Photoactive Cements: A Review
Abstract
:1. Introduction
2. Mechanism of Photocatalysis, Types of Photoactive Cements and Their Preparation
- (a)
- Photocatalytic mechanisms of TiO2Suitable semiconductors are photocatalysts capable of carrying out the photocatalysis process. The most famous semiconductor in the world is titanium dioxide. The supply of energy in the form of light to the semiconductor causes the electrons from the base band to jump to the conduction band, and oxidation and reduction reactions take place in both bands [1]. The mechanism of photocatalytic oxidation is well understood and generally comprises four major steps [6,7,8,9]:
- (1)
- Adsorption of reagents on the surface of the photocatalyst,
- (2)
- Excitation of the photocatalyst with UV radiation and photo-generation of e--h+ pairs (photo-inducted electrons and holes),
- (3)
- Generation of hydroxyl radicals from water molecules adsorbed on the semiconductor surface,
- (4)
- Generated hydroxyl radicals and other structures take part in decomposition of such compounds as organic compounds, nitrogen oxides and other compounds,
- (b)
- Types of photoactive cements.
2.1. Obtaining Photoactive Cements by Incorporation Method
- (a)
- Examples of preparation of the cements by intercalation method using TiO2
- (b)
- Examples of preparation of the cements with modified TiO2
- (c)
- Examples of the preparation of cements with the addition of another type of photocatalyst
2.2. Preparation of Photoactive Cements by Surface Modification
- (a)
- Formation of thin TiO2 films with/without a separation layer
- (b)
- Forming thin films with use of modified TiO2
- (c)
- The formation of coatings using a different type of photocatalyst
2.3. Installation of the Photocatalyst Only in the Top Layer of Photoactive Cement
- (a)
- Examples using TiO2 and modified TiO2
- (b)
- Example using a different type of photocatalyst
3. Test Methods Used for the Evaluation of the Photocatalytic Activity of Cements
- (a)
- Photoactive tests used for determining the photocatalytic efficiency by air purification
- (b)
- Photoactive tests used for determining the self-cleaning properties
- (c)
- Photoactive tests used for determining the antimicrobial activity
4. Results of Mechanical Properties of Modified Cement Mortars
4.1. Effect of Additives on Fresh Properties of Cement-Based Composite Materials—Results
- (a)
- Effect on the workability of modified cement
- (b)
- Effect on the initial and final setting time of modified cement
- (c)
- Effect on hydration process of modified cement
4.2. Effect of Additives on the Properties of Hardened-Cement-Based Composite Materials—Results
4.3. Influence of Introduced Additives on Carbonation of Cement Materials
| Author | Material | Conditions | Photocatalyst Dose (wt.%) | Time of Exposure to Conditions in the Chamber | Results |
|---|---|---|---|---|---|
| Rao et al. [155] | Mortars with the addition of 30 wt.% fly ash, binder: sand ratio 1:1 and 1:2 | 5 ± 1% CO2, RH = 60 ± 5% T = 23 ± 3 °C | 0.5; 0.75, 1% nano-TiO2 (NT) or 0.75%, 1.5%, 3% nano-SiO2 (NS) | At 14, 28, 56, and 91 days, the samples were taken from the chamber, broken into four parts, and the depth of carbonation was measured using the colorimetric method (with 0.1% phenolphthalein content) | 1: 1 blends with 0.5 wt.%, 0.75 wt.%, and 1 wt.%. NT and 0.75% NS show total resistance to carbonation. Mixtures with nano-TiO2 generally showed a lower carbonation depth than blends with nano-SiO2. Similarly, mortars from the 1: 1 family showed a lower depth of carbonation than the mortars from the 1: 2 family. |
| Duan et al. [156] | Geopolymer paste based on fly ash activated in an alkaline sodium silicate solution | 20% CO2, RH = 65 ± 5% T = 24 ± 5 °C | 1, 3, 5% n-TiO2 | At 3, 7, 28, 90, and 180 days, the depth of carbonation was measured along the exposed surface of the split specimens 40 mm long at 12 points using the phenolphthalein spray test | The improvement of the resistance to carbonation was observed only after 28 days, after 180 days, the sample with 1% TiO2 showed the highest resistance |
| Hernandez et al. [42] | Cement mortar | Normal carbonation | Addition of P25 in the amount of 5 and 10 wt.% in the surface layer | Determined with a 1% solution of phenolphthalein in ethanol after 28 days and 365 days | No significant carbonation was observed after 28 days, despite the detection of Ca(OH)2 by thermal analysis. Carbonation was more significant after 365 days, although mortars with/ without an additive of TiO2 were affected to the same extent. |
| Diamanti et al. [159] | Cement mortar w/c = 0.52 or w/c = 0.69 | After 3 days of curing, the samples were moved to the carbonation room: 4% CO2, RH = 65% T = 20 °C | P25 addition in the amount of 2.5 and 5 wt.% | Determined with a solution of phenolphthalein with a concentration of 1% in ethanol after 28 days and 70 days in four points | An increase in the depth of carbonation with an increase in the w/c proportion, the addition of titanium dioxide caused a slight increase in the depth of carbonation, for example, in mixtures with a w/c of 0.69, after 70 days of exposure, the average depth of carbonation increased from about 9 mm to 11 mm and 11, 5 mm in concrete with content of 2.5 and 5% compared to cement TiO2. |
4.4. Influence of TiO2 on Abrasion Resistance
5. Examples of Investments with the Use of Photoactive Composite Materials
6. Conclusions and Future Prospects
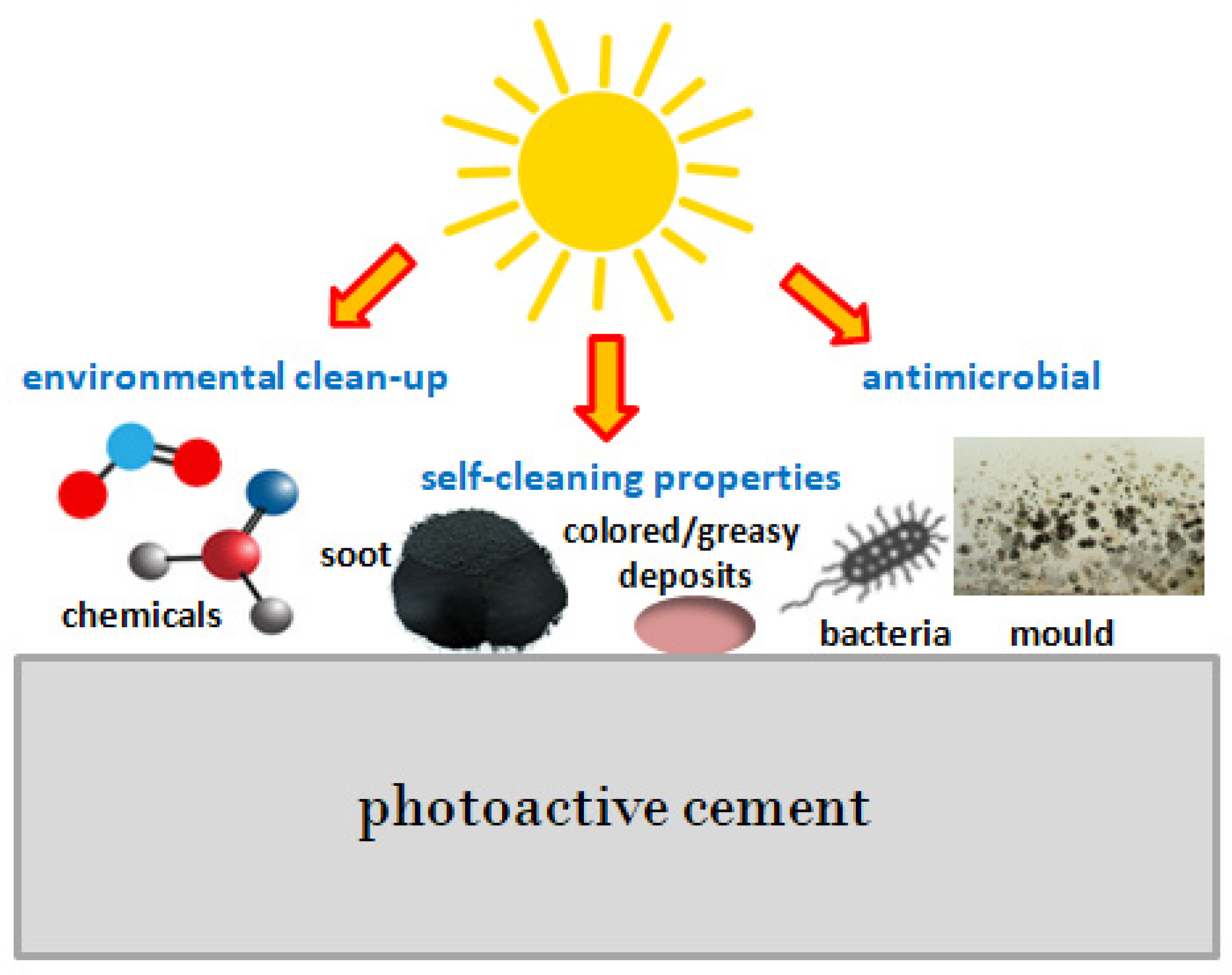
- The presented research shows that the prepared concretes show photocatalytic activity and can clean the air, e.g., removing nitrogen oxides or volatile organic compounds. What is more, they have the ability to degrade soot and microorganisms, maintaining the lasting appearance of the building during its use.
- Photoactive cements used in the production of concrete products are mainly activated by UV radiation; scientists tried to find a photocatalyst active in visible light. Additional studies are needed to demonstrate durability in the field of photocatalysts other than TiO2.
- The procedure for introducing the photocatalytic active ingredient influences the surface concentration of the catalytic active sites, giving preference to the surface coating method over the bulk application of mortar when discussing only the photocatalytic efficiency, but without changing the nature of the active sites. However, the introduction of the active phase of the catalyst to the mass, even with a lower but still acceptable photocatalytic activity, results in a more stable system in terms of mechanical properties of the surface and CSH distribution. Given the need to balance the different photocatalyst performance requirements with the expected overall product yields, the incorporation of the active catalyst phase into the bulk appears to be more promising for sustainable long-term applications.
- 4.
- The issue that cannot be ignored in the research is the place, terrain conditions, and climate where the photocatalytic building, road, pavement, etc. will be located.
- 5.
- The presented research has shown that when designing a photocatalytic mortar, the type of binder, final texture, and microstructure of the material should be carefully selected to meet the performance requirements. Research is also needed to check the performance of the technology in the field.
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Hamidi, F.; Aslani, F. TiO2-based Photocatalytic Cementitious Composites: Materials, Properties, Influential Parameters, and Assessment Techniques. Nanomaterials 2019, 9, 1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona-Quiroga, P.; Martínez-Ramírez, S.; Viles, H. Efficiency and durability of a self-cleaning coating on concrete and stones under both natural and artificial ageing trials. Appl. Surf. Sci. 2018, 433, 312–320. [Google Scholar] [CrossRef]
- Tyukavkina, V.V.; Gerasimova, L.G.; Semushin, V.V. Properties of Compositions Based on Cement and Modified Nanodispersed Titanium Dioxide. Inorg. Mater. Appl. Res. 2019, 10, 122–126. [Google Scholar] [CrossRef]
- Saini, A.; Ratan, J.K. Formulation and evaluation of surface-fluorinated microsized-TiO2 based self-cleaning cement: Characterization, self-cleaning, depollution and antimicrobial study. Chem. Pap. 2022, 76, 3201–3214. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.-S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Rodriguez-Garcia, J.; Castillo, A.; Castellote, M. Characteristics and efficiency of photocatalytic cementitious materials: Type of binder, roughness and microstructure. Cem. Concr. Res. 2015, 71, 124–131. [Google Scholar] [CrossRef]
- Jin, Q.; Hordern, S.L.; Tang, Y.; Kurtis, K.E. NOx sequestration by calcium aluminate cementitious materials. Cem. Concr. Res. 2021, 142, 106381. [Google Scholar] [CrossRef]
- Baltes, L.; Patachia, S.; Tierean, M.; Ekincioglu, O.; Ozkul, H.M. Photoactive glazed polymer-cement composite. Appl. Surf. Sci. 2018, 438, 84–95. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, H.; Wang, S.; Du, P.; Lu, L.; Cheng, X. Assessment of Nano-TiO2 Enhanced Performance for Photocatalytic Polymer-Sulphoaluminate Cement Composite Coating. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2439–2446. [Google Scholar] [CrossRef]
- Guo, S.-Y.; Zhang, X.; Ren, J.; Chen, J.-Z.; Zhao, T.-J.; Li, T.-W.; Zhang, L. Preparation of TiO2/epoxy resin composite and its effect on mechanical and bonding properties of OPC mortars. Constr. Build. Mater. 2020, 272, 121960. [Google Scholar] [CrossRef]
- Binas, V.; Papadaki, D.; Maggos, T.; Katsanaki, A.; Kiriakidis, G. Study of innovative photocatalytic cement based coatings: The effect of supporting materials. Constr. Build. Mater. 2018, 168, 923–930. [Google Scholar] [CrossRef]
- Folli, A.; Jakobsen, U.H.; Guerrini, G.L.; Macphee, D. Rhodamine B Discolouration on TiO2 in the Cement Environment: A Look at Fundamental Aspects of the Self-cleaning Effect in Concretes. J. Adv. Oxid. Technol. 2009, 12, 126–133. [Google Scholar] [CrossRef]
- Ratan, J.K.; Saini, A.; Verma, P. Microsized-titanium dioxide based self-cleaning cement: Incorporation of calcined dolomite for enhancement of photocatalytic activity. Mater. Res. Express 2018, 5, 115509. [Google Scholar] [CrossRef]
- Lucas, S.; Ferreira, V.; de Aguiar, J.B. Incorporation of titanium dioxide nanoparticles in mortars—Influence of microstructure in the hardened state properties and photocatalytic activity. Cem. Concr. Res. 2012, 43, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Folli, A. TiO2 Photocatalysis in Portland Cement Systems: Fundamentals of Self-Cleaning Effect and Air Pollution Mitigation. Ph.D. Thesis, University of Milan, Milan, Italy, 2010. [Google Scholar]
- Chen, J.; Poon, C.-S. Photocatalytic Cementitious Materials: Influence of the Microstructure of Cement Paste on Photocatalytic Pollution Degradation. Environ. Sci. Technol. 2009, 43, 8948–8952. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.-Z.; Poon, C.S. Superior photocatalytic NOx removal of cementitious materials prepared with white cement over ordinary Portland cement and the underlying mechanisms. Cem. Concr. Compos. 2018, 90, 42–49. [Google Scholar] [CrossRef]
- Cassar, L.; Pepe, C.; Tognon, G.; Guerrini, G.L.; Amadelli, R. white cement for architectural concrete, possessing photocatalytic properties. In Proceedings of the 11th International Congress on the Chemistry of Cement, Durban, South Africa, 11–16 May 2003. [Google Scholar]
- Lee, B.Y.; Jayapalan, A.R.; Bergin, M.H.; Kurtis, K.E. Photocatalytic cement exposed to nitrogen oxides: Effect of oxidation and binding. Cem. Concr. Res. 2014, 60, 30–36. [Google Scholar] [CrossRef]
- Bianchi, C.; Gatto, S.; Nucci, S.; Cerrato, G.; Capucci, V. Self-cleaning measurements on tiles manufactured with micro-sized photoactive TiO2. Adv. Mater. Res. 2013, 2, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Cordoni, C.; Munafò, P. Self-cleaning and de-polluting stone surfaces: TiO2 nanoparticles for limestone. Constr. Build. Mater. 2012, 37, 51–57. [Google Scholar] [CrossRef]
- Amor, F.; Diouri, A.; Ellouzi, I.; Ouanji, F. Development of Zn-Al-Ti mixed oxides-modified cement phases for surface photocatalytic performance. Case Stud. Constr. Mater. 2018, 9, e00209. [Google Scholar] [CrossRef]
- Janus, M.; Mądraszewski, S.; Zając, K.; Kusiak-Nejman, E.; Morawski, A.W.; Stephan, D. Photocatalytic Activity and Mechanical Properties of Cements Modified with TiO2/N. Materials 2019, 12, 3756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janus, M.; Zając, K.; Zatorska, J.; Kusiak-Nejman, E.; Czyżewski, A.; Morawski, A.W. Cementitious Plates Containing TiO2-N,C Photocatalysts for NOx Degradation. J. Adv. Oxid. Technol. 2015, 18, 227–232. [Google Scholar] [CrossRef]
- Moreira, M.A.; Heitmann, A.P.; Bezerra, A.C.; Patrício, P.S.; de Oliveira, L.C.; Castro, C.S.; de Souza, P.P. Photocatalytic performance of cementitious materials with addition of red mud and Nb2O5 particles. Constr. Build. Mater. 2020, 259, 119851. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.; Tadé, M. Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 2008, 72, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues: II. options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, L.; Wang, J.; Yu, Y.; Zhang, G.; Cheng, X.; Wang, D. BiOBr Precursor Solutions Modified Cement Paste: The Photocatalytic Performance and Effects. Crystals 2021, 11, 969. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, Z.; Zheng, L.; Yang, S.; Su, W.; Li, B.; Ji, T. Interaction between composition and microstructure of cement paste and polymeric carbon nitride. Constr. Build. Mater. 2022, 335, 127464. [Google Scholar] [CrossRef]
- Wang, D.; Hou, P.; Zhang, L.; Xie, N.; Yang, P.; Cheng, X. Photocatalytic activities and chemically-bonded mechanism of SiO2@ TiO2 nanocomposites coated cement-based materials. Mater. Res. Bull. 2018, 102, 262–268. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Q.; Gauvin, F.; Feng, P.; Qianping, R.; Brouwers, H. Nanodispersed TiO2 hydrosol modified Portland cement paste: The underlying role of hydration on self-cleaning mechanisms. Cem. Concr. Res. 2020, 136, 106156. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, S.H.; Jang, Y.I.; Park, H.M. Evaluation of Reducing NO and SO2 Concentration in Nano SiO2-TiO2 Photocatalytic Concrete Blocks. Materials 2021, 14, 7182. [Google Scholar] [CrossRef]
- Janus, M.; Zając, K. Concretes with Photocatalytic Activity, High Performance Concrete Technology and Applications; INTECH: London, UK, 2016. [Google Scholar]
- Mendoza, C.; Valle, A.; Castellote, M.; Bahamonde, A.; Faraldos, M. TiO2 and TiO2–SiO2 coated cement: Comparison of mechanic and photocatalytic properties. Appl. Catal. B Environ. 2014, 178, 155–164. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Ling, T.-C.; Poon, C.S. Photocatalytic NO x degradation of concrete surface layers intermixed and spray-coated with nano-TiO 2: Influence of experimental factors. Cem. Concr. Compos. 2017, 83, 279–289. [Google Scholar] [CrossRef]
- Vulic, T.; Rudic, O.; Vucetic, S.; Lazar, D.; Ranogajec, J. Photocatalytic activity and stability of TiO2/ZnAl layered double hydroxide based coatings on mortar substrates. Cem. Concr. Compos. 2015, 58, 50–58. [Google Scholar] [CrossRef]
- Wang, D.; Hou, P.; Zhang, L.; Yang, P.; Cheng, X. Photocatalytic and hydrophobic activity of cement-based materials from benzyl-terminated-TiO2 spheres with core-shell structures. Constr. Build. Mater. 2017, 148, 176–183. [Google Scholar] [CrossRef]
- Carrascosa, L.A.M.; Zarzuela, R.; Badreldin, N.; Mosquera, M.J. A Simple, Long-Lasting Treatment for Concrete by Combining Hydrophobic Performance with a Photoinduced Superhydrophilic Surface for Easy Removal of Oil Pollutants. ACS Appl. Mater. Interfaces 2020, 12, 19974–19987. [Google Scholar] [CrossRef]
- Feng, S.; Li, F. Photocatalytic dyes degradation on suspended and cement paste immobilized TiO2/g-C3N4 under simulated solar light. J. Environ. Chem. Eng. 2021, 9, 105488. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, D.; Jiang, C.; Lu, X.; Zhang, L.; Cheng, X. Investigation of visible light catalysis of a graphite-nitride-silica composite material and its cement surface treatment. Crystals 2020, 10, 490. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.J.; Rodríguez, R.S.; Darias, R.; Díaz, O.G.; Luzardo, J.M.P.; Rodríguez, J.M.D.; Melián, E.P. Effect of TiO2 Addition on Mortars: Characterization and Photoactivity. Appl. Sci. 2019, 9, 2598. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.; Dylla, H.; Mohammad, L.N.; Rupnow, T. Evaluation of the durability of titanium dioxide photocatalyst coating for concrete pavement. Constr. Build. Mater. 2010, 24, 1456–1461. [Google Scholar] [CrossRef]
- Huang, M.; Yang, Z.; Lu, L.; Xu, J.; Wang, W.; Yang, C. The Preparation of g-C3N4/CoAl-LDH Nanocomposites and Their Depollution Performances in Cement Mortars under UV-Visible Light. Catalysts 2022, 12, 443. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Castellote, M. Quick assessment of the photocatalytic activity of TiO2 construction materials by nitroblue tetrazolium (NBT) ink. Constr. Build. Mater. 2019, 214, 1–8. [Google Scholar] [CrossRef]
- Baglioni, P.; Cassar, L.; Hashimoto, K.; Filippone, F.; Mattioli, G.; Bonapasta, A.A.; Bianchi, C.L.; Ardizzone, S.; Cappelletti, G.; Pirola, C.; et al. International RILEM Symposium on Photocatalysis, Environment and Construction Materials-TDP 2007; RILEM Publications: Bagneux, France, 2007. [Google Scholar]
- Evans, P.; Mantke, S.; Mills, A.; Robinson, A.; Sheel, D. A comparative study of three techniques for determining photocatalytic activity. J. Photochem. Photobiol. A Chem. 2007, 188, 387–391. [Google Scholar] [CrossRef]
- Bengtsson, N.; Castellote, M. Photocatalytic Activity for NO Degradation by Construction Materials: Parametric Study andMultivariable Correlations. J. Adv. Oxid. Technol. 2010, 13, 341–349. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Castellote, M. Quantification of hydroxyl radicals on cementitious materials by fluorescence spectrophotometry as a method to assess the photocatalytic activity. Cem. Concr. Res. 2015, 74, 108–115. [Google Scholar] [CrossRef]
- Moro, C.; Francioso, V.; Lopez-Arias, M.; Velay-Lizancos, M. Modification of self-cleaning activity on cement pastes containing nano-TiO2 due to CO2 curing. Constr. Build. Mater. 2022, 330, 127185. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef] [Green Version]
- Witkowski, H.; Jackiewicz-Rek, W.; Chilmon, K.; Jarosławski, J.; Tryfon-Bojarska, A.; Gąsiński, A. Air Purification Performance of Photocatalytic Concrete Paving Blocks after Seven Years of Service. Appl. Sci. 2019, 9, 1735. [Google Scholar] [CrossRef] [Green Version]
- Mills, A.; Elouali, S. The nitric oxide ISO photocatalytic reactor system: Measurement of NOx removal activity and capacity. J. Photochem. Photobiol. A Chem. 2015, 305, 29–36. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Luongo, N.; Massari, S.; Spagnolo, S.L.; Daniotti, B.; Pedeferri, M. Durability of self-cleaning cement-based materials. Constr. Build. Mater. 2021, 280, 122442. [Google Scholar] [CrossRef]
- ISO 22197-1:2016; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air-Purification Performance of Semiconducting Photocatalytic Materials—Part 1: Removal of Nitric Oxide. ISO: Geneva, Switzerland, 2016.
- Şahin, O.; Bay, S.; Ilcan, H.; Yıldırım, G.; Şahmaran, M. Influence of mixing methods on the NOx reduction capability and electrical properties of photocatalytic cementitious systems. Cem. Concr. Compos. 2020, 115, 103840. [Google Scholar] [CrossRef]
- Seo, D.; Yun, T.S. NOx removal rate of photocatalytic cementitious materials with TiO 2 in wet condition. Build. Environ. 2017, 112, 233–240. [Google Scholar] [CrossRef]
- Mothes, F.; Ifang, S.; Gallus, M.; Golly, B.; Boréave, A.; Kurtenbach, R.; Kleffmann, J.; George, C.; Herrmann, H. Bed flow photoreactor experiments to assess the photocatalytic nitrogen oxides abatement under simulated atmospheric conditions. Appl. Catal. B Environ. 2018, 231, 161–172. [Google Scholar] [CrossRef]
- ISO 22197-3:2019; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air-Purification Performance of Semiconducting Photocatalytic Materials—Part 3: Removal of Toluene. ISO: Geneva, Switzerland, 2019.
- Martinez, T.; Bertron, A.; Escadeillas, G.; Ringot, E.; Simon, V. BTEX abatement by photocatalytic TiO2-bearing coatings applied to cement mortars. Build. Environ. 2014, 71, 186–192. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Bondioli, F.; D’Orazio, M. Durability of self-cleaning TiO2 coatings on fired clay brick façades: Effects of UV exposure and wet & dry cycles. Build. Environ. 2014, 71, 193–203. [Google Scholar] [CrossRef]
- Hegyi, A.; Lăzărescu, A.-V.; Szilagyi, H.; Grebenişan, E.; Goia, J.; Mircea, A. Influence of TiO2 Nanoparticles on the Resistance of Cementitious Composite Materials to the Action of Bacteria. Materials 2021, 14, 1074. [Google Scholar] [CrossRef]
- Ratan, J.K.; Saini, A. Enhancement of photocatalytic activity of self-cleaning cement. Mater. Lett. 2019, 244, 178–181. [Google Scholar] [CrossRef]
- Wang, D.; Hou, P.; Stephan, D.; Huang, S.; Zhang, L.; Yang, P.; Cheng, X. SiO2/TiO2 composite powders deposited on cement-based materials: Rhodamine B removal and the bonding mechanism. Constr. Build. Mater. 2020, 241, 118124. [Google Scholar] [CrossRef]
- Feng, S.; Song, J.; Liu, F.; Fu, X.; Guo, H.; Zhu, J.; Zeng, Q.; Peng, X.; Wang, X.; Ouyang, Y.; et al. Photocatalytic properties, mechanical strength and durability of TiO2/cement composites prepared by a spraying method for removal of organic pollutants. Chemosphere 2020, 254, 126813. [Google Scholar] [CrossRef]
- Lettieri, M.; Colangiuli, D.; Masieri, M.; Calia, A. Field performances of nanosized TiO2 coated limestone for a self-cleaning building surface in an urban environment. Build. Environ. 2018, 147, 506–516. [Google Scholar] [CrossRef]
- García, L.; Pastor, J.; Peña, J. Self cleaning and depolluting glass reinforced concrete panels: Fabrication, optimization and durability evaluation. Constr. Build. Mater. 2018, 162, 9–19. [Google Scholar] [CrossRef]
- Laplaza, A.; Jimenez-Relinque, E.; Campos, J.; Castellote, M. Photocatalytic behavior of colored mortars containing TiO2 and iron oxide based pigments. Constr. Build. Mater. 2017, 144, 300–310. [Google Scholar] [CrossRef]
- Sikora, P.; Cendrowski, K.; Markowska-Szczupak, A.; Horszczaruk, E.; Mijowska, E. The effects of silica/titania nanocomposite on the mechanical and bactericidal properties of cement mortars. Constr. Build. Mater. 2017, 150, 738–746. [Google Scholar] [CrossRef]
- Taurino, R.; Barbieri, L.; Bondioli, F. Surface properties of new green building material after TiO2–SiO2 coatings deposition. Ceram. Int. 2016, 42, 4866–4874. [Google Scholar] [CrossRef]
- Calia, A.; Lettieri, M.; Masieri, M. Durability assessment of nanostructured TiO2 coatings applied on limestones to enhance building surface with self-cleaning ability. Build. Environ. 2016, 110, 1–10. [Google Scholar] [CrossRef]
- Cohen, J.D.; Gallego, G.A.S.; Tobón, J.I. Evaluation of Photocatalytic Properties of Portland Cement Blended with Titanium Oxynitride (TiO2−xNy) Nanoparticles. Coatings 2015, 5, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Diamanti, M.; Del Curto, B.; Ormellese, M.; Pedeferri, M. Photocatalytic and self-cleaning activity of colored mortars containing TiO2. Constr. Build. Mater. 2013, 46, 167–174. [Google Scholar] [CrossRef]
- Le Pivert, M.; Poupart, R.; Capochichi-Gnambodoe, M.; Martin, N.; Leprince-Wang, Y. Direct growth of ZnO nanowires on civil engineering materials: Smart materials for supported photodegradation. Microsyst. Nanoeng. 2019, 5, 57. [Google Scholar] [CrossRef]
- Calvo, J.G.; Carballosa, P.; Castillo, A.; Revuelta, D.; Gutiérrez, J.; Castellote, M. Expansive concretes with photocatalytic activity for pavements: Enhanced performance and modifications of the expansive hydrates composition. Constr. Build. Mater. 2019, 218, 394–403. [Google Scholar] [CrossRef]
- Koli, V.B.; Mavengere, S.; Kim, J.-S. An efficient one-pot N doped TiO2-SiO2 synthesis and its application for photocatalytic concrete. Appl. Surf. Sci. 2019, 491, 60–66. [Google Scholar] [CrossRef]
- Kapridaki, C.; Xynidis, N.; Vazgiouraki, E.; Kallithrakas-Kontos, N.; Maravelaki-Kalaitzaki, P. Characterization of Photoactive Fe-TiO2 Lime Coatings for Building Protection: The Role of Iron Content. Materials 2019, 12, 1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanfir, A.-V.; Voicu, G.; Bădănoiu, A.-I.; Gogan, D.; Oprea, O.; Vasile, E. Synthesis and characterization of titania-silica fume composites and their influence on the strength of self-cleaning mortar. Compos. Part B Eng. 2018, 140, 157–163. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; D’Orazio, M. The role of roughness and porosity on the self-cleaning and anti-biofouling efficiency of TiO 2 -Cu and TiO 2 -Ag nanocoatings applied on fired bricks. Constr. Build. Mater. 2016, 129, 116–124. [Google Scholar] [CrossRef]
- Cerro-Prada, E.; García-Salgado, S.; Quijano, M.; Varela, F. Controlled Synthesis and Microstructural Properties of Sol-Gel TiO2 Nanoparticles for Photocatalytic Cement Composites. Nanomaterials 2018, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfieri, I.; Lorenzi, A.; Ranzenigo, L.; Lazzarini, L.; Predieri, G.; Lottici, P.P. Synthesis and characterization of photocatalytic hydrophobic hybrid TiO2 -SiO2 coatings for building applications. Build. Environ. 2017, 111, 72–79. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Peng, B.; Chai, L.; Liu, H. Self-cleaning performance of TiO2 -coating cement materials prepared based on solidification/stabilization of electrolytic manganese residue. Constr. Build. Mater. 2016, 106, 236–242. [Google Scholar] [CrossRef]
- Yousefi, A.; Allahverdi, A.; Hejazi, P. Effective dispersion of nano-TiO2 powder for enhancement of photocatalytic properties in cement mixes. Constr. Build. Mater. 2013, 41, 224–230. [Google Scholar] [CrossRef]
- Werle, A.; de Souza, M.; Loh, K.; Ando, R.; John, V. The performance of a self-cleaning cool cementitious surface. Energy Build. 2016, 114, 200–205. [Google Scholar] [CrossRef]
- Mills, A.; Hepburn, J.; Hazafy, D.; O’Rourke, C.; Wells, N.; Krysa, J.; Baudys, M.; Zlamal, M.; Bartkova, H.; Hill, C.E.; et al. Photocatalytic activity indicator inks for probing a wide range of surfaces. J. Photochem. Photobiol. A Chem. 2014, 290, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Mills, A.; O’Rourke, C.; Lawrie, K.; Elouali, S. Assessment of the Activity of Photocatalytic Paint Using a Simple Smart Ink Designed for High Activity Surfaces. ACS Appl. Mater. Interfaces 2013, 6, 545–552. [Google Scholar] [CrossRef]
- Mills, A.; Wells, N.; O’Rourke, C. Correlation between ΔAbs, ΔRGB (red) and stearic acid destruction rates using commercial self-cleaning glass as the photocatalyst. Catal. Today 2014, 230, 245–249. [Google Scholar] [CrossRef]
- Mills, A.; Hill, C.; Robertson, P.K. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A: Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]
- Mills, A. An overview of the methylene blue ISO test for assessing the activities of photocatalytic films. Appl. Catal. B Environ. 2012, 128, 144–149. [Google Scholar] [CrossRef]
- Bolashikov, Z.; Melikov, A.; Bolashikov, Z.; Melikov, A. Methods for air cleaning and protection of building occupants from airborne pathogens. Build. Environ. 2009, 44, 1378–1385. [Google Scholar] [CrossRef]
- Wiszniewska, M.; Walusiak-Skorupa, J.; Gutarowska, B.; Krakowiak, A.; Pałczyński, C. Is the risk of allergic hypersensitivity to fungi increased by indoor exposure to moulds? Int. J. Occup. Med. Environ. Health 2009, 22, 343–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özyıldız, F.; Güden, M.; Uzel, A.; Karaboz, I.; Akil, O.; Bulut, H. Antimicrobial activity of TiO2-coated orthodontic ceramic brackets against Streptococcus mutans and Candida albicans. Biotechnol. Bioprocess Eng. 2010, 15, 680–685. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, P.; Byrne, C.; Breen, A.; Pillai, S.C. Antimicrobial activity of photocatalysts: Fundamentals, mechanisms, kinetics and recent advances. Appl. Catal. B Environ. 2018, 225, 51–75. [Google Scholar] [CrossRef]
- Ganji, N.; Allahverdi, A.; Naeimpoor, F.; Mahinroosta, M. Photocatalytic effect of nano-TiO2 loaded cement on dye decolorization and Escherichia coli inactivation under UV irradiation. Res. Chem. Intermed. 2015, 42, 5395–5412. [Google Scholar] [CrossRef]
- Velez-Pena, E.; Perez-Obando, J.; Pais-Ospina, D.; Marin-Silva, D.A.; Pinotti, A.; Canneva, A.; Rengifo-Herrera, J.A. Self-cleaning and antimicrobial photo-induced properties under indoor lighting irradiation of chitosan films containing Mel-on/TiO2 composites. Appl. Surf. Sci. 2020, 508, 144895. [Google Scholar] [CrossRef]
- Janus, M.; Kusiak-Nejman, E.; Rokicka-Konieczna, P.; Markowska-Szczupak, A.; Zając, K.; Morawski, A.W. Bacterial Inactivation on Concrete Plates Loaded with Modified TiO2 Photocatalysts under Visible Light Irradiation. Molecules 2019, 24, 3026. [Google Scholar] [CrossRef] [Green Version]
- ISO 27447:2019; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Antibacterial Activity of Semiconducting Photocatalytic Materials. ISO: Geneva, Switzerland, 2019.
- Luna, M.; Delgado, J.J.; Gil, M.L.A.; Mosquera, M.J. TiO2-SiO2 Coatings with a Low Content of AuNPs for Producing Self-Cleaning Building Materials. Nanomaterials 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozo-Antonio, J.; Dionísio, A. Self-cleaning property of mortars with TiO2 addition using real diesel exhaust soot. J. Clean. Prod. 2017, 161, 850–859. [Google Scholar] [CrossRef]
- Smits, M.; Chan, C.K.; Tytgat, T.; Craeye, B.; Costarramone, N.; Lacombe, S.; Lenaerts, S. Photocatalytic degradation of soot deposition: Self-cleaning effect on titanium dioxide coated cementitious materials. Chem. Eng. J. 2013, 222, 411–418. [Google Scholar] [CrossRef]
- Luna, M.; Mosquera, M.J.; Vidal, H.; Gatica, J.M. Au-TiO2/SiO2 photocatalysts for building materials: Self-cleaning and de-polluting performance. Build. Environ. 2019, 164, 106347. [Google Scholar] [CrossRef]
- Truppi, A.; Luna, M.; Petronella, F.; Falcicchio, A.; Giannini, C.; Comparelli, R.; Mosquera, M.J. Photocatalytic Activity of TiO2/AuNRs–SiO2 Nanocomposites Applied to Building Materials. Coatings 2018, 8, 296. [Google Scholar] [CrossRef] [Green Version]
- Smits, M.; Huygh, D.; Craeye, B.; Lenaerts, S. Effect of process parameters on the photocatalytic soot degradation on self-cleaning cementitious materials. Catal. Today 2014, 230, 250–255. [Google Scholar] [CrossRef]
- Janus, M.; Mądraszewski, S.; Zając, K.; Kusiak-Nejman, E. A New Preparation Method of Cement with Photocatalytic Activity. Materials 2020, 13, 5540. [Google Scholar] [CrossRef]
- Goyal, R.; Verma, V.K.; Singh, N. Effect of nano TiO2 & ZnO on the hydration properties of Portland cement. Mater. Today Proc. 2022; in press. [Google Scholar] [CrossRef]
- Essawy, A.A.; El Aleem, S.A. Physico-mechanical properties, potent adsorptive and photocatalytic efficacies of sulfate resisting cement blends containing micro silica and nano-TiO2. Constr. Build. Mater. 2014, 52, 73–81. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S. RETRACTED ARTICLE: Effects of Al2O3 nanoparticles on properties of self compacting concrete with ground granulated blast furnace slag (GGBFS) as binder. Sci. China Technol. Sci. 2011, 54, 2327–2338. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.; Biradar, A.V.; Peng, D.-L.; Zboril, R.; Varma, R.S. Core–shell nanoparticles: Synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, G.; Feng, C.; Wang, L.; Zhang, W. Effect of Delaminated MXene (Ti3C2) on the Performance of Cement Paste. J. Nanomater. 2019, 2019, 3074206. [Google Scholar] [CrossRef] [Green Version]
- Rashad, A.M. A synopsis about the effect of nano-titanium dioxide on some properties of cementitious materials-a short guide for civil engineer. Rev. Adv. Mater. Sci. 2015, 40, 72–88. [Google Scholar]
- Hegyi, A.; Szilagyi, H.; Grebenisan, E.; Sandu, A.V.; Lăzărescu, A.-V.; Romila, C. Influence of TiO2 Nanoparticles Addition on the Hydrophilicity of Cementitious Composites Surfaces. Appl. Sci. 2020, 10, 4501. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Mukherjee, S.; Nikraz, H. Effects of nano-Al2O3 on early-age microstructural properties of cement paste. Constr. Build. Mater. 2014, 52, 189–193. [Google Scholar] [CrossRef]
- Hohol, M.; Sanytsky, M.; Kropyvnytska, T.; Barylyak, A.; Bobitski, Y. The effect of sulfur-and carbon-codoped TiO2 nano-composite on the photocatalytic and mechanical properties of cement mortars. East.-Eur. J. Enterp. Technol. 2020, 4, 6–14. [Google Scholar]
- Meng, J.; Zhong, J.; Xiao, H.; Ou, J. Interfacial design of nano-TiO2 modified fly ash-cement based low carbon composites. Constr. Build. Mater. 2020, 270, 121470. [Google Scholar] [CrossRef]
- Grebenişan, E.; Szilagyi, H.; Hegyi, A.-C.; Mircea, C.; Baeră, C. Opportunities regarding the potential use of the self-cleaning concept within urban contemporary architecture in Romania. MATEC Web Conf. 2019, 289, 05003. [Google Scholar] [CrossRef]
- Ng, D.S.; Paul, S.C.; Anggraini, V.; Kong, S.Y.; Qureshi, T.S.; Rodriguez, C.R.; Liu, Q.-F.; Šavija, B. Influence of SiO2, TiO2 and Fe2O3 nanoparticles on the properties of fly ash blended cement mortars. Constr. Build. Mater. 2020, 258, 119627. [Google Scholar] [CrossRef]
- Jalal, M.; Ramezanianpour, A.A.; Pool, M.K. Effects of titanium dioxide nanopowder on rheological properties of self compacting concrete. Am. J. Sci. 2012, 8, 285–288. [Google Scholar]
- Nazari, A.; Riahi, S.; Riahi, S.; Shamekhi, S.F.; Khademno, A. Assessment of the effects of the cement paste composite in presence TiO2 nanoparticles. Am. J. Sci. 2010, 6, 43–46. [Google Scholar]
- Salemi, N.; Behfarnia, K.; Zaree, S.A. Effect of nanoparticles on frost durability of concrete. Asian J. Civ. Eng. BHRC 2014, 15, 411. [Google Scholar]
- Li, H.; Zhang, M.-H.; Ou, J.-P. Flexural fatigue performance of concrete containing nano-particles for pavement. Int. J. Fatigue 2007, 29, 1292–1301. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.-H.; Ou, J.-P. Abrasion resistance of concrete containing nano-particles for pavement. Wear 2006, 260, 1262–1266. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Li, H. Pore structure and chloride permeability of concrete containing nano-particles for pavement. Constr. Build. Mater. 2011, 25, 608–616. [Google Scholar] [CrossRef]
- Meng, T.; Yu, Y.; Qian, X.; Zhan, S.; Qian, K. Effect of nano-TiO2 on the mechanical properties of cement mortar. Constr. Build. Mater. 2012, 29, 241–245. [Google Scholar] [CrossRef]
- Sorathiya, J.; Shah, S.; Kacha, S. Effect on addition of nano “titanium dioxide”(TiO2) on compressive strength of cementitious concrete. Kalpa Publ. Civ. Eng. 2017, 1, 219–225. [Google Scholar]
- Zhang, M.H.; Tanadi, D.; Li, W. Effect of photocatalyst TiO2 on workability, strength, and self-cleaning efficiency of mortars for applications in tropical environment. In Proceedings of the 35th Conference on Our World in Concrete & Structures, Singapore, 25–27 August 2010. [Google Scholar]
- PN-EN 196-3: 2016; Cement Testing Methods—Part 3: Determination of Setting Times and Volume Stability. European Committee for Standarization: Brussels, Belgium, 2016.
- Lee, B.Y. Effect of Titanium Dioxide Nanoparticles on Early Age and Long Term Properties of Cementitious Materials. Ph.D. Thesis, School of Civil & Environmental Engineering, Georgia Institute of Technology, Atlanta, GA, USA, 2012. [Google Scholar]
- Chen, J.; Kou, S.-C.; Poon, C.-S. Hydration and properties of nano-TiO2 blended cement composites. Cem. Concr. Compos. 2012, 34, 642–649. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S.; Riahi, S.; Shamekhi, S.F.; Khademno, A. Improvement the mechanical properties of the cementitious composite by using TiO2 nanoparticles. Am. J. Sci. 2010, 6, 98–101. [Google Scholar]
- Wang, L.; Zhang, H.; Gao, Y. Effect of TiO2 Nanoparticles on Physical and Mechanical Properties of Cement at Low Temperatures. Adv. Mater. Sci. Eng. 2018, 2018, 8934689. [Google Scholar] [CrossRef] [Green Version]
- Zając, K.; Czyżewski, A.; Kaszyńska, M.; Janus, M. Combined Effect of Photocatalyst, Superplasticizer, and Glass Fiber on the Photocatalytic Activity and Technical Parameters of Gypsum. Catalysts 2020, 10, 385. [Google Scholar] [CrossRef] [Green Version]
- Yuenyongsuwan, J.; Sinthupinyo, S.; O’Rear, E.A.; Pongprayoon, T. Hydration accelerator and photocatalyst of nanotitanium dioxide synthesized via surfactant-assisted method in cement mortar. Cem. Concr. Compos. 2018, 96, 182–193. [Google Scholar] [CrossRef]
- Teixeira, K.P.; Rocha, I.P.; Carneiro, L.D.S.; Flores, J.; Dauer, E.A.; Ghahremaninezhad, A. The Effect of Curing Temperature on the Properties of Cement Pastes Modified with TiO2 Nanoparticles. Materials 2016, 9, 952. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jee, H.; Lim, M.; Kim, J.H.; Kwon, S.J.; Lee, K.M.; Nezhad, E.Z.; Bae, S. Photocatalytic Performance Evaluation of Titanium Dioxide Nanotube-Reinforced Cement Paste. Materials 2020, 13, 5423. [Google Scholar] [CrossRef]
- Liu, P.; Gao, Y.; Wang, F.; Zhang, W.; Yang, L.; Yang, J.; Liu, Y. Photocatalytic activity of Portland cement loaded with 3D hierarchical Bi2WO6 microspheres under visible light. Constr. Build. Mater. 2016, 120, 42–47. [Google Scholar] [CrossRef]
- Behfarnia, K.; Azarkeivan, A.; Keivan, A. The effects of TiO2 nad ZnO nanoparticles on physical and mechanical properties of normal conctere. Asian. J. Civil. Eng. 2013, 14, 517–531. [Google Scholar]
- Gawlicki, M.; Czamarska, D. Effect of ZnO on the hydration of Portland cement. J. Therm. Anal. 1992, 38, 2157–2161. [Google Scholar] [CrossRef]
- PN-EN 196-1: 2016-07; Cement Testing Methods. Part 1: Determination of Strength. European Committee for Standarization: Brussels, Belgium, 2016.
- Lawrence, P.; Cyr, M.; Ringot, E. Mineral admixtures in mortars: Effect of inert materials on short-term hydration. Cem. Concr. Res. 2003, 33, 1939–1947. [Google Scholar] [CrossRef]
- Bautista-Gutierrez, K.P.; Herrera-May, A.L.; Santamaría-López, J.M.; Honorato-Moreno, A.; Zamora-Castro, S.A. Recent Progress in Nanomaterials for Modern Concrete Infrastructure: Advantages and Challenges. Materials 2019, 12, 3548. [Google Scholar] [CrossRef] [Green Version]
- Singh, L.; Karade, S.; Bhattacharyya, S.; Yousuf, M.; Ahalawat, S. Beneficial role of nanosilica in cement based materials—A review. Constr. Build. Mater. 2013, 47, 1069–1077. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S. RETRACTED: TiO2 nanoparticles effects on physical, thermal and mechanical properties of self compacting concrete with ground granulated blast furnace slag as binder. Energy Build. 2011, 43, 995–1002. [Google Scholar] [CrossRef]
- Feng, D.; Xie, N.; Gong, C.; Leng, Z.; Xiao, H.; Li, H.; Shi, X. Portland Cement Paste Modified by TiO2 Nanoparticles: A Microstructure Perspective. Ind. Eng. Chem. Res. 2013, 52, 11575–11582. [Google Scholar] [CrossRef]
- Gu, C. Effect of nano-tio₂ on the durability of ultra-high performance concrete with and without a flexural load. Ceram. Silik. 2018, 62, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Mohseni, E.; Miyandehi, B.M.; Yang, J.; Yazdi, M.A. Single and combined effects of nano-SiO2, nano-Al2O3 and nano-TiO2 on The mechanical, rheological and durability properties of self-compacting mortar containing fly ash. Constr. Build. Mater. 2015, 84, 331–340. [Google Scholar] [CrossRef]
- Zhao, A.; Yang, J.; Yang, E.-H. Self-cleaning engineered cementitious composites. Cem. Concr. Compos. 2015, 64, 74–83. [Google Scholar] [CrossRef]
- Ma, B.; Li, H.; Mei, J.; Li, X.; Chen, F. Effects of Nano-TiO2 on the Toughness and Durability of Cement-Based Material. Adv. Mater. Sci. Eng. 2015, 2015, 583106. [Google Scholar] [CrossRef] [Green Version]
- Chinthakunta, R.; Ravella, D.P.; Chand, M.S.R.; Yadav, M.J. Performance evaluation of self-compacting concrete containing fly ash, silica fume and nano titanium oxide. Mater. Today: Proc. 2021, 43, 2348–2354. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, P.; Guo, W.; Bao, J.; Qu, C. Effect of Nano-CaCO3 on the Mechanical Properties and Durability of Concrete Incorporating Fly Ash. Adv. Mater. Sci. Eng. 2020, 2020, 7365862. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Li, Z.; Zhang, L.; Zeng, S.; Yu, X.; Han, B.; Ou, J. Reactive powder concrete reinforced with nano SiO2-coated TiO2. Constr. Build. Mater. 2017, 148, 104–112. [Google Scholar] [CrossRef]
- Sun, J.; Xu, K.; Shi, C.; Ma, J.; Li, W.; Shen, X. Influence of core/shell TiO2@SiO2 nanoparticles on cement hydration. Constr. Build. Mater. 2017, 156, 114–122. [Google Scholar] [CrossRef]
- Shchelokova, E.; Tyukavkina, V.; Tsyryatyeva, A.; Kasikov, A. Synthesis and characterization of SiO2-TiO2 nanoparticles and their effect on the strength of self-cleaning cement composites. Constr. Build. Mater. 2021, 283, 122769. [Google Scholar] [CrossRef]
- Yang, L.; Jia, Z.; Zhang, Y.; Dai, J. Effects of nano-TiO2 on strength, shrinkage and microstructure of alkali activated slag pastes. Cem. Concr. Compos. 2015, 57, 1–7. [Google Scholar] [CrossRef]
- Rahim, A.; Nair, S.R. Influence of nano-materials in high strength concrete. J. Chem. Pharm. Sci. 2016, 3, 15–22. [Google Scholar]
- Rao, S.; Silva, P.; de Brito, J. Experimental study of the mechanical properties and durability of self-compacting mortars with nano materials (SiO2 and TiO2). Constr. Build. Mater. 2015, 96, 508–517. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Luo, W.; Zhou, W. Effects of adding nano-TiO 2 on compressive strength, drying shrinkage, carbonation and microstructure of fluidized bed fly ash based geopolymer paste. Constr. Build. Mater. 2016, 106, 115–125. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, Z.; Zhang, W.; Gu, X.; Dou, X. Numerical analysis of the effect of coarse aggregate distribution on concrete carbonation. Constr. Build. Mater. 2012, 37, 27–35. [Google Scholar] [CrossRef]
- Al Fuhaid, A.F.; Niaz, A. Carbonation and Corrosion Problems in Reinforced Concrete Structures. Buildings 2022, 12, 586. [Google Scholar] [CrossRef]
- Diamanti, M.; Lollini, F.; Pedeferri, M.; Bertolini, L. Mutual interactions between carbonation and titanium dioxide photoactivity in concrete. Build. Environ. 2013, 62, 174–181. [Google Scholar] [CrossRef]
- Moro, C.; Francioso, V.; Velay-Lizancos, M. Impact of nano-TiO2 addition on the reduction of net CO2 emissions of cement pastes after CO2 curing. Cem. Concr. Compos. 2021, 123, 104160. [Google Scholar] [CrossRef]
- Lackhoff, M.; Prieto, X.; Nestle, N.; Dehn, F.; Niessner, R. Photocatalytic activity of semiconductor-modified cement—Influence of semiconductor type and cement ageing. Appl. Catal. B Environ. 2003, 43, 205–216. [Google Scholar] [CrossRef]
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Guo, S.; Wu, Z.; Zhao, W. TiO2-based building materials: Above and beyond traditional applications. Sci. Bull. 2009, 54, 1137–1142. [Google Scholar] [CrossRef] [Green Version]
- Boonen, E.; Beeldens, A. Photocatalytic roads: From lab tests to real scale applications. Eur. Transp. Res. Rev. 2012, 5, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus. Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Witkowski, H.; Jarosławski, J.; Tryfon-Bojarska, A. Application of Photocatalytic Concrete Paving Blocks in Poland—Verification of Effectiveness of Nitric Oxides Reduction and Novel Test Method. Materials 2020, 13, 5183. [Google Scholar] [CrossRef]
- Cardellicchio, L. Building defects in new iconic structures: The technical challenge and the economic impact of restoring the Jubilee Church in Rome. Arch. Eng. Des. Manag. 2020, 17, 146–166. [Google Scholar] [CrossRef]
- Boonen, E.; Akylas, V.; Barmpas, F.; Boréave, A.; Bottalico, L.; Cazaunau, M.; Chen, H.; Daële, V.; De Marco, T.; Doussin, J.F.; et al. Construction of a photocatalytic de-polluting field site in the Leopold II tunnel in Brussels. J. Environ. Manag. 2015, 155, 136–144. [Google Scholar] [CrossRef]
- George, C.; Beeldens, A.; Barmpas, F.; Doussin, J.-F.; Manganelli, G.; Herrmann, H.; Kleffmann, J.; Mellouki, A. Impact of photocatalytic remediation of pollutants on urban air quality. Front. Environ. Sci. Eng. 2016, 10, 2. [Google Scholar] [CrossRef]
- Guerrini, G.L. Some observations regarding in–service performance Photocatalytic paving block surfaces. Betonw. Fert. Teil-Tech. BFT 2009, 5, 16–25. [Google Scholar]
- Guerrini, G.L. Photocatalytic performances in a city tunnel in Rome: NOx monitoring results. Constr. Build. Mater. 2012, 27, 165–175. [Google Scholar] [CrossRef]
- UNI 11247: 2010; Determination of the Degradation of Nitrogen Oxides in the Air by Inorganic Photocatalytic Materials: Continuous Flow Test Method. Ente Nazionale Italiano di Unificazione: Milano, Italy, 2010.
- Folli, A.; Strøm, M.; Madsen, T.P.; Henriksen, T.; Lang, J.; Emenius, J.; Klevebrant, T.; Nilsson, Å. Field study of air purifying paving elements containing TiO2. Atmos. Environ. 2015, 107, 44–51. [Google Scholar] [CrossRef]
- Ballari, M.M.; Brouwers, H.J.H. Full scale demonstration of air-purifying pavement. J. Hazard. Mater. 2013, 254–255, 406–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Zhang, J.; Wang, Z.; Liu, Y.; Wang, P.; Cao, J.J.; Ho, W.K. g-C3N4/TiO2 composite film in the fabrication of a pho-to-catalytic air-purifying pavements. Sol. RRL 2020, 4, 2000170. [Google Scholar] [CrossRef]
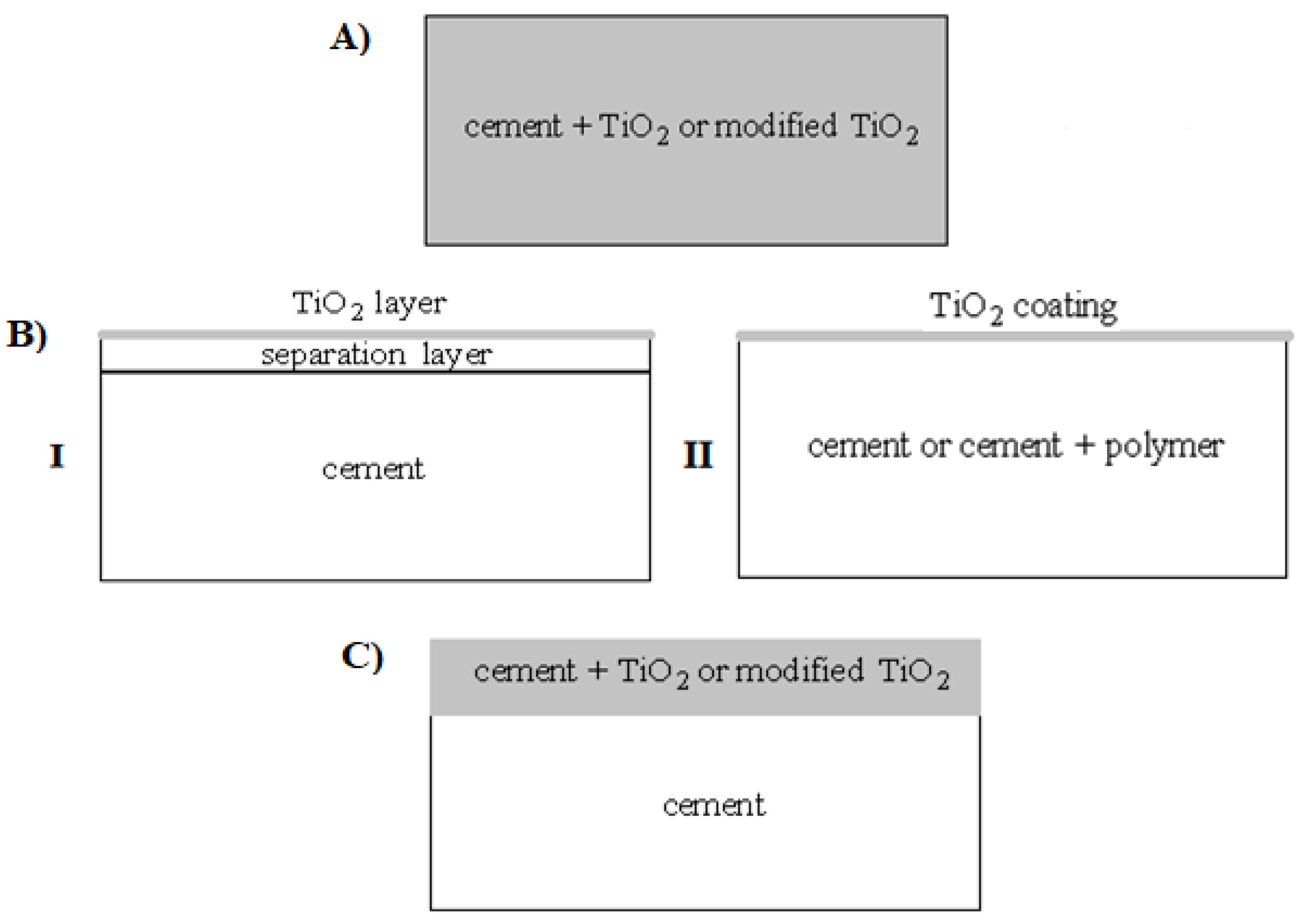





| Type of Method | Short Description |
|---|---|
| (A) Incorporation method. | Adding a photocatalyst during the manufacture of cement; cement replacement by mass with photocatalytic TiO2 particles (micro- or nanosized). |
| (B) Photocatalyst coating technology—cement coating with a thin layer of TiO2 materials with (I) or without (II) a separation layer. | Creating coatings (generally 200 nm thick) by sprinkling with powder, applying paints, enamels, TiO2 suspensions, or special composites, e.g., TiO2/ZnAl. The coatings are applied by techniques such as direct painting on the surface of the cement matrix—by wet coating method; by immersion, spraying, spray pyrolysis, electrodeposition, or chemical vapor deposition (CVD). |
| (C) Addition of the photocatalyst only to the top of the cement mortar layer. | Addition of TiO2 or modified TiO2 to the surface layer; lower layer is unmodified concrete; the top part of concrete consists of cement with photocatalyst. |
| Gas concentration [ppm] | 1.0 |
| Gas flow rate [dm3/min] | 3.0 |
| Duration of the test | 5 h |
| Radiation intensity [mV/cm2] | 1 |
| Sample surface [cm2] | 50 |
| Pretreatment of the sample | 16–24 h of UV irradiation with a power of ≥1.5 mW/cm2 without gas flow |
| Temperature | 25 °C |
| Analytical method | NOx chemiluminescence analyzer |
| Author and Used Photocatalytic Material | Building Material | Age (Days) | Exemplary Dose (wt.%) | Flexural Strength (MPa) | |
|---|---|---|---|---|---|
| Photocatalytic Sample—Description of the Change or Value of the Bending Strength Respective Dose | Reference Sample | ||||
| Wang et al. [130] n-TiO2 (10–25 nm, 200 m2/g) | Cement mortar | 56 | 1, 2, 3 | 12.3, 13.8, 13.6 | 10.7 |
| Meng et al. [114] nano-TiO2 (20–50 nm) 39.91 m2/g | Cement paste, cement + fly ash 20 wt.% | 30 min during ball milling | 1 | increasing bending strength by 37.74% | 9.99 |
| Han et al. [151] nano-SiO2 coated by TiO2 | Reactive concrete powder, w/c = 0.3 | 28 | 1, 3, 5 | 9.60, 12.45, 14.38 | 6.69 |
| Hernandez [42] Aeroxide TiO2 P25 | White and grey cement mortar w/c in layer =1.3 | 28 | 5, 10 | G-8.21 (5%) 8.29 (10%) W-8.70 i 7.53 | G = 9.05 W = 8.65 |
| 365 | G-11.20 (5%) 10.91 (10%) W-10.69 i 10.32 | G = 12.26 W = 12.01 | |||
| Yang et al. [153] TiO2 (20–100 nm) | slag paste activated with alkali | 3 | 0.5 | 7.71 | 6.17 |
| 7 | 12.46 | 10 | |||
| 28 | 17.32 | 12.58 | |||
| Zhang et al. [122] n-TiO2 or n-SiO2 | Concrete | 28 | 1 | n-TiO2: 6.02 n-SiO2: 5.69 | 5.46 |
| Nazari i Riahi [118] nano-TiO2 (15 nm) 155 m2/g | Cement mortar, 1 wt.% superplasticizer, w/c = 0.4 | 28 | 1–5 | increase in bending strength to 4 wt.%, the highest value after 28 days | 4.2 |
| Nazari i Riahi [129] nano-TiO2 (15 nm) | Concrete with 15, 30.45.60 wt.% replacement with blast furnace slag | 28 | 1–4 | up to 3 wt.% TiO2 and 45 wt.% slag bending strength increased | 4.2 |
| 90 | 5.6 | ||||
| Feng et al. [143] nano-TiO2 (20–50 nm) | Cement paste, w/c = 0.4 | 28 | 0.1 | 12.05 | 11.53 |
| 0.5 | 12.45 | ||||
| 1 | 12.48 | ||||
| 5 | 12.30 | ||||
| Lucas et al. [14] P25 (85% anatase, 15% rutile) (21 nm) | Cement, cement-lime, gypsum, or gypsum-lime mortar | 28 | 0.5–5 | Cement and lime-cement mortars show a loss of bending strength in addition to more than 1 wt.%; the gypsum plaster showed a 60% reduction in strength at 0.5% wt., which indicates a greater difficulty for incorporation of nanoparticles | 8.0 |
| Guo et al. [10] nano-TiO2 (100 nm) | Cement mortar modified with epoxy resin TiO2 | 7, 28 | 0, 1, 3 or 5 wt.%. in admixture with pure epoxy resin | increase with increasing dose and curing period | 8.8 9.5 |
| Ma et al. [147] smoky nano-TiO2 50 m2/g | Cement mortar and concrete | 3 28 90 | 1–5 | increase with increasing dose up to 4 wt.% and curing period | 2.82 4.42 6.28 |
| Rahim and Nair [154] nano-TiO2, nano-Al2O3 or nano-SiO2 | Cement mortar partially replaced by fly ash and blast furnace slag | 28 | 2, 3, 4, 5, 6 | After 28 days of hardening to 4 wt.%. nano-TiO2, 3 wt.% Al2O3, and nano-SiO2, an increase in flexural strength was observed | 9.0 |
| Sikora et al. [69] TiO2 P25 (21 nm) n-SiO2 mSiO2/TiO2 (mesoporous silica nanospheres modified with titanium dioxide) | Cement mortar | 28 | 3 | TiO2 P25: 7.0 n-SiO2: 7.0 mSiO2/TiO2: 7.3 | 7.1 |
| Ng et al. [116] TiO2 (15 nm) NT Fe2O3 (20–40 nm) NF SiO2 (20–30 nm) NS | Cement mortar with an admixture of 30 wt.% fly ash w/b = 0.485 | 28 | 1,3,5 | The increase is 19%, 11%, and 10%, respectively, in the NS, NT, and NF samples compared to the control sample. The optimal dose is 3 wt.% for each additive in terms of mechanical properties | NS- circa 4.8 NT- 4.9 NF- circa 4.4 |
| Cerro-Prada et al. [80] TiO2/100% Anataz (20–30 nm) | Cement mortar | 1, 7, 28, 90 | 0.1, 0.2, 0.5, 1—without and with replacement of cement | For samples with cement replacement, in the early and middle age of the mortar (2, 7, and 28), slightly reduced strength is obtained for the substitute content of nano-TiO2 of 0.1%, 0.5%, and 1%. By replacing TiO2 in cement with 0.2%, however, a slight improvement in bending strength (13.7%) is achieved in the long term. In the case of the mortar prepared with the addition of TiO2 without cement replacement, no improvement can be clearly observed for the TiO2 content of 0.2%, 0.5%, and 1% | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, D.; Janus, M. Photoactive Cements: A Review. Materials 2022, 15, 5407. https://doi.org/10.3390/ma15155407
Dudek D, Janus M. Photoactive Cements: A Review. Materials. 2022; 15(15):5407. https://doi.org/10.3390/ma15155407
Chicago/Turabian StyleDudek, Dominika, and Magdalena Janus. 2022. "Photoactive Cements: A Review" Materials 15, no. 15: 5407. https://doi.org/10.3390/ma15155407







