Influence of CeO2 and TiO2 Particles on Physicochemical Properties of Composite Nickel Coatings Electrodeposited at Ambient Temperature
Abstract
:1. Introduction
| Electrolyte Type | Examined Properties | Conditions of Electrodeposition | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Watts | Corrosion | Wear | Hydrophilicity | Photocatalytic | Microroughness | Microhardness, HV | Temperature, °C | Concentration of TiO2, g/L | Particle Size, nm | |
| Watts | √ | √ | √ | 387 | 50 | 12 | 50 | [32] | ||
| 1483 | 30 | 6 | 50 | [43] | ||||||
| √ | 55 | 10–80 | 200–350 | [35] | ||||||
| Watts +surfactant | √ | √ | 640 | 60 ± 5 | 5–15 | >100 | [44] | |||
| √ | √ | 313 | 45 | 1 | 50, 80 | [45] | ||||
| √ | 50 | 45 | 25 | [37] | ||||||
| √ | 50 | 10–25 mL/L | – | [46] | ||||||
| Sulphamate +surfactant | √ | √ | 824 | 50 | 50 | 2–3 μm | [47] | |||
| Sulphamate, pyrophosphate | √ | √ | 620 | 40 | 1–10 | 21 | [33] | |||
| Methansulfonate | √ | 60 | 1–7 | 30 | [48] | |||||
| Tartrate | √ | √ | √ | √ | √ | √ | 22 | 10 | ~11 μm | present contribution |
| Electrolyte Type | Examined Properties | Conditions of Electrodeposition | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Watts | Photocatalytic | Microroughness | Microhardness, HV | Temperature, °C | Concentration of TiO2, g/L | Particle Size, nm | |
| Watts +surfactant | √ | 760 ± 100 | 45 | 10–50 | 15–20 | [38,49] | |
| √ | 560 | 55 | 5–20 | [39] | |||
| √ | 994 | 55 | 1 | 20 | [40] | ||
| 50 | 10 | – | [50] | ||||
| √ | 25 ± 3 | 5 | [51] | ||||
| sulphamate +surfactant | √ | 50 ± 1 | 10–50 | 30 | [52] | ||
| √ | √ | 508 | 100 | 22 | [34] | ||
| √ | 45 | 10–60 | 3 μm | [53] | |||
| Watts | √ | √ | 824 | 50 | 5–20 | 15–25 | [36] |
| acetate+H3BO3 +surfactant | √ | 725 | 30 | 3–12 | – | [41] | |
| Tartrate | √ | √ | √ | 22 | 10 | ~46 μm | present contribution |
2. Materials and Methods
2.1. Materials and Conditions of Deposition
2.2. Material Characterization
3. Results
3.1. Microstructure and Phase Composition of Nickel and Composite Nickel-Based Coatings
3.2. Physicochemical Properties
3.3. Corrosion Properties
3.3.1. Potentiodynamic and EIS Measurements
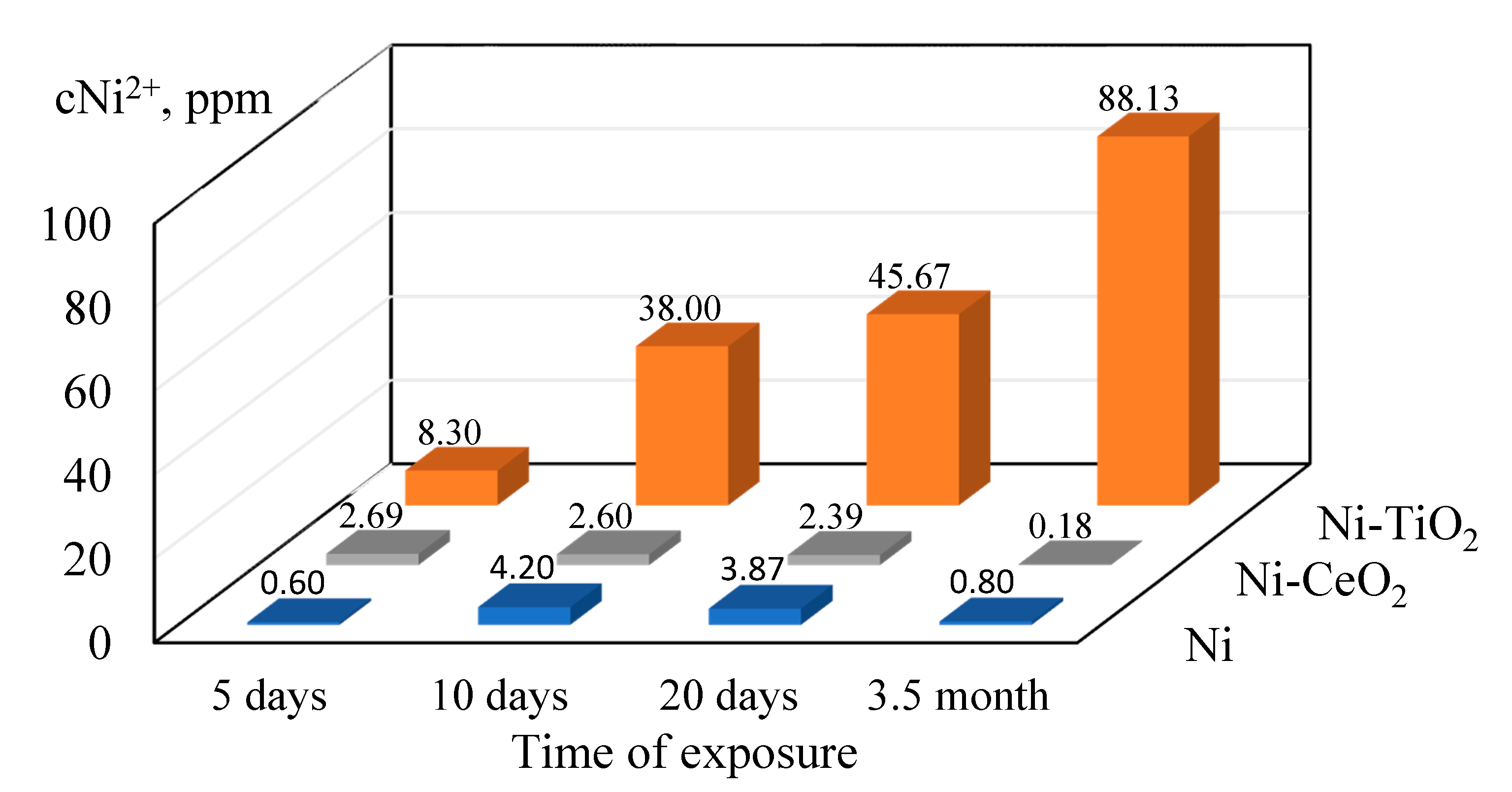
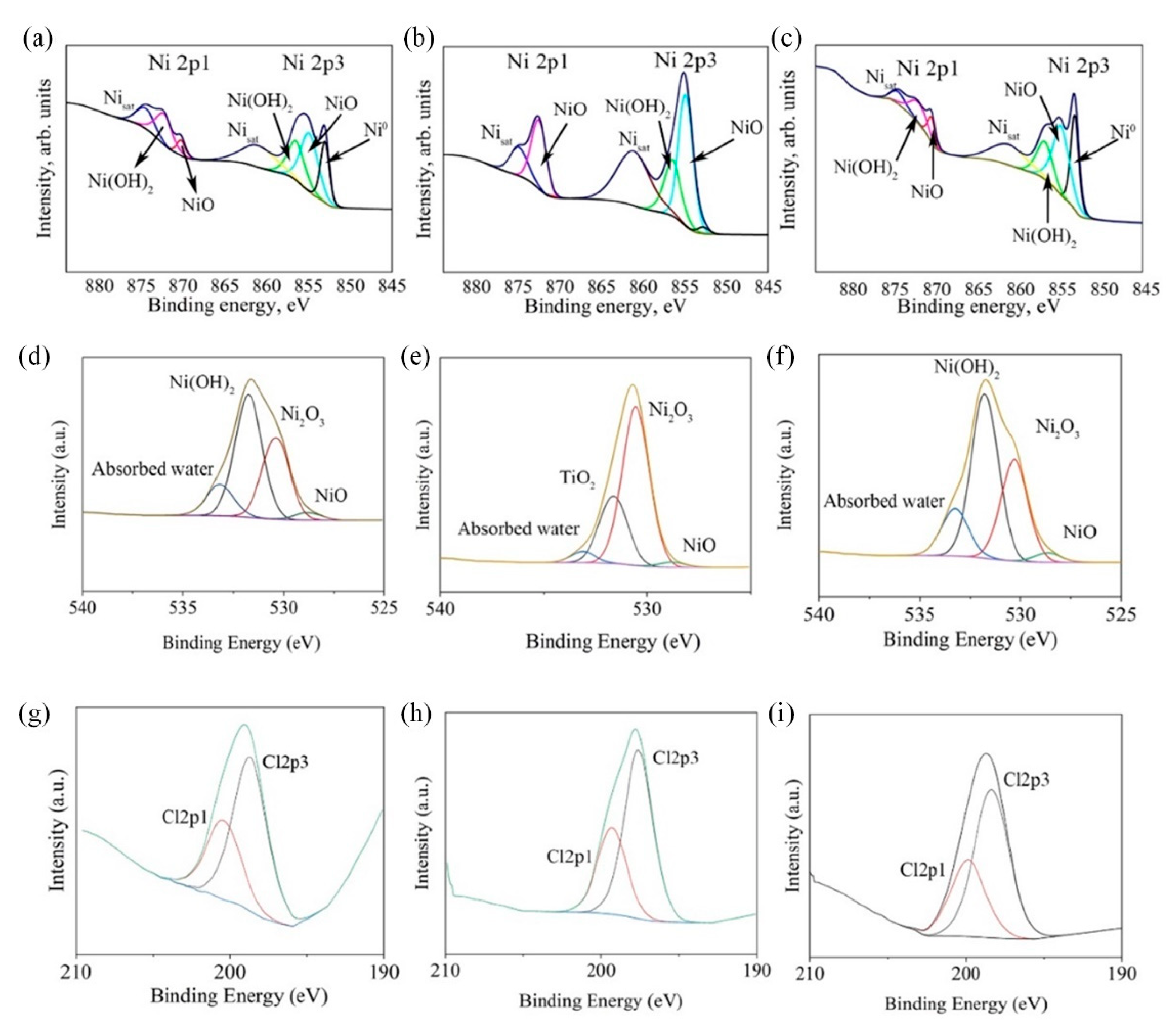
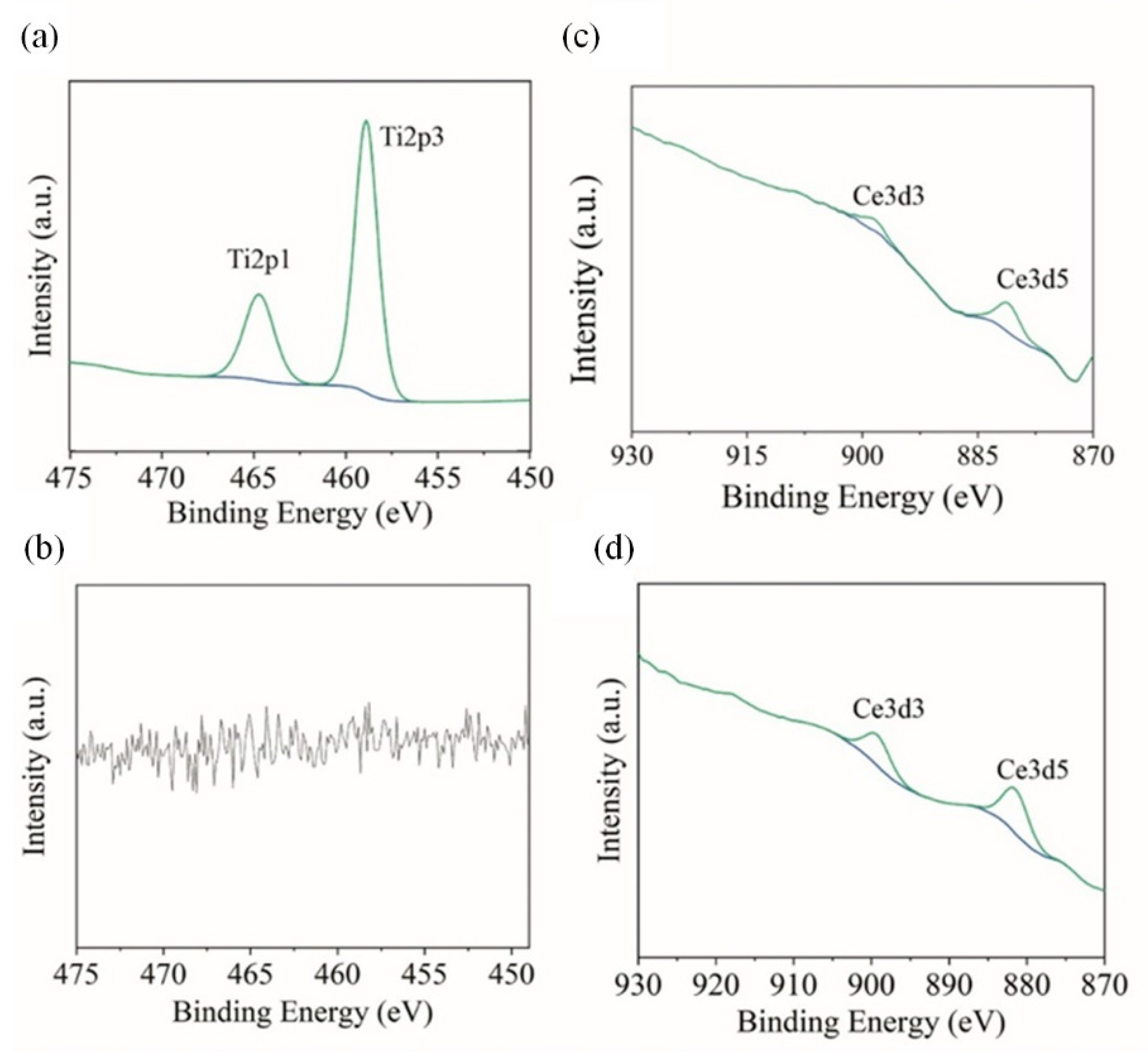
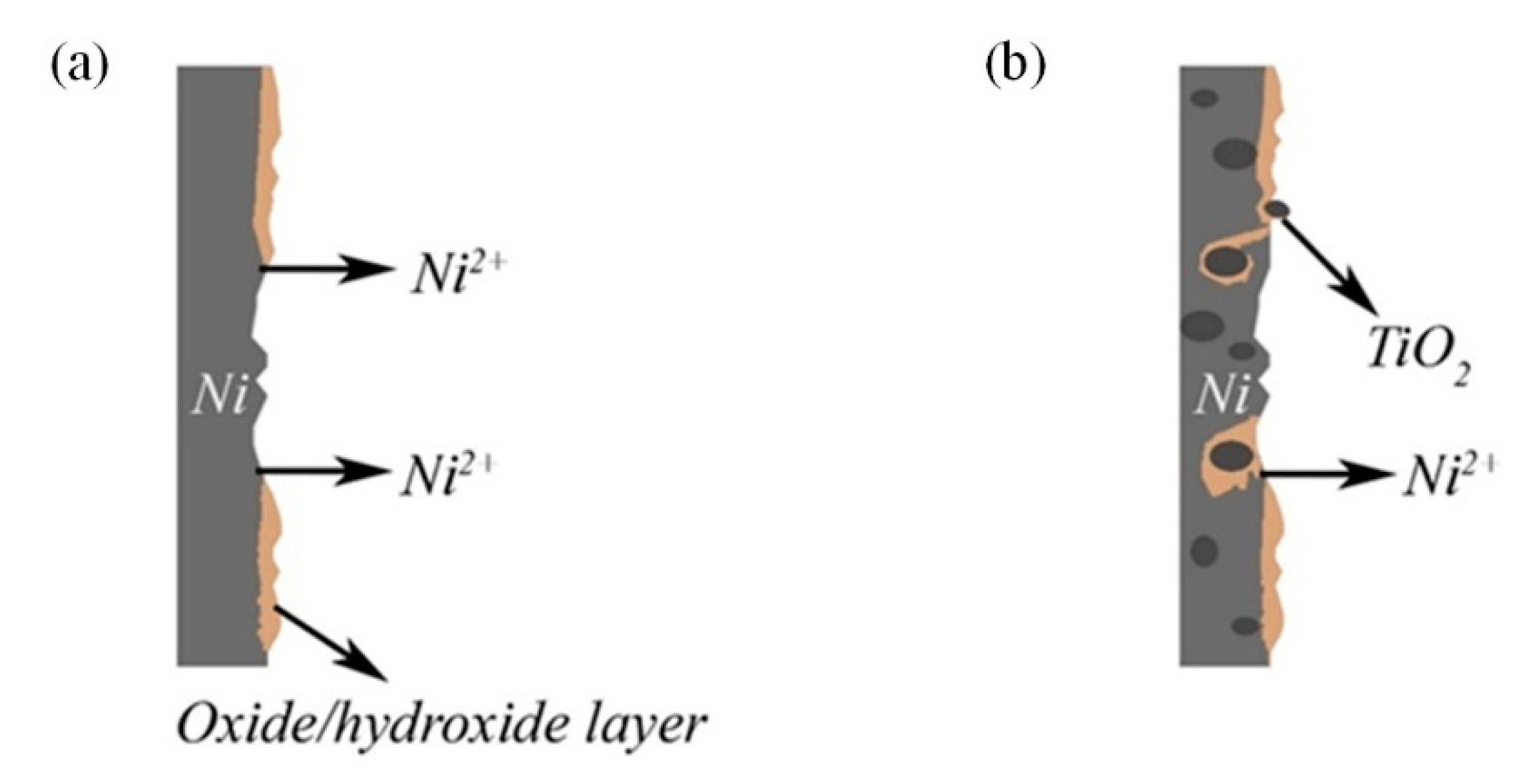
3.3.2. Surface Characterization of Coatings after Corrosion
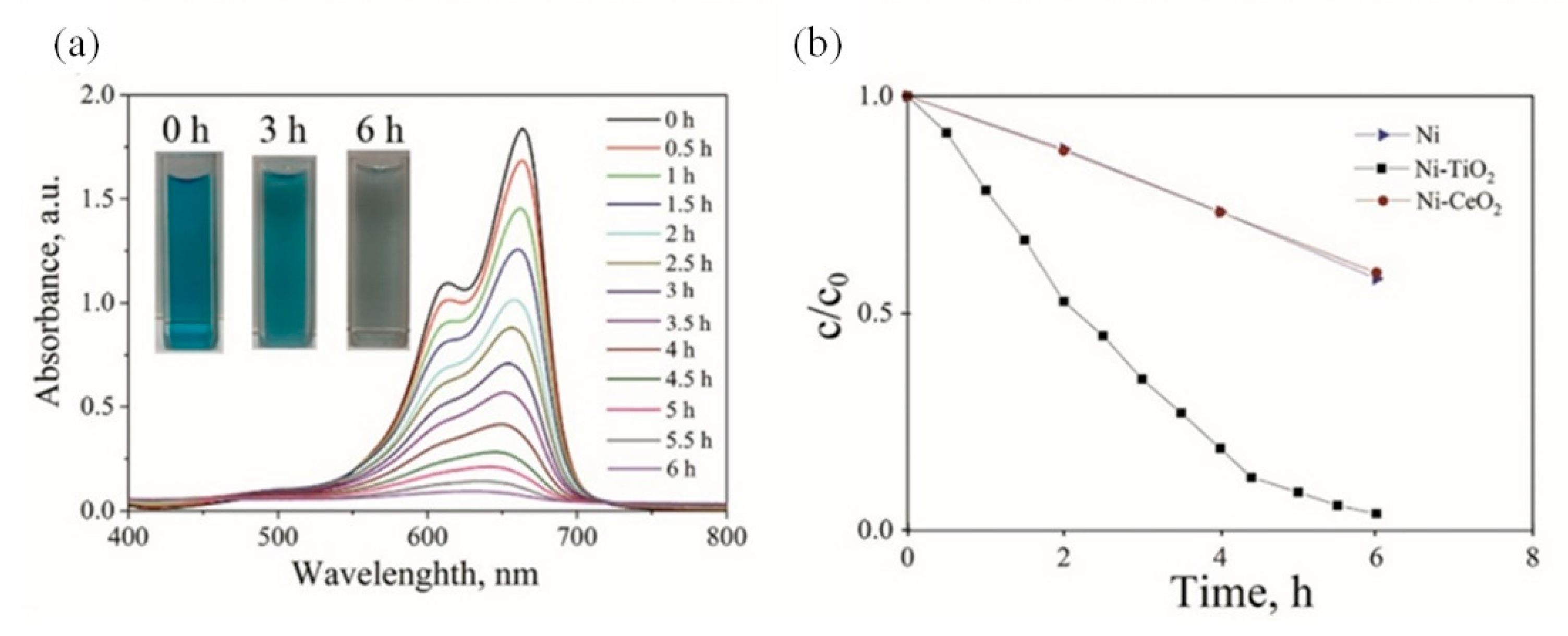
3.4. Photocatalytic Activity
4. Conclusions
- The electrodeposition of pure nickel with a current efficiency of approximately 97% was performed in a simple one-step galvanostatic deposition regime from the solution based on a green buffer agent (Rochelle salt) compared to a traditionally used solution with boric acid;
- The incorporation of CeO2 and TiO2 particles in the Ni matrix increase the wear resistance of the nickel-based composite coatings by 2 times and microhardness by 1.2 and 1.4 times, respectively. The results of this investigation showed that composites possess multifunctional properties that can open new promising exploitation opportunities for these types of materials;
- Corrosion of pure nickel, as well as Ni-CeO2 and Ni-TiO2 composites, were studied by polarization and EIS techniques. Corrosion current density of 0.07 ± 0.072, 0.23 ± 0.095, and 0.29 ± 0.009 μA/cm2 were obtained for Ni, Ni-CeO2, and Ni-TiO2, respectively; thus, the lowest icorr was shown by the pure nickel coating. The presence of the layer of corrosion products in the form of nickel oxides/hydroxides on the surface of coatings was proved by XPS analysis;
- Significant decomposition of methylene blue for Ni-TiO2 composite coating in aqueous solution after 6 h of UV light irradiation demonstrated the outstanding photocatalytic activity of this composite. Moreover, the introduction of the TiO2 particles into the plating solution resulted in an increase in the microroughness of the coating.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Zhang, N.; Fang, F. Fabrication of High-Performance Nickel/Graphene Oxide Composite Coatings Using Ultrasonic-Assisted Electrodeposition. Ultrason. Sonochem. 2020, 62, 104858. [Google Scholar] [CrossRef] [PubMed]
- Bostani, B.; Parvini Ahmadi, N.; Yazdani, S.; Arghavanian, R. Co-Electrodeposition and Properties Evaluation of Functionally Gradient Nickel Coated ZrO2 Composite Coating. Trans. Nonferrous Met. Soc. China 2018, 28, 66–76. [Google Scholar] [CrossRef]
- Xue, Z.; Lei, W.; Wang, Y.; Qian, H.; Li, Q. Effect of Pulse Duty Cycle on Mechanical Properties and Microstructure of Nickel-Graphene Composite Coating Produced by Pulse Electrodeposition under Supercritical Carbon Dioxide. Surf. Coat. Technol. 2017, 325, 417–428. [Google Scholar] [CrossRef]
- Nayana, K.O.; Ranganatha, S.; Shubha, H.N.; Pandurangappa, M. Effect of Sodium Lauryl Sulphate on Microstructure, Corrosion Resistance and Microhardness of Electrodeposition of Ni–Co3O4 Composite Coatings. Trans. Nonferrous Met. Soc. China 2019, 29, 2371–2383. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W. Facile Synthesis and Electrochemical Properties of a Novel Ni-B/TiC Composite Coating via Ultrasonic-Assisted Electrodeposition. Ultrason. Sonochem. 2020, 61, 104837. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, L.; Sun, Y.; Liu, H.; Jiang, C.; Ji, V.; Li, W. Influences of Al and Ti Particles on Microstructure, Internal Stress and Property of Ni Composite Coatings. J. Alloys Compd. 2019, 793, 314–325. [Google Scholar] [CrossRef]
- Yılmaz, E.; Çakıroğlu, B.; Gökçe, A.; Findik, F.; Gulsoy, H.O.; Gulsoy, N.; Mutlu, Ö.; Özacar, M. Novel Hydroxyapatite/Graphene Oxide/Collagen Bioactive Composite Coating on Ti16Nb Alloys by Electrodeposition. Mater. Sci. Eng. C 2019, 101, 292–305. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wang, S.; Zhou, N. The Electrodeposition of Composite Coatings: Diversity, Applications and Challenges. Curr. Opin. Electrochem. 2020, 20, 8–19. [Google Scholar] [CrossRef]
- Lelevic, A.; Walsh, F.C. Electrodeposition of Ni-P Composite Coatings: A Review. Surf. Coat. Technol. 2019, 378, 124803. [Google Scholar] [CrossRef]
- Vaezi, M.R.; Sadrnezhaad, S.K.; Nikzad, L. Electrodeposition of Ni-SiC Nano-Composite Coatings and Evaluation of Wear and Corrosion Resistance and Electroplating Characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 176–182. [Google Scholar] [CrossRef]
- Spanou, S.; Kontos, A.I.; Siokou, A.; Kontos, A.G.; Vaenas, N.; Falaras, P.; Pavlatou, E.A. Self Cleaning Behaviour of Ni/Nano-TiO2 Metal Matrix Composites. Electrochim. Acta 2013, 105, 324–332. [Google Scholar] [CrossRef]
- Faraldos, M.; Bahamonde, A. Multifunctional Photocatalytic Coatings for Construction Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081026410. [Google Scholar]
- Xi, Y.Y.; Huang, B.Q.; Djurišić, A.B.; Chan, C.M.N.; Leung, F.C.C.; Chan, W.K.; Au, D.T.W. Electrodeposition for Antibacterial Nickel-Oxide-Based Coatings. Thin Solid Films 2009, 517, 6527–6530. [Google Scholar] [CrossRef]
- Wu, W.; Huang, J.; Näther, J.; Omar, N.A.B.; Köster, F.; Lampke, T.; Liu, Y.; Pan, H.; Zhang, Y. Texture Orientation, Morphology and Performance of Nanocrystalline Nickel Coatings Electrodeposited from a Watts-Type Bath: Effects of H3BO3 Concentration and Plating Time. Surf. Coat. Technol. 2021, 424, 127648. [Google Scholar] [CrossRef]
- Tsuru, Y.; Nomura, M.; Foulkes, F.R. Effects of Boric Acid on Hydrogen Evolution and Internal Stress in Films Deposited from a Nickel Sulfamate Bath. J. Appl. Electrochem. 2002, 32, 629–634. [Google Scholar] [CrossRef]
- Available online: https://Echa.Europa.Eu/Substance-Information/-/Substanceinfo/100.014.129 (accessed on 9 August 2022).
- Xu, J.; Gao, X.; Chen, G.; Zou, L.; Gao, C. High Performance Boron Removal from Seawater by Two-Pass SWRO System with Different Membranes. Water Sci. Technol. Water Supply 2010, 10, 327–336. [Google Scholar] [CrossRef]
- Brdar-Jokanovi, M. Boron Toxicity and Deficiency in Agricultural Plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef] [PubMed]
- Saito, B.Y.F.; Kishihoto, K.; Nobira, Y.; Kobayakawa, K.; Sato, Y. Nickel Electroplating Bath Using Malic Acid as a Substitute Agent for Boric Acid. Met. Finish. 2007, 105, 34–38. [Google Scholar] [CrossRef]
- Antikhovich, I.V.; Kharitonov, D.S.; Chernik, A.A.; Dobryden’, I.B. Corrosion Resistance of Nickel Coatings Deposited from Low-Temperature Nickel-Plating Electrolytes. Russ. J. Appl. Chem. 2017, 90, 566–573. [Google Scholar] [CrossRef]
- Makarova, I.V.; Kharitonov, D.S.; Dobryden’, I.B.; Chernik, A.A. Corrosion Behavior in Acid and Alkaline Media of Nickel Coatings Deposited at Room Temperature. Russ. J. Appl. Chem. 2018, 91, 1441–1450. [Google Scholar] [CrossRef]
- Makarova, I.; Dobryden, I.; Kharitonov, D.; Kasach, A.; Ryl, J.; Repo, E.; Vuorinen, E. Nickel-Nanodiamond Coatings Electrodeposited from Tartrate Electrolyte at Ambient Temperature. Surf. Coat. Technol. 2019, 380, 125063. [Google Scholar] [CrossRef]
- Bogomazova, N.V.; Antikhovich, I.V.; Chernik, A.A.; Zharskii, I.M. Specific Features of Electrodeposition of Ni-SiO2 Micromounting Composite Coatings from Complex Electrolytes. Russ. J. Appl. Chem. 2014, 87, 1235–1239. [Google Scholar] [CrossRef]
- Mockute, D.; Bernotiene, G.; Vilkaite, R. Reaction Mechanism of Some Benzene Sulfonamide and Saccharin Derivatives during Nickel Electrodeposition in Watts-Type Electrolyte. Surf. Coat. Technol. 2002, 160, 152–157. [Google Scholar] [CrossRef]
- Antikhovich, I.V.; Chernik, A.A.; Zharskii, I.M.; Bolvako, A.K. Electrodeposition of a Nickel Coating from a Low-Temperature Acetate-Chloride Nickel-Plating Electrolyte. Russ. J. Electrochem. 2015, 51, 281–285. [Google Scholar] [CrossRef]
- Antihovich, I.V.; Ablazhey, N.M.; Chernik, A.A.; Zharsky, I.M. Electrodeposition of Nickel and Composite Nickel-Fullerenol Coatings from Low-Temperature Sulphate-Chloride-Isobutyrate Electrolyte. Procedia Chem. 2014, 10, 373–377. [Google Scholar] [CrossRef]
- Lu, A.; Jin, T.; Guo, Z.; Qu, M.; Chang, Y.; Liu, Q.; Zhang, C. Characterization of the Tool Influence Function in a Dual-Axis Wheel Polishing Process to Achieve High Material Removal Rates. Precis. Eng. 2018, 52, 276–290. [Google Scholar] [CrossRef]
- Tang, H.; Yang, W.; Liu, W.; Ma, J.; Luo, X. Characteristic of Fixed Abrasive Polishing for Fused Silica in Anhydrous Environment. Optik 2020, 202, 163623. [Google Scholar] [CrossRef]
- Pyanko, A.V.; Makarova, I.V.; Kharitonov, D.S.; Makeeva, I.S.; Sergievich, D.S.; Chernik, A.A. Physicochemical and Biocidal Properties of Nickel–Tin and Nickel–Tin—Titania Coatings. Prot. Met. Phys. Chem. Surf. 2021, 57, 88–95. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Kasach, A.A.; Sergievich, D.S.; Wrzesinska, A.; Bobowska, I.; Darowicki, K.; Zielinski, A.; Ryl, J.; Kurilo, I.I. Ultrasonics Sonochemistry Ultrasonic-Assisted Electrodeposition of Cu-Sn-TiO2 Nanocomposite Coatings with Enhanced Antibacterial Activity. Ultrason. Sonochem. 2021, 75, 105593. [Google Scholar] [CrossRef] [PubMed]
- Azizi, H.; Pourafshari, M.; Haghighi, A.; Esmaeili, M.; Pihlajamäki, A.; Kallioinen, M.; Mänttäri, M. Ultra-Desulfurization of Sulfur Recovery Unit Wastewater Using Thin Film Nanocomposite Membrane. Sep. Purif. Technol. 2019, 221, 211–225. [Google Scholar] [CrossRef]
- Shao, W.; Nabb, D.; Renevier, N.; Sherrington, I.; Fu, Y.; Luo, J. Mechanical and Anti-Corrosion Properties of TiO2 Nanoparticle Reinforced Ni Coating by Electrodeposition. J. Electrochem. Soc. 2012, 159, D671–D676. [Google Scholar] [CrossRef]
- Thiemig, D.; Bund, A. Characterization of Electrodeposited Ni-TiO2 Nanocomposite Coatings. Surf. Coat. Technol. 2008, 202, 2976–2984. [Google Scholar] [CrossRef]
- Aruna, S.T.; Bindu, C.N.; Ezhil Selvi, V.; William Grips, V.K.; Rajam, K.S. Synthesis and Properties of Electrodeposited Ni/Ceria Nanocomposite Coatings. Surf. Coat. Technol. 2006, 200, 6871–6880. [Google Scholar] [CrossRef]
- Stanković, V.; Gojo, M.; Grekulovic, V.; Pajkić, N.; Cigula, T. Surface Quality of the Ni-TiO2 Composite Coatings Produced by Electroplating Produced by Electroplating. J. Min. Met. Sect. B-Metall 2017, 53, 341–348. [Google Scholar] [CrossRef]
- Shanmugasamy, S.; Balakrishnan, K.; Subasri, A.; Ramalingam, S.; Subramania, A. Development of CeO2 Nanorods Reinforced Electrodeposited Nickel Nanocomposite Coating and Its Tribological and Corrosion Resistance Properties. J. Rare Earths 2018, 36, 1319–1325. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, S.; Huang, S.; Pan, W. Fabrication of Superhydrophobic Surfaces of Titanium Dioxide and Nickel through Electrochemical Deposition on Stainless Steel Substrate. Key Eng. Mater. 2010, 434–435, 496–498. [Google Scholar] [CrossRef]
- Sen, R.; Das, S.; Das, K. Synthesis and Properties of Pulse Electrodeposited Ni-CeO2 Nanocomposite. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 3809–3823. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, Y.; Hu, B.; Chen, L.; Tay, S.L.; Xu, A.; Gao, W. Microstructure and Properties of Electrodeposited Ni-CeO2 Coatings. Int. J. Electrochem. Sci. 2016, 11, 906–914. [Google Scholar]
- Jin, H.; Wang, Y.Y.; Wang, Y.T.; Yang, H.B. Synthesis and Properties of Electrodeposited Ni–CeO2 Nano-Composite Coatings. Rare Met. 2018, 37, 148–153. [Google Scholar] [CrossRef]
- Kasturibai, S.; Kalaignan, G.P. Characterizations of Electrodeposited Ni-CeO2 Nanocomposite Coatings. Mater. Chem. Phys. 2014, 147, 1042–1048. [Google Scholar] [CrossRef]
- Xue, Y.J.; Li, J.S.; Ma, W.; De Duan, M.; Lan, M.M. Fabrication and Wear Resistance of Ni-CeO2 Nanocomposite Coatings by Electrodeposition under Ultrasound Condition. Int. J. Surf. Sci. Eng. 2010, 4, 202–213. [Google Scholar] [CrossRef]
- Bhogal, S.S.; Kumar, V.; Dhami, S.S.; Pabla, B.S. Preparation and Properties of Electrodeposited Ni-TiO2 Composite Coating. J. Electrochem. Sci. Eng. 2015, 5, 37–45. [Google Scholar] [CrossRef]
- Parida, G.; Chaira, D.; Chopkar, M.; Basu, A. Synthesis and Characterization of Ni-TiO2 Composite Coatings By. Surf. Coat. Technol. 2011, 205, 4871–4879. [Google Scholar] [CrossRef]
- Birlik, I.; Ak Azem, N.F.; Toparli, M.; Celik, E.; Koc Delice, T.; Yildirim, S.; Bardakcioglu, O.; Dikici, T. Preparation and Characterization of Ni-TiO2 Nanocomposite Coatings Produced by Electrodeposition Technique. Front. Mater. 2016, 3, 46. [Google Scholar] [CrossRef]
- Mohajery, B.; Dolati, A. An Investigation on the Electrodeposition Mechanism of Ni-TiO2 Nanocomposite Coatings. J. Ultrafine Grained Nanostructured Mater. 2016, 49, 51–63. [Google Scholar] [CrossRef]
- Lin, C.S.; Lee, C.Y.; Chang, C.F.; Chang, C.H. Annealing Behavior of Electrodeposited Ni-TiO2 Composite Coatings. Surf. Coatings Technol. 2006, 200, 3690–3697. [Google Scholar] [CrossRef]
- Sknar, Y.E.; Sknar, I.V.; Savchuk, A.A.; Bezik, A.A.; Danilov, F.I. Danilov Photocatalytic Activity of Ni-TiO2 Composites Electrodeposited on a Nickel Matrix with Developed Surface. Vopr. Khimii I Khimicheskoi Tekhnologi 2017, 6, 50–56. [Google Scholar]
- Sen, R.; Das, S.; Das, K. Effect of Stirring Rate on the Microstructure and Microhardness of Ni-CeO2 Nanocomposite Coating and Investigation of the Corrosion Property. Surf. Coat. Technol. 2011, 205, 3847–3855. [Google Scholar] [CrossRef]
- Li, Y.; Geng, S.; Chen, G. Electrodeposited Ni/CeO2 Mulriple Coating on SUS 430 Steel Interconnect. Int. J. Hydrogen Energy 2018, 43, 12811–12816. [Google Scholar] [CrossRef]
- Phala, M.F.; Popoola, A.P.I.; Fayomi, O.S.I. Corrosion and Properties of Developed Ni-CeO2 Co-Deposited on Mild Steel at Varying Current Densities. Procedia Manuf. 2017, 7, 543–548. [Google Scholar] [CrossRef]
- Ningsong, Q. Synthesis of Ni-CeO2 Nanocomposite Coatings by Electroforming. Appl. Mech. Mater. 2013, 248, 48–53. [Google Scholar] [CrossRef]
- Xue, Y.-J.; Jia, X.-Z.; Zhou, Y.-W.; Ma, W.; Li, J.-S. Tribological Performance of Ni–CeO2 Composite Coatings by Electrodeposition. Surf. Coat. Technol. 2006, 200, 5677–5681. [Google Scholar] [CrossRef]
- Hou, C.; Hu, B.; Zhu, J. Photocatalytic Degradation of Methylene Blue over TiO2 Pretreated with Varying Concentrations of NaOH. Catalysts 2018, 8, 575. [Google Scholar] [CrossRef]
- Zuo, R.; Du, G.; Zhang, W.; Liu, L.; Liu, Y.; Mei, L.; Li, Z. Photocatalytic Degradation of Methylene Blue Using TiO2 Impregnated Diatomite. Adv. Mater. Sci. Eng. 2014, 2014, 170148. [Google Scholar] [CrossRef]
- Burduhos-Nergis, D.P.; Vizureanu, P.; Sandu, A.V.; Bejinariu, C. Phosphate Surface Treatment for Improving the Corrosion Resistance of the C45 Carbon Steel Used in Carabiners Manufacturing. Materials 2020, 13, 3410. [Google Scholar] [CrossRef]
- Hou, F.; Wang, W.; Guo, H. Effect of the Dispersibility of ZrO2 Nanoparticles in Ni-ZrO2 Electroplated Nanocomposite Coatings on the Mechanical Properties of Nanocomposite Coatings. Appl. Surf. Sci. 2006, 252, 3812–3817. [Google Scholar] [CrossRef]
- Aruna, S.T.; Muniprakash, M.; William Grips, V.K. Effect of Titania Particles Preparation on the Properties of Ni–TiO2 Electrodeposited Composite Coatings. J. Appl. Electrochem. 2013, 43, 805–815. [Google Scholar] [CrossRef]
- Guan, B.; Yu, J.; Guo, S.; Yu, S.; Han, S. Porous Nickel Doped Titanium Dioxide Nanoparticles with Improved Visible Light Photocatalytic Activity. Nanoscale Adv. 2020, 2, 1352–1357. [Google Scholar] [CrossRef]
- Azimi, G.; Dhiman, R.; Kwon, H.-M.; Paxson, A.T.; Varanasi, K.K. Hydrophobicity of Rare-Earth Oxide Ceramics. Nat. Mater. 2013, 12, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wang, S. Fabrication of Co-Ni Based Superhydrophobic Coating and Its Wear Resistance, Durability and Corrosion Resistance. In Surfaces, Interfaces and Coatings Technology; Bosia, A., Ed.; Vide Leaf: Hyderabad, India, 2021; pp. 1–26. [Google Scholar]
- Xue, Y.; Wang, S.; Xue, Y.; Cao, L.; Nie, M.; Jin, Y. Robust Self-Cleaning and Marine Anticorrosion Super-Hydrophobic Co-Ni/CeO2 Composite Coating. Adv. Eng. Mater. 2020, 22, 2000402. [Google Scholar] [CrossRef]
- Moharana, M.; Mallik, A. Nickel Electrocrystallization in Different Electrolytes: An in-Process and Post Synthesis Analysis. Electrochim. Acta 2013, 98, 1–10. [Google Scholar] [CrossRef]
- Kasach, A.A.; Kharitonov, D.S.; Wrzesińska, A.; Bobowska, I.; Predko, A.A.; Romanovskii, V.I.; Zharskii, I.M.; Kurilo, I.I. The Effect of Ultrasound Treatment on Physicochemical and Tribological Properties of Electrolytic Cu–Sn–TiO2 Coatings. Prot. Met. Phys. Chem. Surf. 2020, 56, 385–391. [Google Scholar] [CrossRef]
- Mattli, M.R.; Matli, P.R.; Khan, A.; Abdelatty, R.H.; Yusuf, M.; Al Ashraf, A.; Kotalo, R.G.; Shakoor, R.A. Study of Microstructural and Mechanical Properties of Al/SiC/TiO2 Hybrid Nanocomposites Developed by Microwave Sintering. Crystals 2021, 11, 1078. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Latha, S.M.; Ramakrishna, M.; Rao, D.S.; Sundararajan, G. Pulsed Electrodeposition and Mechanical Properties of Ni-W/SiC Nano-Composite Coatings. Mater. Des. 2016, 112, 140–150. [Google Scholar] [CrossRef]
- Badarulzaman, N.A.; Mohamad, A.A.; Puwadaria, S.; Ahmad, Z.A. The Evaluation of Nickel Deposit Obtained via Watts Electrolyte at Ambient Temperature. J. Coat. Technol. Res. 2010, 7, 815–820. [Google Scholar] [CrossRef]
- Shozib, I.A.; Ahmad, A.; Abdul-Rani, A.M.; Beheshti, M.; Aliyu, A.A. A Review on the Corrosion Resistance of Electroless Ni-P Based Composite Coatings and Electrochemical Corrosion Testing Methods. Corros. Rev. 2022, 40, 1–37. [Google Scholar] [CrossRef]
- Kasach, A.A.; Kharytonau, D.S.; Paspelau, A.V.; Ryl, J.; Sergievich, D.S.; Zharskii, I.M.; Kurilo, I.I. Effect of TiO2 Concentration on Microstructure and Properties of Composite Cu–Sn–TiO2 Coatings Obtained by Electrodeposition. Materials 2021, 14, 6179. [Google Scholar] [CrossRef]
- Montemor, M.F.; Ferreira, M.G.S. Cerium Salt Activated Nanoparticles as Fillers for Silane Films: Evaluation of the Corrosion Inhibition Performance on Galvanised Steel Substrates. Electrochim. Acta 2007, 52, 6976–6987. [Google Scholar] [CrossRef]
- Sharma, A.; Das, S.; Das, K. Electrochemical Corrosion Behavior of CeO2 Nanoparticle Reinforced Sn-Ag Based Lead Free Nanocomposite Solders in 3.5wt.% NaCl Bath. Surf. Coat. Technol. 2015, 261, 235–243. [Google Scholar] [CrossRef]
- Cruz, M.J.; Makarova, I.V.; Kharitonov, D.S.; Ushak, S.; Chernik, A.A.; Grageda, M. Corrosion Properties of Nickel Coatings Obtained from Aqueous and Nonaqueous Electrolytes. Surf. Interface Anal. 2019, 51, 943–953. [Google Scholar] [CrossRef]
- Huang, C.C.; Li, H.M.; Li, D.H.; Lin, S.Y. The Performance of Titanium Composite Coatings Obtained through Thermal Spraying and Microarc Oxidation. Compos. Adv. Mater. 2021, 30, 1–11. [Google Scholar] [CrossRef]
- Mansour, A.N.; Mansour, A.N. Characterization of ΒNi(OH)2 by XPS. Surf. Sci. Spectra 1994, 3, 239. [Google Scholar] [CrossRef]
- Diebold, U.; Madey, T.E. TiO2 by XPS. Surf. Sci. Spectra 1996, 4, 227–231. [Google Scholar] [CrossRef]
- Deng, S.-H.; Lu, H.; Li, D. Effect of UV Light Illumination on the Corrosion Behavior of Electrodeposited TiO2 -Ni Composite Foils. Appl. Surf. Sci. 2018, 462, 291–302. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Cui, K.; Jia, A.; Hu, G.; Jiao, Z. Role of Surface Ni and Ce Species of Ni / CeO2 Catalyst in CO2 Methanation. Appl. Surf. Sci. 2016, 383, 248–252. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, F. Influence of Grain Size on the Corrosion Behavior of a Ni-Based Superalloy Nanocrystalline Coating in NaCl Acidic Solution. Electrochim. Acta 2008, 53, 2453–2462. [Google Scholar] [CrossRef]
- Pineau, N.; Minot, C.; Maurice, V.; Marcus, P. Density Functional Theory Study of the Interaction of Cl− with Passivated Nickel Surfaces. Electrochem. Solid-State Lett. 2003, 6, B47. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of Oxygen Vacancies and Ti3+ State in TiO2 Thin Film and Enhanced Optical Properties by Air Plasma Treatment. Sci. Rep. 2016, 6, srep32355. [Google Scholar] [CrossRef]
- Beche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce3d XPS Investigation of Cerium Oxides and Mixed Cerium Oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; St, R.; Smart, C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Sharma, R.; Raghav, S.; Nair, M.; Kumar, D. Kinetics and Adsorption Studies of Mercury and Lead by Ceria Nanoparticles Entrapped in Tamarind Powder. ACS Omega 2018, 3, 14606–14619. [Google Scholar] [CrossRef]
- Dvoranová, D.; Brezová, V.; Mazúr, M.; Malati, M.A. Investigations of Metal-Doped Titanium Dioxide Photocatalysts. Appl. Catal. B Environ. 2002, 37, 91–105. [Google Scholar] [CrossRef]
- Fujishima, A.; Tryk, A.; RaoDonald, T.N. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 112666. [Google Scholar] [CrossRef]
- Ahmed, M.A. Synthesis and Structural Features of Mesoporous NiO/TiO2 Nanocomposites Prepared by Sol-Gel Method for Photodegradation of Methylene Blue Dye. J. Photochem. Photobiol. A Chem. 2012, 238, 63–70. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Tabrizi, N.S. Kinetics of Heterogeneous Photocatalytic Degradation of Reactive Dyes in an Immobilized TiO2 Photocatalytic Reactor. J. Colloid Interface Sci. 2006, 295, 159–164. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters Affecting the Photocatalytic Degradation of Dyes Using TiO2-Based Photocatalysts: A Review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Wang, J.; Wu, N.; Sheng, H.; Chen, C.; Zhao, J. An unprecedent hydride transfer pathway for selective photocatalytic reduction of CO2 to formic acid on TiO2. Appl. Catal. B Environ. 2021, 284, 119692. [Google Scholar] [CrossRef]
- Arifin, K.; Yunus, R.M.; Minggu, L.J.; Kassim, M.B. Improvement of TiO2 nanotubes for photoelectrochemical water splitting: Review. Int. J. Hydrogen Energy 2021, 46, 4998–5024. [Google Scholar] [CrossRef]
- Elghniji, K.; Ksibi, M.; Elaloui, E. Sol-gel reverse micelle preparation and characterization of n-doped Tio2: Efficient photocatalytic degradation of methylene blue in water under visible light. J. Ind. Eng. Chem. 2012, 18, 178–182. [Google Scholar] [CrossRef]
- Zimmerman, A.J.; Gutierrez, D.G.; Campos, V.M.; Weindorf, D.C.; Deb, S.K.; Chacón, S.U.; Landrot, G.; Flores, N.G.G.; Siebecker, M.G. Arsenic speciation in titanium dioxide (TiO2) waste produced via drinking water filtration: Potential environmental implications for soils, sediments, and human health. Environ. Adv. 2021, 3, 100036. [Google Scholar] [CrossRef]
- Anwar, D.I.; Mulyadi, D. Synthesis of Fe-TiO2 Composite as a photocatalyst for degradation of methylene blue. Procedia Chem. 2015, 17, 49–54. [Google Scholar] [CrossRef]
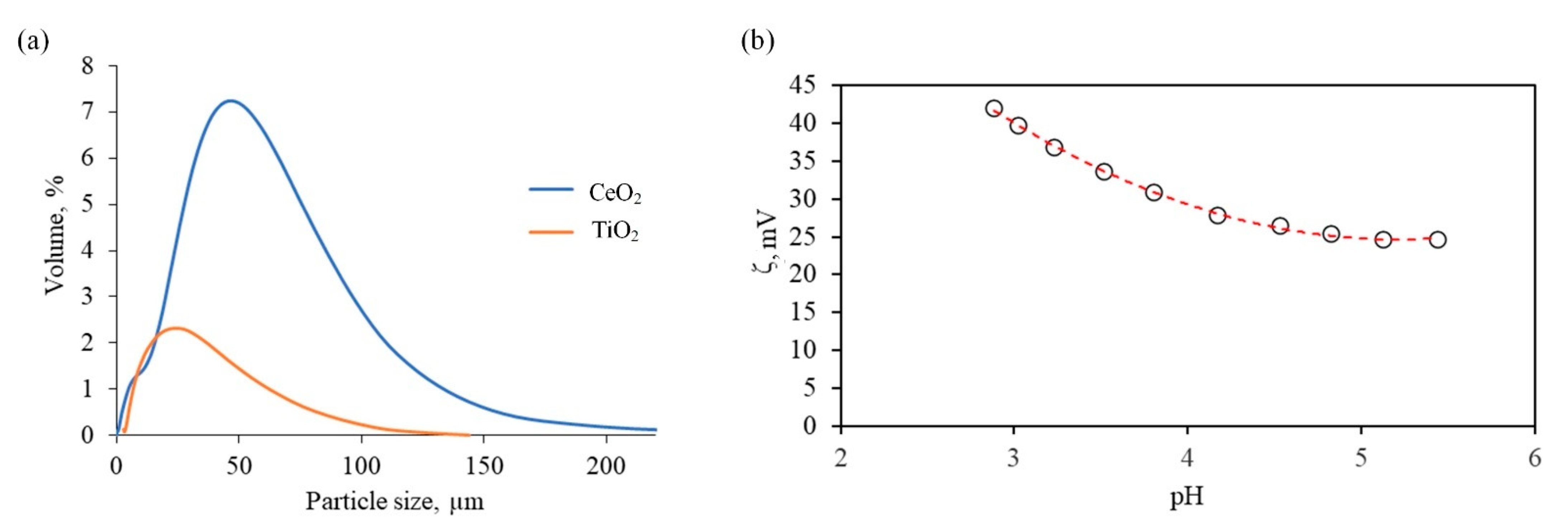
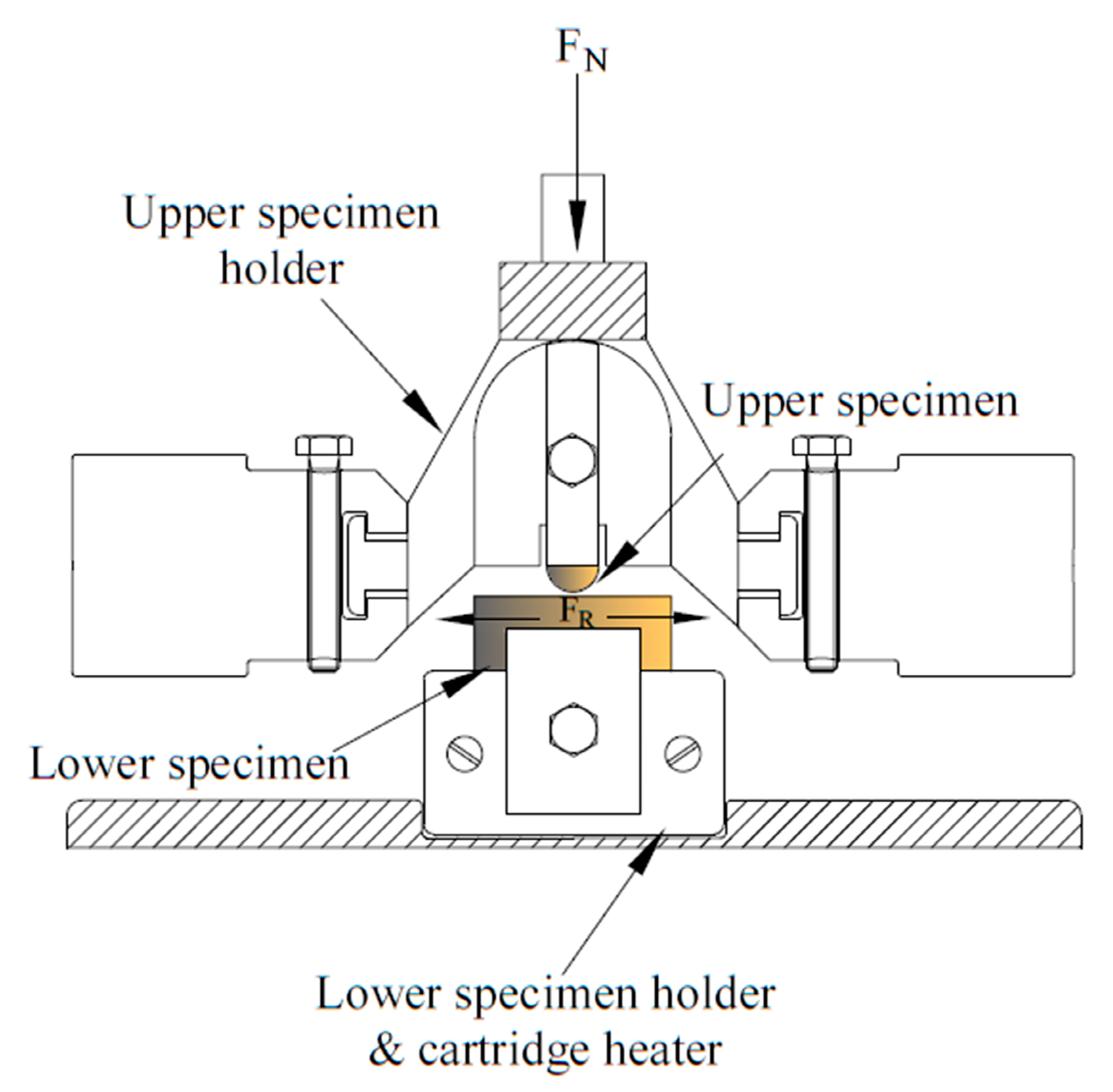
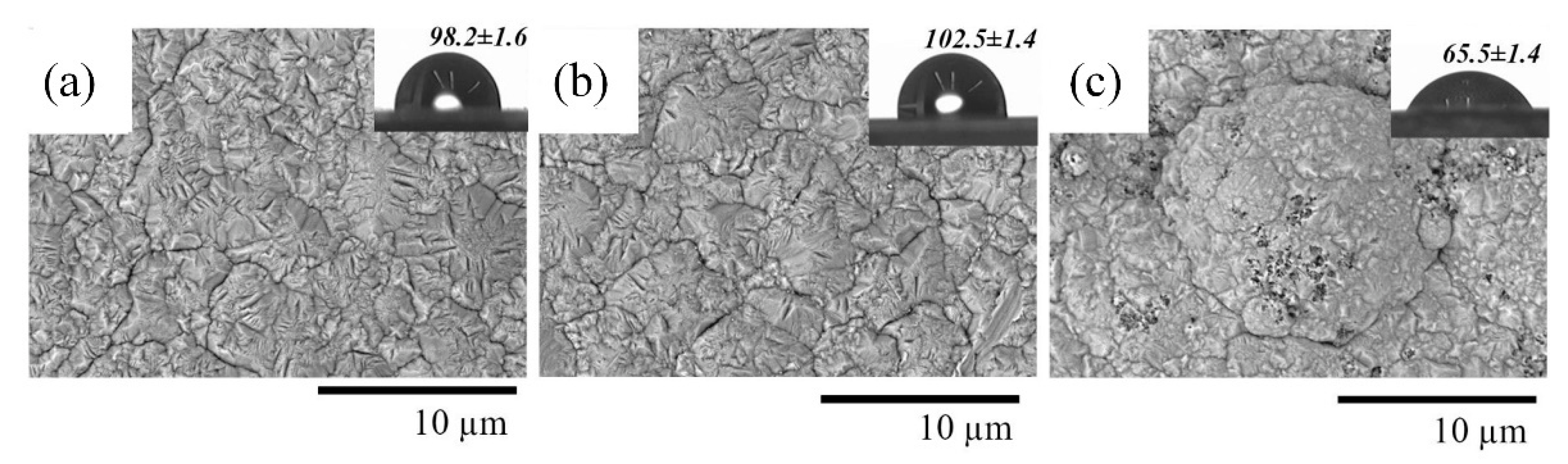
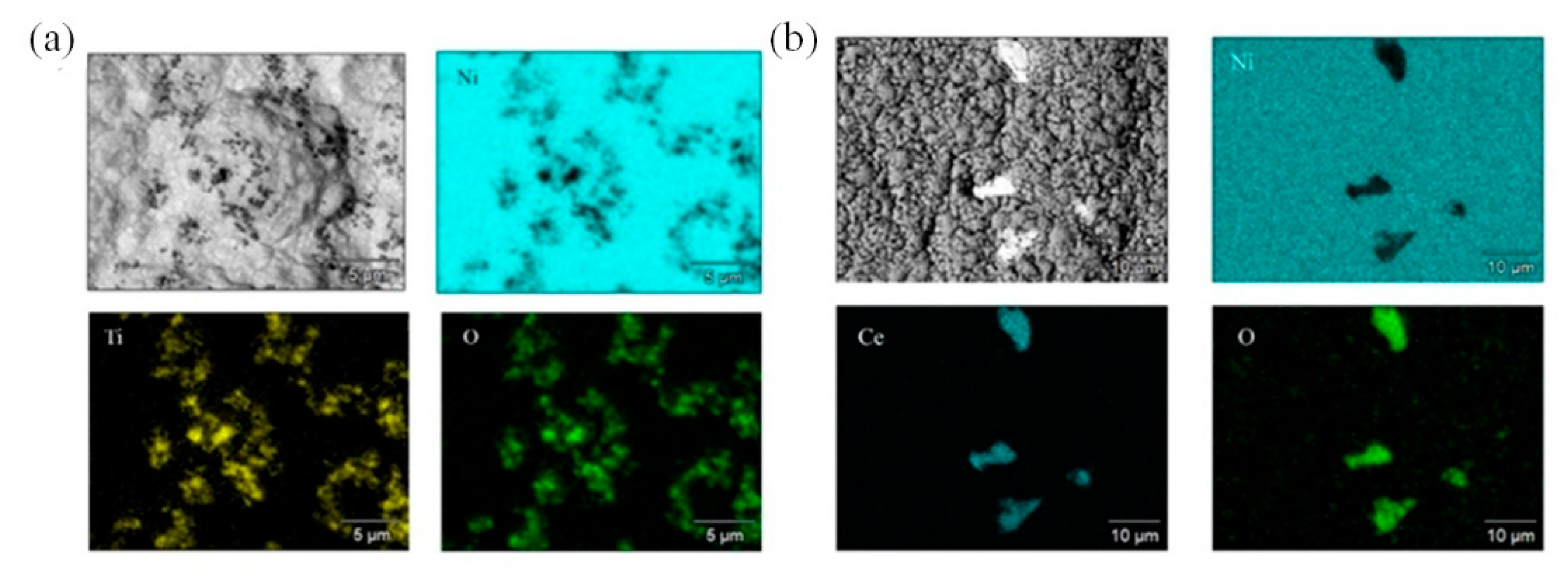
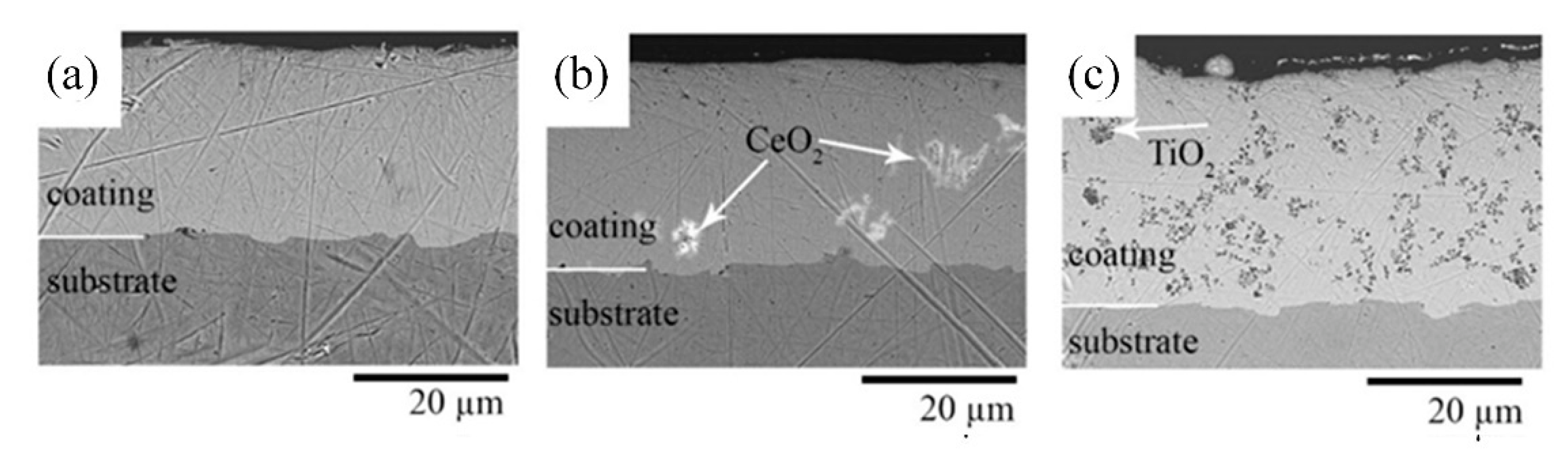
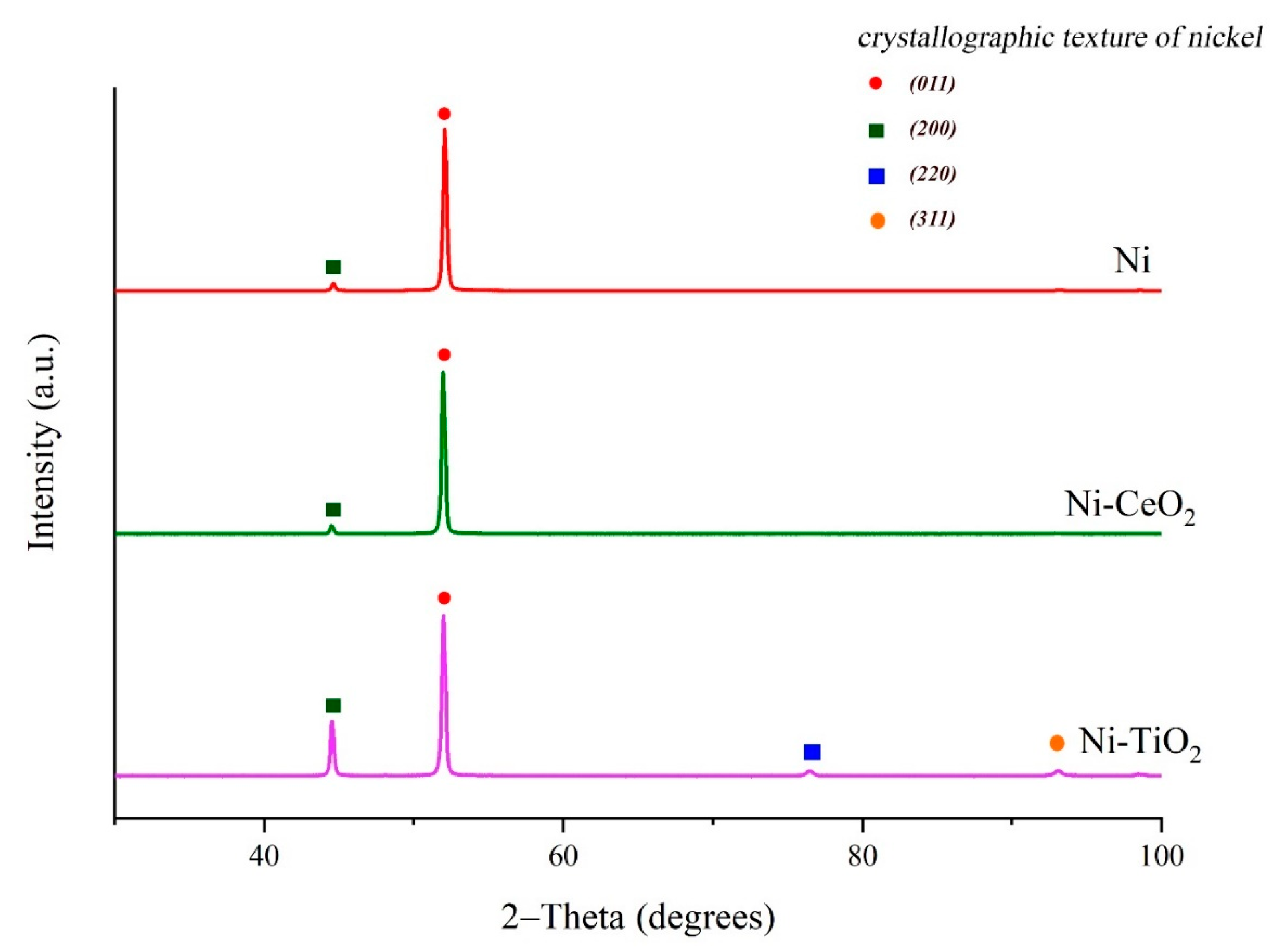
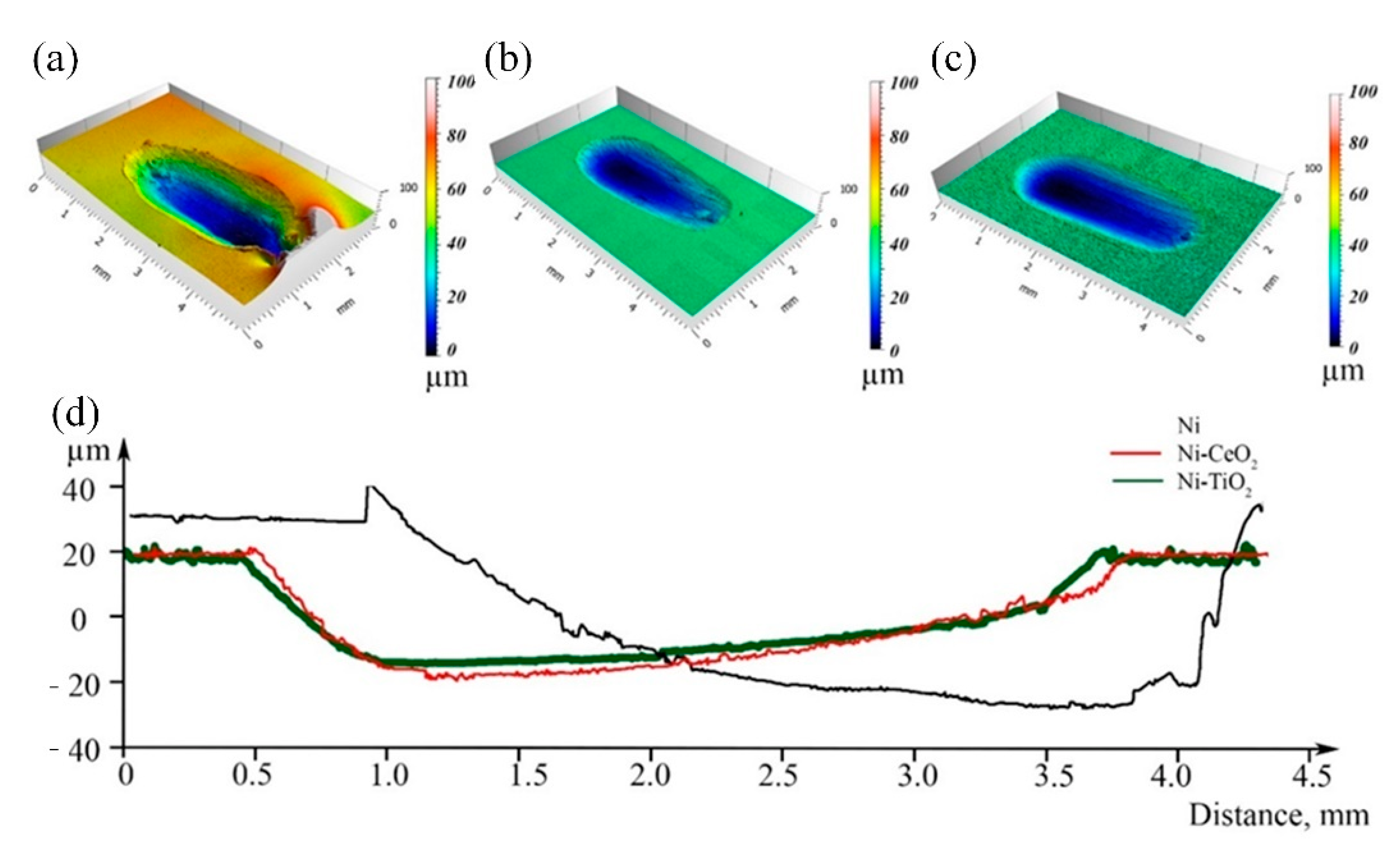
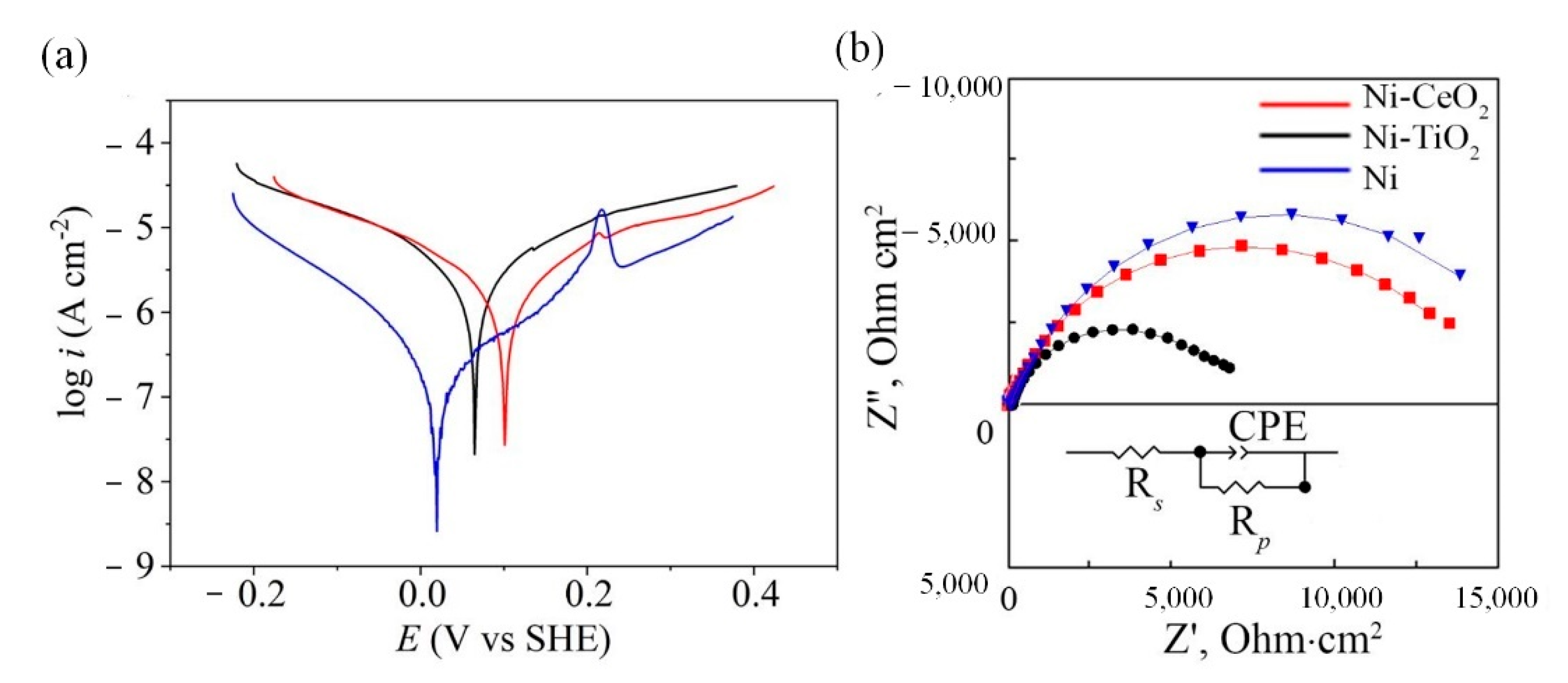

| Chemical/Parameter | Value | Purity | Suppliers |
|---|---|---|---|
| NiSO4·7H2O | 0.8 mol/L | 99.0% | Sigma-Aldrich |
| NiCl2·6H2O | 0.2 mol/L | 99.9% | Sigma-Aldrich |
| KNaC4H4O6·4H2O. | 0.2 mol/L | 99.0% | Sigma-Aldrich |
| CeO2 | 10 g/L | ≥99.0% | Sigma-Aldrich |
| TiO2 | 10 g/L | ≥99.5% | Sigma-Aldrich |
| Stirring Rate | 400 rpm | ||
| Current Density | 200 A/m2 | ||
| pH | 3 ± 0.1 | ||
| Temperature | 22 ± 1 °C |
| Element, wt.% | Ni | Ni-CeO2 | Ni-TiO2 |
|---|---|---|---|
| Ni | 100 | 98.9 | 96.7 |
| O | − | 0.7 | 1.2 |
| Ce | − | 0.4 | − |
| Ti | − | − | 2.1 |
| Type of Coating | Ni | Ni-CeO2 | Ni-TiO2 | Ni (Watts Electrolyte) |
|---|---|---|---|---|
| Microhardness, HV | 191 ± 10 | 230 ± 15 | 270 ± 5 | 270 ± 12 [14] |
| Microroughness Sa, μm | 0.26 ± 0.09 | 0.26 ± 0.02 | 0.86 ± 0.03 | 0.41 [63] |
| Specific wear rate × 1014, m3/m | 4.12 | 2.58 | 2.01 | 50–80 [14] |
| Coating | |bc|, V/dec | ba V/dec | icorr, μA/cm2 | vcorr, mm/year | Ecorr, V |
|---|---|---|---|---|---|
| Ni | 0.0229 | 0.0354 | 0.07 ± 0.072 | 0.00075 | 0.019 ± 0.003 |
| Ni-CeO2 | 0.0216 | 0.0326 | 0.23 ± 0.095 | 0.00248 | 0.101 ± 0.023 |
| Ni-TiO2 | 0.0258 | 0.0234 | 0.29 ± 0.009 | 0.00313 | 0.066 ± 0.015 |
| Type of Coating | |||
|---|---|---|---|
| Ni | Ni-TiO2 | Ni-CeO2 | |
| Rs, Ohm∙cm2 | 65.32 | 51.62 | 54.50 |
| Y1, sn/ (Ohm∙cm2) | 1.19 × 10−5 | 1.50 × 10−4 | 1.03 × 10−5 |
| n1 | 0.79 | 0.74 | 0.74 |
| Rp, k Ohm∙cm2 | 16.00 | 6.90 | 14.41 |
| Time of Immersion | Type of Coating | ||
|---|---|---|---|
| Ni | Ni-TiO2 | Ni-CeO2 | |
| 5 days | 6.6 | 6.6 | 6.5 |
| 10 days | 6.9 | 7.1 | 7.2 |
| 20 days | 7.2 | 7.4 | 7.2 |
| 3.5 month | 7.6 | 7.5 | 8.1 |
| Coating | Composition (at.%) | ||||
|---|---|---|---|---|---|
| Ni0 | NiOx | Ni(OH)2 | TiO2 | CeO2 | |
| Ni | 24.0 | 45.2 | 30.8 | − | − |
| Ni-TiO2 | 2.4 | 64.3 | 33.3 | 0 | − |
| Ni-CeO2 | 29.8 | 47.0 | 23.1 | − | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarava, I.; Esmaeili, M.; Kharytonau, D.S.; Pelcastre, L.; Ryl, J.; Bilesan, M.R.; Vuorinen, E.; Repo, E. Influence of CeO2 and TiO2 Particles on Physicochemical Properties of Composite Nickel Coatings Electrodeposited at Ambient Temperature. Materials 2022, 15, 5550. https://doi.org/10.3390/ma15165550
Makarava I, Esmaeili M, Kharytonau DS, Pelcastre L, Ryl J, Bilesan MR, Vuorinen E, Repo E. Influence of CeO2 and TiO2 Particles on Physicochemical Properties of Composite Nickel Coatings Electrodeposited at Ambient Temperature. Materials. 2022; 15(16):5550. https://doi.org/10.3390/ma15165550
Chicago/Turabian StyleMakarava, Iryna, Mohammadamin Esmaeili, Dzmitry S. Kharytonau, Leonardo Pelcastre, Jacek Ryl, Mohammad Reza Bilesan, Esa Vuorinen, and Eveliina Repo. 2022. "Influence of CeO2 and TiO2 Particles on Physicochemical Properties of Composite Nickel Coatings Electrodeposited at Ambient Temperature" Materials 15, no. 16: 5550. https://doi.org/10.3390/ma15165550
APA StyleMakarava, I., Esmaeili, M., Kharytonau, D. S., Pelcastre, L., Ryl, J., Bilesan, M. R., Vuorinen, E., & Repo, E. (2022). Influence of CeO2 and TiO2 Particles on Physicochemical Properties of Composite Nickel Coatings Electrodeposited at Ambient Temperature. Materials, 15(16), 5550. https://doi.org/10.3390/ma15165550











