirubin TOC glucose | Serum | [44] |
| S. No. | Coating | Technique Used | LOD (nM) | Range (nm) | Interference | Sample | Reference |
|---|---|---|---|---|---|---|---|
| 1. | MWCNT/DHP | CV DPV | 5 | 20–5000 | DA AA UA | Human Serum | [3] |
| 2. | SWCNT | SWV | 32 | 100–100,000 | HIAA DA UA AA | Human urine | [56] |
| 3. | MWCNT/IL gel | CV DPV | 8 | 20–7000 | AA glucose NaCl UA purine His Cys | Human serum | [50] |
| 4. | MWCNT/CHT | CV DPV CA | 80 | 500–130,000 | L-Dopa AA Trp Tyr Ala glutamic acid His aspartic acid UA OA glucose Cys | Human serum and urine | [53] |
| 5. | MWCNT/CHT | CV DPV | 50 | 50–16,000 | AA DA UA | Blood serum | [51] |
| 6. | MWCNT/CHT/Poly (p-ABSA) | DPV | 80 | 100–100,000 | AA DA UA | Artificial urine | [52] |
| 7. | Nafion/Ni(OH)2/MWCNT | CV DPV CA | 3 | 8–10,000 | DOPAC citric acid GC glucose NaCl KCl MgCl2 CaCl2 Ca(NO3)2 oxalate | Human serum | [53] |
| 8. | Reduced graphene nano-sheets | CV DPV CA | 32 | 1000–100,000 | AA DA | Human serum | [54] |
| 9. | G-g-PLA-Pd | CV Amp | 80 | 100–100,000 | UA DA AA H2O | Human serum | [55] |
| 10. | Porphyrin derivative-GO | DPV Amp | 4.9 | 100–300,000 | UA AA Na+ Mg2+ Zn2+ Fe2+ Fe3+ Cl− | Human serum | [4] |
| S. No. | Coating | Technique Used | LOD (nM) | Range (nM) | Interference | Sample | Reference |
|---|---|---|---|---|---|---|---|
| 1. | RGO/Co3O4—nanocomposite | CV DPV | 48.7 | 100–51,000 | DA AA EP UA Trp Cys vitamin B 6 vitamin B 9 Tyr guanine-cytosine NO2 NO3 KCl | Human serum | [62] |
| 2. | PEDOT/RGO/Ag-hybrid nanocomposite | CV DPV CA | 0.1 | 1–500,000 | UA AA Tyr L -Cys L -Trp L -Ala DA Ep NE glucose | BAM solution | [60] |
| 3. | MWCNT doped with nickel, zinc and iron oxide NPs | CV SWV | 118 (NI) | 5.9–62,800 | DA AA | Human urine | [63] |
| 4. | Fe3O4 NPs–MWCNT–poly BCG) | DPV | 80 | 500–100,000 | DA UA AD Trp L -Cys glucose | Human serum | [57] |
| 5. | DDF-CNTs-TiO2/IL | CV DPV | 154 | 1000–650,000 | IP Cl− F− Br− S2− CO32− K+ Ca2+ Mg2+ NH4+ NO3− CH3COO− L-lysine glucose fructose sucrose L-asparagines GA L-Cys L-Trp AA UA D-penicilamine | Human serum | [33] |
| 6. | RGO/PANI/AuNPs/MIPs | CV DPV | 11.7 | 200–10,000 | AA DA UA EP | Human serum | [60] |
| 7. | L-Cys/AuNPs | CV CC SWV | 20 | 60–6000 | EP DA AA FA | Human serum | [59] |
| 8. | Nano-Au/oxidized-PPyox | DPV | 1 | 70–2200 | DOPAC UA AA DA Glucose oxalate KCl MgCl2 NaCl CaCl2 Ca(NO3)2 | Human serum | [64] |
| S. No. | Bare Electrode | Coating | Technique Used | LOD (nM) | Range (nM) | Interference | Sample | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | Graphite | Intercalating CNT | CV DPV | 200 | 1000–15,000 | DOPAC AA DA UA oxalate glucose | Rabbit brain homogenate | [68] |
| 2. | EPPGE | No coating | CV DPV | 60 | 100–100,000 | DA AA | Horse blood | [66] |
| 3. | EPPGE | Poly-melamine | CV SWV | 30 | 100–100,000 | AA xanthine DA hypoxanthine | Human serum and urine | [67] |
| 4. | PGE | Poly(pyrrole−3-carboxylic acid) | CV DPV ADSV | 2.5 | 10–1000 | DA AA UA NaCl KCl Urea Citric acid TA Glucose | Blood serum and urine | [69] |
| 5. | CPE | Non-ionic PAMT film | CV DPV Amp | 0.4 | 20–1560 | AA DA Na+ K+ Mg2+ CH3 CO− SO42− Ca2+ Cl− PO43− BO33− | Pharmaceutical samples | [58] |
| 6. | CILE | Nafion/Co(OH)2- MWCNTs | CV DPV | 23 | 50–75,000 | L-Dopa DOPAC citric acid UA AA Glucose GA NaCl Ca(NO3)2 CaCl2 | Human serum | [53] |
| 7. | Graphite | Polyrutin modified paraffin | CV DPV | 100 | 300–9000 | EP AA | - | [70] |
| S. No. | Coating | Technique Used | LOD (nM) | Range (µM) | Interference | Sample | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Nafion | FSCV | 1 | 1000–100,000 | - | Rat brain (in vivo) | [78] |
| 2. | PPyox/CT-DNA | CV DPV | 7 | 10–1000 | AA DE DOPAC UA glucose oxalate | Human serum | [79] |
| 3. | CNTs/Nafion | DPV | 1 | 5–200 | - | Zebra-fish (in vivo) | [78] |
| 4. | SWCNTs | FSCV | - | - | DA | Striatum of rat | [77] |
| 5. | Au-NPs/CT-DNA | CV DPV | 800 | 80–200,000 | DA AA glucose-fructose purine citric acid GA NH4NO3 | - | [68] |
| S. No. | Electrode | Coating | Technique Used | LOD (nM) | Range (µM) | Interference | Sample | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | ITO | Gold NPs | DPV SWV | 3 | 10–250,000 | DA AA UA NaCl KCl caffeine TA | Human serum and urine | [86] |
| 2. | ITO | Ag ion irradiated MWCNTs | CV SWV | 0.75 | 10–105,000 | AA DA UA | Human serum and urine | [87] |
| 3. | FTO | PEDOT: PSS/ 3IP-TPyP | CV | 230 | 1700–138,000 | DA EP AA Urea, D- Glucose L-Dopa | - | [88] |
| 4. | ITO | AuAg-GR nanohybrid | CV Amp | 1.6 | 2.7–4820 | UA AA Glucose KCl | Human serum | [89] |
| 5. | Acupuncture needle | PEDOT/CNTs | DPV Amp | 78 | 1000–100,000 | His bradykinin AA UA Prostaglandin E 2 | Rat (in vivo) | [90] |
| 6. | Pt | MWCNT/PPy/ Colloidal AgNPs | DPV CA | 150 | 500–5000 | AA UA | Artificial plasmatic serum | [91] |
| 7. | Gold | Captopril/ thiophenol | DPV EIS | 28 | 4000–250,000 2–3500 | AA DA Trp Glutamate 5-HTP NE ACh Asp Gly Tryptamine His Adenosine | Human serum | [92] |
| 8. | Carbon-SPE | SWCNTs or MWCNTs or Graphene | DPV | 400 | 1000–2,500,000 | UA MEL Tyramine Tryptamine Ep | Commercial drugs | [83] |
| 9. | Graphite based SPE | PPy NPs-Au NPs | CV SWV | 33.22 | 100–15,000 | DA NE Paracetamol AA Acetylsalicylic acid Glucose | Human serum | [85] |
| 10. | CGSPE | Nafion | CV DPV | 5 | 20–500,000 | UA DA AA Glucose NaCl TA oxalate | Brain homogenate and platelet-rich plasma of mice | [82] |
| 11. | SPE | MWCNTs-ZnO/CHT composites | CV SWV | 10 | 50–1000 | AA UA NE glucose Ca Mg citric acid | Rat cerebrospinal fluid | [84] |
| 12. | CP | ZMCPE | CV DPV | 585 | 10,000–50,000 | DA | Real samples | [80] |
| S. No. | Electrode | Technique Used | pH | LOD (nM) | Range (nM) | Sensitivity (mA-mM−1) | Sample | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | Modified CNT carbon paste electrode | DPV | 7 | 70 | 100–35,000 35,000–750,000 | 0.019 0.152 | - | [93] |
| 2. | MWCNT-PANI-TiO2 | DPV | 7 | 0.16 | 4900–76,900 | 785.7 | Pharmaceutical sample | [94] |
| 3. | MWCNT-PANI-RuO2 | DPV | 7 | 0.18 | 79,226 | - | [94] | |
| 5. | Electropolymerized MIP | Amp | - | 9 | 300–100,000 | - | - | [96] |
| 6. | Hemin modified MIP | DPV | 7 | 12 | 50–40,000 | - | Human blood serum and injection samples | [95] |
| 7. | PLG-Ag/GCE | CV DPV | 7.5 | 800 | 3000–100,000 | - | - | [98] |
| 8. | L-GlutamicAcid-Graphene/GCE | DPV CV | 4 | 30 | 100–1,000,000 | 0.2 | Human urine | [97] |
| 9. | TiO2/RGO-MCPE | CV DPV | 7 | 3 | 5–100 100–2000 | - | - | [99] |
| S. No. | Electrode | Technique Used | pH | LOD (nM) | Range (nM) | Sample | Reference |
|---|---|---|---|---|---|---|---|
| 1. | GR/Pd/GCE | CV DPV | 7.2 | 0.064 | 5000–500,000 | Pharmaceutical dosage forms and human urine | [103] |
| 2. | DMSA/Au/GE | CV | 7 | 0.055 | 100–700,000 | - | [104] |
| 3. | MWNT/EPPGE | DPV | 7.2 | 0.0009 | 5–100 | Human blood plasma and urine | [105] |
| 4. | CTAB-SnO2/GCE | CV | 5 | 0.006 0.010 | 100–1000 1000–300,000 | Human urine | [102] |
| 5. | graphene-modified glassy carbon electrodes (GME) | CV | 7 | 4000 | 600–120,000 | Injection | [106] |
| 6. | MoO3 NWs/GCE | CV | 7 | 0.11 | - | - | [101] |
| 7. | GC/MWCNT/FCo98 | CV | 7 | 760 | 160–1910 | ~Drug | [100] |
| 8. | bare BDD electrode | SWV CV | 2 | 254 | 4900–490,000 | Drug | [107] |
| S. No. | Analyte | Electrode | Range (nM) | LOD (nM) | Sample | Reference |
|---|---|---|---|---|---|---|
| 1. | NE | ME/Au SAMs | 2000–100,000 | 700 | Drug | [108] |
| 2. | NE | p-ATD/GCE | 40–250 | 1700 | Drug | [109] |
| 3. | NE, AA, UA | PAAMWCNTs/SPCE | 1000–10,000 | 130 | - | [110] |
| 4. | NE | CACE/GCE | 550–9700 | 280 | Drug | [111] |
| 5. | NE | p-TMP/GCE | 5000–100,000 | 80 | Drug | [112] |
| 6. | NE, DA | PL-Asp/GCE | 30–16,000 | 4310 | Drug | [113] |
| 7. | NE, AC, FA | 5ADMBCNPEs | 15,000–1,000,000 | 8000 | - | [114] |
| 8. | NE | BDD | 4900–490,000 | 1200 | Drug | [107] |
| S. No. | Electrode | Technique Used | Modifier | LDR (nM) | LOD (nM) | pH | Sample | Reference |
|---|---|---|---|---|---|---|---|---|
| 1. | MIP | CV | Molecularly imprinted polymer-coated PdNPs | 500–80,000 | 100 | - | Real samples | [117] |
| 2. | Glassy carbon | SWSV | Graphene quantum dots/gold nanoparticles | 500–7500 | 150 | 7 | Rat brain tissue | [116] |
| 3. | Carbon paste | DPV | Graphene quantum dots/ionic liquid | 20–400,000 | 60 | 7 | Urine | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.; Soni, I.; Jayaprakash, G.K.; Flores-Moreno, R. Studies of Monoamine Neurotransmitters at Nanomolar Levels Using Carbon Material Electrodes: A Review. Materials 2022, 15, 5782. https://doi.org/10.3390/ma15165782
Kumar P, Soni I, Jayaprakash GK, Flores-Moreno R. Studies of Monoamine Neurotransmitters at Nanomolar Levels Using Carbon Material Electrodes: A Review. Materials. 2022; 15(16):5782. https://doi.org/10.3390/ma15165782
Chicago/Turabian StyleKumar, Pankaj, Isha Soni, Gururaj Kudur Jayaprakash, and Roberto Flores-Moreno. 2022. "Studies of Monoamine Neurotransmitters at Nanomolar Levels Using Carbon Material Electrodes: A Review" Materials 15, no. 16: 5782. https://doi.org/10.3390/ma15165782
APA StyleKumar, P., Soni, I., Jayaprakash, G. K., & Flores-Moreno, R. (2022). Studies of Monoamine Neurotransmitters at Nanomolar Levels Using Carbon Material Electrodes: A Review. Materials, 15(16), 5782. https://doi.org/10.3390/ma15165782








