Inerting Waste Al Alloy Dust with Natural High Polymers: Sustainability of Industrial Waste
Abstract
:1. Introduction
2. Materials and Methods
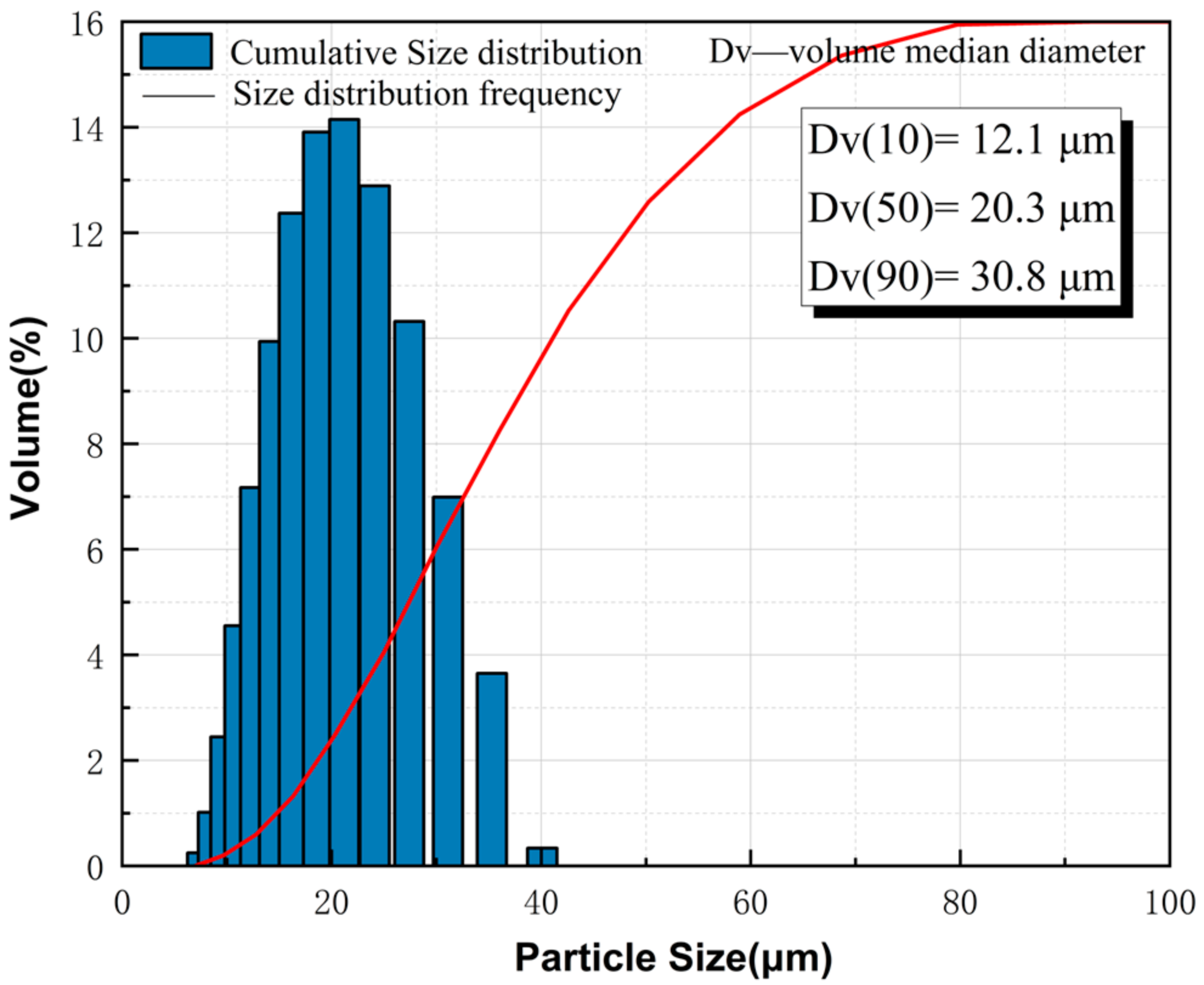
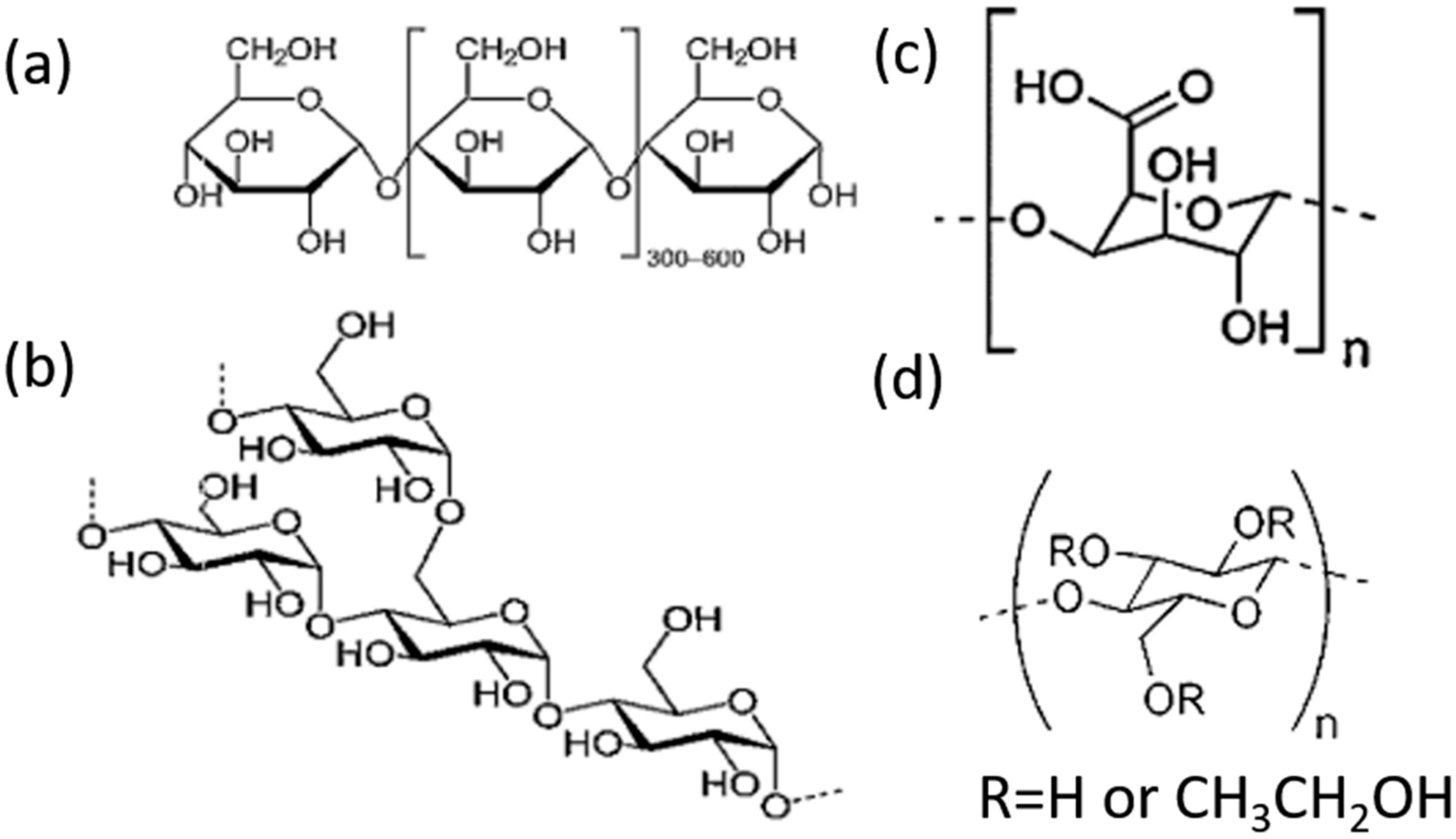
2.1. Materials
2.2. Inerting Efficiency Measurement
2.3. Characterization Test
2.4. Industrial Verification
3. Results and Discussion
3.1. Inerting Efficiency
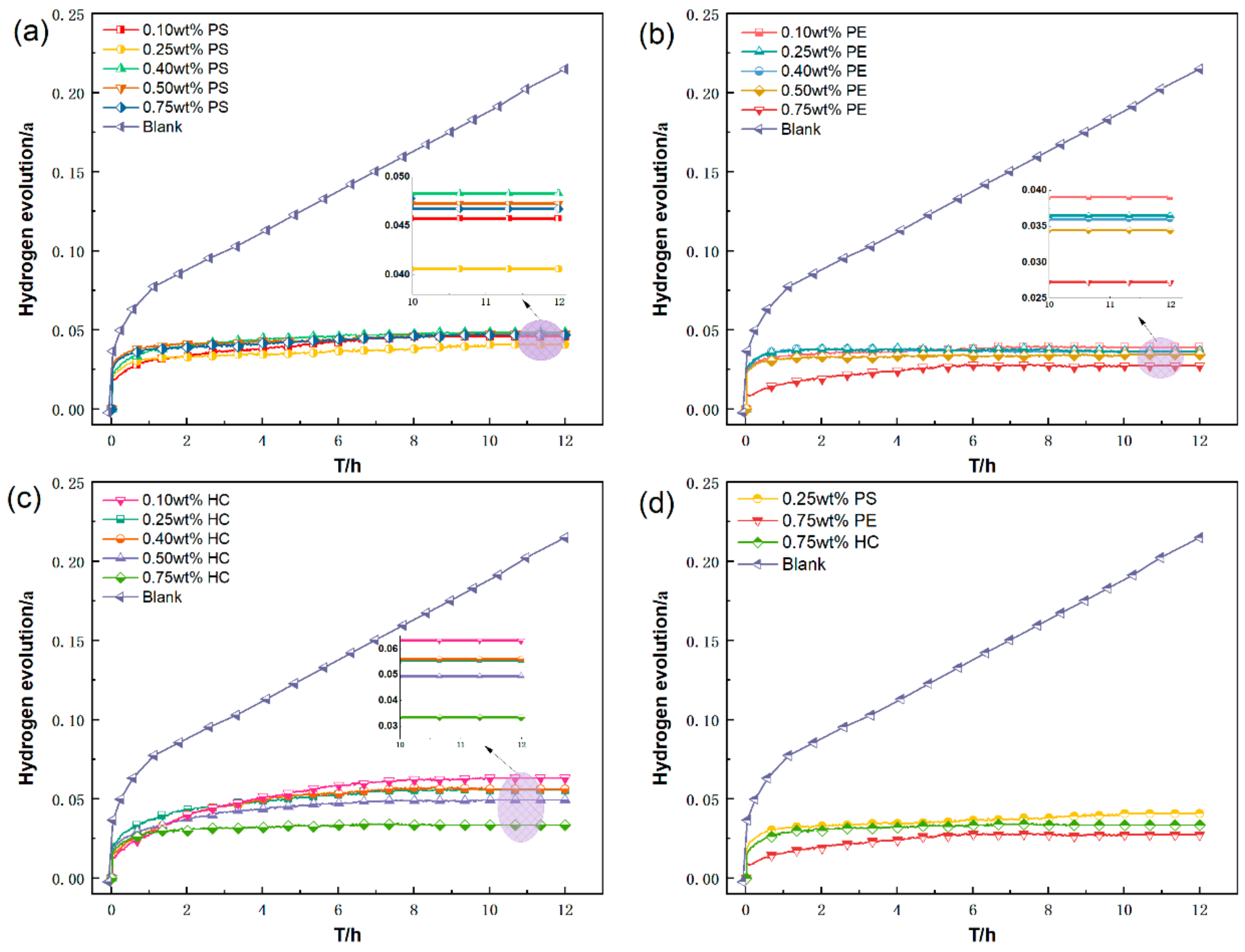
3.1.1. Effect of Inerting Agent Concentration
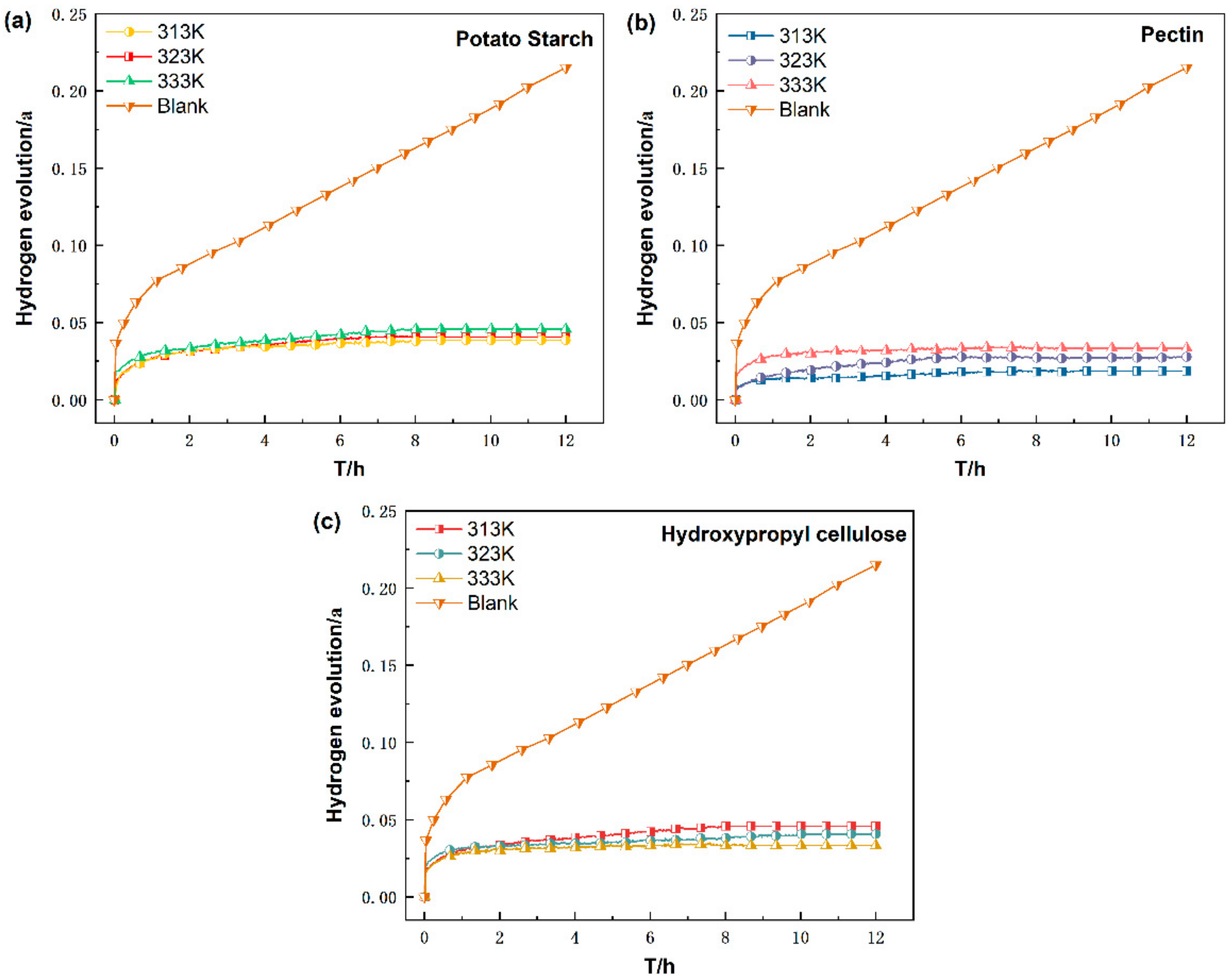
3.1.2. Effect of Temperature
3.1.3. Chemical Kinetics Model
3.1.4. Adsorption Isotherm
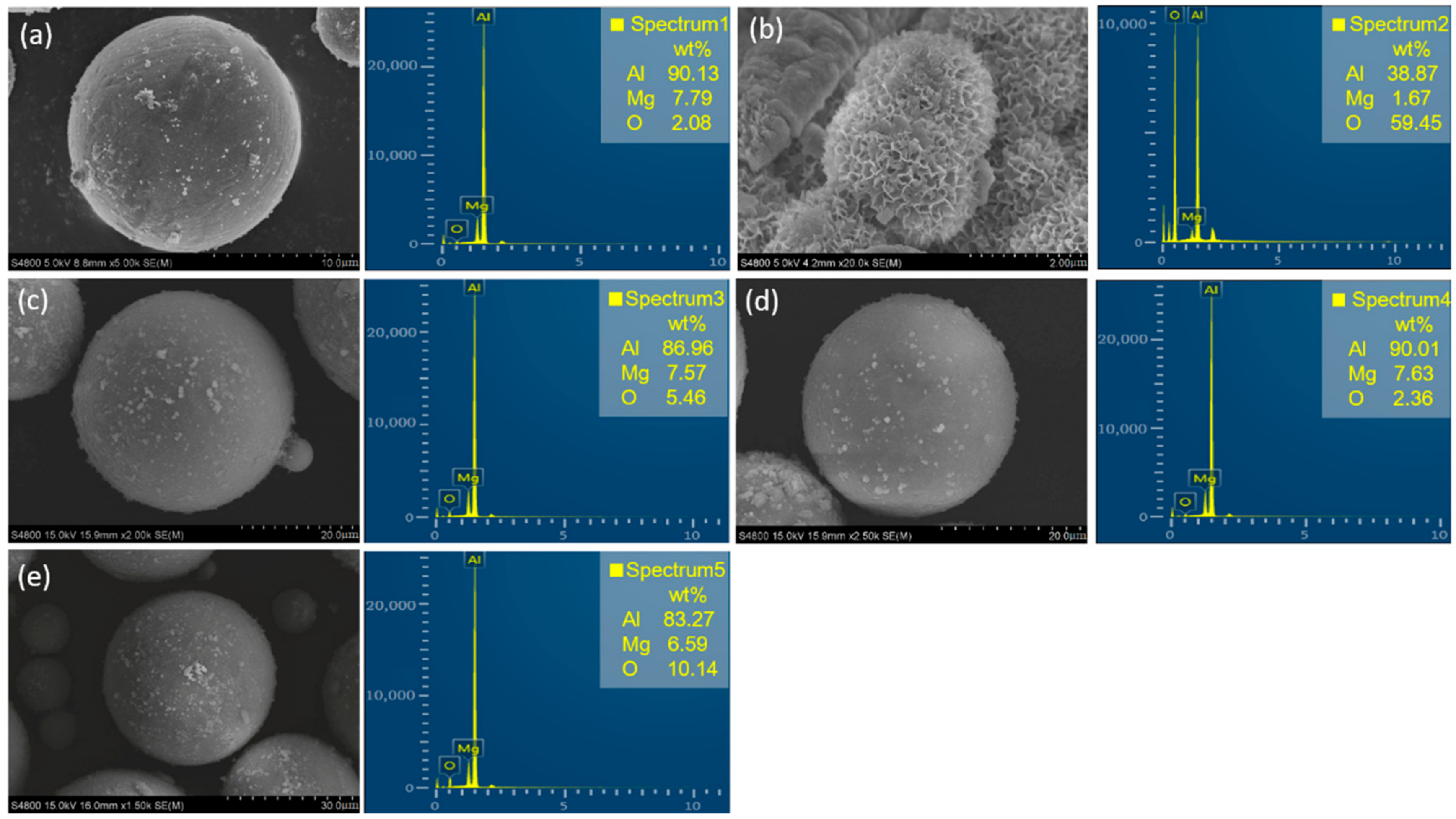
3.2. SEM and EDS
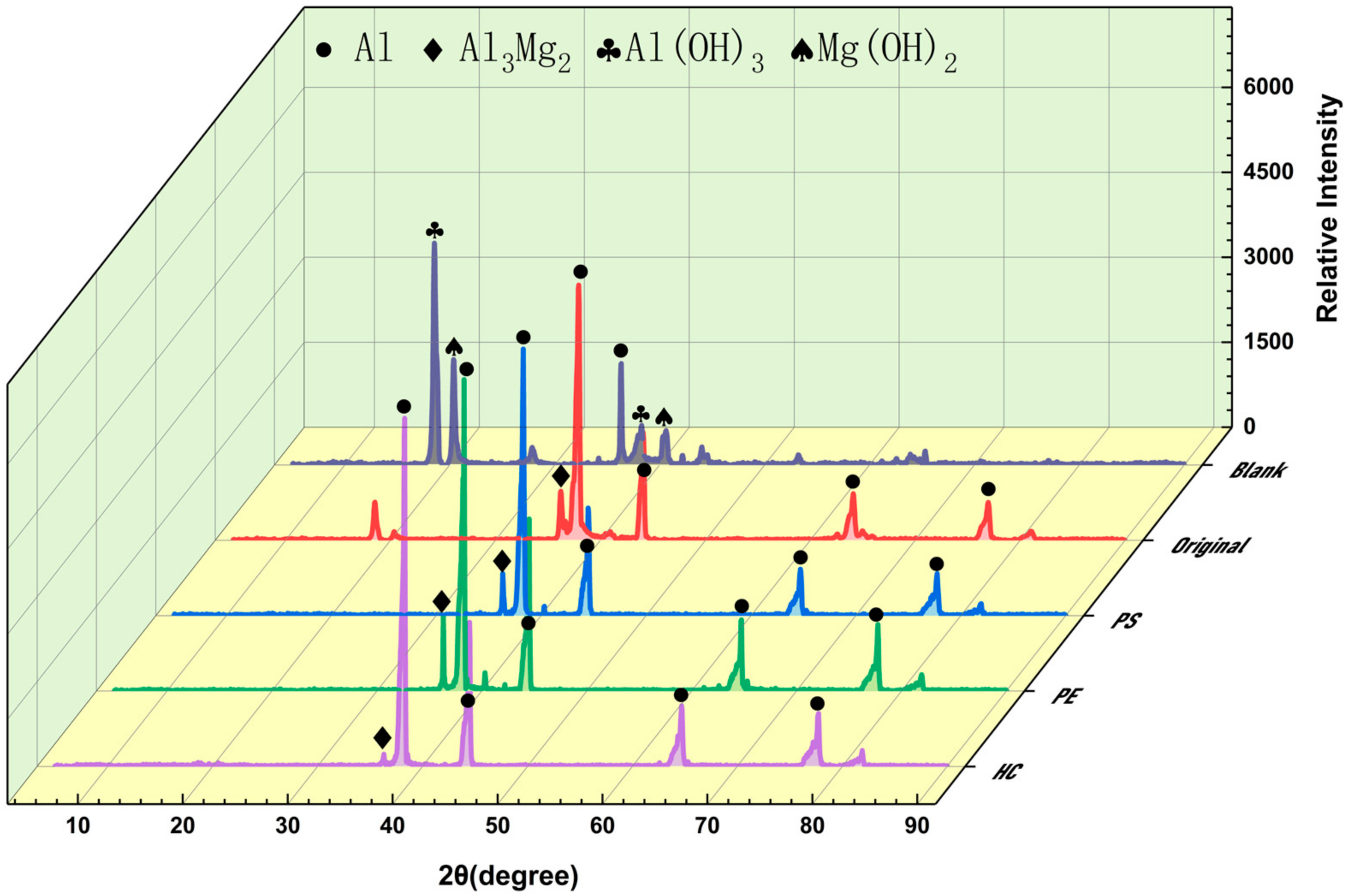
3.3. Inerting Product
3.4. Inerting Mechanism
3.5. Industrial Verification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, G.; Orrù, R. Self-Propagating Reactions for Environmental Protection: State of the Art and Future Directions. Chem. Eng. J. 2002, 87, 239–249. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Xu, K.; Li, J.; Yao, X.; Wu, C.; Li, S.; Yan, F.; Zhang, J.; Xu, Q. A New Accident Causation Theory Based on Systems Thinking and Its Systemic Accident Analysis Method of Work Systems. Process Saf. Environ. Prot. 2022, 158, 644–660. [Google Scholar] [CrossRef]
- Yoon, C.-W.; Yoon, Y.-S.; Hong, S.-Y.; Jeon, T.; Shin, S.-K. Hazardous Characteristics of Dust Waste from Metal Manufacturing Industries in South Korea. Waste Manag. Res. 2021, 39, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, T.; Akiyama, T. Exergetic Life Cycle Assessment of New Waste Aluminium Treatment System with Co-Production of Pressurized Hydrogen and Aluminium Hydroxide. Int. J. Hydrogen Energy 2009, 34, 153–161. [Google Scholar] [CrossRef]
- Gil, A.; Korili, S.A. Management and Valorization of Aluminum Saline Slags: Current Status and Future Trends. Chem. Eng. J. 2016, 289, 74–84. [Google Scholar] [CrossRef]
- Martínez, S.S.; Albañil Sánchez, L.; Álvarez Gallegos, A.A.; Sebastian, P.J. Coupling a PEM Fuel Cell and the Hydrogen Generation from Aluminum Waste Cans. Int. J. Hydrogen Energy 2007, 32, 3159–3162. [Google Scholar] [CrossRef]
- Park, J.T.; Xu, X.R.; Wang, J.; Zhu, X.J. A Small-Scale and Portable 50 W PEMFC System That Automatically Generates Hydrogen from a Mixture of Al, CaO, NaOH and Sodium CMC in Water without External Power Supply. Int. J. Hydrogen Energy 2013, 38, 10511–10518. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, K.; Liu, B.; Ge, J. Conversion Coating Technology for Recovery and Reuse of Waste Mg Alloy Polishing Powder. J. Clean. Prod. 2022, 368, 133181. [Google Scholar] [CrossRef]
- Uan, J.Y.; Cho, C.Y.; Liu, K.T. Generation of Hydrogen from Magnesium Alloy Scraps Catalyzed by Platinum-Coated Titanium Net in NaCl Aqueous Solution. Int. J. Hydrogen Energy 2007, 32, 2337–2343. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, K.; Li, J.; Liu, B.; Wang, B. Hydrogen Inhibition Effect of Chitosan and Sodium Phosphate on ZK60 Waste Dust in a Wet Dust Removal System: A Feasible Way to Control Hydrogen Explosion. J. Magnes. Alloy. 2021, in press. [Google Scholar] [CrossRef]
- Ogawa, M.; Kayama, F. A Study of the Association between Urinary Aluminum Concentration and Pre-Clinical Findings among Aluminum-Handling and Non-Handling Workers. J. Occup. Med. Toxicol. 2015, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Exley, C. Why Industry Propaganda and Political Interference Cannot Disguise the Inevitable Role Played by Human Exposure to Aluminium in Neurodegenerative Diseases, Including Alzheimer’s Disease. Front. Neurol. 2014, 5, 1–5. [Google Scholar] [CrossRef]
- Traoré, M.; Dufaud, O.; Perrin, L.; Chazelet, S.; Thomas, D. Dust Explosions: How Should the Influence of Humidity Be Taken into Account? Process Saf. Environ. Prot. 2009, 87, 14–20. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M. Application of Carbohydrate Polymers as Corrosion Inhibitors for Metal Substrates in Different Media: A Review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef] [PubMed]
- Umoren, S.A.; Solomon, M.M.; Madhankumar, A.; Obot, I.B. Exploration of Natural Polymers for Use as Green Corrosion Inhibitors for AZ31 Magnesium Alloy in Saline Environment. Carbohydr. Polym. 2020, 230, 115466. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, V.; Kesavan, D.; Gopiraman, M.; Viswanathamurthi, P. Physicochemical Studies of Glucose, Gellan Gum, and Hydroxypropyl Cellulose—Inhibition of Cast Iron Corrosion. Carbohydr. Polym. 2013, 95, 288–294. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Ali, S.A.; Dafalla, H.D.M. Synthesis, Characterization, and Utilization of a Diallylmethylamine-Based Cyclopolymer for Corrosion Mitigation in Simulated Acidizing Environment. Mater. Sci. Eng. C 2019, 100, 897–914. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, K.; Ge, J.; Liu, B. Study on Hydrogen Evolution Risk and Suppression Methods of Mg–Al/Mg–Zn Alloy Waste Dust in Wet Dust Collector. Process Saf. Environ. Prot. 2022, 163, 321–329. [Google Scholar] [CrossRef]
- Xu, X.; Wang, B.; Xu, K.; Wang, Y. Prevention of a Hydrogen Explosion Accident in the Wet Aluminum Waste Dust Collection Process Based on L-Malic Acid. Powder Technol. 2021, 382, 126–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, K.; Li, M.; Liu, B.; Wang, B.; Li, J.; Pei, X. Hydrogen Inhibition in Wet Dust Removal Systems by Using L-Aspartic: A Feasible Way of Hydrogen Explosion Control Measures. J. Loss Prev. Process Ind. 2021, 73, 104612. [Google Scholar] [CrossRef]
- Brindha, T.; Mallika, J.; Sathyanarayana Moorthy, V. Synergistic Effect between Starch and Substituted Piperidin-4-One on the Corrosion Inhibition of Mild Steel in Acidic Medium. J. Mater. Environ. Sci. 2015, 6, 191–200. [Google Scholar]
- Jaehne, E.; Kowalik, T.; Adler, H.J.P.; Plagge, A.; Stratmann, M. Ultra-Thin Layers of Phosphorylated Cellulose Derivatives on Metal Surfaces. Macromol. Symp. 2002, 177, 97–110. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, K.; Wang, Y.; Shen, R.; Wang, Q. Study of Hydrogen Explosion Control Measures by Using L-Phenylalanine for Aluminum Wet Dust Removal Systems. RSC Adv. 2018, 8, 41308–41316. [Google Scholar] [CrossRef]
- Wang, B.; Xu, K.; Wang, Y. Using Sodium D-Gluconate to Suppress Hydrogen Production in Wet Aluminium Waste Dust Collection Systems. J. Hazard. Mater. 2020, 397, 122780. [Google Scholar] [CrossRef] [PubMed]
- El-Haddad, M.N. Hydroxyethylcellulose Used as an Eco-Friendly Inhibitor for 1018 c-Steel Corrosion in 3.5% NaCl Solution. Carbohydr. Polym. 2014, 112, 595–602. [Google Scholar] [CrossRef]
- Hassan, R.M.; Ibrahim, S.M. Performance and Efficiency of Methyl-Cellulose Polysaccharide as a Green Promising Inhibitor for Inhibition of Corrosion of Magnesium in Acidic Solutions. J. Mol. Struct. 2021, 1246, 131180. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Liu, X.; Li, W.; Han, T. Microstructure and Phase Analysis of Mg-Al Alloy Bulk Material by Cold Spraying. Welded Pipe Tube 2019, 42, 25–31. [Google Scholar]
- Zhang, Y.; Liu, B.; Wang, B.; Pei, X.; Li, J.; Li, M.; Xu, K. Study on the Influence of Mg Content on the Risk of Hydrogen Production from Waste Alloy Dust in Wet Dust Collector. Int. J. Hydrogen Energy 2021, 46, 38563–38573. [Google Scholar] [CrossRef]
- Rosliza, R.; Wan Nik, W.B. Improvement of Corrosion Resistance of AA6061 Alloy by Tapioca Starch in Seawater. Curr. Appl. Phys. 2010, 10, 221–229. [Google Scholar] [CrossRef]
- Fellah, A.; Anjukandi, P.; Waterland, M.R.; Williams, M.A.K. Determining the Degree of Methylesterification of Pectin by ATR/FT-IR: Methodology Optimisation and Comparison with Theoretical Calculations. Carbohydr. Polym. 2009, 78, 847–853. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Determination of Pectin Degree of Esterification by Diffuse Reflectance. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Haladu, S.A.; Umoren, S.A.; Ali, S.A.; Solomon, M.M.; Mohammed, A.R.I. Synthesis, Characterization and Electrochemical Evaluation of Anticorrosion Property of a Tetrapolymer for Carbon Steel in Strong Acid Media. Chin. J. Chem. Eng. 2019, 27, 965–978. [Google Scholar] [CrossRef]
- Dindodi, N.; Shetty, A.N. Stearate as a Green Corrosion Inhibitor of Magnesium Alloy ZE41 in Sulfate Medium. Arab. J. Chem. 2019, 12, 1277–1289. [Google Scholar] [CrossRef]



| Corrosion Inhibitor | Concentration (wt%) | k (h−1) |
|---|---|---|
| PS | 0.1 wt% | 8.9009 × 10−4 |
| 0.25 wt% | 8.8889 × 10−4 | |
| 0.4 wt% | 0.00159 | |
| 0.5 wt% | 0.00156 | |
| 0.75 wt% | 9.4697 × 10−4 | |
| PE | 0.1 wt% | 5.4730 × 10−4 |
| 0.25 wt% | 3.2068 × 10−4 | |
| 0.4 wt% | 2.4066 × 10−4 | |
| 0.5 wt% | 8.6672 × 10−5 | |
| 0.75 wt% | 4.6800 × 10−5 | |
| HC | 0.1 wt% | 0.00181 |
| 0.25 wt% | 0.00144 | |
| 0.4 wt% | 0.00106 | |
| 0.5 wt% | 9.7387 × 10−4 | |
| 0.75 wt% | 3.6469 × 10−4 |
| Hardness | Tensile Strength | Maximum Compressive Strength | Elastic Modulus | |
|---|---|---|---|---|
| Standard Al alloy fittings | 91.6 HB | 175.94 MPa | 172.38 KN | 39.68% |
| Inerted Al alloy fittings | 90.5 HB | 175.62 MPa | 170.54 KN | 38.99% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Yin, W.; Xu, K.; Zhang, Y. Inerting Waste Al Alloy Dust with Natural High Polymers: Sustainability of Industrial Waste. Materials 2022, 15, 5540. https://doi.org/10.3390/ma15165540
Liu B, Yin W, Xu K, Zhang Y. Inerting Waste Al Alloy Dust with Natural High Polymers: Sustainability of Industrial Waste. Materials. 2022; 15(16):5540. https://doi.org/10.3390/ma15165540
Chicago/Turabian StyleLiu, Bo, Wenjing Yin, Kaili Xu, and Yuyuan Zhang. 2022. "Inerting Waste Al Alloy Dust with Natural High Polymers: Sustainability of Industrial Waste" Materials 15, no. 16: 5540. https://doi.org/10.3390/ma15165540
APA StyleLiu, B., Yin, W., Xu, K., & Zhang, Y. (2022). Inerting Waste Al Alloy Dust with Natural High Polymers: Sustainability of Industrial Waste. Materials, 15(16), 5540. https://doi.org/10.3390/ma15165540






