Preparation, Structural Characterization of Anti-Cancer Drugs-Mediated Self-Assembly from the Pluronic Copolymers through Synchrotron SAXS Investigation
Abstract
1. Introduction
2. Materials and Methods
3. Results
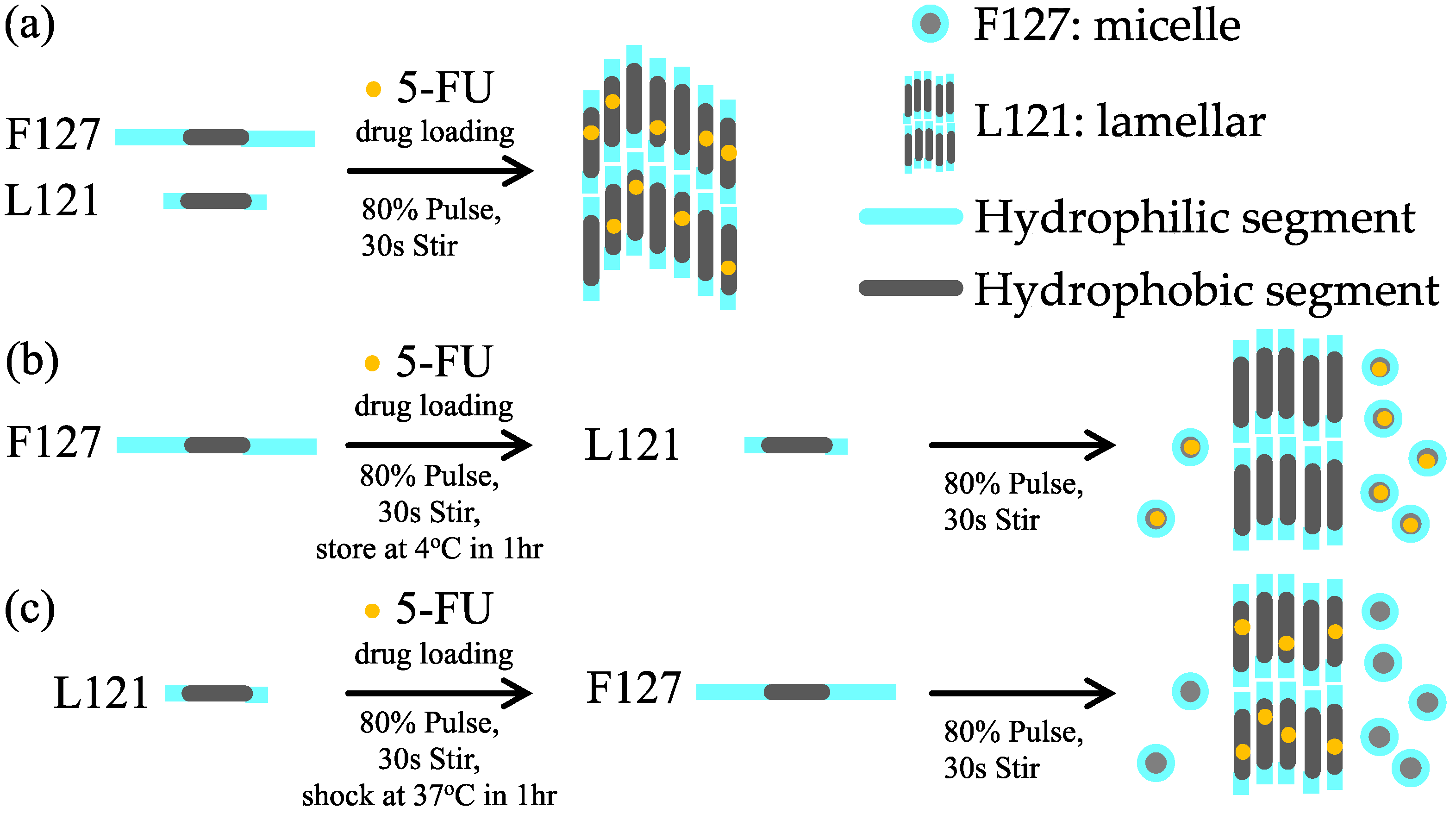
3.1. Preparation of Pluronic Biomedical Hydrogels
3.2. Self-Assembly and Characteristics of the Pluronic Biomedical Hydrogels
3.3. 5-FU Drug Releasing of the Pluronic Biomedical Hydrogels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles-From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development. J. Colloid Interface Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Cai, Y.; Zhang, Y.; Tian, Y.; Wang, L.; Gu, H.; Chen, W. Pluronic® F127 Micelles Template Biomimetic Assembly and Optical Properties of Red Fluorescent Silica Nanocapsules. Sci. Adv. Mater. 2017, 9, 608–615. [Google Scholar] [CrossRef]
- Dunphy, D.R.; Sheth, P.H.; Garcia, F.L.; Brinker, C.J. Enlarged Pore Size in Mesoporous Silica Films Templated by Pluronic F127: Use of Poloxamer Mixtures and Increased Template/SiO2 Ratios in Materials Synthesized by Evaporation-Induced Self-Assembly. Chem. Mater. 2014, 27, 75–84. [Google Scholar] [CrossRef]
- Fermino, T.Z.; Awano, C.M.; Moreno, L.X.; Vollet, D.R.; de Vicente, F.S. Structure and thermal stability in hydrophobic Pluronic F127-modified silica aerogels. Microporous Mesoporous Mater. 2018, 267, 242–248. [Google Scholar] [CrossRef]
- Liu, D.; Zou, D.; Zhu, H.; Zhang, J. Mesoporous Metal-Organic Frameworks: Synthetic Strategies and Emerging Applications. Small 2018, 14, 1801454. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantu, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Boonrat, O.; Tantishaiyakul, V.; Hirun, N.; Rugmai, S.; Soontaranon, S. Structural characterization using SAXS and rheological behaviors of pluronic F127 and methylcellulose blends. Polym. Bull. 2020, 78, 1175–1187. [Google Scholar] [CrossRef]
- Qavi, S.; Bandegi, A.; Firestone, M.; Foudazi, R. Polymerization in soft nanoconfinement of lamellar and reverse hexagonal mesophases. Soft Matter 2019, 15, 8238–8250. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cui, Y.; Ling, P.; Li, L.B. Preparation and evaluation of folate-modified cationic pluronic micelles for poorly soluble anticancer drug. Drug Deliv. 2012, 19, 208–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oh, K.T.; Bronich, T.K.; Kabanov, A.V. Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic Pluronic block copolymers. J. Control. Release 2004, 94, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Oh, Y.T.; Youn, Y.S.; Nam, M.; Park, B.; Yun, J.; Kim, J.H.; Song, H.T.; Oh, K.T. Binary mixing of micelles using Pluronics for a nano-sized drug delivery system. Colloids Surf. B Biointerfaces 2011, 82, 190–195. [Google Scholar] [CrossRef]
- Newby, G.E.; Hamley, I.W.; King, S.M.; Martin, C.M.; Terrill, N.J. Structure, rheology and shear alignment of Pluronic block copolymer mixtures. J. Colloid. Interface Sci. 2009, 329, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Torchio, A.; Cassino, C.; Lavella, M.; Gallina, A.; Stefani, A.; Boffito, M.; Ciardelli, G. Injectable supramolecular hydrogels based on custom-made poly(ether urethane)s and alpha-cyclodextrins as efficient delivery vehicles of curcumin. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112194. [Google Scholar] [CrossRef]
- Ezhilrani, V.C.; Karunanithi, P.; Sarangi, B.; Joshi, R.G.; Dash, S. Hydrophilic-hydrophilic mixed micellar system: Effect on solubilization of drug. SN Appl. Sci. 2021, 3, 371. [Google Scholar] [CrossRef]
- Oshiro, A.; da Silva, D.C.; de Mello, J.C.; de Moraes, V.W.; Cavalcanti, L.P.; Franco, M.K.; Alkschbirs, M.I.; Fraceto, L.F.; Yokaichiya, F.; Rodrigues, T.; et al. Pluronics F-127/L-81 binary hydrogels as drug-delivery systems: Influence of physicochemical aspects on release kinetics and cytotoxicity. Langmuir 2014, 30, 13689–13698. [Google Scholar] [CrossRef]
- Mike Motloung, B.; Babu, B.; Prinsloo, E.; Nyokong, T. The photophysicochemical properties and photodynamic therapy activity of In and Zn phthalocyanines when incorporated into individual or mixed Pluronic® micelles. Polyhedron 2020, 188, 114683. [Google Scholar] [CrossRef]
- Lim, C.; Moon, J.; Sim, T.; Hoang, N.H.; Won, W.R.; Lee, E.S.; Youn, Y.S.; Choi, H.G.; Oh, K.; Oh, K.T. Cyclic RGD-conjugated Pluronic® blending system for active, targeted drug delivery. Int. J. Nanomed. 2018, 13, 4627–4639. [Google Scholar] [CrossRef]
- Patel, D.; Patel, D.; Ray, D.; Kuperkar, K.; Aswal, V.K.; Bahadur, P. Single and mixed Pluronics® micelles with solubilized hydrophobic additives: Underscoring the aqueous solution demeanor and micellar transition. J. Mol. Liq. 2021, 343, 117625. [Google Scholar] [CrossRef]
- Cheng, X.; Lv, X.; Xu, J.; Zheng, Y.; Wang, X.; Tang, R. Pluronic micelles with suppressing doxorubicin efflux and detoxification for efficiently reversing breast cancer resistance. Eur. J. Pharm. Sci. 2020, 146, 105275. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Hao, J.; Yuan, S.; Li, Y.; Juan, W.; Sha, X.; Fang, X. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: Formulation, optimization and in vitro characterization. Int. J. Pharm. 2009, 376, 176–185. [Google Scholar] [CrossRef]
- Russo, A.; Pellosi, D.S.; Pagliara, V.; Milone, M.R.; Pucci, B.; Caetano, W.; Hioka, N.; Budillon, A.; Ungaro, F.; Russo, G.; et al. Biotin-targeted Pluronic® P123/F127 mixed micelles delivering niclosamide: A repositioning strategy to treat drug-resistant lung cancer cells. Int. J. Pharm. 2016, 511, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Jeng, U.S.; Su, C.H.; Su, C.-J.; Liao, K.-F.; Chuang, W.-T.; Lai, Y.-H.; Chang, J.-W.; Chen, Y.-J.; Huang, Y.-S.; Lee, M.-T.; et al. A small/wide-angle X-ray scattering instrument for structural characterization of air–liquid interfaces, thin films and bulk specimens. J. Appl. Crystallogr. 2009, 43, 110–121. [Google Scholar] [CrossRef]
- Naskar, B.; Ghosh, S.; Moulik, S.P. Solution behavior of normal and reverse triblock copolymers (pluronic L44 and 10R5) individually and in binary mixture. Langmuir 2012, 28, 7134–7146. [Google Scholar] [CrossRef]
- Yang, T.F.; Chen, C.N.; Chen, M.C.; Lai, C.H.; Liang, H.F.; Sung, H.W. Shell-crosslinked Pluronic L121 micelles as a drug delivery vehicle. Biomaterials 2007, 28, 725–734. [Google Scholar] [CrossRef]
- Suman, K.; Sourav, S.; Joshi, Y.M. Rheological signatures of gel–glass transition and a revised phase diagram of an aqueous triblock copolymer solution of Pluronic F127. Phys. Fluids 2021, 33, 073610. [Google Scholar] [CrossRef]
- Lee, C.F.; Yang, C.H.; Lin, T.L.; Bahadur, P.; Chen, L.J. Role of molecular weight and hydrophobicity of amphiphilic tri-block copolymers in temperature-dependent co-micellization process and drug solubility. Colloids Surf. B Biointerfaces 2019, 183, 110461. [Google Scholar] [CrossRef]
- Chiappim, W., Jr.; Awano, C.M.; Donatti, D.A.; de Vicente, F.S.; Vollet, D.R. Structure of hydrophobic ambient-pressure-dried aerogels prepared by sonohydrolysis of tetraethoxysilane with additions of N,N-dimethylformamide. Langmuir 2014, 30, 1151–1159. [Google Scholar] [CrossRef]
- Pepic, I.; Lovric, J.; Hafner, A.; Filipovic-Grcic, J. Powder form and stability of Pluronic mixed micelle dispersions for drug delivery applications. Drug Dev. Ind. Pharm. 2014, 40, 944–951. [Google Scholar] [CrossRef] [PubMed]





| Sample Code | F127 in H2O (25 wt%) | L121 in H2O (100 wt%) | 5-FU in DMSO (0.2 M) |
|---|---|---|---|
| PBH-all | 100 | 300 | 24 |
| PBH-1 | 100 1 | 300 | 24 |
| PBH-2 | 100 | 300 2 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-F.; Wang, W.-C.; Zeng, X.-Y.; Lu, Y.-X.; Shih, P.-J. Preparation, Structural Characterization of Anti-Cancer Drugs-Mediated Self-Assembly from the Pluronic Copolymers through Synchrotron SAXS Investigation. Materials 2022, 15, 5387. https://doi.org/10.3390/ma15155387
Lin T-F, Wang W-C, Zeng X-Y, Lu Y-X, Shih P-J. Preparation, Structural Characterization of Anti-Cancer Drugs-Mediated Self-Assembly from the Pluronic Copolymers through Synchrotron SAXS Investigation. Materials. 2022; 15(15):5387. https://doi.org/10.3390/ma15155387
Chicago/Turabian StyleLin, Tz-Feng, Wei-Chieh Wang, Xin-Yu Zeng, Yi-Xian Lu, and Pei-Jung Shih. 2022. "Preparation, Structural Characterization of Anti-Cancer Drugs-Mediated Self-Assembly from the Pluronic Copolymers through Synchrotron SAXS Investigation" Materials 15, no. 15: 5387. https://doi.org/10.3390/ma15155387
APA StyleLin, T.-F., Wang, W.-C., Zeng, X.-Y., Lu, Y.-X., & Shih, P.-J. (2022). Preparation, Structural Characterization of Anti-Cancer Drugs-Mediated Self-Assembly from the Pluronic Copolymers through Synchrotron SAXS Investigation. Materials, 15(15), 5387. https://doi.org/10.3390/ma15155387







