Resveratrol-Loaded Diacetate Fiber by Supercritical CO2 Fluid Assisted Impregnation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Impregnation of Diacetate Fiber with Resveratrol in SCF-CO2
2.3. Quantitative Evaluation of LA of Resveratrol onto Diacetate Fiber in SCF-CO2
2.4. Free Radical Inhibiting Ratio Assessment of Resveratrol
2.5. Characterization and Analysis of Diacetate Fiber
3. Results and Discussion
3.1. The Effect of SCF-CO2 Treatment Time on LA of Resveratrol onto Diacetate Fiber
3.2. The Effect of SCF-CO2 Treatment Temperature and Pressure on LA of Resveratrol onto Diacetate Fiber
3.3. Free Radical Inhibiting Ratio of Resveratrol to DPPH after Being Treated by SCF-CO2
3.4. Structure and Property of Resveratrol-Loaded Diacetate Fiber
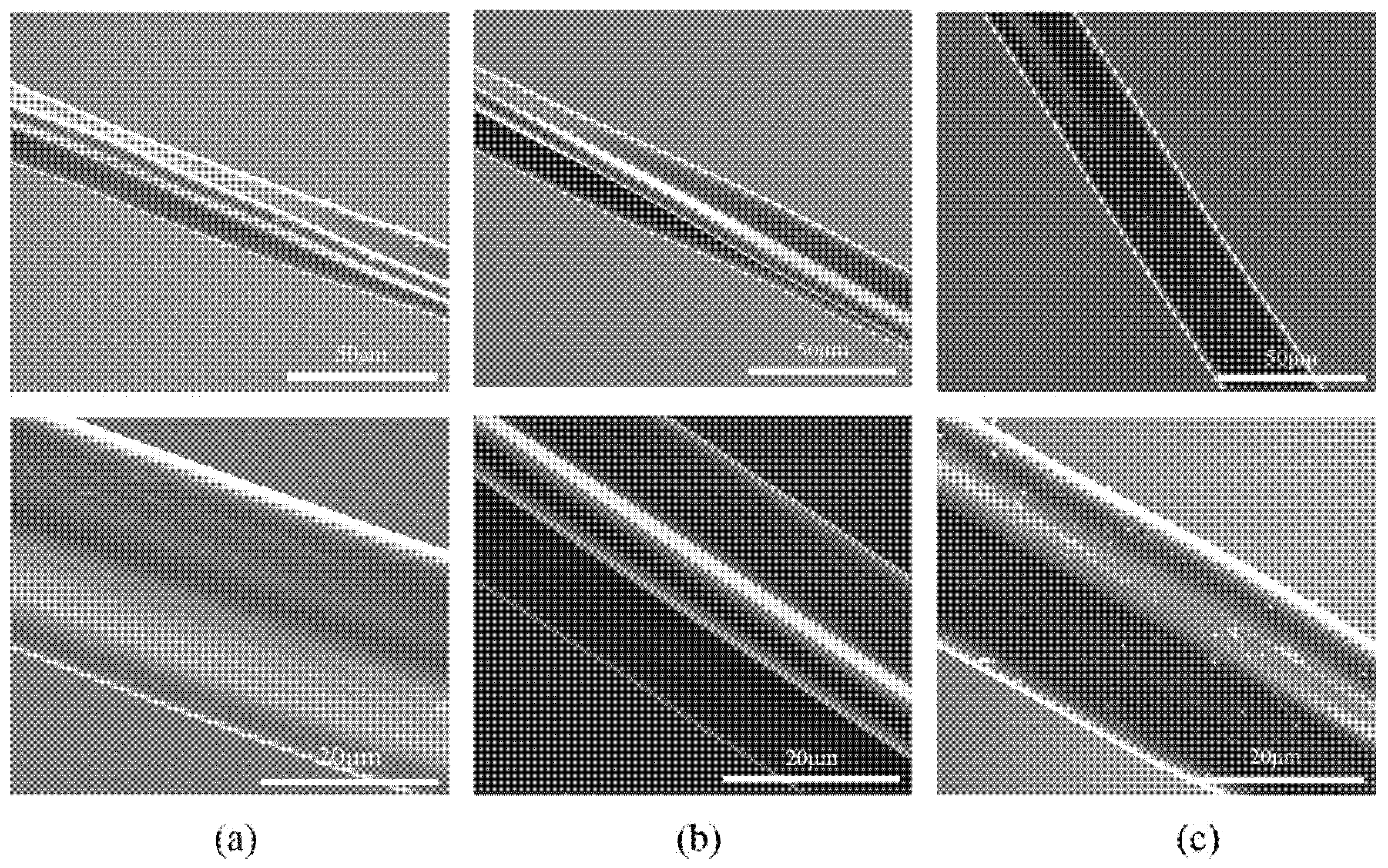
3.4.1. Distribution Pattern of Resveratrol on the Surface of Diacetate Fiber
3.4.2. Chemical Structure of Resveratrol-Loaded Diacetate Fiber
3.4.3. Aggregation Structure of Resveratrol-Loaded Diacetate Fiber
3.4.4. Thermal Degradation of Resveratrol-Loaded Diacetate Fiber
3.4.5. Tensile Breaking Strength of Resveratrol-Loaded Diacetate Fiber
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, Z.; Wang, F.; Bai, X.E. Research Progress of Antibacterial Fiber and Fabric Used in Clothing. Adv. Mater. Res. 2011, 332–334, 1790–1793. [Google Scholar] [CrossRef]
- Ghaheh, F.S.; Khoddami, A.; Alihosseini, F.; Jing, S.; Ribeiro, A.; Cavaco-Paulo, A.; Silva, C. Antioxidant cosmetotextiles: Cotton coating with nanoparticles containing vitamin E. Process Biochem. 2017, 59, 46–51. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Yuen, M.C.W.; Kan, C.W.; Cheuk, K.K.L.; Chui, C.H.; Lam, K.H. Cosmetic textiles with biological benefits: Gelatin microcapsules containing vitamin C. Int. J. Mol. Med. 2009, 24, 411–419. [Google Scholar] [PubMed]
- Walentowska, J.; Foksowicz-Flaczyk, J. Thyme essential oil for antimicrobial protection of natural textiles. Int. Biodeterior. Biodegrad. 2013, 84, 407–411. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Anbumani, N.; Priyal, V.V. Analysis of eco-friendly medicinal herb extracts and essential oil applications on textile products for healthcare applications. Indian J. Tradit. Knowl. 2015, 14, 481–487. [Google Scholar]
- Mathis, R.; Mehling, A. 6—Textiles with cosmetic effects A2. In Handbook of Medical Textiles; Bartels, V.T., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 153–172. [Google Scholar]
- Son, K.; Yoo, D.I.; Shin, Y. Fixation of vitamin E microcapsules on dyed cotton fabrics. Chem. Eng. J. 2014, 239, 284–289. [Google Scholar] [CrossRef]
- Fei, B.; Xin, J.H. N,N-diethyl-m-toluamide–Containing Microcapsules for Bio-Cloth Finishing. Am. J. Trop. Med. Hyg. 2007, 77, 52–57. [Google Scholar] [CrossRef]
- Teixeira, C.S.N.R.; Martins, I.M.D.; Mata, V.L.G.; Filipe Barreiro, M.F.; Rodrigues, A.E. Characterization and evaluation of commercial fragrance microcapsules for textile application. J. Text. Inst. 2012, 103, 269–282. [Google Scholar]
- Sousa, V.I.; Parente, J.F.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Microencapsulation of Essential Oils: A Review. Polymers 2022, 14, 1730. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, L.; Wang, J.; Wei, N. Preparation and Properties Research on Electrostatic Spinning Acetate/Chitosan Composite Fiber. In Advanced Graphic Communications, Packaging Technology and Materials; Ouyang, Y., Xu, M., Yang, L., Ouyang, Y., Eds.; Springer: Singapore, 2016; pp. 801–808. [Google Scholar]
- Phiriyawirut, M.; Phaechamud, T. Gallic Acid-loaded Cellulose Acetate Electrospun Nanofibers: Thermal Properties, Mechanical Properties, and Drug Release Behavior. Open J. Polym. Chem. 2012, 2, 21–29. [Google Scholar] [CrossRef]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur. J. Pharm. Biopharm. 2007, 67, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Champeau, M.; Thomassin, J.; Tassaing, T.; Jerome, C. Drug loading of polymer implants by supercritical CO2 assisted impregnation: A review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef]
- Milovanovic, S.; Hollermann, G.; Errenst, C.; Pajnik, J.; Frerich, S.; Kroll, S.; Rezwan, K.; Ivanovic, J. Supercritical CO2 impregnation of PLA/PCL films with natural substances for bacterial growth control in food packaging. Food Res. Int. 2018, 107, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Fan, Y.; Zhang, C.; Cai, C.; Long, J.; Shi, M. Impregnation of viscose substrate with nicotinamide in supercritical carbon dioxide. Text. Res. J. 2018, 89, 3475–3483. [Google Scholar] [CrossRef]
- Costa, V.P.; Braga, M.E.; Duarte, C.M.; Alvarez-Lorenzo, C.; Concheiro, A.; Gil, M.H.; de Sousa, H.C. Anti-glaucoma drug-loaded contact lenses prepared using supercritical solvent impregnation. J. Supercrit. Fluids 2010, 53, 165–173. [Google Scholar] [CrossRef]
- Zhu, W.; Shi, W.; Shi, M.; Long, J.-J. Solubility and loading ability of benzene derivative drugs onto viscose substrate in supercritical carbon dioxide and their release behavior in solvent. J. Clean. Prod. 2020, 255, 120200. [Google Scholar] [CrossRef]
- Workt, R.W. The Effect of Variations in Degree of Structural Order on Some Physical Properties of Cellulose and Cellulose Acetate Yarns. Text. Res. J. 1949, 19, 381–393. [Google Scholar] [CrossRef]
- Nikitin, L.N.; Gallyamov, M.O.; Vinokur, R.A.; Nikolaec, A.Y.; Said-Galiyev, E.E.; Khokhlov, A.R.; Jespersen, H.T.; Schaumburg, K. Swelling and impregnation of polystyrene using supercritical carbon dioxide. J. Supercrit. Fluids 2003, 26, 263–273. [Google Scholar] [CrossRef]
- Kazarian, S.G. Supercritical fluid impregnation of polymers for drug delivery. In Supercritical Fluid Technology for Drug Product Development; CRC Press: Boca Raton, FL, USA, 2004; pp. 343–364. [Google Scholar]
- Kazarian, S.G.; Brantley, N.H.; West, B.L.; Vincent, M.F.; Eckert, C.A. In situ Spectroscopy of Polymers Subjected to Supercritical CO2: Plasticization and Dye Impregnation. Appl. Spectrosc. 1997, 51, 491–494. [Google Scholar] [CrossRef]
- Chimowitz, E.; Pennisi, K. Process synthesis concepts for supercritical gas extraction in the crossover region. AIChE J. 1986, 32, 1665–1676. [Google Scholar] [CrossRef]
- Marsal, A.; Celma, P.J.; Cot, J.; Cequier, M. Supercritical CO2 extraction as a clean degreasing process in the leather industry. J. Supercrit. Fluids 2000, 16, 217–223. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, J.; Meng, T.; Zhang, J.; He, J.; Li, H.; Zhang, Y. Acetone-soluble cellulose acetates prepared by one-step homogeneous acetylation of cornhusk cellulose in an ionic liquid 1-allyl-3-methylimidazolium chloride (AmimCl). Carbohydr. Polym. 2007, 69, 665–672. [Google Scholar] [CrossRef]
- Ramesh, S.; Shanti, R.; Morris, E. Plasticizing effect of 1-allyl-3-methylimidazolium chloride in cellulose acetate based polymer electrolytes. Carbohydr. Polym. 2012, 87, 2624–2629. [Google Scholar] [CrossRef]


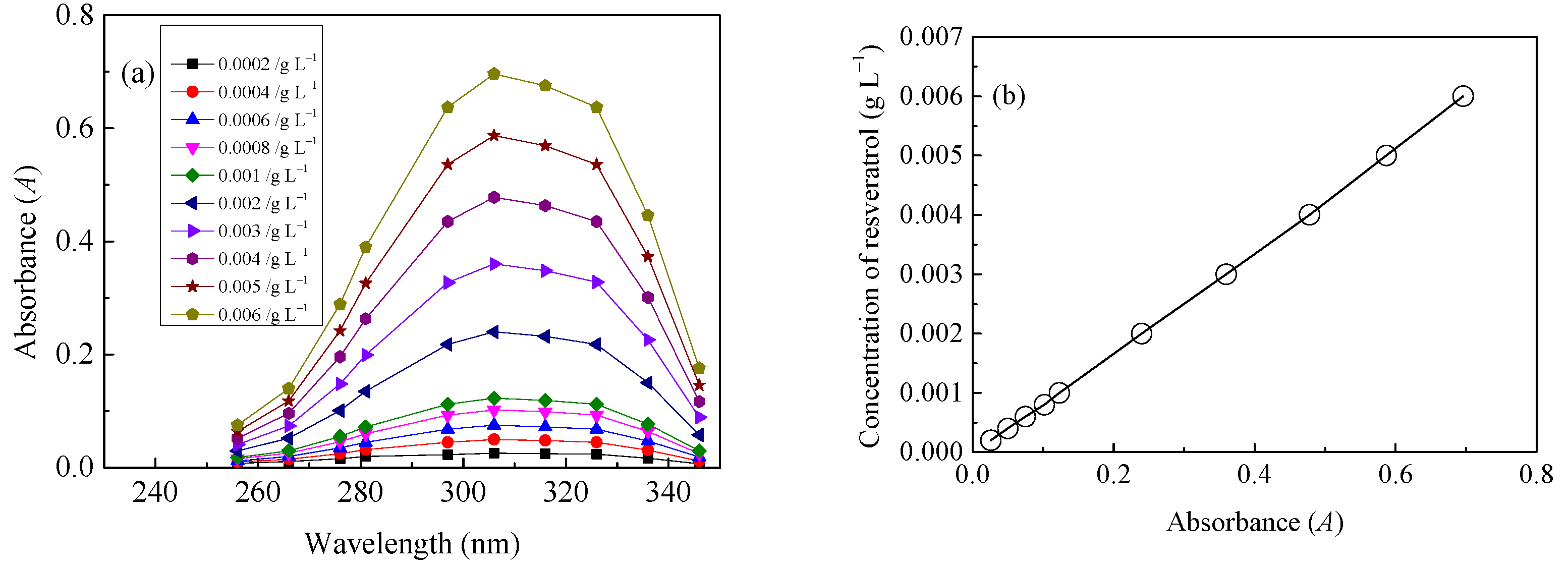
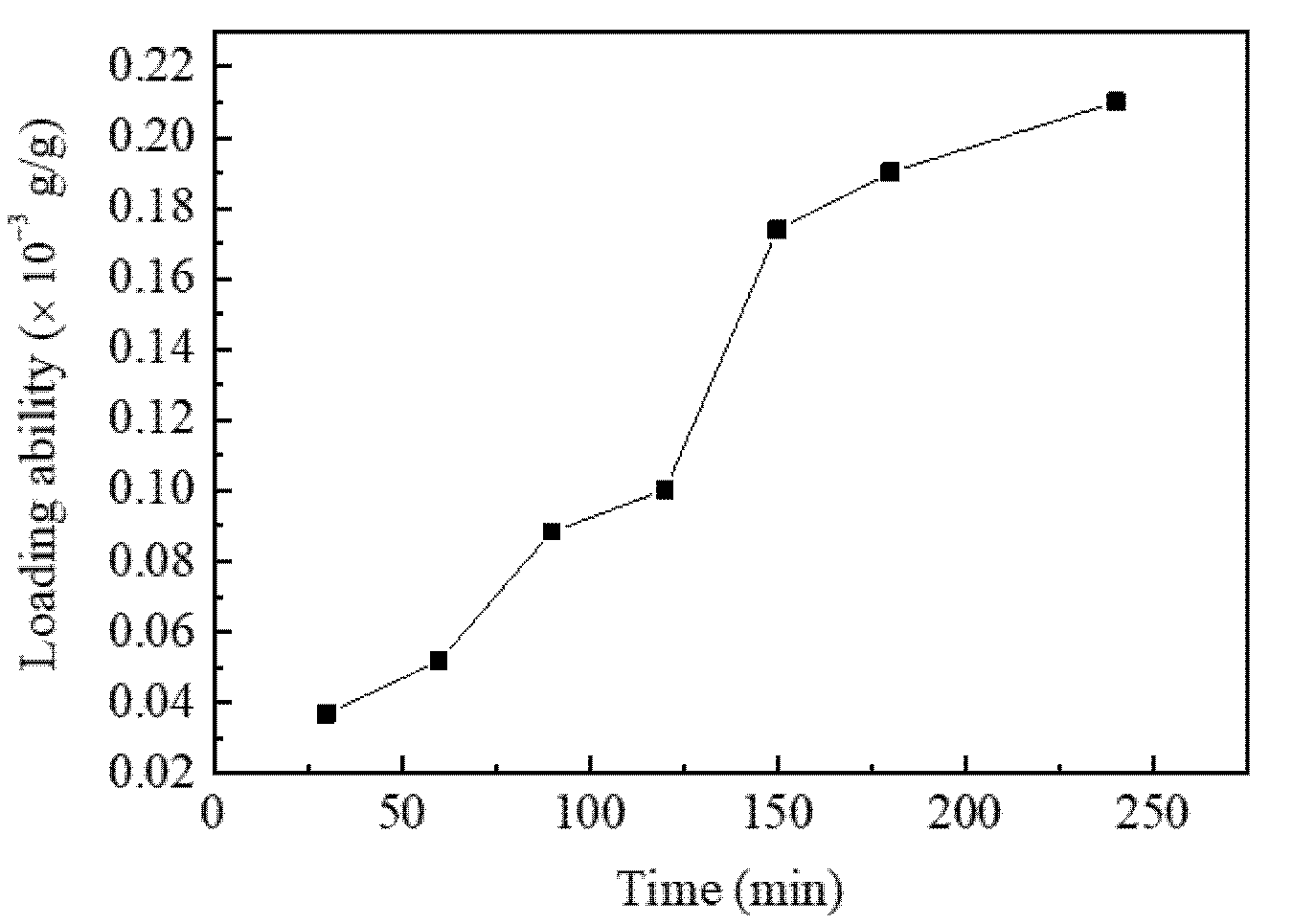
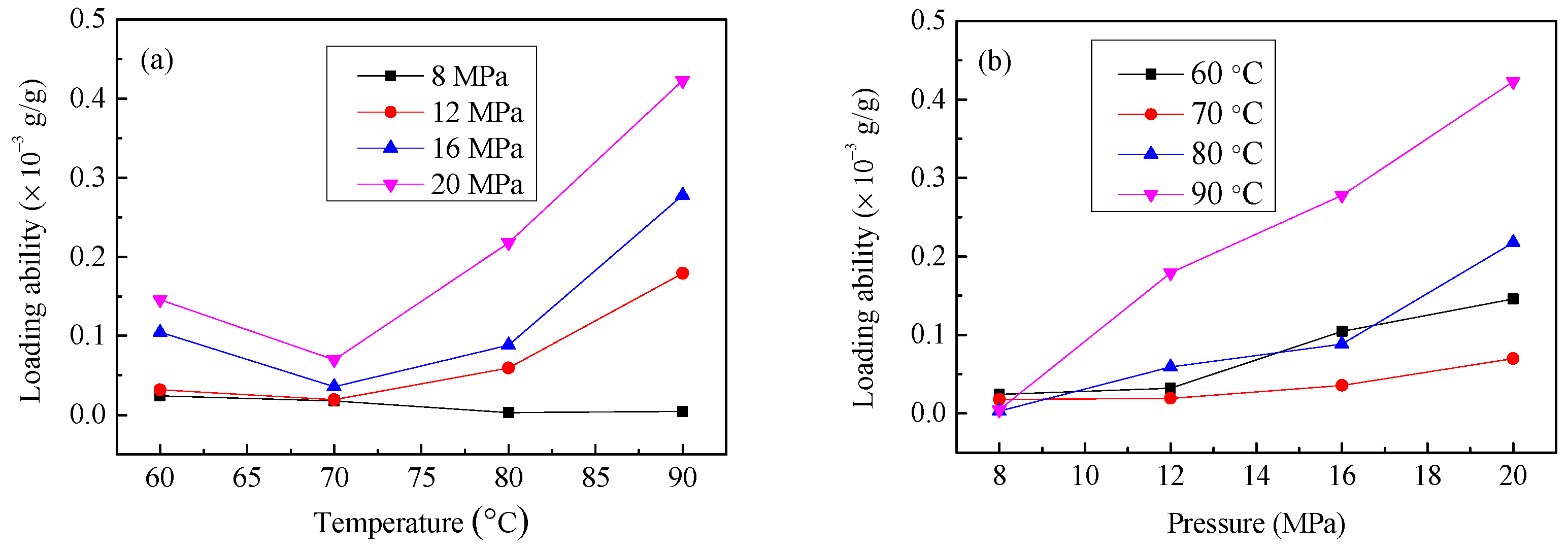
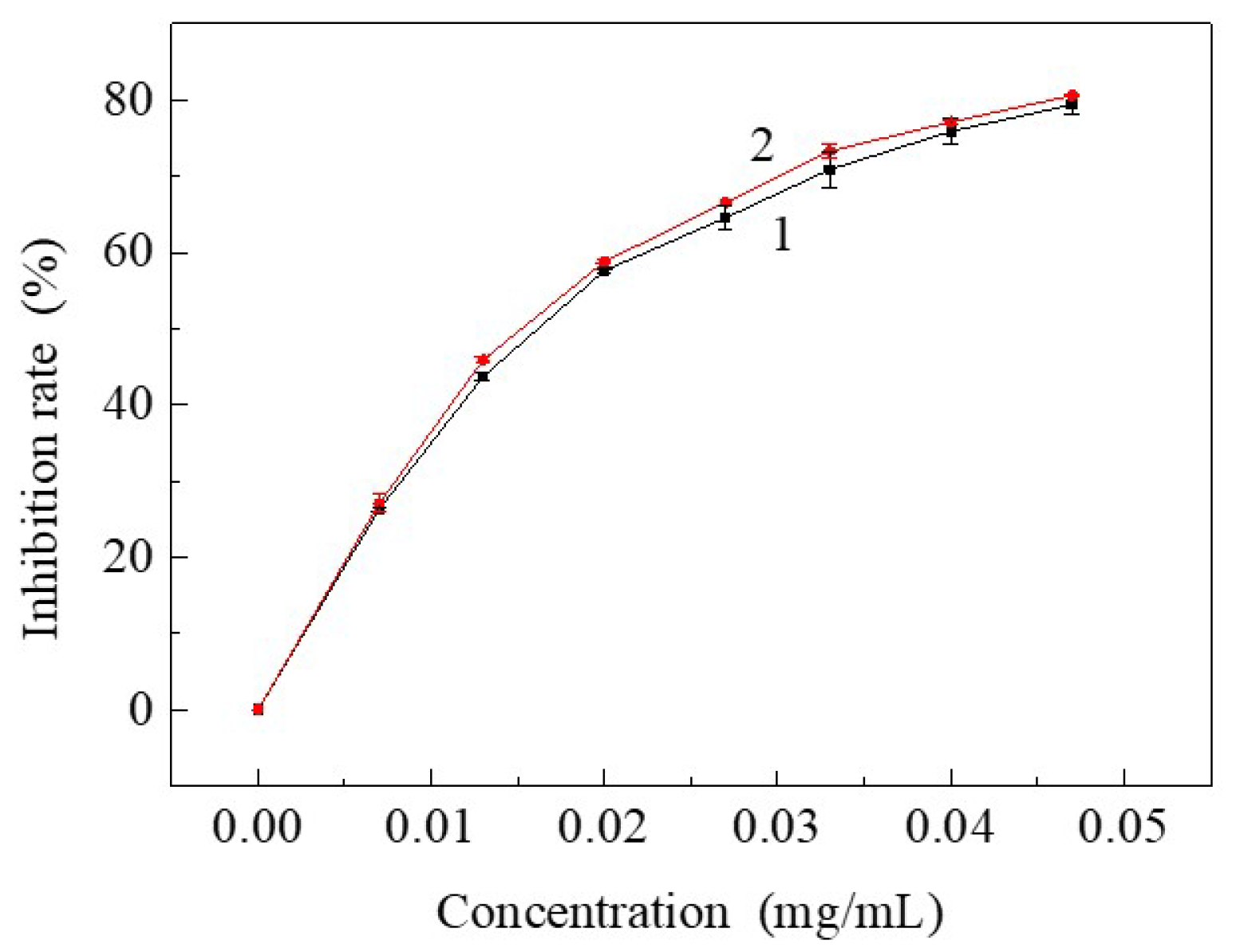



| Sample | Tensile Breaking Strength (cN) | Variation Rate (%) |
|---|---|---|
| (1) | 3.20 | |
| (2) | 2.97 | −7.19 |
| (3) | 2.07 | −35.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Long, J.; Shi, M. Resveratrol-Loaded Diacetate Fiber by Supercritical CO2 Fluid Assisted Impregnation. Materials 2022, 15, 5552. https://doi.org/10.3390/ma15165552
Zhu W, Long J, Shi M. Resveratrol-Loaded Diacetate Fiber by Supercritical CO2 Fluid Assisted Impregnation. Materials. 2022; 15(16):5552. https://doi.org/10.3390/ma15165552
Chicago/Turabian StyleZhu, Weiwei, Jiajie Long, and Meiwu Shi. 2022. "Resveratrol-Loaded Diacetate Fiber by Supercritical CO2 Fluid Assisted Impregnation" Materials 15, no. 16: 5552. https://doi.org/10.3390/ma15165552
APA StyleZhu, W., Long, J., & Shi, M. (2022). Resveratrol-Loaded Diacetate Fiber by Supercritical CO2 Fluid Assisted Impregnation. Materials, 15(16), 5552. https://doi.org/10.3390/ma15165552






