Evaluation of the Usefulness of Sorbents in the Remediation of Soil Exposed to the Pressure of Cadmium and Cobalt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Procedure for Setting Up and Conducting the Experiment
2.3. Characteristics of Sorbents
2.4. Microbiological Analyses
2.5. Enzymatic Analyses
2.6. Physicochemical and Chemical Soil Analyses
2.7. Statistical Analyses
- IFAd—index of the influence of the adsorbent.
- IFHm—index of the influence of heavy metals.
- Po—the activity of enzymes in the soil or the yield of plants after the use of sorbents/contaminated with heavy metals.
- Co—enzyme activity or plant yield in unpolluted soil (no heavy metals) and without sorbents.
3. Results
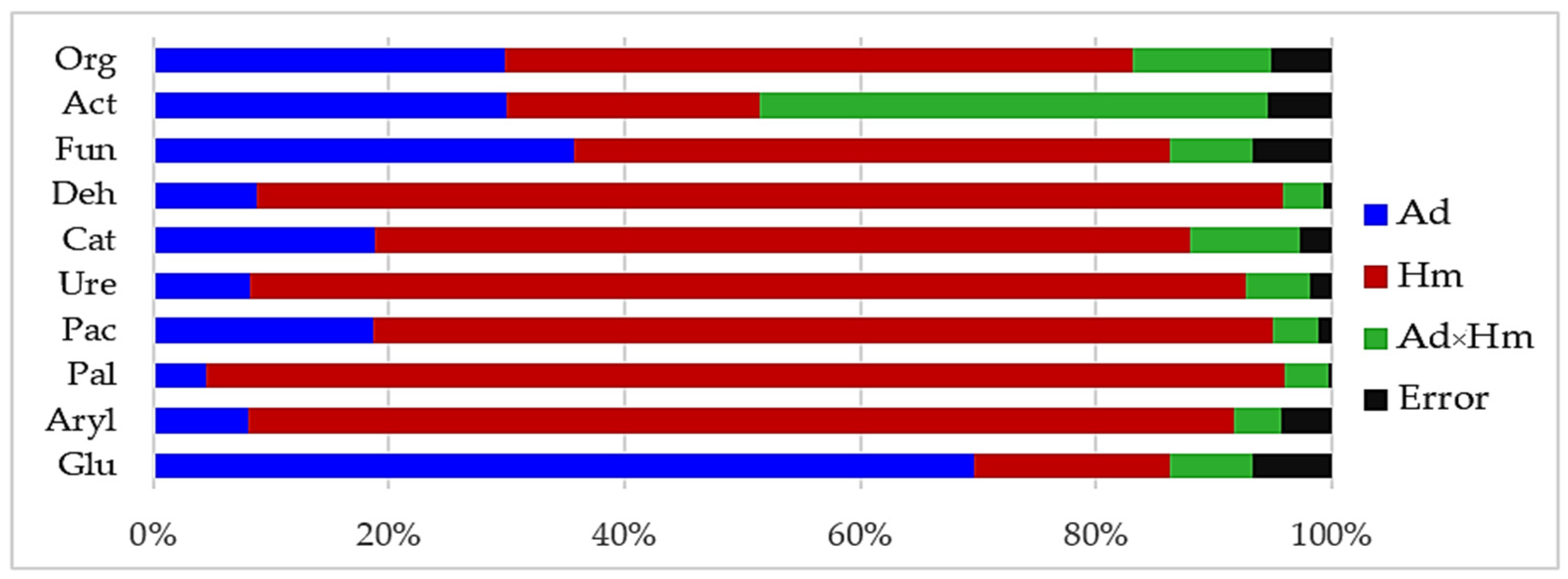
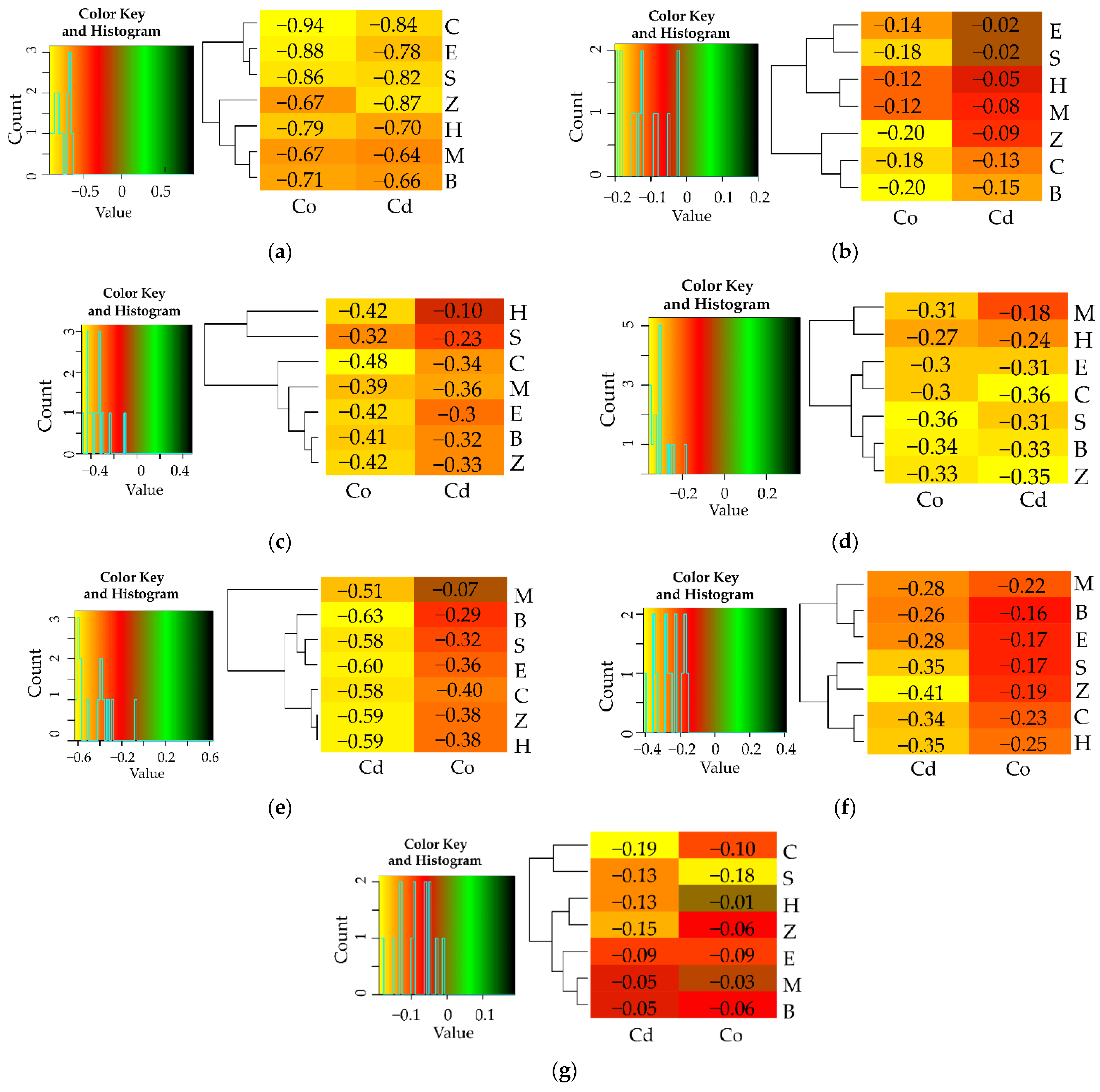
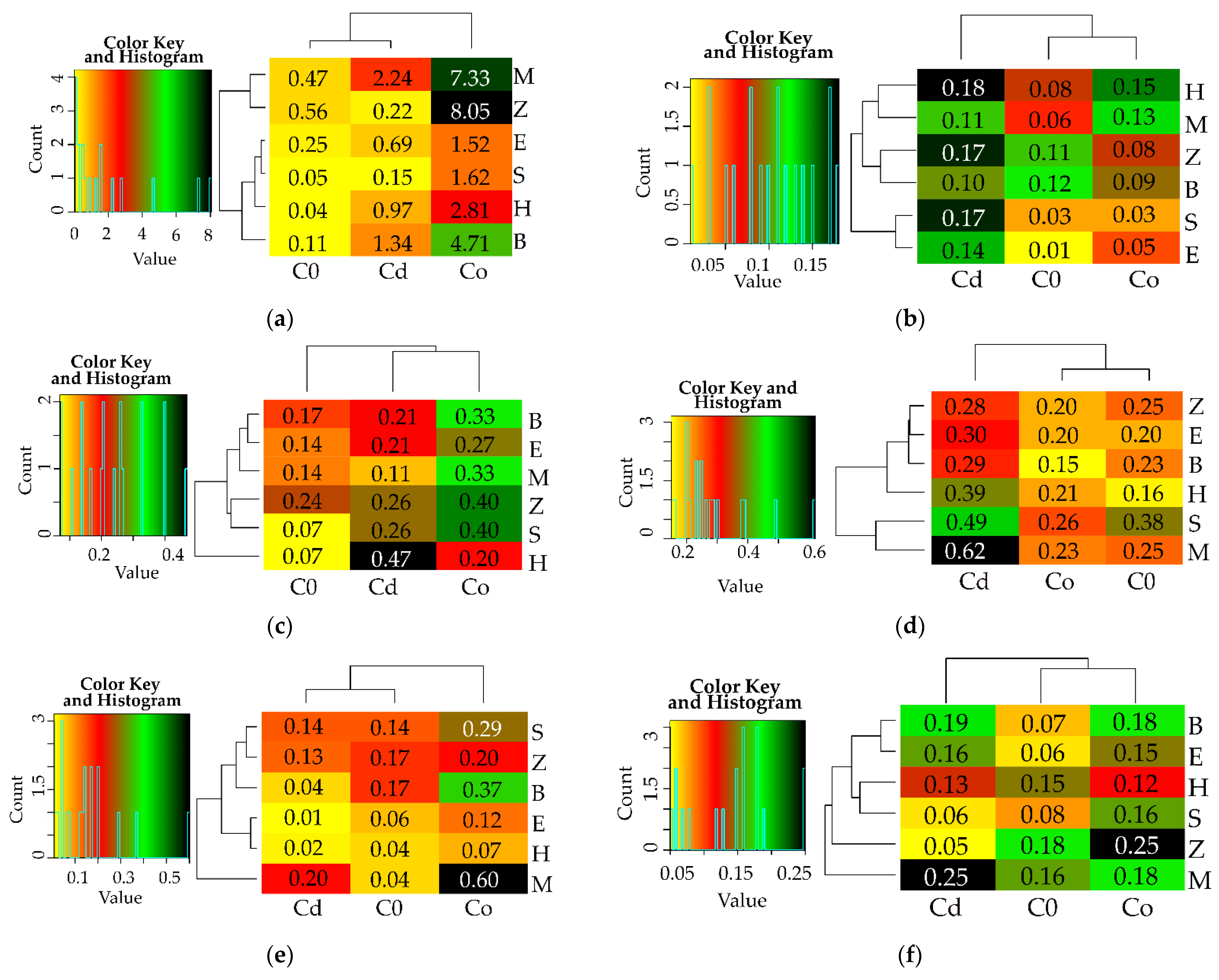
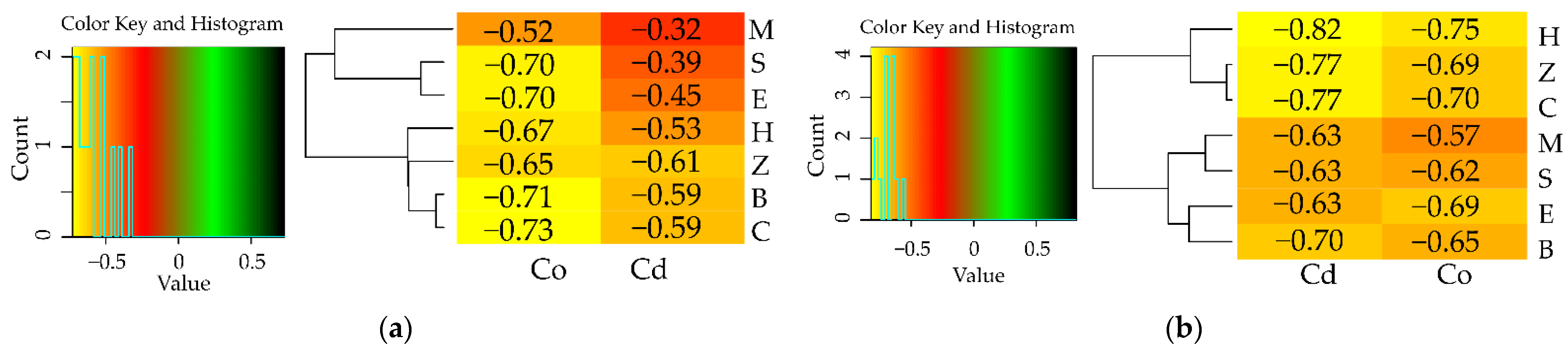
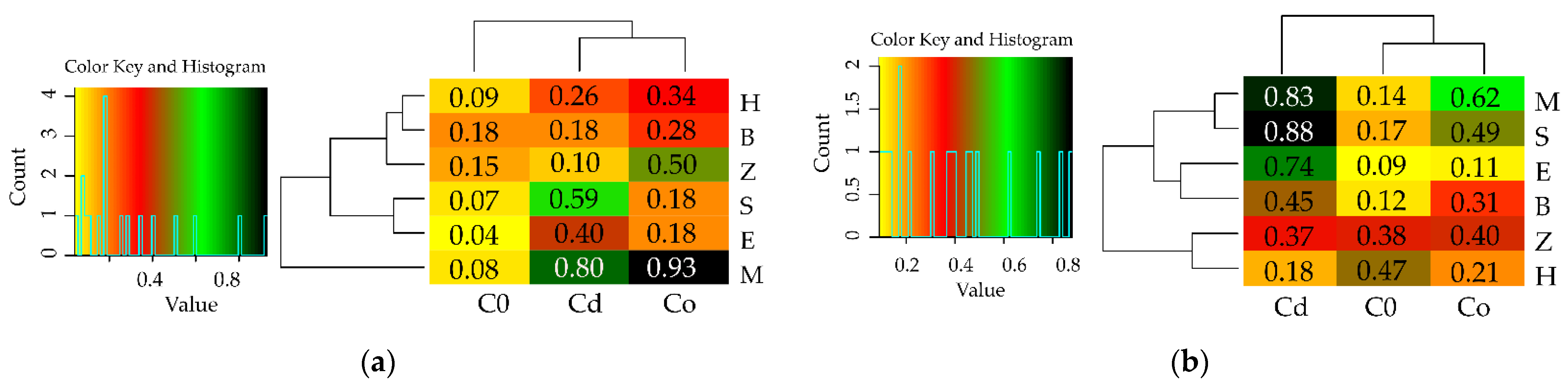
3.1. Counts of Microorganisms
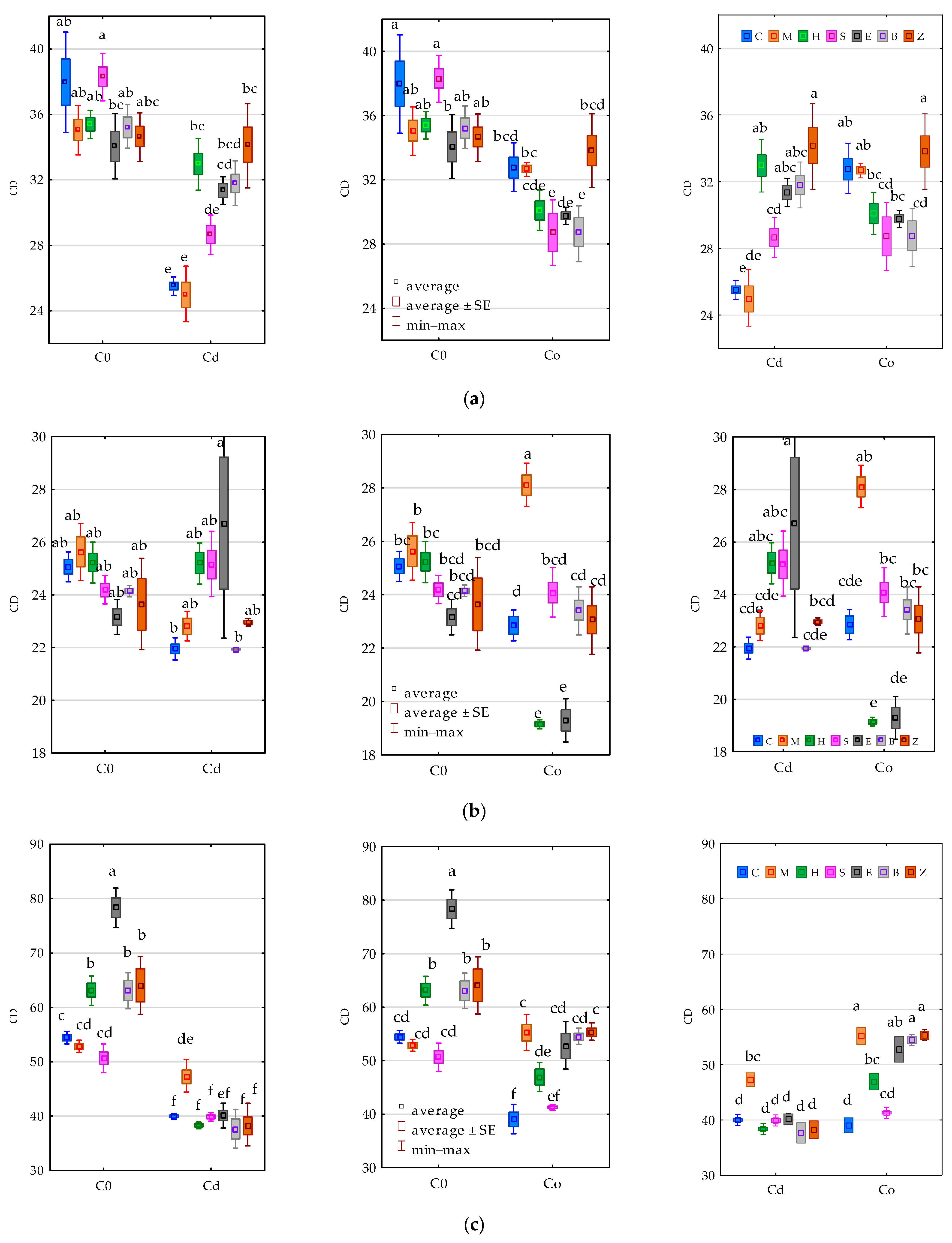
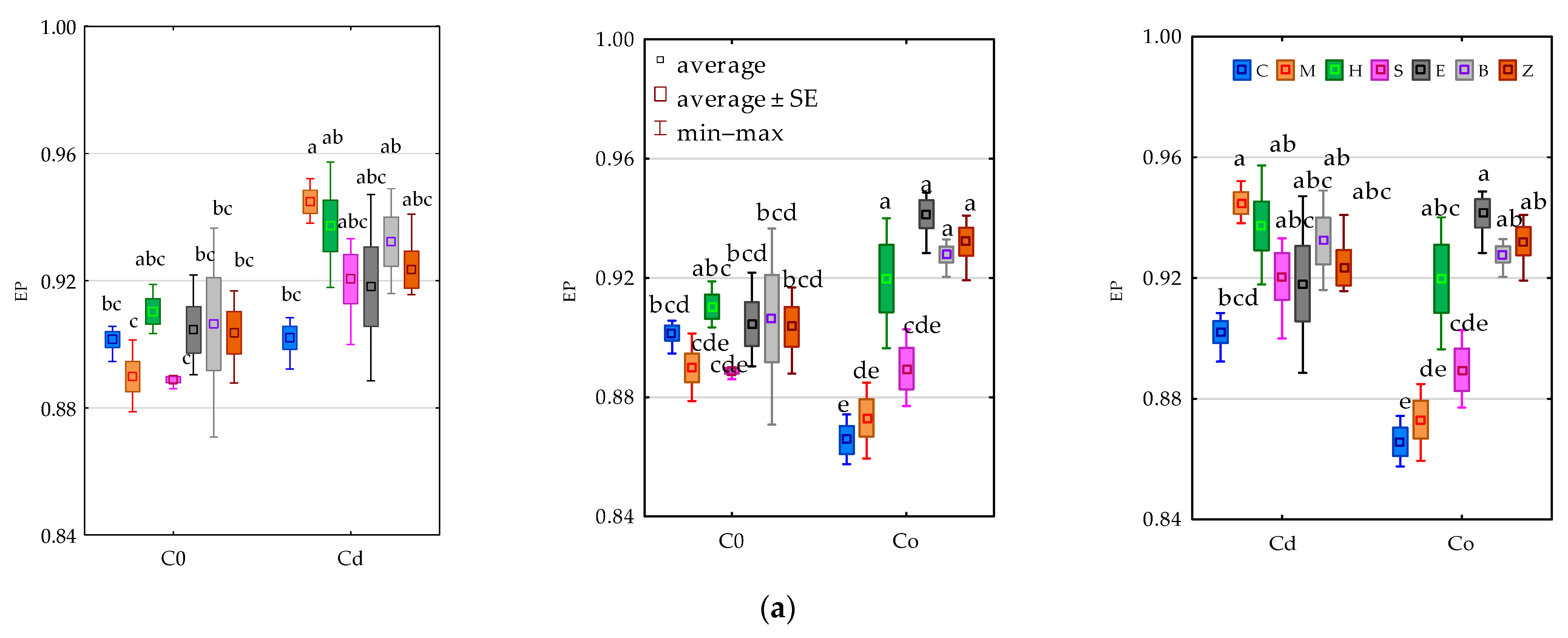
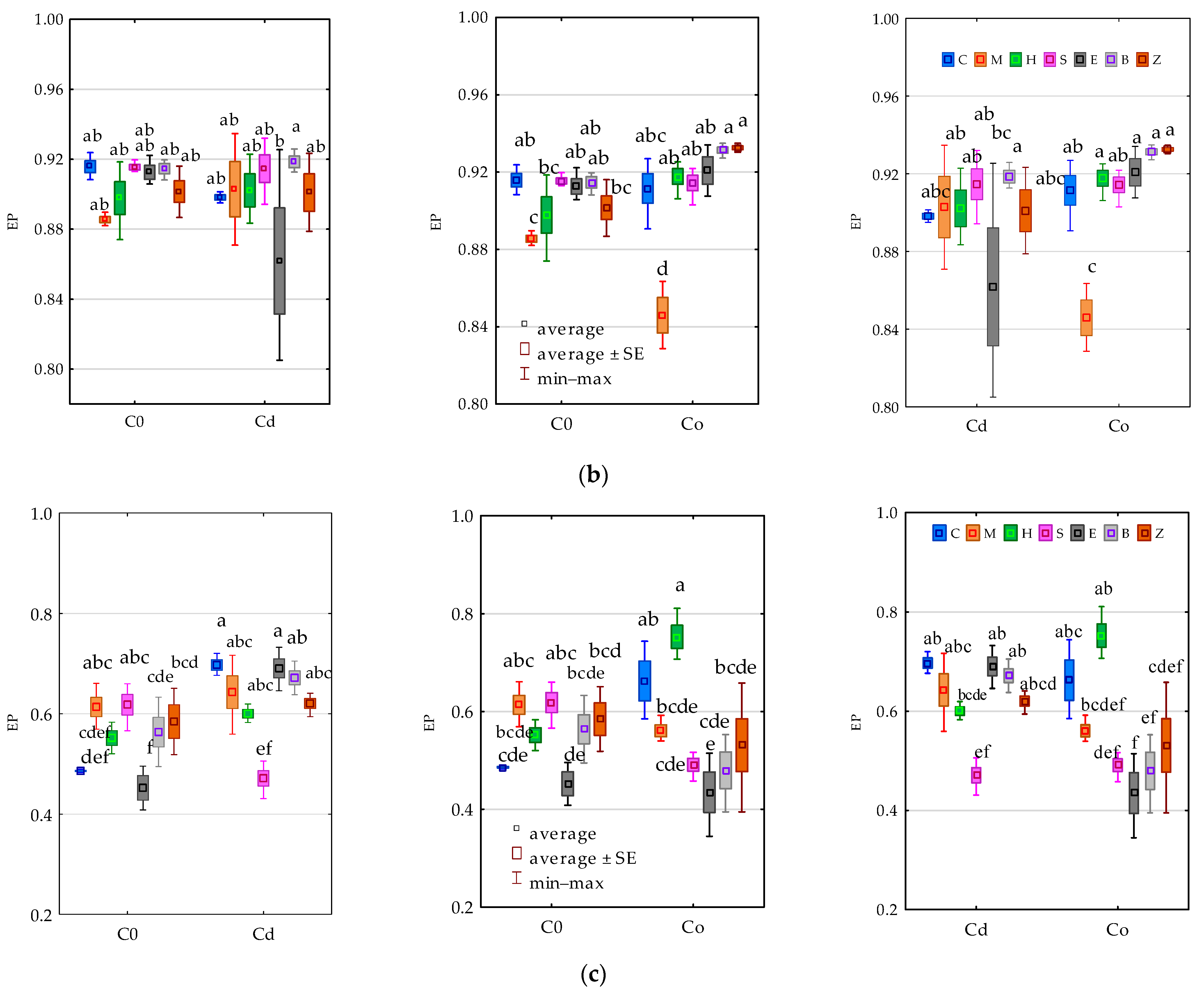
3.2. Enzyme Activity
4. Discussion
4.1. Microorganisms
4.2. Soil Enzymes
4.3. Sunflower (Helianthus annuus L.) against Soil Contamination with Co2+ and Cd2+
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Barra Caracciolo, A.; Terenzi, V. Rhizosphere microbial communities and heavy metal. Microorganisms 2021, 9, 1462. [Google Scholar] [CrossRef] [PubMed]
- Karlen, D.L.; Rice, C.W. Soil degradation: Will humankind ever learn? Sustainability 2015, 7, 12490–12501. [Google Scholar] [CrossRef]
- Hossain, A.; Krupnik, T.J.; Timsina, J.; Mahboob, M.G.; Chaki, A.K.; Farooq, M. Agricultural land degradation: Processes and problems undermining future food security. In Environment, Climate, Plant and Vegetation Growth; Fahad, S., Hasanuzzaman, M., Alam, M., Ullah, H., Saeed, M., Ali Khan, I., Adnan, M., Eds.; Springer International Publishing: Cham, Germany, 2020; pp. 17–61. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Navarrete, M.; Debaeke, P.; Souchere, V.; Alberola, C.; Menassieu, J. Agronomy for sustainable agriculture. A review. Agron. Sustain. Dev. 2009, 29, 1–6. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Awan, S.A.; Ilyas, N.; Khan, I.; Raza, M.A.; Rehman, A.U.; Rizwan, M.; Rastogi, A.; Tariq, R.; Brestic, M. Bacillus siamensis reduces cadmium accumulation and improves growth and antioxidant defense system in two wheat (Triticum aestivum L.) varieties. Plants (Basel, Switz.). Plants 2020, 9, 878. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Xu, J.; Hu, C.; Wang, M.; Zhao, Z.; Zhao, X.; Cao, L.; Lu, Y.; Cai, X. Changeable effects of coexisting heavy metals on transfer of cadmium from soils to wheat grains. J. Hazard. Mater. 2022, 423, 127182. [Google Scholar] [CrossRef]
- Kusvuran, S.; Kiran, S.; Ellialtioglu, S.S. Antioxidant enzyme activities and abiotic stress tolerance relationship in vegetable crops. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives, 1st ed.; InTech: Rijeka, Croatia, 2016; pp. 481–503. [Google Scholar] [CrossRef]
- Mashhadikhan, S.; Amooghin, A.E.; Sanaeepur, H.; Shirazi, M.M.A. A critical review on cadmium recovery from wastewater towards environmental sustainability. Desalination 2022, 535, 115815. [Google Scholar] [CrossRef]
- Rigby, H.; Smith, S.R. The significance of cadmium entering the human food chain via livestock ingestion from the agricultural use of biosolids, with special reference to the UK. Environ. Int. 2020, 143, 105844. [Google Scholar] [CrossRef]
- European Commission. Study on the Review of the List of Critical Raw Materials-Final Report; European Commission: Brussels, Belgium, 2020; p. 158. ISBN1 978-92-79-72119-9. Available online: http://hytechcycling.eu/wp-content/uploads/Study-on-the-review-of-the-list-of-Critical-Raw-Materials.pdf (accessed on 18 May 2022)ISBN2 978-92-79-72119-9.
- Casado, M.; Anawar, H.M.; Garcia-Sanchez, A.; Santa Regina, I. Cadmium and zinc in polluted mining soils and uptake by plants (El Losar mine, Spain). Int. Environ. Pollut. 2008, 33, 146–159. [Google Scholar] [CrossRef]
- Thompson, J.; Bannigan, J. Cadmium: Toxic effects on the reproductive system and the embryo. Reproduct. Toxicol. 2008, 25, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.T.; Nauman, M.; Nazir, N.; Ali, S.; Bangash, N. Environmental hazards of cadmium: Past, present, and future. In Cadmium Toxicity and Tolerance in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 163–183. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- ATSDR. Substance Priority List (SPL); Agency for Toxic Substances and Disease Registry (ATSDR): Atlanta, GA, USA, 2017; p. 2019.
- Dehaine, Q.; Tijsseling, L.T.; Glass, H.J.; Törmänen, T.; Butcher, A.R. Geometallurgy of cobalt ores: A review. Miner. Eng. 2021, 160, 106656. [Google Scholar] [CrossRef]
- Johnson, D.B.; Dybowska, A.; Schofield, P.F.; Herrington, R.J.; Smith, S.L.; Santos, A.L. Bioleaching of arsenic-rich cobalt mineral resources, and evidence for concurrent biomineralisation of scorodite during oxidative bio-processing of skutterudite. Hydrometallurgy 2020, 195, 105395. [Google Scholar] [CrossRef]
- USGS. Mineral Commodity Summaries—Cobalt. Reston, Virginia. 2020. Available online: https://doi.org/10.3133/mcs2020 (accessed on 10 May 2022).
- Darton Commodities Ltd. Cobalt Market Review 2019–2020. Guildford. 2020. Available online: https://www.dartoncommodities.co.uk/cobalt/ (accessed on 10 May 2022).
- Gil, A.; Santamaría, L.; Korili, S.A.; Vicente, M.A.; Barbosa, L.V.; de Souza, S.D.; Marçal, L.; de Faria, E.H.; Ciuffi, K.J. A review of organic-inorganic hybrid clay based adsorbents for contaminants removal: Synthesis, perspectives and applications. J. Environ. Chem. Eng. 2021, 9, 105808. [Google Scholar] [CrossRef]
- Oustriere, N.; Marchand, L.; Lottier, N.; Motelica, M.; Mench, M. Long-term Cu stabilization and biomass yields of Giant reed and poplar after adding a biochar, alone or with iron grit, into a contaminated soil from a wood preservation site. Sci. Total Environ. 2017, 579, 620–627. [Google Scholar] [CrossRef]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.W. Role of organic amendments on enhanced bioremediation of heavy metal (loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef]
- Cao, F.; Lian, C.; Yu, J.; Yang, H.; Lin, S. Study on the adsorption performance and competitive mechanism for heavy metal contaminants removal using novel multipore activated carbons derived from recyclable long-root Eichhornia crassipes. Bioresour. Technol. 2019, 276, 211–218. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Ragone, P.; Fiore, S. Immobilization of Zn and Pb in polluted soil by in-situ crystallization zeolites from fly ash. Water Air Soil Pollut. 2012, 223, 5357–5364. [Google Scholar] [CrossRef]
- Okoye, E.A.; Bocca, B.; Ruggieri, F.; Ezejiofor, A.N.; Nwaogazie, I.L.; Domingo, J.L.; Rovira, J.; Frazzoli, C.; Orisakwe, O.E. Metal pollution of soil, plants, feed and food in the Niger Delta, Nigeria: Health risk assessment through meat and fish consumption. Environ. Res. 2021, 198, 111273. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.S.; Xi, S.H. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, J.; Zhang, J.; Cai, J.; Pi, J.; Xu, J.F. Inspirations of cobalt oxide nanoparticle based anticancer therapeutics. Pharmaceutics 2021, 13, 1599. [Google Scholar] [CrossRef] [PubMed]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Amari, T.; Ghnaya, T.; Abdelly, C. Nickel, cadmium and lead phytotoxicity and potential of halophytic plants in heavy metal extraction. S. Afr. J. Bot. 2017, 111, 99–110. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.R.; Idoko, G.O.; Akabuogo, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef]
- Levy, D.B.; Barbarick, K.A.; Siemer, E.G.; Sommers, L.E. Distribution and partitioning of trace metals in contaminated soils near Leadville, Colorado. J. Environ. Qual. 1992, 21, 185–195. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, S.; Rehman, M.Z.; Qayyum, M.F.; Nawaz, R.; Ahmad, A.; Asrar, M.; Ahmad, S.R.; Alsahli, A.A. Combined use of different nanoparticles effectively decreased cadmium (Cd) concentration in grains of wheat grown in a field contaminated with Cd. Ecotoxicol. Environ. Saf. 2021, 215, 112139. [Google Scholar] [CrossRef]
- Qianqian, M.; Haider, F.U.; Farooq, M.; Adeel, M.; Shakoor, N.; Jun, W.; Jiaying, X.; Wang, X.W.; Panjun, L.; Liqun, C. Selenium treated Foliage and biochar treated soil for improved lettuce (Lactuca sativa L.) growth in Cd-polluted soil. J. Clean. Prod. 2022, 335, 130267. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Grobelak, A.; Hiller, J. Bacterial siderophores promote plant growth: Screening of catechol and hydroxamate siderophores. Int. J. Phytoremediat. 2017, 19, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Kumari, S.; Rath, S.; Priyadarshanee, M.; Das, S. Diversity, structure and regulation of microbial metallothionein: Metal resistance and possible applications in sequestration of toxic metals. Matallomics 2020, 12, 1637–1655. [Google Scholar] [CrossRef] [PubMed]
- Rono, J.K.; Le Wang, L.; Wu, X.C.; Cao, W.H.; Zhao, Y.N.; Khan, I.U.; Yang, Z.M. Identification of a new function of metallothioneinlike gene OsMT1e for cadmium detoxification and potential phytoremediation. Chemosphere 2021, 265, 129136. [Google Scholar] [CrossRef]
- Balan, B.; Dhaulaniya, A.S.; Varma, D.A. Microbial biofilm ecology, in silico study of quorum sensing receptor-ligand interactions and biofilm mediated bioremediation. Arch. Microbiol. 2021, 203, 13–30. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Grenni, P.; Garbini, G.L.; Rolando, L.; Campanale, C.; Aimola, G.; Fernandez-Lopez, M.; Fernandez-Gonzalez, A.J.; Villadas, P.J.; Ancona, V. Characterization of the belowground microbial community in a poplar-phytoremediation strategy of a multi-contaminated soil. Front. Microb. 2020, 11, 2073. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Boros-Lajszner, E.; Kucharski, J. Calorific value of Festuca rubra biomass in the phytostabilization of soil contaminated with nickel, cobalt and cadmium which disrupt the microbiological and biochemical properties of soil. Energies 2022, 15, 3445. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, J. Microbiological study in petrol-spiked soil. Molecules 2021, 26, 2664. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, M.; Cao, Y.; Wang, J.; Tang, M.; Ban, Y. Comparisons of soil properties, enzyme activities and microbial communities in heavy metal contaminated bulk and rhizosphere soils of Robinia pseudoacacia L. in the Northern foot of Qinling Mountain. Forests 2017, 8, 430. [Google Scholar] [CrossRef]
- Govarthanana, M.; Mythilib, R.; Selvankumarb, T.; Kamala-Kannanc, S.; Kima, H. Myco-phytoremediation of arsenic- and lead-contaminated soils by Helianthus annuus and wood rot fungi, Trichoderma sp. isolated from decayed wood. Ecotoxicol. Environ. Saf. 2018, 151, 279–284. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources 2006; World Soil Research Report No.103; FAO: Rome, Italy, 2014. [Google Scholar]
- ISO 11261; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. ISO: Geneva, Switzerland, 1995.
- Nelson, D.; Sommers, L. Total carbon, organic carbon, and organic matter. In Method of Soil Analysis: Chemical Methods; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- Egner, H.; Riehm, H.; Domingo, W. Untersuchungen Über Die Chemische Bodenanalyse Als Grundlage Für Die Beurteilung Des Nährstoffzustandes Der böden. II. Chemische extractionsmethoden zur phospor- und kaliumbestimmung. Ann. Royal Agricult. Coll. Swed. 1960, 26, 199–215. [Google Scholar]
- Schlichting, E.; Blume, H.; Stahr, K. Bodenkundliches praktikum. Pareys studientexte 81; Blackwell Wissenschafts-Verlag: Berlin, Germany, 1995. [Google Scholar]
- ISO 11047:1998; Soil quality—Determination of cadmium, chromium, cobalt, copper, lead, manganese, nickel and zinc—Flame and electrothermal atomic absorption spectrometric methods. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 10390; In Soil Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- Klute, A. Methods of Soil Analysis; American Society of Agronomy, Agronomy Monograph 9: Madison, WI, USA, 1996. [Google Scholar]
- Bunt, J.S.; Rovira, A.D. Microbiological studies of some subantarctic soils. J. Soil Sci. 1955, 6, 119–128. [Google Scholar] [CrossRef]
- Parkinson, D.; Gray, T.R.G.; Williams, S.T. Methods for Studying the Ecology of Soil Microorganisms; IBP Handbook 19; Blackwell Scientific Publication: Oxford, UK, 1971. [Google Scholar]
- Martin, J. Use of acid rose bengal and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Öhlinger, R. Dehydrogenase activity with the substrate TTC. In Methods in Soil Biology; Schinner, F., Ohlinger, R., Kandler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 241–243. [Google Scholar]
- Johnson, J.L.; Temple, K.L. Some variables affecting the measurement of “catalase activity” in soil. Soil Sci. Soc. Am. J. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. (Eds.) Methods in Applied Soil Microbiology and Biochemistry; Academic London: London, UK, 1988; pp. 316–365. [Google Scholar]
- Keeling, J.L. The Mineralogy, Geology and Occurrences of Halloysite; Apple Academic Press: Oakville, ON, Canada, 2015; Available online: https://www.researchgate.net/publication/272481304_The_mineralogy_geology_and_occurrences_of_halloysite (accessed on 20 June 2022).
- Sun, Y.; Sun, G.; Xu, Y.; Liu, W.; Liang, X.; Wang, L. Evaluation of the effectiveness of sepiolite, bentonite, and phosphate amendments on the stabilization remediation of cadmium contaminated soils. J. Environ. Manag. 2016, 166, 204–210. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Liu, L.; Ulhassan, Z.; He, Z.; Yang, X. Sepiolite clay: A review of its applications to immobilize toxic metals in contaminated soils and its implications in soil–plant system. Environ. Technol. Innov. 2021, 23, 101598. [Google Scholar] [CrossRef]
- Ayaz, M.; Feizienė, D.; Tilvikienė, V.; Akhtar, K.; Stulpinaitė, U.; Iqbal, R. Biochar role in the sustainability of agriculture and environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Strachel, R.; Wyszkowska, J.; Baćmaga, M. An evaluation of the effectiveness of sorbents in the remediation of soil contaminated with zinc. Water Air Soil Pollut. 2018, 229, 235. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Use of zeolite to neutralise nickel in a soil environment. Environ. Monit. Assess. 2018, 190, 54. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Remediation of soil contaminated with diesel oil. J. Elem. 2018, 23, 767–788. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Use of a zeolite and molecular sieve to restore homeostasis of soil contaminated with cobalt. Minerals 2020, 10, 53. [Google Scholar] [CrossRef]
- ISO 7218:2007/AMD 1:2013; Microbiology of food and animal feeding stuffs—General requirements and guidance for microbiological examina-tions—Amendment 1. ISO: Geneva, Switzerland, 2013.
- De Leji, F.A.M.; Whipps, M.; Lynch, J.M. The use for colony development for the characterization of bacterial communities in soil and on roots. Microb. Ecol. 1993, 27, 81–97. [Google Scholar] [CrossRef]
- Statistica (Data Analysis Software System). version 13; Tibco Softwere Inc.: Palo Alto, CA, USA, 2017; Available online: http://statistica.io (accessed on 10 May 2022).
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 10 May 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 10 May 2022).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, M.; Moeller, S.; et al. Various R Programming Tools for Plotting Data. R Package Version 2.17.0. 2020. Available online: https://CRAN.R-project.org/package=gplots (accessed on 23 February 2020).
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Heavy metal pollution from gold mines: Environmental effects and bacterial strategies for resistance. Int. J. Environ. Res. Public Health 2016, 13, 1047. [Google Scholar] [CrossRef]
- Mathivanan, K.; Chandirika, J.U.; Vinothkanna, A.; Yin, H.; Liu, X.; Meng, D. Bacterial adaptive strategies to cope with metal toxicity in the contaminated environment—A review. Ecotoxicol. Environ. Saf. 2021, 226, 112863. [Google Scholar] [CrossRef]
- Pal, C.; Asiani, K.; Arya, S.; Rensing, C.; Stekel, D.J.; Larsson, D.G.J.; Hobman, J.L. Metal resistance and its association with antibiotic resistance. Adv. Microb. Physiol. 2017, 70, 261–313. [Google Scholar] [CrossRef]
- Shelton, A.N.; Seth, E.C.; Mok, K.C.; Han, A.W.; Jackson, S.N.; Haft, D.R.; Taga, M.E. Uneven distribution of cobamide biosynthesis and dependence in bacteria predicted by comparative genomics. ISME J. 2019, 13, 789–804. [Google Scholar] [CrossRef]
- Toledano, M.B.; Huang, B. Microbial 2-Cys peroxiredoxins: Insights into their complex physiological roles. Mol. Cells 2016, 39, 31–39. [Google Scholar] [CrossRef]
- Kosiorek, M.; Wyszkowski, M. Effect of cobalt on the environment and living organisms—A review. Appl. Ecol. Environ. Res. 2019, 17, 11419–11449. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Phytoremediation of soil contaminated with nickel, cadmium and cobalt. Int. J. Phytoremediat. 2021, 23, 252–262. [Google Scholar] [CrossRef]
- Zaborowska, M.; Kucharski, J.; Wyszkowska, J. Biological activity of soil contaminated with cobalt, tin, and molybdenum. Environ. Monit. Assess. 2016, 188, 398. [Google Scholar] [CrossRef]
- Barras, F.; Fontecave, M. Cobalt stress in Escherichia coli and Salmonella enterica: Molecular bases for toxicity and resistance. Metallomics 2011, 3, 1130–1134. [Google Scholar] [CrossRef]
- Zaborowska, M.; Kucharski, J.; Wyszkowska, J. Brown algae and basalt meal in maintaining the activity of arylsulfatase of soil polluted with cadmium. Water Air Soil Pollut. 2017, 228, 267. [Google Scholar] [CrossRef]
- Muhammad, H.; Wei, T.; Cao, G.; Yu, S.H.; Ren, X.H.; Jia, H.L.; Saleem, A.; Hua, L.; Guo, J.K.; Li, Y. Study of soil microorganisms modified wheat straw and biochar for reducing cadmium leaching potential and bioavailability. Chemosphere 2021, 273, 129644. [Google Scholar] [CrossRef]
- Gunina, A.; Kuzyakov, Y. Sugars in soil and sweets for microorganisms: Review of origin, content, composition and fate. Soil Biol. Biochem. 2015, 90, 87–100. [Google Scholar] [CrossRef]
- Virk, A.L.; Kan, Z.-R.; Liu, B.-Y.; Qi, J.-Y.; He, C.; Liu, Q.-Y.; Zhao, X.; Zhang, H.-L. Impact of biochar water extract addition on soil organic carbon mineralization and C fractions in different tillage systems. Environ. Technol. Innov. 2020, 21, 101193. [Google Scholar] [CrossRef]
- Sandhu, S.S.; Dan, U.; Kumar, S.; Chintala, R.; Papiernik, S.K.; Malo, D.D.; Schumacher, T.E. Analyzing the impacts of three types of biochar on soil carbon fractions and physiochemical properties in a corn-soybean rotation. Chemosphere 2017, 184, 473–481. [Google Scholar] [CrossRef]
- Steiner, C.; Das, K.C.; Garcia, M.; Förster, B.; Zech, W. Charcoal and smoke extract stimulate the soil microbial community in a highly weathered xanthic Ferralsol. Pedobiologia 2008, 51, 359–366. [Google Scholar] [CrossRef]
- Ma, C.; Ci, K.; Zhu, J.; Sun, Z.; Liu, Z.; Li, X.; Zhu, Y.; Tang, C.; Wang, P.; Liu, Z. Impacts of exogenous mineral silicon on cadmium migration and transformation in the soil-rice system and on soil health. Sci. Total Environ. 2021, 759, 143501. [Google Scholar] [CrossRef]
- Roces, E.; Muñiz-Menéndez, M.; González-Galindo, J.; Estaire, J. Lightweight expanded clay aggregate properties based on laboratory testing. Constr. Build. Mater. 2021, 313, 27–125486. [Google Scholar] [CrossRef]
- Mlih, R.; Bydalek, F.; Klumpp, E.; Yaghi, N.; Bol, R.; Wenk, J. Light-expanded clay aggregate (LECA) as a substrate in constructed wetlands—A review. Ecol. Eng. 2020, 148, 105783. [Google Scholar] [CrossRef]
- Zwikel, S.; Lavee, H.; Sarah, P. Temporal dynamics in arylsulfatase enzyme activity in various microenvironments along a climatic transect in Israel. Geoderma 2007, 140, 30–41. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Burns, R.G. Ecology of extracellular enzyme activities and organic matter degradation in soil: A complex community-driven process. In Methods of Soil Enzymology; Dick, R.P., Ed.; Soil Science Society of America: Madison, WI, USA, 2011; pp. 35–55. [Google Scholar]
- Yuan, B.; Yue, D. Soil microbial and enzymatic activities across a chronosequence of Chinese pine plantation development on the loess plateau of China. Pedosphere 2012, 22, 1–12. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, W.; Liang, Y.; Liu, S.; Wang, K. Increased associated effects of topography and litter and soil nutrients on soil enzyme activities and microbial biomass along vegetation successions in karst ecosystem, southwestern China. Environ. Sci. Pollut. Res. 2018, 25, 16979–16990. [Google Scholar] [CrossRef]
- Moeskops, B.; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; de Neve, S. Soil microbial communities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112120. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, J.; Shi, D.; Li, S.; Zhang, F.; Zhang, F. Syntheses, urease inhibition activities, and fluorescent properties of transition metal complexes. J. Coord. Chem. 2013, 66, 1616–1625. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, D.; Xu, Y.; Wang, L.; Liang, X.; Shen, Y. Effects of sepiolite on stabilization remediation of heavy metal-contaminated soil and its ecological evaluation. Front. Environ. Sci. Eng. 2016, 10, 85–92. [Google Scholar] [CrossRef]
- Chao, H.P.; Chen, S.H. Adsorption characteristics of both cationic and oxyanionic metal ions on hexadecyltrimethylammonium bromide-modified NaY zeolite. Chem. Eng. J. 2012, 193–194, 283–289. [Google Scholar] [CrossRef]
- Jimenez-Castaneda, M.E.; Medina, D.I. Use of surfactant modified zeolites and clays for the removal of heavy metals from water. Water 2017, 9, 235. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112, 75–93. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, G.; Xu, Y.; Wang, L.; Liang, X.; Lin, D. Assessment of sepiolite for immobilization of cadmiumcontaminated soils. Geoderma 2013, 193–194, 149–155. [Google Scholar] [CrossRef]
- Abad-Valle, P.; Álvarez-Ayuso, E.; Murciego, A.; Pellitero, E. Assessment of the use of sepiolite amendment to restore heavy metal polluted mine soil. Geoderma 2016, 280, 57–66. [Google Scholar] [CrossRef]
- Forte, J.; Mutiti, S. Phytoremediation potential of Helianthus annuus and Hydrangea paniculata in copper and lead-contaminated soil. Water Air Soil Pollut. 2017, 228, 29954–29966. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rizvi, H.; Rinklebe, J.; Tsang, D.C.W.; Meers, E.; Ok, Y.S.; Ishaque, W. Phytomanagement of heavy metals in contaminate soils using sunflower—A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1498–1528. [Google Scholar] [CrossRef]
- Palit, S.; Sharma, A.; Talukder, G. Effects of cobalt on plants. Bot. Rev. 1994, 60, 149–181. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Rehman, S.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef]
- Bashir, S.; Zhu, J.; Fu, Q.; Hu, H. Cadmium mobility, uptake and anti-oxidative response of water spinach (Ipomoea aquatic) under rice straw biochar, zeolite and rock phosphate as amendments. Chemosphere 2018, 194, 579–587. [Google Scholar] [CrossRef]
- Ouariti, O.; Gouia, H.; Ghorbal, M.H. Responses of bean and tomato plants to cadmium—growth, mineral nutrition, and nitrate reduction. Plant Physiol. Biochem. 1997, 35, 347–354. [Google Scholar]
- Weryszko-Chmielewska, E.; Chwil, M. Lead-Induced histological and ultrastructural changes in the leaves of soybean (Glycine max L.) Merr.). J. Plant Nutr. Soil Sci. 2005, 51, 203–212. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Oh, S.; Park, Y.-K. Overview of biochar production from preservative treated wood with detailed analysis of biochar characteristics, heavy metal behavior, and their ecotoxicity. J. Hazard. Mater. 2019, 384, 121356. [Google Scholar] [CrossRef]


| Abbreviation | Properties | Unit | Soil | Methodical Literature |
|---|---|---|---|---|
| Chemical and physicochemical properties | ||||
| Ntot | total nitrogen | g kg−1 d.m. | 1.07 | [49] |
| Corg | organic carbon | g kg−1 d.m. | 14.69 | [50] |
| P | phosphorus | mg kg−1 d.m. | 166.72 | [51] |
| K | potassium | mg kg−1 d.m. | 171.31 | [51] |
| Mg | magnesium | mg kg−1 d.m. | 443.21 | [52] |
| Cd | cadmium | mg kg−1 d.m. | 0.56 | [53] |
| Co | cobalt | mg kg−1 d.m. | 7.21 | [53] |
| pH | pHKCl—soil reaction | - | 6.00 | [54] |
| EBC | sum of exchangeable base cations | mmol (+) kg−1 d.m. | 145.00 | [55] |
| HAC | hydrolytic acidity | mmol (+) kg−1 d.m. | 13.50 | [55] |
| CEC | cation exchange capacity | mmol (+) kg−1 d.m. | 158.50 | [55] |
| ACS | alkaline cation saturation | % | 91.49 | [55] |
| Microorganisms number per 1 kg d.m. | ||||
| Org | organotrophic bacteria | cfu | 18.192 × 109 | [56] |
| Act | actinomyces | cfu | 11.931 × 109 | [57] |
| Fun | fungi | cfu | 5.374 × 107 | [58] |
| Enzymatic activity per 1 kg d.m. h−1 | ||||
| Deh | dehydrogenases | µmol TPF | 3.124 | [59] |
| Cat | catalase | mol O2 | 0.304 | [60] |
| Ure | urease | mmol N-NH4 | 0.492 | [61] |
| Pac | acid phosphatase | mmol PN | 1.168 | [61] |
| Pal | alkaline phosphatase | mmol PN | 1.014 | [61] |
| Glu | β-glucosidase | mmol PN | 0.492 | [61] |
| Aryl | arylsulphatase | mmol PN | 0.140 | [61] |
| Object | C0 | Cd | Co |
|---|---|---|---|
| Control | 35.988 ab | 21.625 d–f | 4.450 g |
| Molecular sieve | 34.713 a | 29.563 bc | 28.313 b–d |
| Halloysite | 38.638 a | 19.588 ef | 21.325 d–f |
| Sepiolite | 35.175 ab | 25.413 c–e | 22.963 c–e |
| Expanded clay | 35.238 ab | 24.275 c–e | 14.563 f |
| Biochar | 39.138 a | 22.975 c–e | 19.438 ef |
| Zeolite | 33.488 a | 20.213 ef | 14.525 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Evaluation of the Usefulness of Sorbents in the Remediation of Soil Exposed to the Pressure of Cadmium and Cobalt. Materials 2022, 15, 5738. https://doi.org/10.3390/ma15165738
Wyszkowska J, Borowik A, Zaborowska M, Kucharski J. Evaluation of the Usefulness of Sorbents in the Remediation of Soil Exposed to the Pressure of Cadmium and Cobalt. Materials. 2022; 15(16):5738. https://doi.org/10.3390/ma15165738
Chicago/Turabian StyleWyszkowska, Jadwiga, Agata Borowik, Magdalena Zaborowska, and Jan Kucharski. 2022. "Evaluation of the Usefulness of Sorbents in the Remediation of Soil Exposed to the Pressure of Cadmium and Cobalt" Materials 15, no. 16: 5738. https://doi.org/10.3390/ma15165738
APA StyleWyszkowska, J., Borowik, A., Zaborowska, M., & Kucharski, J. (2022). Evaluation of the Usefulness of Sorbents in the Remediation of Soil Exposed to the Pressure of Cadmium and Cobalt. Materials, 15(16), 5738. https://doi.org/10.3390/ma15165738









