Electrochemical Evaluation of Protective Coatings with Ti Additions on Mild Steel Substrate with Potential Application for PEM Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Powder and Powder Deposition
2.2. Characterization of NiCr(Ti) Protective Coatings
3. Results and Discussion
3.1. Microstructure and Phase Analysis
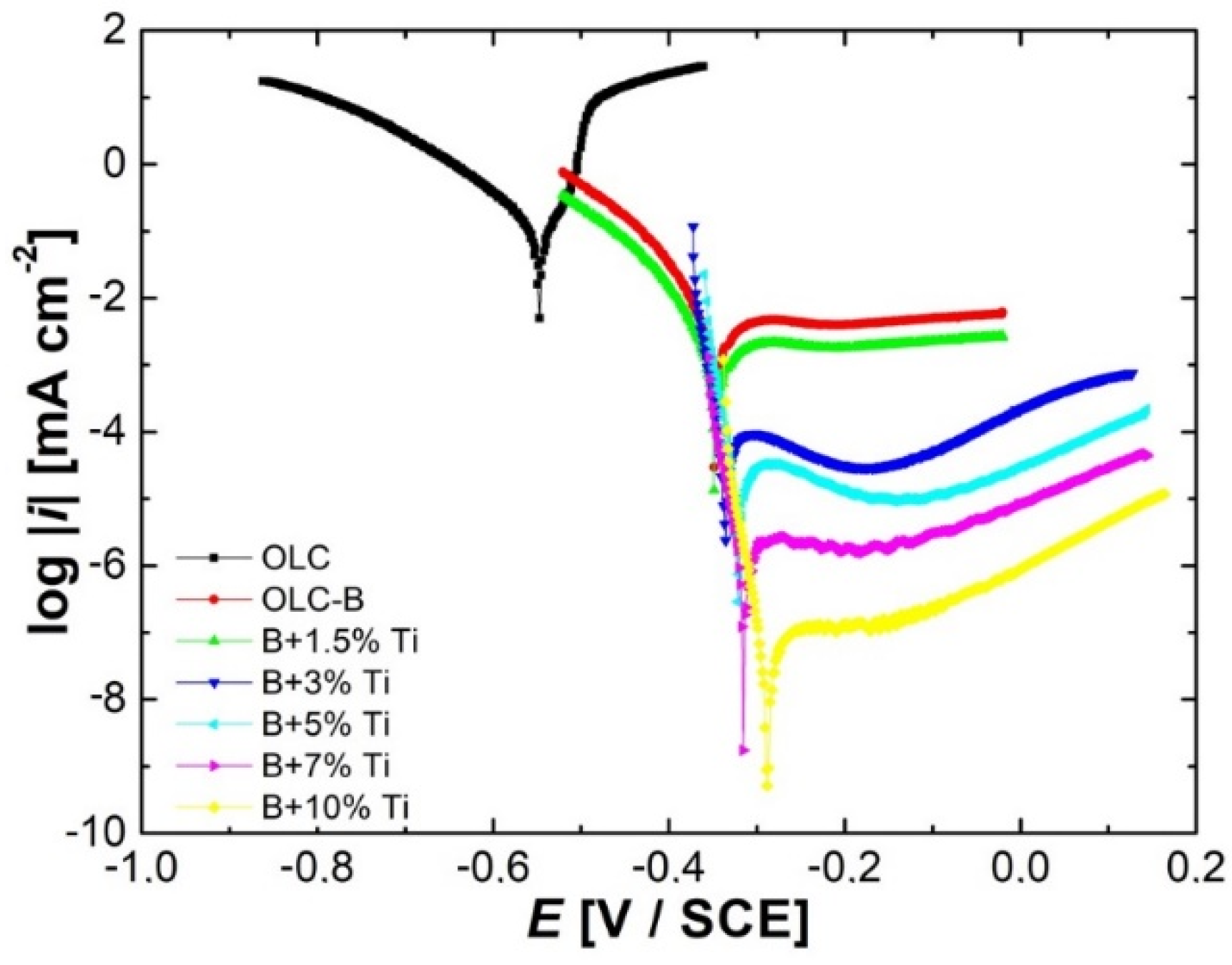
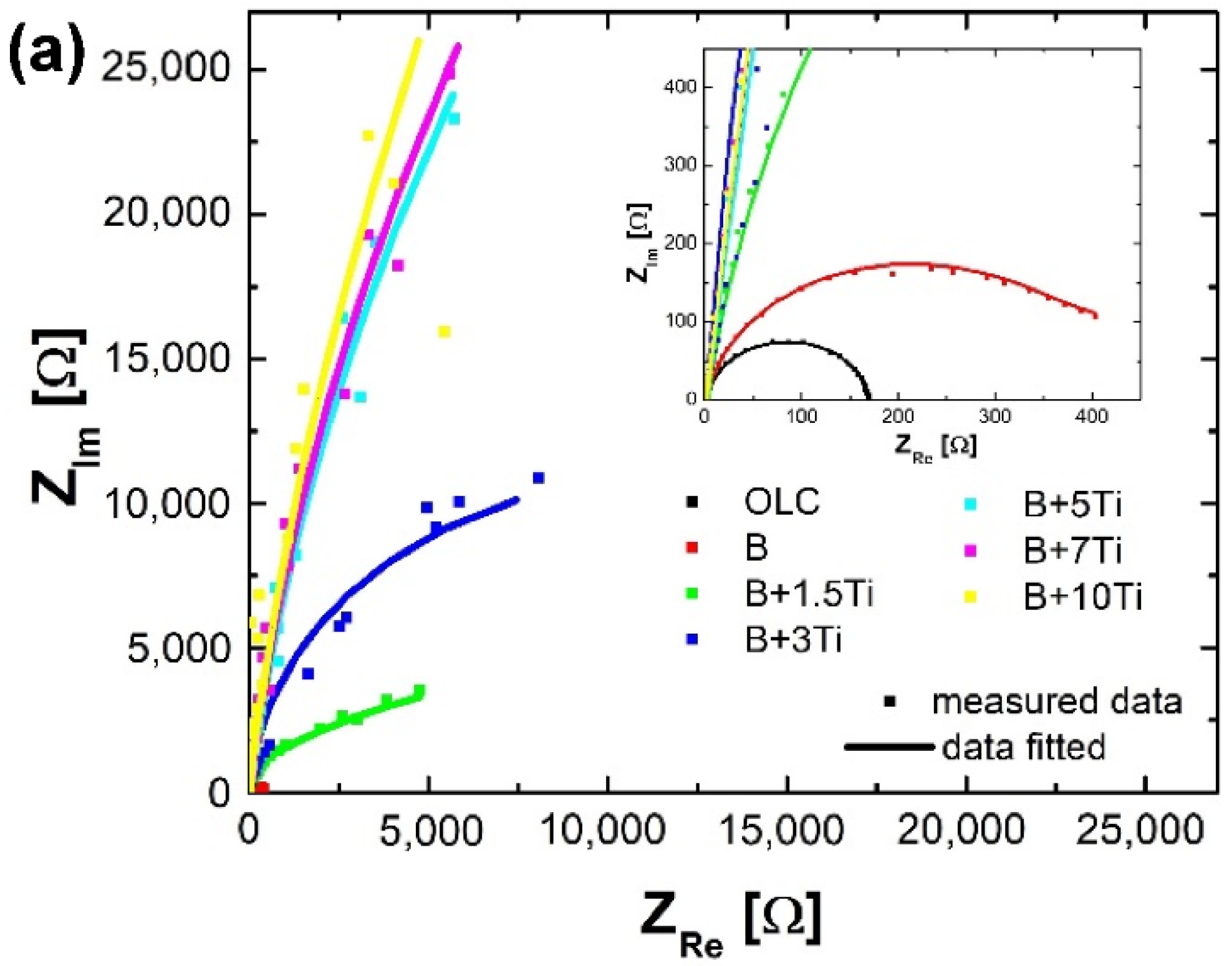
3.2. Electrochemical Behavior
4. Conclusions
- All the analyzed coatings show a dendritic microstructure; the size of dendrites increased with the Ti addition while the volume fraction of the NiCr-ɣ phase diminished;
- The dendritic region is rich in Ni, Cr and Mo with traces of Fe and Ti, while the inter-dendritic area is rich in Ni, Cr, Mo and Nb, also with traces of Ti and Fe;
- In the coatings manufactured with Ti addition, the presence of Ti within the coatings was confirmed by XRD; titanium is dissolved into the FCC Ni-Cr phase and the new Ni-Cr-Ti phase is formed;
- For all the coatings on mild steel, the OCP changes to more positive values in time, which is typically attributed to the growth and stabilization of a passive film on the surface of the samples;
- The decrease in the anodic current densities with the increase in titanium addition in the NiCr(Ti) coatings indicates an increase in corrosion resistance in the aggressive environment used in experimental tests; the values of the cathodic current densities decrease proportionally with the amount of titanium added in coatings, which means that in the acidic environment used to carry out the tests, titanium acts as an inhibitor of the cathodic process, thus limiting the hydrogen evolution reaction;
- The protective efficiency increases with the increase in Ti content due to the formation of a stable oxide film on the surface that enhances the corrosion resistance in an aggressive acidic medium;
- The corrosion rates decrease with the increase in Ti content due to the formation of the thin oxide film developed on the coating’s surface;
- As the titanium content increases more than 3%, the formed film thickens and has capacitive behavior, a process which is linked to a decrease in capacitance;
- The coatings with 5% Ti, 7% Ti and 10%Ti addition comply with the conditions of the US DOE regarding corrosion performance to be used as materials for the manufacture of the bipolar plates.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vielstich, W.; Lamm, A.; Gasteiger, H. Handbook of Fuel Cells: Fundamentals, Technology, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Hoogers, G. Fuel Cell Technology Handbook; CRC Press: Milton Park, UK; Routledge: London, UK, 2002; ISBN 0429120478. [Google Scholar]
- Madadi, F.; Rezaeian, A.; Edris, H.; Zhiani, M. Influence of surface roughness and hydrophobicity of bipolar plates on PEM performance. Surf. Coat. Technol. 2020, 389, 125676. [Google Scholar] [CrossRef]
- Hinds, G.; Brightman, E. Towards more representative test methods for corrosion resistance of PEMFC metallic bipolar plates. Int. J. Hydrogen Energy 2015, 40, 2785–2791. [Google Scholar] [CrossRef]
- Husby, H.; Kongstein, O.E.; Oedegaard, A.; Seland, F. Carbon-polymer composite coatings for PEM fuel cell bipolar plates. Int. J. Hydrogen Energy 2014, 39, 951–957. [Google Scholar] [CrossRef]
- Cells, T.F.; Cell, F.; Office, T.; Cells, F.; Office, T.; Cells, T.F. Fuel Cell 2016 Multi-Year Research, Development, and Demonstration Plan. Dep. Energy Multi-Year Res. Dev. Demonstr. Plan 2016, 2015, 1–58. [Google Scholar]
- André, J.; Antoni, L.; Petit, J.-P.; De Vito, E.; Montani, A. Electrical contact resistance between stainless steel bipolar plate and carbon felt in PEFC: A comprehensive study. Int. J. Hydrogen Energy 2009, 34, 3125–3133. [Google Scholar] [CrossRef]
- Fukutsuka, T.; Yamaguchi, T.; Miyano, S.-I.; Matsuo, Y.; Sugie, Y.; Ogumi, Z. Carbon-coated stainless steel as PEFC bipolar plate material. J. Power Sources 2007, 174, 199–205. [Google Scholar] [CrossRef]
- Shi, K.; Li, X.; Zhao, Y.; Li, W.-W.; Wang, S.-B.; Xie, X.-F.; Yao, L.; Jensen, J.O.; Li, Q.-F. Corrosion Behavior and Conductivity of TiNb and TiNbN Coated Steel for Metallic Bipolar Plates. Appl. Sci. 2019, 9, 2568. [Google Scholar] [CrossRef] [Green Version]
- Mani, S.P.; Rikhari, B.; Agilan, P.; Rajendran, N. Evaluation of the corrosion behavior of a TiN-coated 316L SS bipolar plate using dynamic electrochemical impedance spectroscopy. New J. Chem. 2018, 42, 14394–14409. [Google Scholar] [CrossRef]
- Yun, Y.H. Deposition of gold-titanium and gold-nickel coatings on electropolished 316L stainless steel bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2010, 35, 1713–1718. [Google Scholar] [CrossRef]
- Wu, B.; Lin, G.; Fu, Y.; Hou, M.; Yi, B. Chromium-containing carbon film on stainless steel as bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2010, 35, 13255–13261. [Google Scholar] [CrossRef]
- Rendón-Belmonte, M.; Pérez-Quiroz, J.T.; Terán-Guillén, J.; Porcayo-Calderón, J.; Torres-Acosta, A.; Orozco-Gamboa, G. Evaluation of a Cr3C2(NiCr) Coating Deposited on s4400 by Means of an HVOF Process and Used for Flow Plates of PEM Fuel. Int. J. Electrochem. Sci. 2012, 7, 1079–1092. [Google Scholar]
- Kim, S.C.; Yamaura, S.I.; Shimizu, Y.; Nakashima, K.; Igarashi, T.; Makino, A.; Inoue, A. Production of Ni65Cr15P16B4 metallic glass-coated bipolar plate for fuel cell by high velocity oxy-fuel (HVOF) spray coating method. Mater. Trans. 2010, 51, 1609–1613. [Google Scholar] [CrossRef] [Green Version]
- Pathak, D.; Bedi, R.K.; Kaur, D. Characterization of laser ablated AgInSe2 films. Mater. Sci. Pol. 2010, 28, 199–205. [Google Scholar]
- Pathak, D.; Bedi, R.K.; Kaushal, A.; Kaur, D. Crystalline AgInSe2 films on glass by laser ablation. Int. J. Mod. Phys. B 2010, 24, 5379–5385. [Google Scholar] [CrossRef]
- Xu, X.J.; Mi, G.; Xiong, L.; Jiang, P.; Shao, X.; Wang, C. Morphologies, microstructures and properties of TiC particle reinforced Inconel 625 coatings obtained by laser cladding with wire. J. Alloy. Compd. 2018, 740, 16–27. [Google Scholar] [CrossRef]
- Pascu, A.; Rosca, J.M.; Stanciu, E.M. Laser cladding: From experimental research to industrial applications. In Proceedings of the 11th International Conference on Materials Science & Engineering, BraMat Brasov, Romania, 13–16 March 2019. [Google Scholar]
- Siddiqui, A.A.; Dubey, A.K. Recent trends in laser cladding and surface alloying. Opt. Laser Technol. 2021, 134, 106619. [Google Scholar] [CrossRef]
- Stanciu, E.M.; Pascu, A.; Ţierean, M.H.; Voiculescu, I.; Roată, I.C.; Croitoru, C.; Hulka, I. Dual Coating Laser Cladding of NiCrBSi and Inconel 718. Mater. Manuf. Process. 2016, 31, 1556–1564. [Google Scholar] [CrossRef]
- Hulka, I.; Uțu, I.D.; Avram, D.; Dan, M.L.; Pascu, A.; Stanciu, E.M.; Roată, I.C. Influence of the Laser Cladding Parameters on the Morphology, Wear and Corrosion Resistance of WC-Co/NiCrBSi Composite Coatings. Materials 2021, 14, 5583. [Google Scholar] [CrossRef] [PubMed]
- Abioye, T.E.; McCartney, D.G.; Clare, A.T. Laser cladding of Inconel 625 wire for corrosion protection. J. Mater. Process. Technol. 2015, 217, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Perdomo-Socorro, P.P.; Florido-Suárez, N.R.; Verdú-Vázquez, A.; Mirza-Rosca, J.C. Comparative EIS study of titanium-based materials in high corrosive environments. Int. J. Surf. Sci. Eng. 2021, 15, 152–164. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Z.; Zhao, S.; Wang, Y.; Chen, H.; Lin, X. Effect of a small addition of Ti on the Fe-based coating by laser cladding. Surf. Coat. Technol. 2016, 291, 423–429. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, C.; Gong, Z.; Wang, G.; Sun, H.; Yang, H. Effect of Ti on T15M composite coating fabricated by laser cladding technology. Surf. Coat. Technol. 2017, 325, 643–649. [Google Scholar] [CrossRef]
- Hulka, I.; Utu, D.; Serban, V.A.; Negrea, P.; Lukáč, F.; Chráska, T. Effect of Ti addition on microstructure and corrosion properties of laser cladded WC-Co/NiCrBSi(Ti) coatings. Appl. Surf. Sci. 2020, 504, 144349. [Google Scholar] [CrossRef]
- Jin, J.; Zhu, Z.; Zheng, D. Influence of Ti content on the corrosion properties and contact resistance of CrTiN coating in simulated proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2017, 42, 11758–11770. [Google Scholar] [CrossRef]
- Huang, L.; Luo, Z.; Huang, X.; Wang, Y.; Yan, J.; Liu, W.; Guo, Y.; Babu Arulmani, S.R.; Shao, M.; Zhang, H. Applications of biomass-based materials to remove fluoride from wastewater: A review. Chemosphere 2022, 301, 134679. [Google Scholar] [CrossRef] [PubMed]
- Socorro-Perdomo, P.P.; Florido-Suárez, N.R.; Voiculescu, I.; Mirza-Rosca, J.C. Comparative eis study of alxcocrfeni alloys in ringer’s solution for medical instruments. Metals 2021, 11, 928. [Google Scholar] [CrossRef]
- Socorro-Perdomo, P.P.; Florido-Suárez, N.R.; Mirza-Rosca, J.C.; Saceleanu, M.V. EIS Characterization of Ti Alloys in Relation to Alloying Additions of Ta. Materials 2022, 15, 476. [Google Scholar] [CrossRef] [PubMed]
- Alvarães, C.P.; Sandes, S.S.; Jorge, J.C.F.; de Souza, L.F.G.; Araújo, L.S.; Mendes, M.C.; Dille, J. Microstructural Characterization of Inconel 625 Nickel-Based Alloy Weld Cladding Obtained by Electroslag Welding Process. J. Mater. Eng. Perform. 2020, 29, 3004–3015. [Google Scholar] [CrossRef]
- Ibriş, N.; Mirza Rosca, J.C. EIS study of Ti and its alloys in biological media. J. Electroanal. Chem. 2002, 526, 53–62. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Nonlinear Least Squares Fit Procedure for Analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Ugalde-Reyes, O.; Liu, H.B.; Roquero, P.; Alvarez-Ramirez, J.; Sosa-Hernández, E. EIS and relaxation times study for CO adsorbed on bimetallic Pt-Mo catalysts during the methanol oxidation reaction. Electrochim. Acta 2022, 418, 140309. [Google Scholar] [CrossRef]
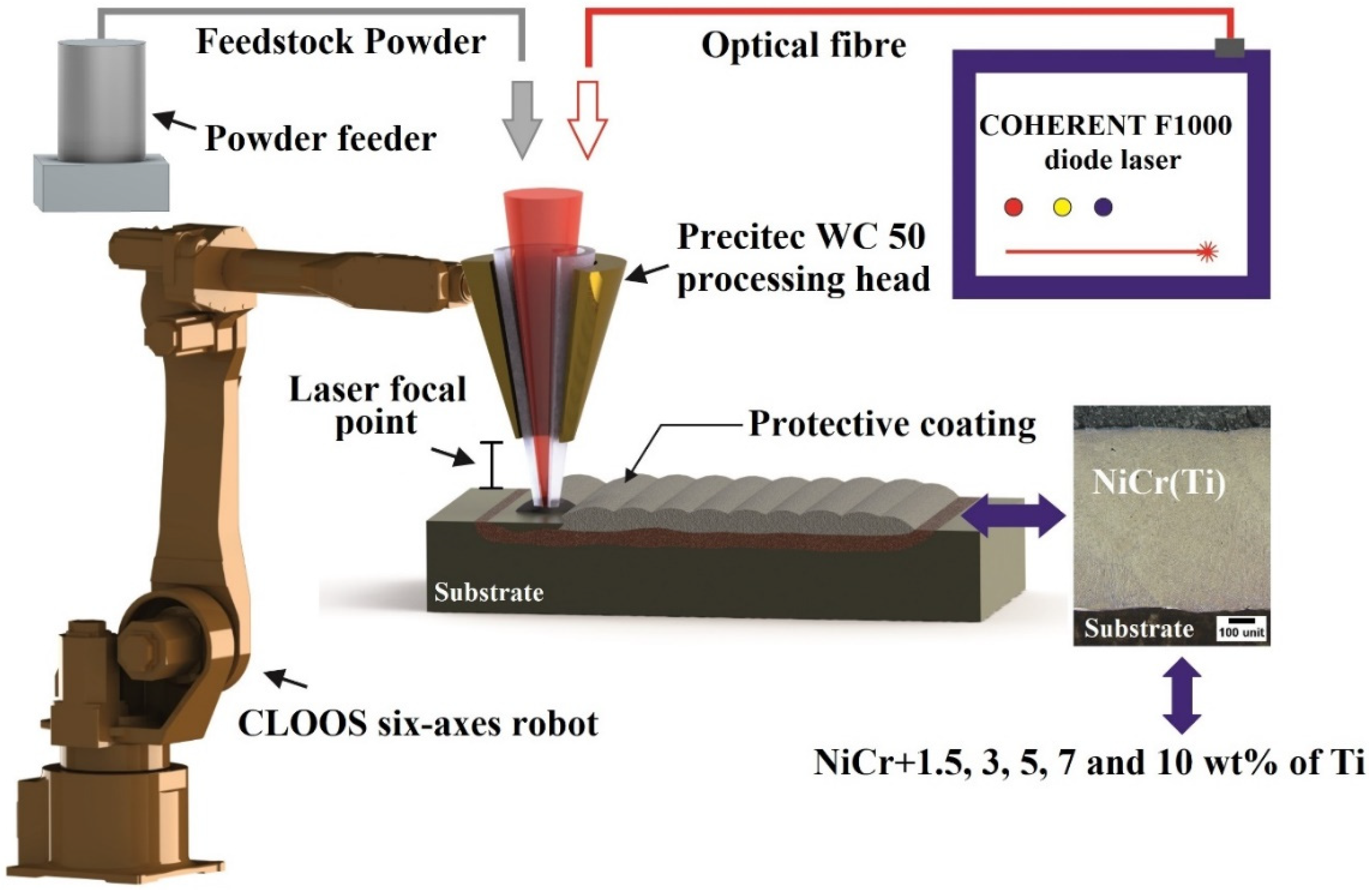

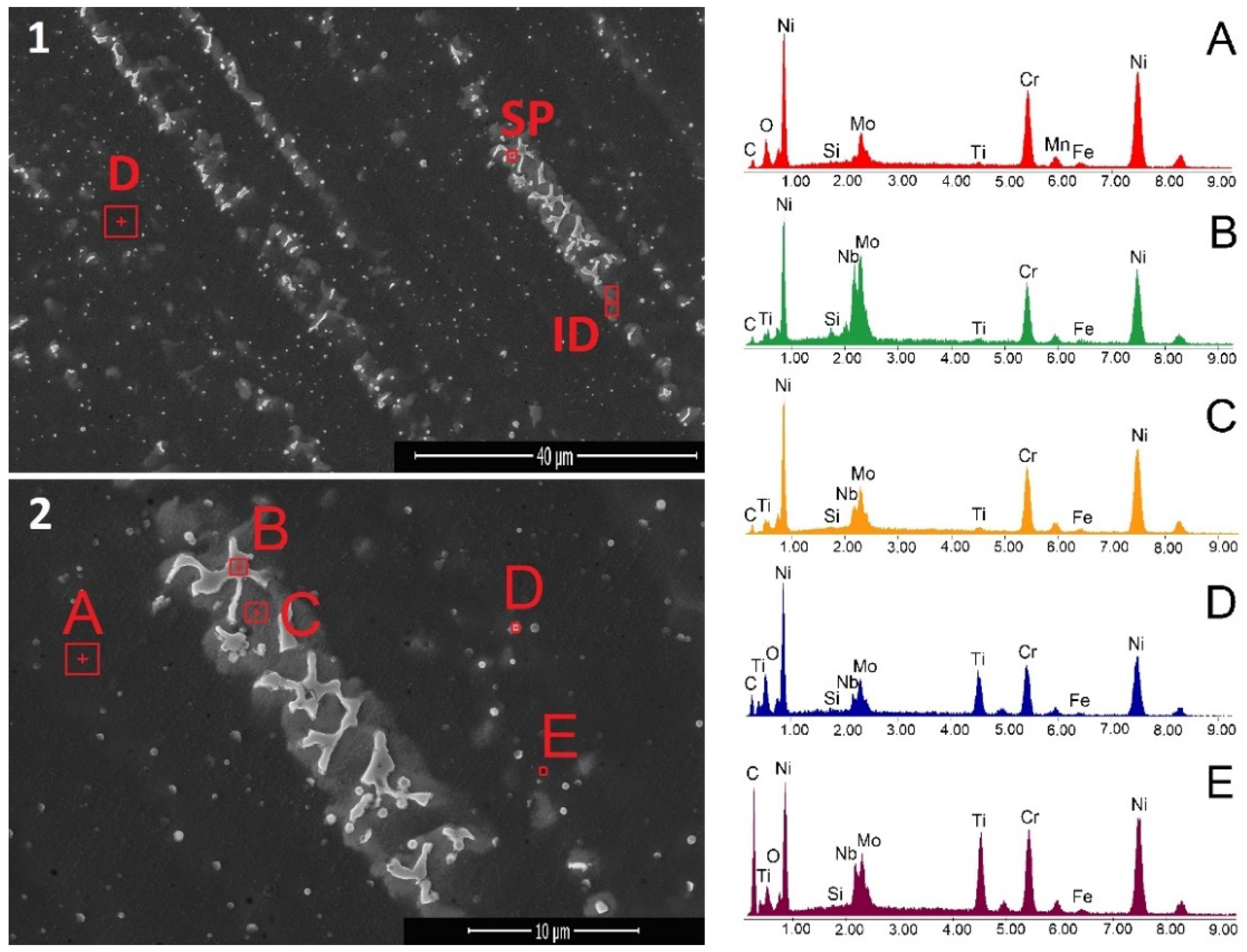




| Samples | Powder Preheating [°C] | Type of Laser | Power [W] | Focal Distance [mm] | Stand-Off Distance [mm] | Deposition Speed [cm/min] | Gas Flow [L/min] |
|---|---|---|---|---|---|---|---|
| B B + 1.5% Ti B + 3% Ti B + 5% Ti B + 7%Ti B + 10% Ti | 70 | continuous | 859 | 200 | 11.7 | 40 | 15.1 |
| Samples | icorr [μA·cm−2] | Ecorr [mV/SCE] | −bc [mV·dec−1] | ba [mV·dec−1] | Rp [kΩ·cm2] |
|---|---|---|---|---|---|
| OLC | 46.31 | −575 | 75.2 | 50 | 0.28 |
| OLC-B (B) | 9.84 | −395 | 72.1 | 451 | 2.74 |
| B + 1.5% Ti | 2.41 | −325 | 29.1 | 422 | 4.91 |
| B + 3% Ti | 1.72 | −292 | 27.7 | 410 | 6.55 |
| B + 5% Ti | 0.189 | −239 | 25.4 | 394 | 54.82 |
| B + 7% Ti | 0.085 | −216 | 23.2 | 385 | 111.77 |
| B + 10% Ti | 0.071 | −206 | 21.3 | 373 | 123.22 |
| Samples | PE [%] | CR [mm/yr] |
|---|---|---|
| OLC | - | 0.5423 |
| OLC-B (B) | 78.7 | 0.0980 |
| B + 1.5%Ti | 94.8 | 0.0235 |
| B + 3%Ti | 96.3 | 0.0163 |
| B + 5%Ti | 99.5 | 0.0017 |
| B + 7%Ti | 99.8 | 0.0007 |
| B + 10%Ti | 99.8 | 0.0006 |
| Samples | Rs [Ω·cm2] | Q [S·cm−2·sn] | n | Rct [Ω·cm2] | W [S·cm−2·s0.5] | Chi2 |
|---|---|---|---|---|---|---|
| OLC | 2.073 | 12.11 × 10−5 | 0.9251 | 168 | 7.958 × 10−9 | 1.84 × 10−3 |
| OLC-B (B) | 1.364 | 8.61 × 10−5 | 0.9005 | 385 | 5.946 × 10−3 | 1.26 × 10−3 |
| B + 1.5% Ti | 2.784 | 6.01 × 10−5 | 0.9251 | 4005 | 1.789 × 10−3 | 2.90 × 10−3 |
| B + 3% Ti | 1.807 | 5.13 × 10−5 | 0.9634 | 2.293 × 104 | 9.696 × 10−5 | 4.22 × 10−2 |
| B + 5% Ti | 1.661 | 4.61 × 10−5 | 0.9404 | 2.694 × 104 | 2.694 × 10−5 | 5.63 × 10−3 |
| B + 7% Ti | 1.589 | 4.54 × 10−5 | 0.9397 | 1.861 × 105 | 4.301 × 10−5 | 2.51 × 10−3 |
| B + 10% Ti | 1.972 | 4.87 × 10−5 | 0.9329 | 1.909 × 105 | 1.587 × 10−4 | 2.25 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avram, D.N.; Davidescu, C.M.; Dan, M.L.; Mirza-Rosca, J.C.; Hulka, I.; Pascu, A.; Stanciu, E.M. Electrochemical Evaluation of Protective Coatings with Ti Additions on Mild Steel Substrate with Potential Application for PEM Fuel Cells. Materials 2022, 15, 5364. https://doi.org/10.3390/ma15155364
Avram DN, Davidescu CM, Dan ML, Mirza-Rosca JC, Hulka I, Pascu A, Stanciu EM. Electrochemical Evaluation of Protective Coatings with Ti Additions on Mild Steel Substrate with Potential Application for PEM Fuel Cells. Materials. 2022; 15(15):5364. https://doi.org/10.3390/ma15155364
Chicago/Turabian StyleAvram, Diana N., Corneliu M. Davidescu, Mircea L. Dan, Julia C. Mirza-Rosca, Iosif Hulka, Alexandru Pascu, and Elena M. Stanciu. 2022. "Electrochemical Evaluation of Protective Coatings with Ti Additions on Mild Steel Substrate with Potential Application for PEM Fuel Cells" Materials 15, no. 15: 5364. https://doi.org/10.3390/ma15155364
APA StyleAvram, D. N., Davidescu, C. M., Dan, M. L., Mirza-Rosca, J. C., Hulka, I., Pascu, A., & Stanciu, E. M. (2022). Electrochemical Evaluation of Protective Coatings with Ti Additions on Mild Steel Substrate with Potential Application for PEM Fuel Cells. Materials, 15(15), 5364. https://doi.org/10.3390/ma15155364








