Inhibition Effect and Mechanism Explanation of Perilla Seed Extract as a Green Corrosion Inhibitor on Q235 Carbon Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Corrosion Inhibitor Solution and Simulated Concrete Pore Solution
2.3. Potentiodynamic Polarization Curve Test
2.4. Detection and Characterization Methods of Corrosion Inhibition Effect
2.4.1. HPLC-MS Test
2.4.2. ATR-FTIR Test
2.4.3. XPS Test
2.5. Chemistry Calculation
2.5.1. Quantum Chemical Calculation
2.5.2. Molecular Dynamics Simulation
3. Results and Discussion
3.1. Evaluation of Corrosion Inhibition Efficiency
3.1.1. Influence of Corrosion Inhibitor Concentration on Corrosion Inhibition Efficiency
3.1.2. Adsorption Behavior of Perilla Seed Corrosion Inhibitor on Carbon Steel Surface
3.2. Determination of Chemical Constituents in Perilla Seed Extract
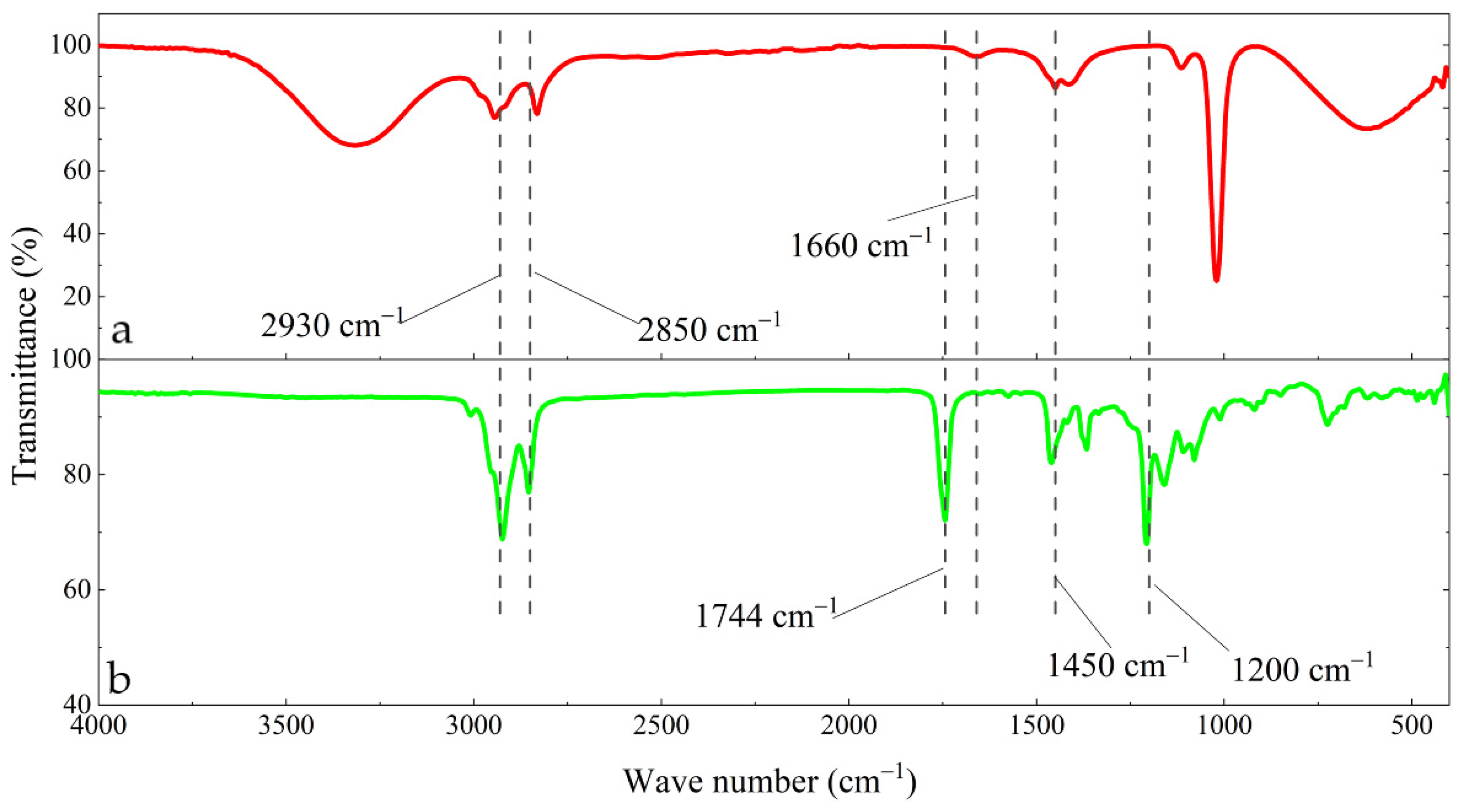
3.3. Changes in Functional Groups in the Surface Film
3.4. Effect of Effective Anti-Corrosion Ingredients on the Surface Film
3.5. Analysis of Quantum Chemistry Calculation
3.5.1. Frontier Orbit Distribution
3.5.2. Local Reactivity
3.6. Analysis of Molecular Dynamics Simulation
4. Conclusions
- (1)
- The corrosion inhibitor solution prepared by perilla seed extract belongs to mixed corrosion inhibitor, its adsorption behavior accords with Langmuir adsorption theory and its adsorption free energy is −22.70 kJ/mol.
- (2)
- In the corrosion inhibitor solution, luteolin and apigenin are mainly adsorbed parallel to the surface of carbon steel to form a film. The adsorption mechanism is that carbonyl O atoms in luteolin and apigenin hybridize with the 3 d empty orbit of Fe.
- (3)
- From the point of view of quantum chemistry, the smaller the HOMO value and the energy gap value, the better the adsorption of the corrosion inhibitor on the surface of carbon steel. From the point of view of molecular dynamics simulation, the greater the absolute value of adsorption energy, the better the adsorption of the corrosion inhibitor on carbon steel surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koushkbaghi, M.; Kazemi, M.J.; Mosavi, H.; Mohseni, E. Acid resistance and durability properties of steel fiber-reinforced concrete incorporating rice husk ash and recycled aggregate. Constr. Build. Mater. 2019, 202, 266–275. [Google Scholar] [CrossRef]
- Huang, B.-T.; Yu, J.; Wu, J.-Q.; Dai, J.-G.; Leung, C.K. Seawater sea-sand Engineered Cementitious Composites (SS-ECC) for marine and coastal applications. Compos. Commun. 2020, 20, 100353. [Google Scholar] [CrossRef]
- Yeau, K.Y.; Kim, E.K. An experimental study on corrosion resistance of concrete with ground granulate blast-furnace slag. Cem. Concr. Res. 2005, 35, 1391–1399. [Google Scholar] [CrossRef]
- Jin, Z.; Chang, H.; Du, F.; Zhao, T.; Jiang, Y.; Chen, Y. Influence of SAP on the chloride penetration and corrosion behavior of steel bar in concrete. Corros. Sci. 2020, 171, 108714. [Google Scholar] [CrossRef]
- Coronelli, D.; Gambarova, P. Structural assessment of corroded reinforced concrete beams: Modeling guidelines. J. Struct. Eng. 2004, 130, 1214–1224. [Google Scholar] [CrossRef]
- Goni, K.; Mazumder, M.A. Green corrosion inhibitors. Corros. Inhib. 2019, 1–18. [Google Scholar]
- Cui, L.; Hang, M.; Huang, H.; Gao, X. Experimental study on multi-component corrosion inhibitor for steel bar in chloride environment. Constr. Build. Mater. 2021, 313, 125533. [Google Scholar] [CrossRef]
- Abdulrahman, A.; Ismail, M.; Hussain, M.S. Corrosion inhibitors for steel reinforcement in concrete: A review. Sci. Res. Essays 2011, 6, 4152–4162. [Google Scholar] [CrossRef] [Green Version]
- Dariva, C.G.; Galio, A.F. Corrosion inhibitors–principles, mechanisms and applications. Dev. Corros. Prot. 2014, 16, 365–378. [Google Scholar]
- Singh, A.; Quraishi, M.A. Acidizing corrosion inhibitors: A review. J. Mater. Environ. Sci 2015, 6, 224–235. [Google Scholar]
- Wu, Y.; Guo, L.; Tan, B.; Li, W.; Zhang, F.; Zheng, X. 5-Mercapto-1-phenyltetrazole as a high-efficiency corrosion inhibitor for Q235 steel in acidic environment. J. Mol. Liq. 2021, 325, 115132. [Google Scholar] [CrossRef]
- Han, C.; Li, W.; Liu, H.K.; Dou, S.; Wang, J. Principals and strategies for constructing a highly reversible zinc metal anode in aqueous batteries. Nano Energy 2020, 74, 104880. [Google Scholar] [CrossRef]
- Li, J.-H.; Zhao, B.; Hu, J.; Zhang, H.; Dong, S.-G.; Du, R.-G.; Lin, C.-J. Corrosion inhibition effect of D-sodium gluconate on reinforcing steel in chloride-contaminated simulated concrete pore solution. Int. J. Electrochem. Sci. 2015, 10, 956–968. [Google Scholar]
- Tommaselli, M.; Mariano, N.; Kuri, S. Effectiveness of corrosion inhibitors in saturated calcium hydroxide solutions acidified by acid rain components. Constr. Build. Mater. 2009, 23, 328–333. [Google Scholar] [CrossRef]
- Guo, L.; Obot, I.B.; Zheng, X.; Shen, X.; Qiang, Y.; Kaya, S.; Kaya, C. Theoretical insight into an empirical rule about organic corrosion inhibitors containing nitrogen, oxygen, and sulfur atoms. Appl. Surf. Sci. 2017, 406, 301–306. [Google Scholar] [CrossRef]
- Salleh, S.Z.; Yusoff, A.H.; Zakaria, S.K.; Taib, M.A.A.; Seman, A.A.; Masri, M.N.; Mohamad, M.; Mamat, S.; Sobri, S.A.; Ali, A. Plant extracts as green corrosion inhibitor for ferrous metal alloys: A review. J. Clean. Prod. 2021, 304, 127030. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Probing molecular adsorption/interactions and anti-corrosion performance of poppy extract in acidic environments. J. Mol. Liq. 2020, 304, 112750. [Google Scholar]
- Lu, Y.; Feng, H.; Xu, H. Studying Corrosion and Adhesion Performance of a Phytic Acid Based Conversion Coating Post-Treated with Garlic Extract on Q235 Steel. Int. J. Electrochem. Sci. 2021, 16, 210448. [Google Scholar] [CrossRef]
- Cheng-an, J. Research on Anti-corrosion Action of Pepper Extracts. J. Anhui Agric. Sci. 2012, 40, 126–127. [Google Scholar]
- Pradipta, I.; Kong, D.; Tan, J.B.L. Natural organic antioxidants from green tea inhibit corrosion of steel reinforcing bars embedded in mortar. Constr. Build. Mater. 2019, 227, 117058. [Google Scholar] [CrossRef]
- Bui, H.T.; Dang, T.-D.; Le, H.T.; Hoang, T.T. Comparative study on corrosion inhibition of vietnam orange peel essential oil with urotropine and insight of corrosion inhibition mechanism for mild steel in hydrochloric solution. J. Electrochem. Sci. Technol. 2019, 10, 69–81. [Google Scholar]
- Elabbasy, H.M.; Zidan, S.M.; El-Aziz, A.F.S. Inhibitive behavior of Ambrosia Maritima extract as an eco-friendly corrosion inhibitor for carbon steel in 1M HCI. Zaštita Mater. 2019, 60, 129–146. [Google Scholar] [CrossRef] [Green Version]
- Salhi, A.; Bouyanzer, A.; Chetouani, A.; El Barkany, S.; Amhamdi, H.; Hamdani, I.; Zarrouk, A.; Hammouti, B.; Desjobert, J.; Costa, J. Chemical composition of essential oil and antioxidant and anti-corrosion activity of extract and essential oil of Pennyroyal Mint (Mentha pulegium, MP). Moroc. J. Chem. 2017, 5, 59–71. [Google Scholar]
- Czepirski, L.; Balys, M.R.; Komorowska-Czepirska, E. Some generalization of Langmuir adsorption isotherm. Internet J. Chem. 2000, 3, 1099–8292. [Google Scholar]
- Vigdorowitsch, M.; Tsygankova, L.E.; Bernatsky, P.N. To the mathematical theory of the temkin adsorption model. J. Appl. Solut. Chem. Modeling 2020, 9, 6–12. [Google Scholar] [CrossRef]
- Kolev, V.; Danov, K.; Kralchevsky, P.; Broze, G.; Mehreteab, A. Comparison of the van der Waals and Frumkin adsorption isotherms for sodium dodecyl sulfate at various salt concentrations. Langmuir 2002, 18, 9106–9109. [Google Scholar] [CrossRef]
- Shalabi, K.; Nazeer, A.A. Ethoxylates nonionic surfactants as promising environmentally safe inhibitors for corrosion protection of reinforcing steel in 3.5% NaCl saturated with Ca(OH)2 solution. J. Mol. Struct. 2019, 1195, 863–876. [Google Scholar] [CrossRef]
- Messali, M.; Larouj, M.; Lgaz, H.; Rezki, N.; Al-Blewi, F.; Aouad, M.; Chaouiki, A.; Salghi, R.; Chung, I.-M. A new schiff base derivative as an effective corrosion inhibitor for mild steel in acidic media: Experimental and computer simulations studies. J. Mol. Struct. 2018, 1168, 39–48. [Google Scholar] [CrossRef]
- Wei, M.; Shi, S.; Wang, J.; Li, Y.; Duan, X. Studies on the intercalation of naproxen into layered double hydroxide and its thermal decomposition by in situ FT-IR and in situ HT-XRD. J. Solid State Chem. 2004, 177, 2534–2541. [Google Scholar] [CrossRef]
- Kagarise, R. Relation between the electronegativities of adjacent substitutents and the stretching frequency of the carbonyl group. J. Am. Chem. Soc. 1955, 77, 1377–1379. [Google Scholar] [CrossRef]
- Ryu, S.R.; Bae, W.M.; Hong, W.J.; Ihn, K.J.; Jung, Y.M. Characterization of chain transfer reaction during radical polymerization of silver nanocomposite polyvinylpyrrolidone by using 2D hetero-spectral IR/NMR correlation spectroscopy. Vib. Spectrosc. 2012, 60, 168–172. [Google Scholar] [CrossRef]
- Bilba, K.; Ouensanga, A. Fourier transform infrared spectroscopic study of thermal degradation of sugar cane bagasse. J. Anal. Appl. Pyrolysis 1996, 38, 61–73. [Google Scholar] [CrossRef]
- Pisareva, A.; Shilov, G.; Karelin, A.; Dobrovolsky, Y.A. The structure and properties of phenol-2, 4-disulfonic acid dihydrate. Russ. J. Phys. Chem. A Focus Chem. 2008, 82, 355–363. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Lee, W.; Lee, J.; Reucroft, P. XPS study of carbon fiber surfaces treated by thermal oxidation in a gas mixture of O2/(O2 + N2). Appl. Surf. Sci. 2001, 171, 136–142. [Google Scholar] [CrossRef]
- López, G.P.; Castner, D.G.; Ratner, B.D. XPS O 1s binding energies for polymers containing hydroxyl, ether, ketone and ester groups. Surf. Interface Anal. 1991, 17, 267–272. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Xie, A.; Yu, X.; Qiu, L.; Zhang, L.; Zhang, Q. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007, 9, 852–858. [Google Scholar] [CrossRef]
- Kaya, S.; Tüzün, B.; Kaya, C.; Obot, I.B. Determination of corrosion inhibition effects of amino acids: Quantum chemical and molecular dynamic simulation study. J. Taiwan Inst. Chem. Eng. 2016, 58, 528–535. [Google Scholar] [CrossRef]
- Rauk, A. Orbital Interaction Theory of Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]

















| Inhibitor Concentration (g/L) | Ecorr (mV) | icorr (A/cm2) | IE% |
|---|---|---|---|
| 0 | −931 | 1.11 × 10−4 | - |
| 1 | −922 | 3.84 × 10−5 | 65.41 |
| 2 | −900 | 3.16 × 10−5 | 71.49 |
| 3 | −884 | 1.52 × 10−5 | 86.27 |
| 4 | −875 | 1.18 × 10−5 | 89.39 |
| Component | EHOMO (eV) | ELUMO (eV) | ΔE (eV) |
|---|---|---|---|
| luteolin | −5.045 | −2.474 | 2.571 |
| apigenin | −5.429 | −2.779 | 2.650 |
| α-linolenic acid | −5.505 | −1.058 | 4.447 |
| Atom | fi+ | fi− | Atom | fi+ | fi− |
|---|---|---|---|---|---|
| C1 | 0.033 | 0.031 | O7 | 0.033 | 0.037 |
| C2 | 0.026 | 0.011 | O8 | 0.024 | 0.015 |
| C3 | 0.008 | 0.019 | O9 | 0.027 | 0.034 |
| C4 | 0.007 | 0.019 | O13 | 0.092 | 0.280 |
| C5 | 0.023 | 0.028 | O20 | 0.028 | 0.026 |
| C6 | 0.030 | 0.031 | O21 | 0.052 | 0.041 |
| C10 | 0.070 | 0.045 | H22 | 0.023 | 0.025 |
| C11 | 0.055 | 0.024 | H23 | 0.016 | 0.022 |
| C12 | 0.057 | 0.061 | H24 | 0.015 | 0.018 |
| C14 | 0.027 | 0.005 | H25 | 0.020 | 0.026 |
| C15 | 0.043 | 0.016 | H26 | 0.026 | 0.022 |
| C16 | 0.027 | 0.022 | H27 | 0.022 | 0.010 |
| C17 | 0.056 | 0.034 | H28 | 0.026 | 0.019 |
| C18 | 0.035 | 0.023 | H29 | 0.021 | 0.009 |
| C19 | 0.039 | 0.019 | H30 | 0.016 | 0.012 |
| H31 | 0.021 | 0.016 |
| Atom | fi+ | fi− | Atom | fi+ | fi− |
|---|---|---|---|---|---|
| C1 | 0.032 | 0.053 | O7 | 0.040 | 0.104 |
| C2 | 0.031 | 0.036 | O8 | 0.034 | 0.027 |
| C3 | 0.024 | 0.104 | O9 | 0.034 | 0.046 |
| C4 | 0.009 | 0.026 | O20 | 0.050 | 0.039 |
| C5 | 0.012 | 0.037 | O21 | 0.079 | 0.043 |
| C6 | 0.025 | 0.046 | H22 | 0.024 | 0.033 |
| C10 | 0.058 | 0.017 | H23 | 0.018 | 0.040 |
| C11 | 0.051 | 0.056 | H24 | 0.014 | 0.023 |
| C12 | 0.074 | 0.029 | H25 | 0.018 | 0.025 |
| C14 | 0.024 | 0.006 | H26 | 0.026 | 0.027 |
| C15 | 0.041 | 0.018 | H27 | 0.021 | 0.010 |
| C16 | 0.034 | 0.024 | H28 | 0.026 | 0.018 |
| C17 | 0.056 | 0.031 | H29 | 0.026 | 0.018 |
| C18 | 0.035 | 0.026 | H30 | 0.022 | 0.007 |
| Atom | fi+ | fi− | Atom | fi+ | fi− |
|---|---|---|---|---|---|
| O1 | 0.096 | 0.006 | H26 | 0.008 | 0.000 |
| C2 | 0.177 | 0.001 | H27 | 0.008 | 0.000 |
| O3 | 0.179 | 0.006 | H28 | 0.007 | 0.005 |
| C4 | 0.033 | 0.001 | H29 | 0.007 | 0.005 |
| C5 | 0.005 | 0.002 | H30 | 0.002 | 0.000 |
| C6 | 0.006 | 0.001 | H31 | 0.002 | 0.000 |
| C7 | 0.005 | 0.003 | H32 | 0.005 | 0.010 |
| C8 | 0.002 | 0.002 | H33 | 0.005 | 0.010 |
| C9 | 0.003 | 0.005 | H34 | 0.001 | 0.012 |
| C10 | 0.002 | 0.008 | H35 | 0.001 | 0.012 |
| C11 | 0.008 | 0.079 | H36 | 0.007 | 0.037 |
| C12 | 0.008 | 0.047 | H37 | 0.008 | 0.027 |
| C13 | 0.004 | 0.021 | H38 | 0.002 | 0.036 |
| C14 | 0.024 | 0.093 | H39 | 0.003 | 0.036 |
| C15 | 0.019 | 0.094 | H40 | 0.014 | 0.042 |
| C16 | 0.009 | 0.021 | H41 | 0.013 | 0.042 |
| C17 | 0.030 | 0.042 | H42 | 0.012 | 0.036 |
| C18 | 0.034 | 0.073 | H43 | 0.012 | 0.035 |
| C19 | 0.005 | 0.009 | H44 | 0.016 | 0.025 |
| C20 | 0.004 | 0.009 | H45 | 0.017 | 0.034 |
| H21 | 0.045 | 0.001 | H46 | 0.005 | 0.014 |
| H22 | 0.052 | 0.000 | H47 | 0.005 | 0.013 |
| H23 | 0.053 | 0.000 | H48 | 0.006 | 0.012 |
| H24 | 0.012 | 0.003 | H49 | 0.006 | 0.012 |
| H25 | 0.011 | 0.003 | H50 | 0.003 | 0.015 |
| Adsorption Surface | Luteolin (kJ/mol) | Apigenin (kJ/mol) | α-Linolenic Acid (kJ/mol) |
|---|---|---|---|
| Fe (110) | −144.175 | −141.949 | −133.046 |
| γ-FeOOH (010) | −33.293 | −33.608 | −27.304 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xu, W.; Lai, J.; Qiang, S. Inhibition Effect and Mechanism Explanation of Perilla Seed Extract as a Green Corrosion Inhibitor on Q235 Carbon Steel. Materials 2022, 15, 5394. https://doi.org/10.3390/ma15155394
Li Y, Xu W, Lai J, Qiang S. Inhibition Effect and Mechanism Explanation of Perilla Seed Extract as a Green Corrosion Inhibitor on Q235 Carbon Steel. Materials. 2022; 15(15):5394. https://doi.org/10.3390/ma15155394
Chicago/Turabian StyleLi, Yu, Wenqiang Xu, Jiayu Lai, and Sheng Qiang. 2022. "Inhibition Effect and Mechanism Explanation of Perilla Seed Extract as a Green Corrosion Inhibitor on Q235 Carbon Steel" Materials 15, no. 15: 5394. https://doi.org/10.3390/ma15155394
APA StyleLi, Y., Xu, W., Lai, J., & Qiang, S. (2022). Inhibition Effect and Mechanism Explanation of Perilla Seed Extract as a Green Corrosion Inhibitor on Q235 Carbon Steel. Materials, 15(15), 5394. https://doi.org/10.3390/ma15155394







