Antimicrobial Efficacy of Silver Nanoparticles against Candida Albicans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reporting Format
2.2. Research Question
2.3. Eligibility Criteria
2.4. Search Strategy and Study Selection
2.5. Data Collection
2.6. Quality Assessment and Risk of Bias
2.6.1. Risk of Bias Tool for Clinical Studies
2.6.2. Quality of Assessment of Laboratory Studies
2.7. Data Synthesis
3. Results
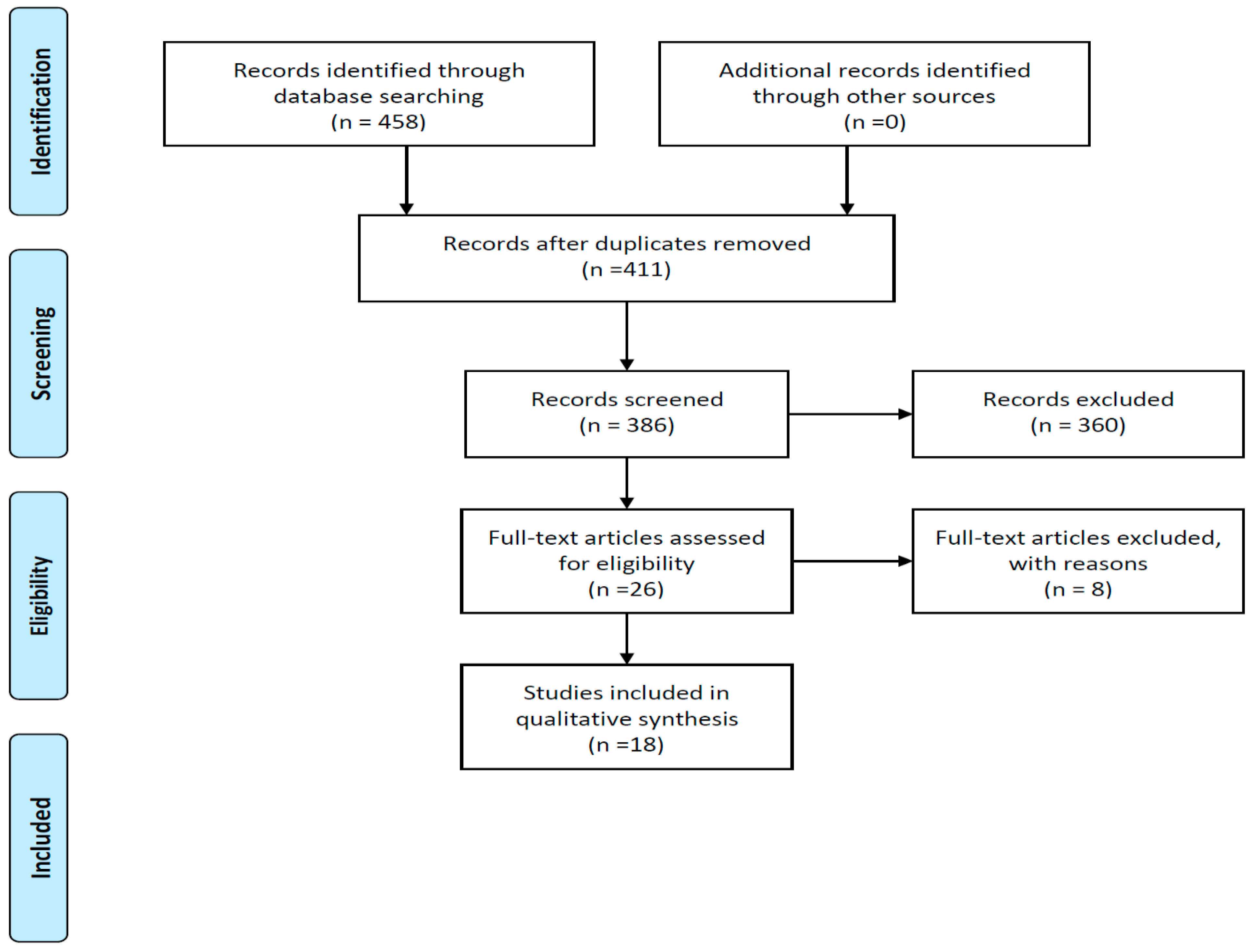
3.1. Study Selection
3.2. Study Methods and Characteristics
3.3. Quality Assessment and Risk of Bias
- (a)
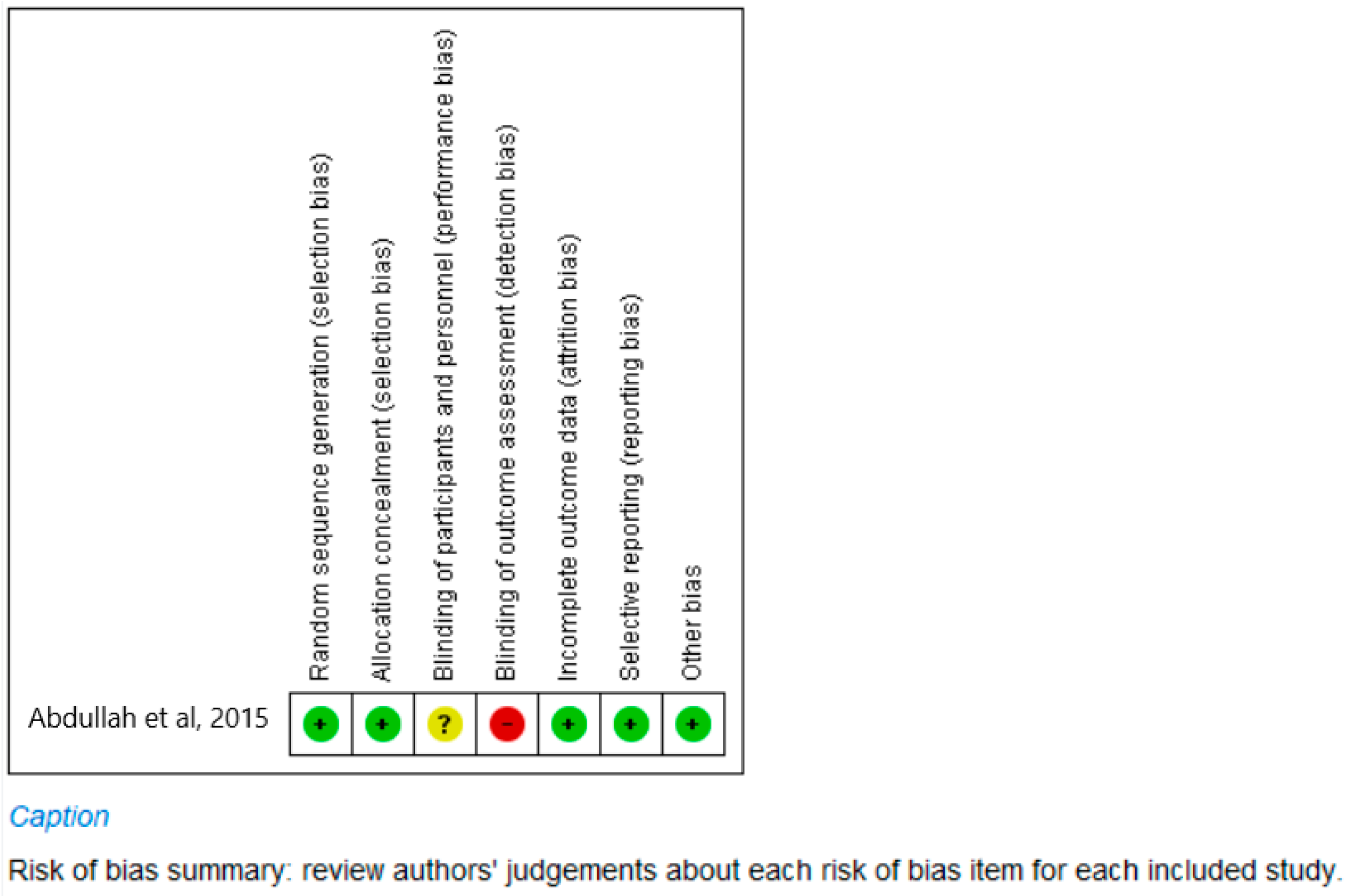
- Clinical study (Figure 2)
- (b)
- In vitro Laboratory studies (Table 3)
3.4. Effectiveness of AgNPs against Candida Albicans in Denture Acrylic
3.5. Effectiveness of AgNPs against Candida Albicans in Denture Liners
3.6. Excluded Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral candidiasis: A disease of opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Gendreau, L.; Loewy, Z.G. Epidemiology and Etiology of Denture Stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A.O.; Williams, D.W. Diagnosis and management of oral candidosis. Br. Dent. J. 2017, 223, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Ikawa, S.; Kitano, K.; Maeda, N. A proposal of remedies for oral diseases caused by Candida: A mini review. Front. Microbiol. 2018, 1522. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Albanese, G.; De Giorgi, M.L.; Corsalini, M.; Rinaldi, R. Silver nanoparticles addition in poly(methyl methacrylate) dental matrix: Topographic and antimycotic studies. Int. J. Mol. Sci. 2019, 20, 4691. [Google Scholar] [CrossRef]
- Subramani, K.; Ahmed, W.; Hartsfield, J.K. Nanobiomaterials in Clinical Dentistry, Nanobiomaterials in Clinical Dentistry; William Andrew: Norwich, NY, USA, 2012. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibacterial Nanoparticles in Endodontics: A Review. J. Endod. 2016, 1417–1426. [Google Scholar] [CrossRef]
- Jandt, K.D.; Watts, D.C. Nanotechnology in dentistry: Present and future perspectives on dental nanomaterials. Dent. Mater. 2020, 36, 1365–1378. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Adam, R.Z.; Khan, S.B. Antimicrobial efficacy of silver nanoparticles against Candida albicans: A systematic review protocol. PLoS ONE 2021, 16, e0245811. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.W.V. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Kreve, S.; Oliveira, V.C.; Bachmann, L.; Alves, O.L.; Dos Reis, A.C. Influence of AgVO3 incorporation on antimicrobial properties, hardness, roughness and adhesion of a soft denture liner. Sci. Rep. 2019, 9, 11889. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Takamiya, A.S.; Feresin, L.P.; Gorup, L.F.; de Camargo, E.R.; Delbem, A.C.B.; Henriques, M.; Barbosa, D.B. Silver colloidal nanoparticle stability: Influence on Candida biofilms formed on denture acrylic. Med. Mycol. 2014, 52, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Wady, A.; Machado, A.; Zucolotto, V.; Zamperini, C.; Berni, E.; Vergani, C. Evaluation of Candida albicans adhesion and biofilm formation on a denture base acrylic resin containing silver nanoparticles. J. Appl. Microbiol. 2012, 112, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- De Castro, D.T.; Valente, M.L.C.; da Silva, C.H.L.; Watanabe, E.; Siqueira, R.L.; Schiavon, M.A.; Alves, O.L.; Dos Reis, A.C. Evaluation of antibiofilm and mechanical properties of new nanocomposites based on acrylic resins and silver vanadate nanoparticles. Arch. Oral Biol. 2016, 67, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, J.; Lan, J.; Qi, Q. Effect of a denture base acrylic resin containing silver nanoparticles on Candida albicans adhesion and biofilm formation. Gerodontology 2016, 33, 209–216. [Google Scholar] [CrossRef]
- Han, Z.; Zhu, B.; Chen, R.; Huang, Z.; Zhu, C.; Zhang, X. Effect of silver-supported materials on the mechanical and antibacterial properties of reinforced acrylic resin composites. Mater. Des. 2015, 65, 1245–1252. [Google Scholar] [CrossRef]
- Abdallah, R.; Emera, R.; Sh Gebreil, A. Antimicrobial Activity of Silver Nanoparticles. Egypt. Dent. J. 2015, 61, 1039–1052. [Google Scholar]
- Suganya, S.; Ahila, S.C.; Kumar, B.M.; Kumar, M.V. Evaluation and comparison of anti-Candida effect of heat cure polymethylmethacrylate resin enforced with silver nanoparticles and conventional heat cure resins: An in vitro study. Indian J. Dent. Res. 2014, 25, 204. [Google Scholar] [CrossRef]
- Ghahremanloo, A.; Movahedzadeh, M. The Effect of Silver Nano Particles on Candida Albicans and Streptococcus Mutans in Denture Acrylic Resins. J. Dent. Mater. Tech. 2015, 5, 23–30. [Google Scholar]
- Mousavi, S.A.; Ghotaslou, R.; Akbarzadeh, A.; Azima, N.; Aeinfar, A.; Khorramdel, A. Evaluation of antibacterial and antifungal properties of a tissue conditioner used in complete dentures after incorporation of ZnO-Ag nanoparticles. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 11–18. [Google Scholar] [CrossRef]
- Kamikawa, Y.; Hirabayashi, D.; Nagayama, T.; Fujisaki, J.; Hamada, T.; Sakamoto, R.; Kamikawa, Y.; Sugihara, K. In vitro antifungal activity against oral Candida species using a denture base coated with silver nanoparticles. J. Nanomater. 2014, 1–7. [Google Scholar] [CrossRef]
- Nam, K.Y. In vitro antimicrobial effect of the tissue conditioner containing silver nanoparticles. J. Adv. Prosthodont. 2011, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y.; Lee, C.J.; Lee, C.H. Antifungal and physical characteristics of modified denture base acrylic incorporated with silver nanoparticles. Gerodontology 2012, 29, e413–e419. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, M.; Nam, K.Y. Inhibitory effect of PMMA denture acrylic impregnated by silver nano-particles for Candida albicans. J. Korean Chem. Soc. 2008, 52, 380–386. [Google Scholar]
- Acosta-Torres, L.S.; Mendieta, I.; Nuñez-Anita, R.E.; Cajero-Juárez, M.; Castaño, V.M. Cytocompatible Antifungal Acrylic Resin Containing Silver Nanoparticles for Dentures Background: Inhibition of Candida Albicans on Denture Resins could Play a Significant Role. Int. J. Nanomed. 2012, 7, 4777–4786. [Google Scholar]
- Chladek, G.; Mertas, A.; Barszczewska-Rybarek, I.; Nalewajek, T.; Żmudzki, J.; Król, W.; Łukaszczyk, J. Antifungal activity of denture soft lining material modified by silver nanoparticles—A pilot study. Int. J. Mol. Sci. 2011, 12, 4735–4744. [Google Scholar] [CrossRef] [PubMed]
- Nikola, G.; Milena, K.; Ana, T.; Ljubiša, N.; Vesna, N. Antimikrobna svojstva akrilatnih smola za stomatološke proteze impregniranih nanočesticama srebra. Acta Stomatol. Naissi 2017, 32, 1696–1702. [Google Scholar]
- Li, C.; Wang, X.; Chen, F.; Zhang, C.; Zhi, X.; Wang, K.; Cui, D. The antifungal activity of graphene oxide-silver nanocomposites. Biomaterials 2013, 34, 3882–3890. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Silva, S.; Negri, M.; Gorup, L.; de Camargo, E.; Oliveira, R.; Barbosa, D.; Henriques, M. Silver colloidal nanoparticles: Effect on matrix composition and structure of Candida albicans and Candida glabrata biofilms. J. Appl. Microbiol. 2013, 114, 1175–1183. [Google Scholar] [CrossRef]
- Chladek, G.; Kasperski, J.; Barszczewska-Rybarek, I.; Żmudzki, J. Sorption, solubility, bond strength and hardness of denture soft lining incorporated with silver nanoparticles. Int. J. Mol. Sci. 2013, 14, 563–574. [Google Scholar] [CrossRef]
- de Castro, D.T.; da Costa Valente, M.L.; Aires, C.P.; Alves, O.L.; Dos Reis, Z.C. Elemental ion release and cytotoxicity of antimicrobial acrylic resins incorporated with nanomaterial. Gerodontology 2017, 34, 320–325. [Google Scholar] [CrossRef]
- Malic, S.; Rai, S.; Redfern, J.; Pritchett, J.; Liauw, C.M.; Verran, J.; Tosheva, L. Zeolite-embedded silver extends antimicrobial activity of dental acrylics. Colloids Surf. B Biointerfaces 2019, 173, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.-K.; El-Fiqi, A.; Lee, J.-H.; Kim, D.-A.; Kim, H.-W.; Lee, H.-H. Rechargeable microbial anti-adhesive polymethyl methacrylate incorporating silver sulfadiazine-loaded mesoporous silica nanocarriers. Dent. Mater. 2017, 33, e361–e372. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, M.C.; Negrutiu, M.; Hogea, E.; Freiman, P.C. Bacteriostatic effect of silver nanoparticles over acrylic resin and composite dental materials. Mater. Plast. 2014, 51, 414–416. [Google Scholar]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.F.B.; Guidelli, É.J.; Jafari, S.M.; Ramos, A.P. Green synthesis of metal nanoparticles by plant extracts and biopolymers. In Handbook of Food Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 257–278. [Google Scholar]
| Study | Year | Country | Study Design | Type of Material | Organism |
|---|---|---|---|---|---|
| Abdallah et al., 2015 [18] | 2015 | Egypt | RCT | Heat-cured acrylic resin (Acrostone; Acrostone Dental factory, under exclusive license of England, Egypt) | Alternaria, Aspergillus, Aureobasidium, Candida, Cladosporium, Claviceps, Cryptococcus, Eurotium, Fusarium, Nigrospora, Pyrenophora, Saccharomyces, Schizophyllum, and Zygosaccharomyces. |
| De Matteis et al., 2019 [5] | 2019 | Italy | In vitro | Resin Paladon 65 (Kulzer) | Candida albicans |
| Kamikawa et al., 2014 [22] | 2014 | Japan | In vitro | Heat-cured acrylic resin for denture base, Acron | Candida albicans and Candida glabrata |
| Kreve et al., 2019 [12] | 2019 | Brazil | In vitro | Resin denture liner (Trusoft) | Staphylococcus aureus (ATCC 6538), Pseudomonas aeruginosa (ATCC 27853), Candida albicans (ATCC 90028), and Enterococcus faecalis |
| Lee et al., 2008 [25] | 2008 | Korea | In vitro | Heat-polymerized denture resin | Candida albicans (American type culture collection (ATCC) 66026) |
| Monteiro et al., 2014 [13] | 2014 | Brazil | In vitro | Denture acrylic resin | C. albicans (American Type Culture Collection (ATCC) 10231) and C. glabrata (ATCC90030), two Candida oral clinical isolates were used, namely, C. albicans 324LA/94 (obtained from the culture collection of Cardiff Dental School, Cardiff, UK) and C. glabrata D1 |
| Wady et al., 2012 [14] | 2012 | Brazil | In vitro | Microwave denture base acrylic (Vipi wave) | Candida albicans strain ATCC 90028 |
| Nam, 2011 [23] | 2011 | Korea | In vitro | GC Soft liner | Candida albicans ATCC 14053 |
| Nam et al., 2012 [24] | 2012 | Korea | In vitro | Acrylic denture powder (Lucitone 199) | Candida albicans strain, ATCC 66026 |
| Nikola et al. 2017 [28] | 2017 | Serbia | In vitro | Cold polymerized acrylic powder (Triplex Cold, Ivoclar Vivadent). | Staphylococcus aureus ATCC 25923 and fungus Candida albicans ATCC 2091 |
| Acosta Torres et al., 2012 [26] | 2012 | Mexico | In vitro | Denture acrylic (Nature cryl) | C. albicans strain (90026) |
| Li et al., 2016 [16] | 2016 | China | In vitro | Denture base acrylic resin | C. albicans conference strain 3153A |
| Ghahremanloo et al., 2015 [20] | 2015 | Iran | In vitro | Denture acrylic resin | Candida albicans ATCC 10231; Streptococcus mutans ATCC 35668; Candida albicans hospital isolated |
| Suganya et al., 2014 [19] | 2013 | India | In vitro | Heat cure polymethylmethacrylate (DPI heat cure material) | Candida albicans |
| De Castro et al., 2016 [15] | 2016 | Brazil | In vitro | Dencor Lay Autopolymerizable (SC); and Clássico thermopolymerizable (TR) (Clássico Artigos Odontológicos1) acrylic resins were used | C. albicans (ATCC 10231) and S. mutans (ATCC 25175). |
| Chladek et al., 2011 [27] | 2011 | Poland | In vitro | (Ufi Gel SC): chemically cured silicone soft liner | Candida albicans (ATCC 10231) |
| Mousavi et al., 2019 [21] | 2018 | Iran | In vitro | Tissue conditioner GC soft liner | S. aureus (ATCC6538), P. aeruginosa (ATCC9027), C. albicans (ATCC10231), E. faecalis (ATCC29212) |
| Han et al., 2014 [17] | 2014 | China | In vitro | PMMA powder | C. albicans (76615), S. mutans (UA159) |
| Study | Concentration of AgNPs | Size of AgNPs | Evaluation Method | Time Period | Sample Size |
|---|---|---|---|---|---|
| Abdallah et al., 2015 [18] | 0.05 wt% and 0.2 wt% | 30 patients | |||
| De Matteis et al., 2019 [5] | 3 wt% and 3.5 wt% | 20 nm | Miles and Misra assay (CFU) | 24 and 48 h | Not indicated |
| Kamikawa et al., 2014 [22] | Not indicated | SEM and CFU | 1, 3, 8, and 12 h | n = 40 | |
| Kreve et al., 2019 [12] | 1, 2.5, 10% | Agar diffusion | 24 h | n = 60 | |
| Lee et al., 2008 [25] | 0.002, 0.01, and 0.05 M | CFU | 24 h | No information | |
| Monteiro et al., 2014 [13] | 54 mg/L | 5 nm | CV assay, total biomass, and CFU | 5 h and 24 h | |
| Nam, 2011 [23] | 0.1, 0.5, 1.0, 2.0 to 3.0 vol/vol% | 100–120 nm | CFU | 72 h | n = 162 |
| Wady et al., 2012 [14] | 1000, 750, 500, 250, and 30 ppm | 9 nm | MIC, MFC, XTT | 48 h (MFC, MIC); 3 h (XTT) | n = 360 |
| Nam et al., 2012 [24] | 1.0, 5.0, 10.0, 20.0–30.0 wt% | CFU | 24 h | n = 90 | |
| Nikola et al., 2017 [28] | 2, 5, 10% | Disc diffusion | 24 h for bacteria; 48 h for fungi | ||
| Acosta Torres et al., 2012 [26] | 10–20 nm | Microbial cell viability assay based on luminescent ATP measurement (Bac Titer-Glo™, Promega, Fitchburg, WI, USA) | 24 h | n = 18 | |
| Li et al., 2016 [16] | adhesion (24 h); biofilm assays (72 h) | ||||
| Ghahremanloo et al., 2015 [20] | 2.5, 5, and 10 w/w % | 22 nm | CFU | 1, 6, 24 h | n = 160 |
| Suganya et al., 2014 [19] | 2.5%, 3%, and 5% | 20–100 nm | Agar diffusion/CFU | 24 h | n = 40 |
| De Castro et al., 2016 [15] | 0.5, 1, 2.5, 5, and 10% wt% | XTT and CFU | 48 h | n = 80 | |
| Chladek et al., 2011 [27] | 10, 20, 40, 80, 120, and 200 ppm | 10–30 nm | CFU, AFE | 17 h | |
| Mousavi et al., 2019 [21] | 0.625, 1.25, 2.5, 5, 10, and 20 wt% | 20 nm | MIC, CFU | 24 and 48 h | n = 168 |
| Han et al., 2014 [17] | 1, 2, 3, 4, 5, 6 wt% | CFU | 48 h | n = 36 |
| Scores for Risk of Bias Criteria | |||||||
|---|---|---|---|---|---|---|---|
| Standardization of Sampling Procedures | Description of Sample Size Calculation | Calibration of Samples before Testing\Standards | Measured Outcomes in Valid, Reliable Manner | Appropriate Statistical Analyses Used | Summary Score | ||
| 1 | Nam et al., 2012 [24] | 0 | 2 | 1 | 0 | 0 | 3 |
| 2 | Nikola et al., 2017 [28] | 0 | 2 | 2 | 2 | 2 | 8 |
| 3 | Acosta-Torres et al., 2012 [26] | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | Li et al., 2016 [16] | 0 | 2 | 1 | 1 | 0 | 4 |
| 5 | Ghahremanloo et al., 2016 [20] | 0 | 2 | 2 | 2 | 0 | 6 |
| 6 | Han et al., 2015 [17] | 0 | 2 | 0 | 0 | 0 | 2 |
| 7 | Suganya et al., 2014 [19] | 0 | 2 | 1 | 2 | 2 | 7 |
| 8 | de Castro et al., 2016 [15] | 1 | 2 | 1 | 0 | 0 | 4 |
| 9 | Chladek et al., 2011 [27] | 0 | 2 | 2 | 1 | 2 | 7 |
| 10 | Mousavi et al., 2019 [21] | 0 | 0 | 1 | 1 | 0 | 2 |
| 11 | Abdallah et al., 2015 [18] | 0 | 2 | 2 | 1 | 0 | 5 |
| 12 | De Matteis et al., 2019 [5] | 2 | 2 | 2 | 2 | 2 | 10 |
| 13 | Kamikawa et al., 2014 [22] | 0 | 2 | 2 | 2 | 0 | 6 |
| 14 | Kreve et al., 2019 [12] | 1 | 2 | 1 | 1 | 0 | 5 |
| 15 | Lee et al., 2008 [25] | 2 | 2 | 0 | 1 | 0 | 5 |
| 16 | Monteiro et al., 2014 [13] | 0 | 2 | 2 | 2 | 0 | 6 |
| 17 | Nam, K. Y. (2011) [23] | 0 | 2 | 2 | 2 | 0 | 6 |
| 18 | Wady et al., 2012 [14] | 0 | 2 | 2 | 2 | 0 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, R.Z.; Khan, S.B. Antimicrobial Efficacy of Silver Nanoparticles against Candida Albicans. Materials 2022, 15, 5666. https://doi.org/10.3390/ma15165666
Adam RZ, Khan SB. Antimicrobial Efficacy of Silver Nanoparticles against Candida Albicans. Materials. 2022; 15(16):5666. https://doi.org/10.3390/ma15165666
Chicago/Turabian StyleAdam, Razia Z., and Saadika B. Khan. 2022. "Antimicrobial Efficacy of Silver Nanoparticles against Candida Albicans" Materials 15, no. 16: 5666. https://doi.org/10.3390/ma15165666
APA StyleAdam, R. Z., & Khan, S. B. (2022). Antimicrobial Efficacy of Silver Nanoparticles against Candida Albicans. Materials, 15(16), 5666. https://doi.org/10.3390/ma15165666






