N′-Substituted 4-Phenylpicolinohydrazonamides with Thiosemicarbazone Moiety as New Potential Antitubercular Agents: Synthesis, Structure and Evaluation of Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
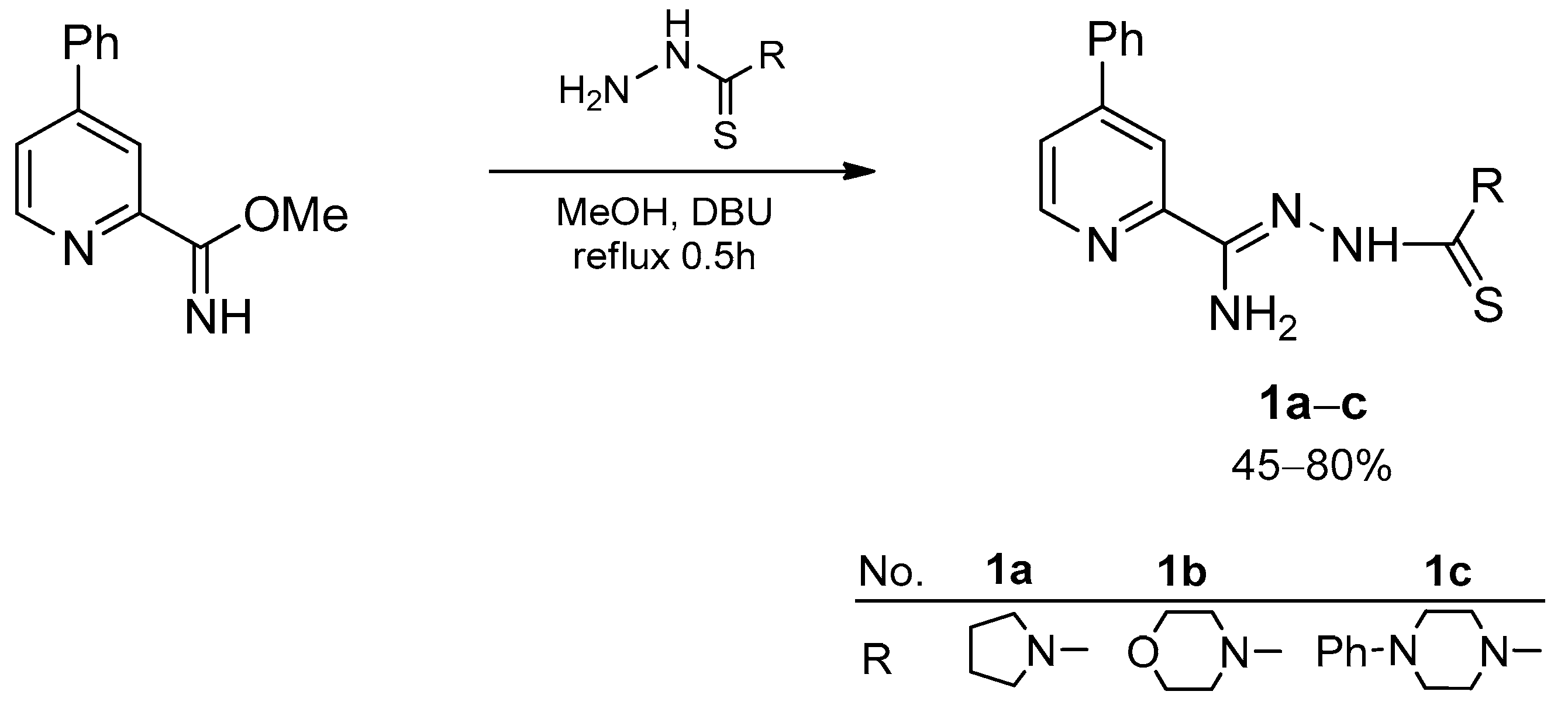
2.1.1. General Procedure for the Synthesis of Hydrazonamides (1a–c)
4-Phenyl-N′-(pyrrolidine-1-carbonothioyl) Picolinohydrazonamide (1a)
N′-(Morpholine-4-carbonothioyl)-4-phenylpicolinohydrazonamide (1b)
4-Phenyl-N′-(4-phenylpiperazine-1-carbonothioyl) Picolinohydrazonamide (1c)
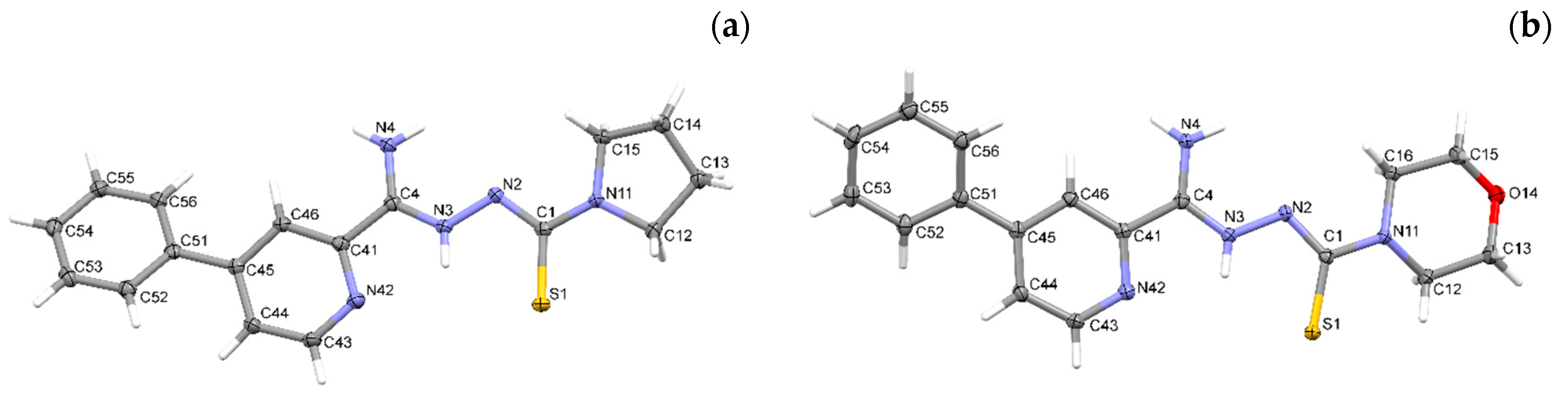
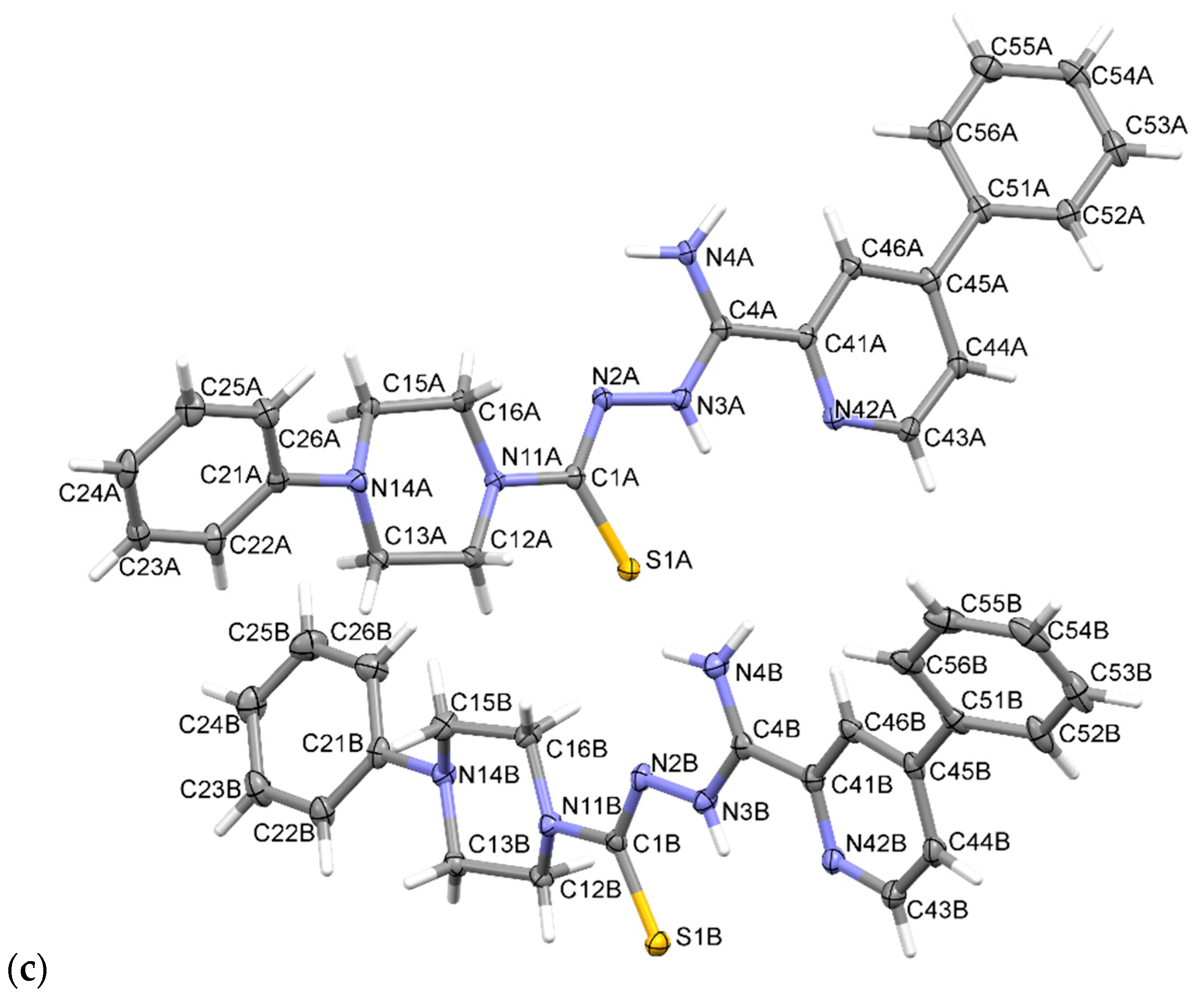
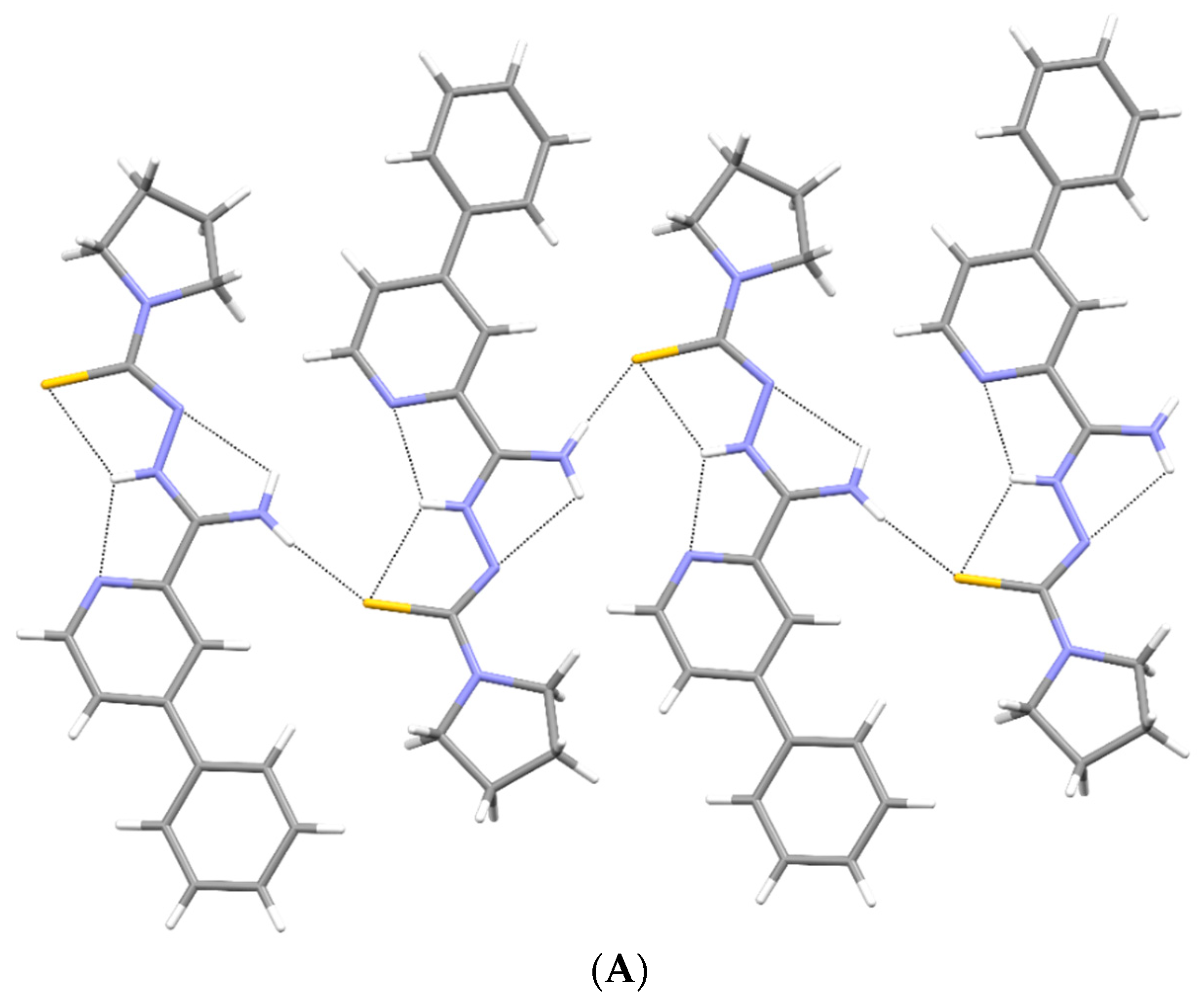
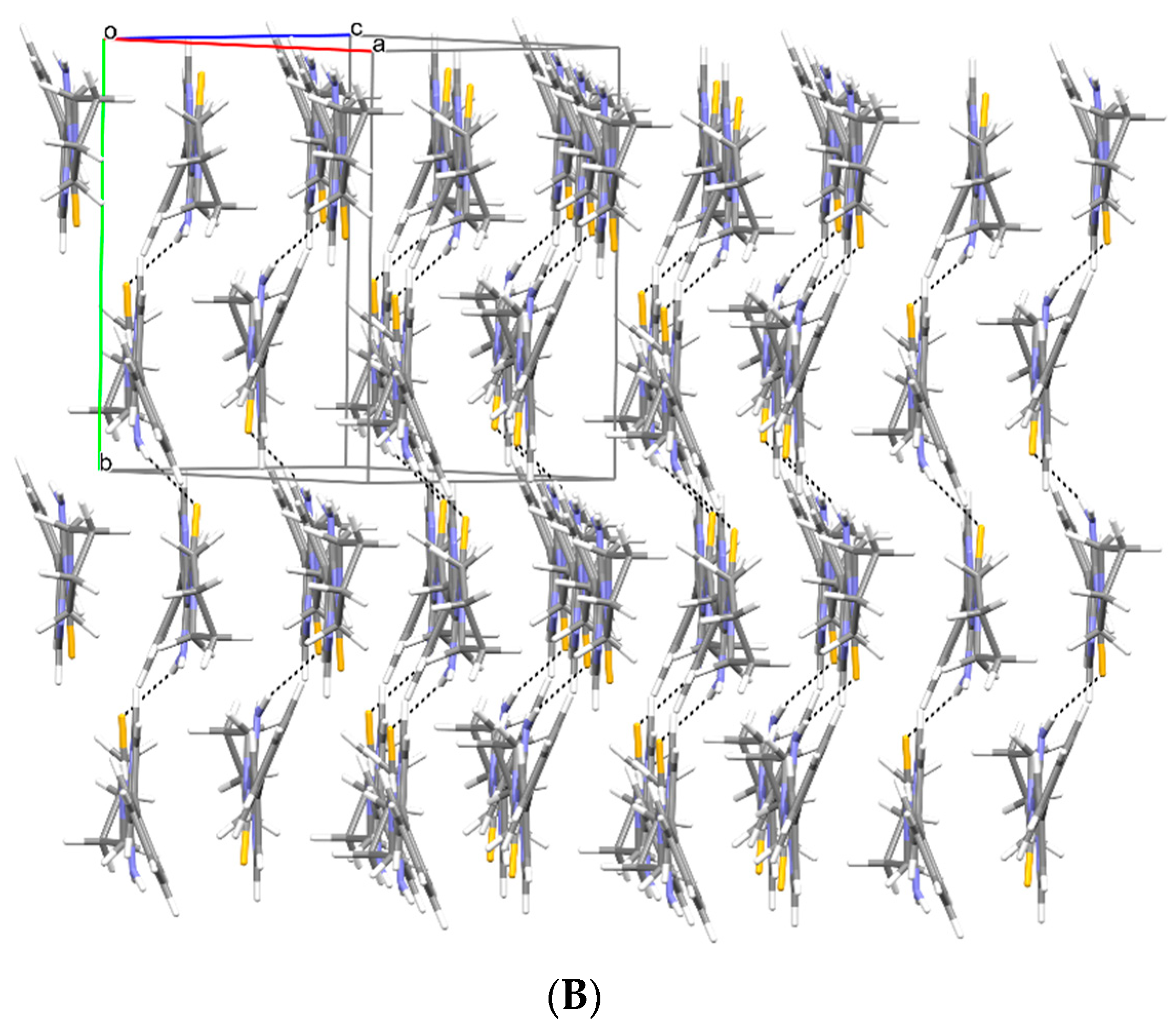
2.2. X-ray Study
2.3. ADME
2.4. Tuberculostatic Activity Assay
2.5. In Vitro Antibacterial Activity Assay
3. Results and Discussion
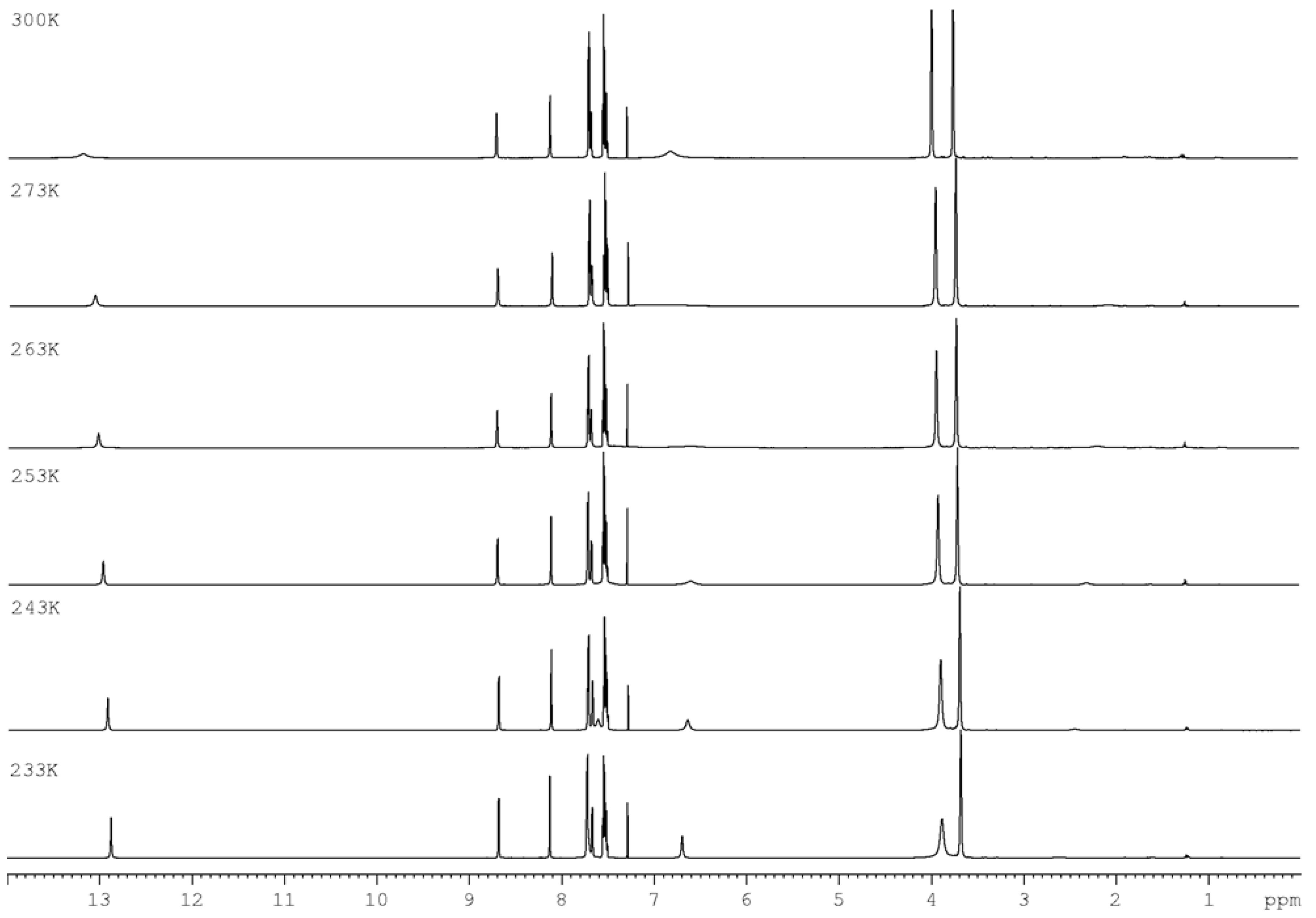
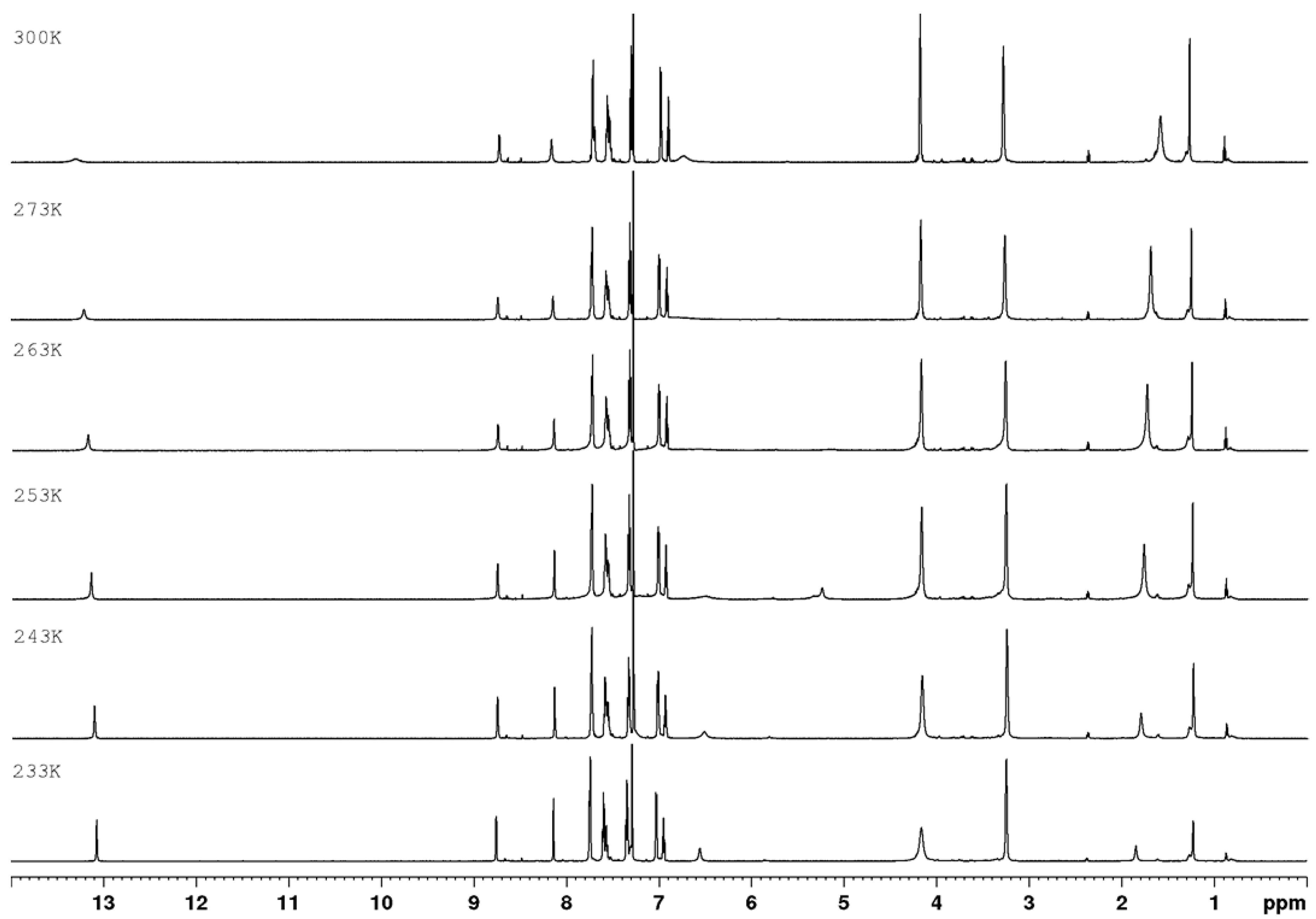
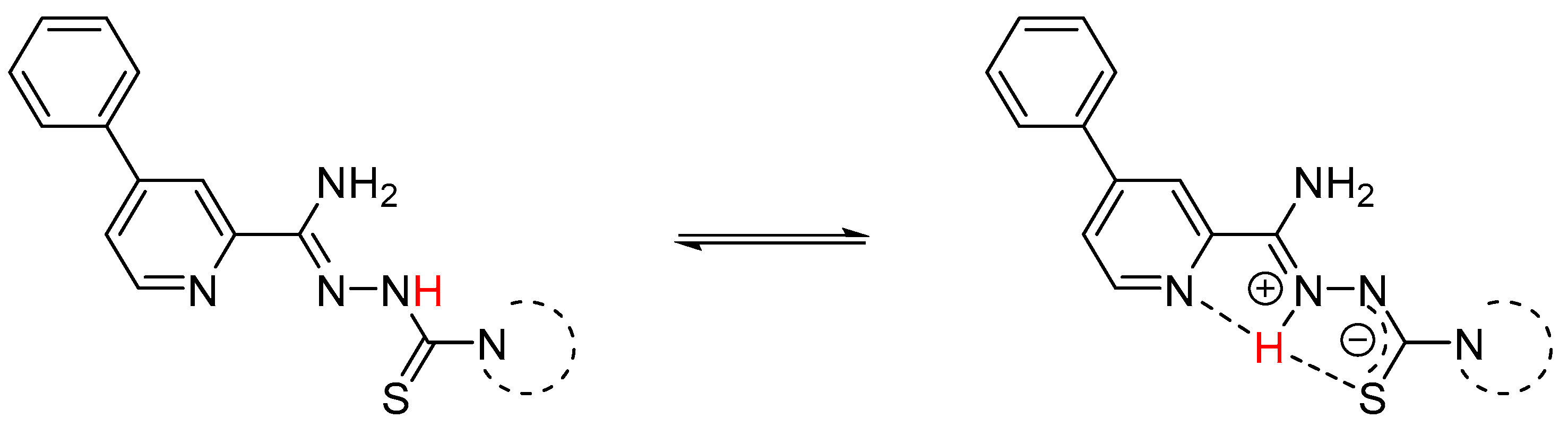
3.1. Chemistry
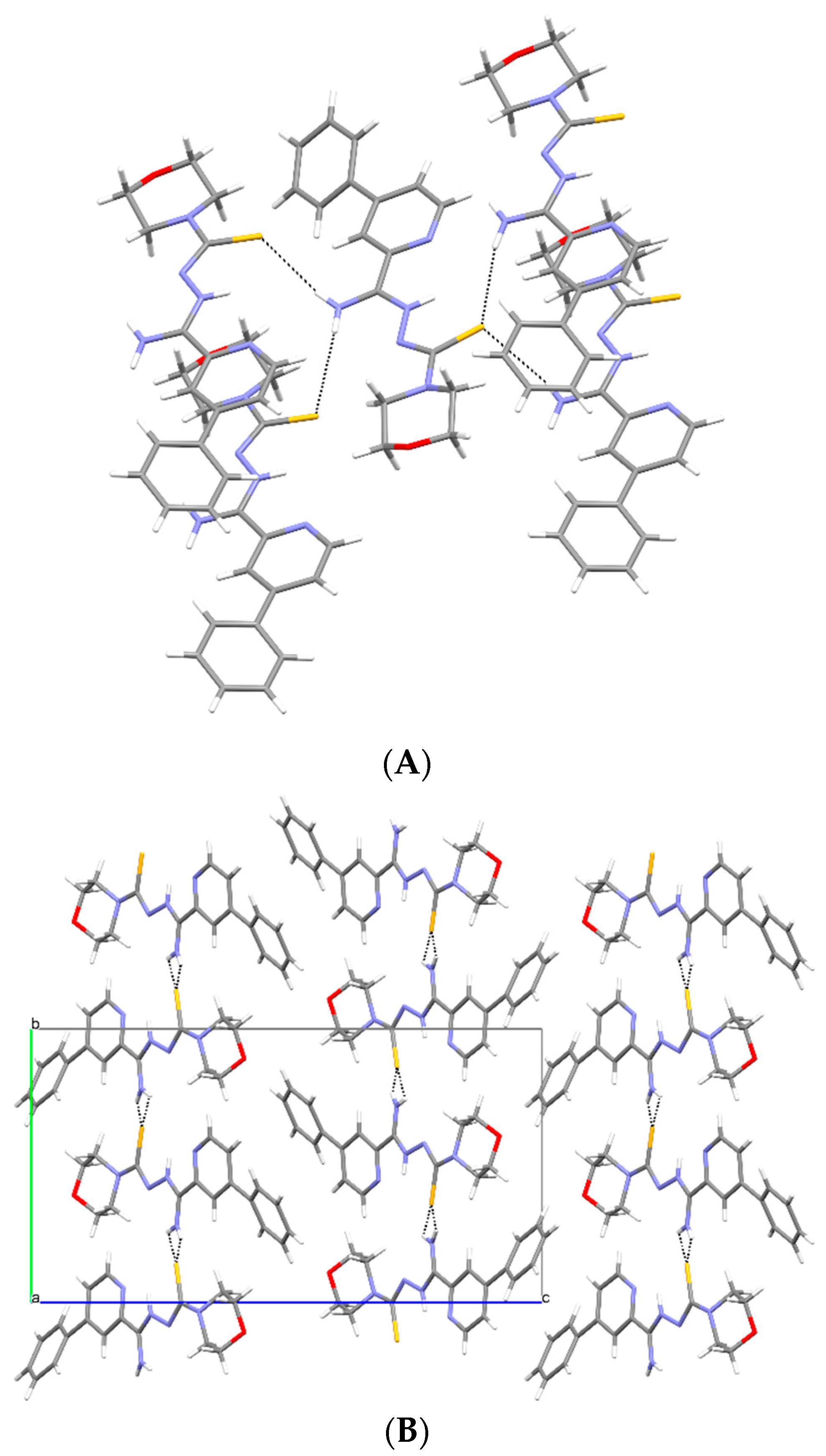
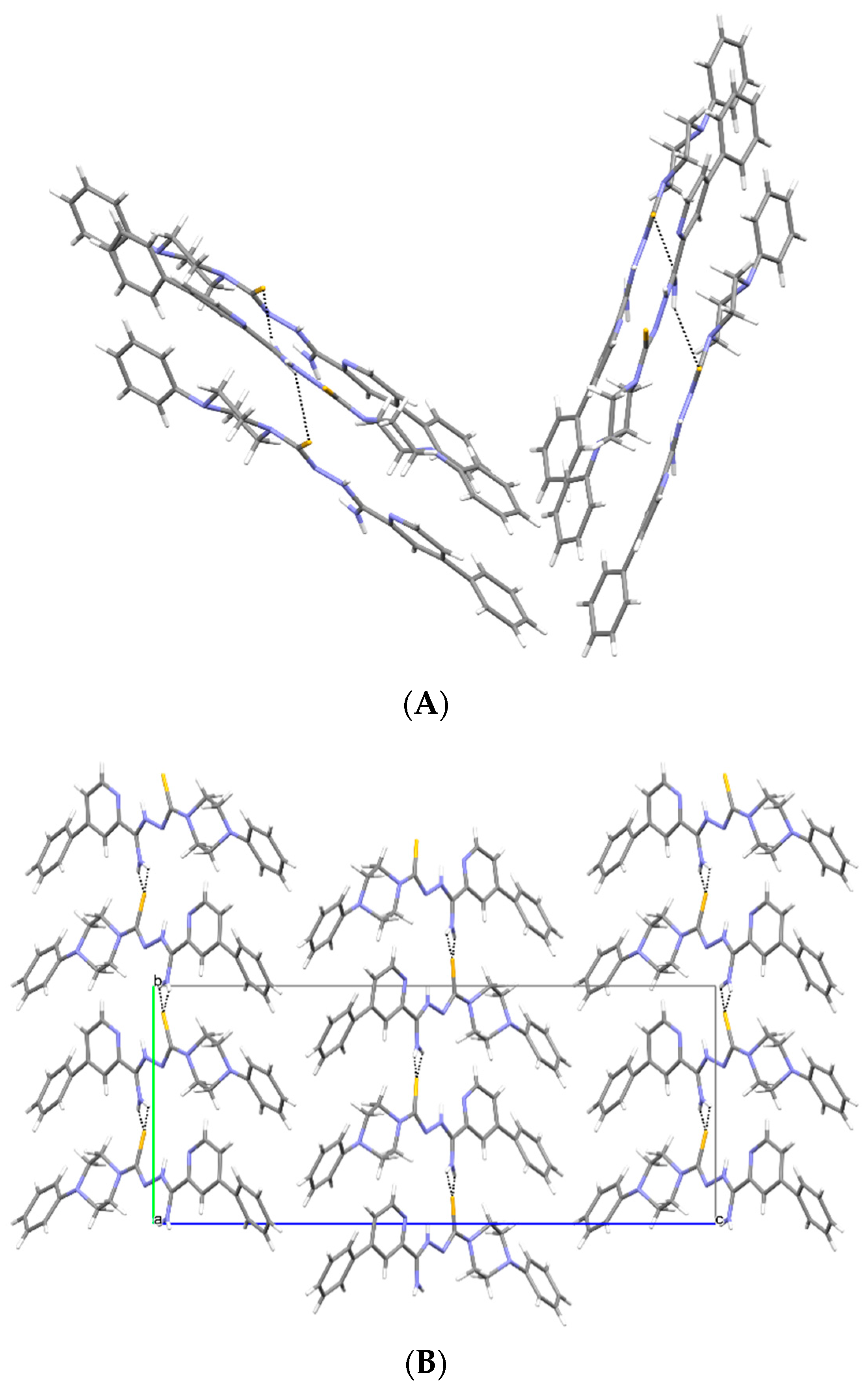
3.2. X-ray Study
3.3. ADME
3.4. Tuberculostatic Activity
3.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| a, b, c (Å) | unit cell parameters in angstroms |
| ACSc-5 | (Z)-6-methyl-N′-(4-phenylpiperazine-1-carbonothioyl) picolinohydrazonamide |
| ADME | absorption, distribution, metabolism, and excretion |
| AIDS | acquired immune deficiency syndrome |
| ATCC | American Type Culture Collection |
| CCDC | Cambridge Crystallographic Data Centre |
| Cip | ciprofloxacin |
| COVID-19 | Coronavirus Disease 2019 |
| DBU | 1,8-diazabicyclo [5.4.0]undec-7-ene |
| DMK-15 | (Z)-N′-(morpholine-4-carbonothioyl)-4-(pyrrolidin-1-yl)picolinohydrazonamide |
| DMSO-d6 | isotopologue of dimethyl sulfoxide |
| ETB | ethambutol |
| F2 | structure factor squared |
| FLEX | flexibility |
| FT-IR | Fourier-transform infrared spectroscopy |
| H37Rv | standard M. tuberculosis strain |
| HDF | human dermal fibroblast line |
| HIV | human immunodeficiency virus |
| IC50 | half-maximal inhibitory concentration |
| INH | isoniazid |
| INSATU | insaturation |
| INSOLU | insolubility |
| IR | infrared radiation |
| M. tuberculosis | Mycobacterium tuberculosis |
| MBC | minimal bactericidal concentration |
| MFC | minimal fungicidal concentration |
| MIC | minimal inhibition concentration |
| Mr | molecular mass |
| NMR | nuclear magnetic resonance |
| Nys | nystatin |
| PAS | p-aminosalicylic acid |
| POLAR | polarity |
| PZA | pyrazinamide |
| R | index based on structure factors describing compatibility of the model with X-ray diffraction data |
| RMP | rifampicin |
| S | goodness of fit of the refined X-ray structure |
| Spec. 192 | strain fully sensitive to the administrated tuberculostatic drugs |
| Spec. 210 | strain isolated from tuberculosis patients resistant to PAS, INH, ETB, and RMP |
| tPSA | polarity |
| UV | ultraviolet |
| V (Å3) | volume of a unit cell in cube angstroms |
| Van | vancomycin |
| WHO | World Health Organization |
| WLOGP | lipophilicity |
References
- The End TB Strategy. Available online: https://www.who.int/teams/global-tuberculosis-programme/the-end-tb-strategy (accessed on 14 July 2022).
- Global Tuberculosis Report 2021. World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 14 October 2021).
- Trend in Tuberculosis. 2020. Available online: https://www.cdc.gov/tb/publications/factsheets/statistics/tbtrends.htm (accessed on 14 July 2022).
- Tiberi, S.; Utjesanovic, N.; Galvin, J.; Centis, R.; D’Ambrosio, L.; Van den Boom, M.; Zumla, A.; Migliori, G.B. Drug resistant TB—Latest developments in epidemiology, diagnostics and management. Int. J. Infect. Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Drug-Resistant, T.B. Available online: https://www.cdc.gov/tb/topic/drtb/ (accessed on 14 July 2022).
- Dean, A.S.; Tosas Auguet, O.; Glaziou, P.; Zignol, M.; Ismail, N.; Kasaeva, T.; Floyd, K. 25 years of surveillance of drug-resistant tuberculosis: Achievements, challenges, and way forward. Lancet Infect. Dis. 2022, 22, e191–e196. [Google Scholar] [CrossRef]
- Bhatt, R.; Chopra, K.; Vashisht, R. Impact of integrated psycho-socio-economic support on treatment outcome in drug resistant tuberculosis—A retrospective cohort study. Indian J. Tuberc. 2019, 66, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Dooley, K.E.; Rosenkranz, S.L.; Conradie, F.; Moran, L.; Hafner, R.; Groote-Bidlingmaier, F.; Lama, J.R.; Shenje, J.; De Los Rios, J.; Comins, K.; et al. QT effects of bedaquiline, delamanid, or both in patients with rifampicin-resistant tuberculosis: A phase 2, open-label, randomised, controlled trial. Lancet Infect. Dis. 2021, 21, 975–983. [Google Scholar] [CrossRef]
- Kim, S.; Louie, A.; Drusano, G.L.; Almoslem, M.; Kim, S.; Myrick, J.; Nole, J.; Duncanson, B.; Peloquin, C.A.; Scanga, C.A.; et al. Evaluating the effect of clofazimine against Mycobacterium tuberculosis given alone or in combination with pretomanid, bedaquiline or linezolid. Int. J. Antimicrob. Agents 2022, 59, 106509. [Google Scholar] [CrossRef]
- Santos, F.; Duarte, A.R.C. Combination drug delivery approaches for tuberculosis. In Combination Drug Delivery Approach as an Effective Therapy for Various Diseases; Kesharwani, P., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 173–210. [Google Scholar]
- Krause, M.; Foks, H.; Ziembicka, D.; Augustynowicz-Kopeć, E.; Głogowska, A.; Korona-Głowniak, I.; Bojanowski, K.; Siluk, D.; Gobis, K. 4-substituted picolinohydrazonamides as a new class of potential antitubercular agents. Eur. J. Med. Chem. 2020, 190, 1–10. [Google Scholar] [CrossRef]
- Szczesio, M.; Gobis, K.; Korona-Głowniak, I.; Mazerant-Politowicz, I.; Ziembicka, D.; Foks, H.; Główka, M.; Olczak, A. Synthesis, structure and biological activity of four new picolinohydrazonamide derivatives. Acta Cryst. C 2020, C76, 673–680. [Google Scholar] [CrossRef]
- Gobis, K.; Szczesio, M.; Olczak, A.; Korona-Głowniak, I.; Augustynowicz-Kopeć, E.; Mazernt-Politowicz, I.; Ziembicka, D.; Główka, M.L. Differences in the Structure and Antimicrobial Activity of Hydrazones Derived from Methyl 4-Phenylpicolinimidate. Materials 2022, 15, 3085. [Google Scholar] [CrossRef]
- Brunner, H.; Störiko, R.; Rominger, F. Novel Chiral Oxazoline Ligands for Potential Charge-Transfer Effects in the Rh(I) -Catalysed Enantioselective Hydrosilylation. Eur. J. Inorg. Chem. 1998, 6, 771–781. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Westrip, S.P. publCIF: Software for editing, validating and formatting crystallographic information files. J. Appl. Cryst. 2010, 43, 920–925. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Zoete, V. A Boiled-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Krause, M.; Foks, H.; Augustynowicz-Kopeć, E.; Napiórkowska, A.; Szczesio, M.; Gobis, K. Synthesis and Tuberculostatic Activity Evaluation of Novel Benzazoles with Alkyl, Cycloalkyl or Pyridine Moiety. Molecules 2018, 23, 985. [Google Scholar] [CrossRef] [Green Version]
- Gacki, M.; Kafarska, K.; Pietrzak, A.; Korona-Głowniak, I.; Wolf, W.M. Quasi-Isostructural Co(II) and Ni(II) Complexes with Mefenamato Ligand: Synthesis, Characterization, and Biological Activity. Molecules 2020, 25, 3099. [Google Scholar] [CrossRef]
- Gobis, K.; Foks, H.; Zwolska, Z.; Augustynowicz-Kopec, E. Synthesis of 2-aminoaryl-5-substituted-1,3,4-thiadiazoles in a thermal 1,3-dipolar cycloaddition reaction. Phosphorus Sulfur 2005, 180, 2653–2666. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Cryst. B 2013, 69, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendolski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 3, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1946. [Google Scholar] [CrossRef]
- O’Donnell, F.; Smyth, T.J.P.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents 2009, 35, 30–38. [Google Scholar] [CrossRef] [Green Version]





| 1a | 1b | 1c | |
|---|---|---|---|
| Crystal Data | |||
| Chemical formula | C17H19N5S | C17H19N5OS | 2(C23H24N6S) |
| Mr | 325.43 | 341.43 | 833.08 |
| Space group | P21/c | P21/c | P21 |
| a, b, c (Å) | 13.3591 (4), 12.0691 (4), 9.9817 (3) | 5.9419 (4), 12.1487 (7), 22.9938 (13) | 6.2510 (3), 11.9035 (5), 27.8679 (12) |
| β (°) | 101.248 (2) | 96.549 (2) | 93.696 (2) |
| V (Å3) | 1578.46 (9) | 1649.01 (17) | 2069.30 (16) |
| Z | 4 | 4 | 2 |
| μ (mm−1) | 1.87 | 1.86 | 1.56 |
| Crystal size (mm) | 0.87 × 0.24 × 0.20 | 1.24 × 0.18 × 0.14 | 1.0 × 0.41 × 0.13 |
| Data Collection | |||
| No. of measured, independent and observed [I > 2σ(I)] reflections | 21324, 3119, 2979 | 17762, 3213, 3185 | 8030, 8030, 8024 |
| (sin θ/λ)max (Å−1) | 0.618 | 0.618 | 0.618 |
| Refinement | |||
| R[F2 > 2σ(F2)], wR(F2), S | 0.028, 0.076, 1.05 | 0.027, 0.073, 1.04 | 0.026, 0.074, 1.13 |
| No. of reflections | 3119 | 3213 | 8030 |
| No. of parameters | 220 | 230 | 568 |
| No. of restraints | 0 | 0 | 1 |
| Δmax, Δmin (e Å−3) | 0.22, −0.32 | 0.34, −0.18 | 0.20, −0.19 |
| Absolute structure | – | – | Flack x determined using 3129 quotients [(I+) − (I-)]/[(I+) + (I-)] [26] |
| Absolute structure parameter | – | – | 0.046 (4) |
| Compd. | MIC 1 [µg/mL] | ||
|---|---|---|---|
| H37Rv 2 | Spec. 192 | Spec. 210 | |
| 1a | 6.2 | 12.5 | 6.2 |
| 1b | 3.1 | 3.1 | 3.1 |
| 1c | 50 | 50 | 50 |
| INH 3 | 12.5 | 12.5 | 25 |
| PZA 3 | 25 | 25 | >400 |
| Mycobacterium Strain | MIC [µg/mL] |
|---|---|
| M. tuberculosis H37Rv | 3.1 |
| M. bovis | 3.1 |
| M. kanssasi | 12.5 |
| M. intracellulare | 6.2 |
| M. scrafulaceum | 12.5 |
| 1a | 1b | 1c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MBC/MIC Ratio | MIC | MBC | MBC/MIC Ratio | MIC | MBC | MBC/MIC Ratio | MIC | |
| Gram-Positive Bacteria | Van | |||||||||
| S. aureus ATCC 25923 | 1.95 | 15.6 | 8 | 1.95 | 1.95 | 1 | 0.98 | 31.3 | 32 | 0.98 |
| S. aureus ATCC 43300 | 3.9 | 125 | 32 | 1.95 | 250 | 128 | 0.49 | 31.3 | 64 | 0.49 |
| S. aureus ATCC 6538 | 3.9 | 62.5 | 16 | 1.95 | 125 | 64 | 1.95 | 31.3 | 16 | 0.49 |
| S. epidermidis ATCC 12228 | 1.95 | 125 | 64 | 0.98 | 31.3 | 16 | 0.49 | 31.3 | 64 | 0.98 |
| M. luteus ATCC 10240 | 0.49 | 15.6 | 32 | 1.95 | 1.95 | 1 | 0.06 | 15.6 | 260 | 0.12 |
| B. cereus ATCC 10876 | 3.9 | 31.3 | 8 | 7.8 | >1000 | >128 | 31.3 | 31.3 | 1 | 0.98 |
| B. subtilis ATCC 6633 | 3.9 | 62.5 | 16 | 0.98 | 0.98 | 1 | 31.3 | 31.3 | 1 | 0.24 |
| S. pyogenes ATCC 19615 | 7.8 | 15.6 | 2 | 15.6 | 31.3 | 2 | 62.5 | 125 | 2 | 0.24 |
| S. mutans ATCC 25175 | 3.9 | 15.6 | 4 | 31.3 | >1000 | >32 | 62.5 | 62.5 | 1 | 0.98 |
| S. pneumoniae ATCC 49619 | 7.8 | 31.3 | 4 | 31.3 | 31.3 | 1 | 31.3 | 125 | 4 | 0.24 |
| Gram-Negative Bacteria | Cip | |||||||||
| E. coli ATCC 25922 | 1000 | >1000 | Nd | 125 | >1000 | >8 | 125 | >1000 | >8 | 0.015 |
| S. typhimurium ATCC 14028 | 250 | >1000 | >4 | 125 | >1000 | >8 | 125 | >1000 | >8 | 0.061 |
| K. pneumoniae ATCC 13883 | >1000 | >1000 | Nd | 500 | >1000 | Nd | 125 | >1000 | >8 | 0.122 |
| P. mirabilis ATCC 12453 | 125 | >1000 | >8 | 31.3 | >1000 | >32 | 62.5 | >1000 | >16 | 0.030 |
| P. aeruginosa ATCC 9027 | 500 | >1000 | Nd | 31.3 | >1000 | >32 | 62.5 | >1000 | >16 | 0.488 |
| Yeasts | MIC | MFC | MIC | MFC | MIC | MFC | Nys | |||
| C. albicans ATCC 102231 | 15.6 | 62.5 | 4 | 15.6 | 62.5 | 2 | 7.8 | 7.8 | 1 | 0.48 |
| C. parapsilosis ATCC 22019 | 62.5 | 62.5 | 1 | 15.6 | 125 | 8 | 7.8 | 31.3 | 4 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobis, K.; Szczesio, M.; Olczak, A.; Mazerant-Politowicz, I.; Ziembicka, D.; Pacholczyk-Sienicka, B.; Augustynowicz-Kopeć, E.; Głogowska, A.; Korona-Głowniak, I.; Fruziński, A. N′-Substituted 4-Phenylpicolinohydrazonamides with Thiosemicarbazone Moiety as New Potential Antitubercular Agents: Synthesis, Structure and Evaluation of Antimicrobial Activity. Materials 2022, 15, 5513. https://doi.org/10.3390/ma15165513
Gobis K, Szczesio M, Olczak A, Mazerant-Politowicz I, Ziembicka D, Pacholczyk-Sienicka B, Augustynowicz-Kopeć E, Głogowska A, Korona-Głowniak I, Fruziński A. N′-Substituted 4-Phenylpicolinohydrazonamides with Thiosemicarbazone Moiety as New Potential Antitubercular Agents: Synthesis, Structure and Evaluation of Antimicrobial Activity. Materials. 2022; 15(16):5513. https://doi.org/10.3390/ma15165513
Chicago/Turabian StyleGobis, Katarzyna, Małgorzata Szczesio, Andrzej Olczak, Ida Mazerant-Politowicz, Dagmara Ziembicka, Barbara Pacholczyk-Sienicka, Ewa Augustynowicz-Kopeć, Agnieszka Głogowska, Izabela Korona-Głowniak, and Andrzej Fruziński. 2022. "N′-Substituted 4-Phenylpicolinohydrazonamides with Thiosemicarbazone Moiety as New Potential Antitubercular Agents: Synthesis, Structure and Evaluation of Antimicrobial Activity" Materials 15, no. 16: 5513. https://doi.org/10.3390/ma15165513
APA StyleGobis, K., Szczesio, M., Olczak, A., Mazerant-Politowicz, I., Ziembicka, D., Pacholczyk-Sienicka, B., Augustynowicz-Kopeć, E., Głogowska, A., Korona-Głowniak, I., & Fruziński, A. (2022). N′-Substituted 4-Phenylpicolinohydrazonamides with Thiosemicarbazone Moiety as New Potential Antitubercular Agents: Synthesis, Structure and Evaluation of Antimicrobial Activity. Materials, 15(16), 5513. https://doi.org/10.3390/ma15165513









