Mid-Infrared Laser Generation of Zn1−xMnxSe and Zn1−xMgxSe (x ≈ 0.3) Single Crystals Co-Doped by Cr2+ and Fe2+ Ions—Comparison of Different Excitation Wavelengths
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pumping Laser Systems
3. Results
3.1. Cr,Fe:ZnMnSe and Cr,Fe:ZnMgSe (x ≈ 0.3) Spectroscopic Properties
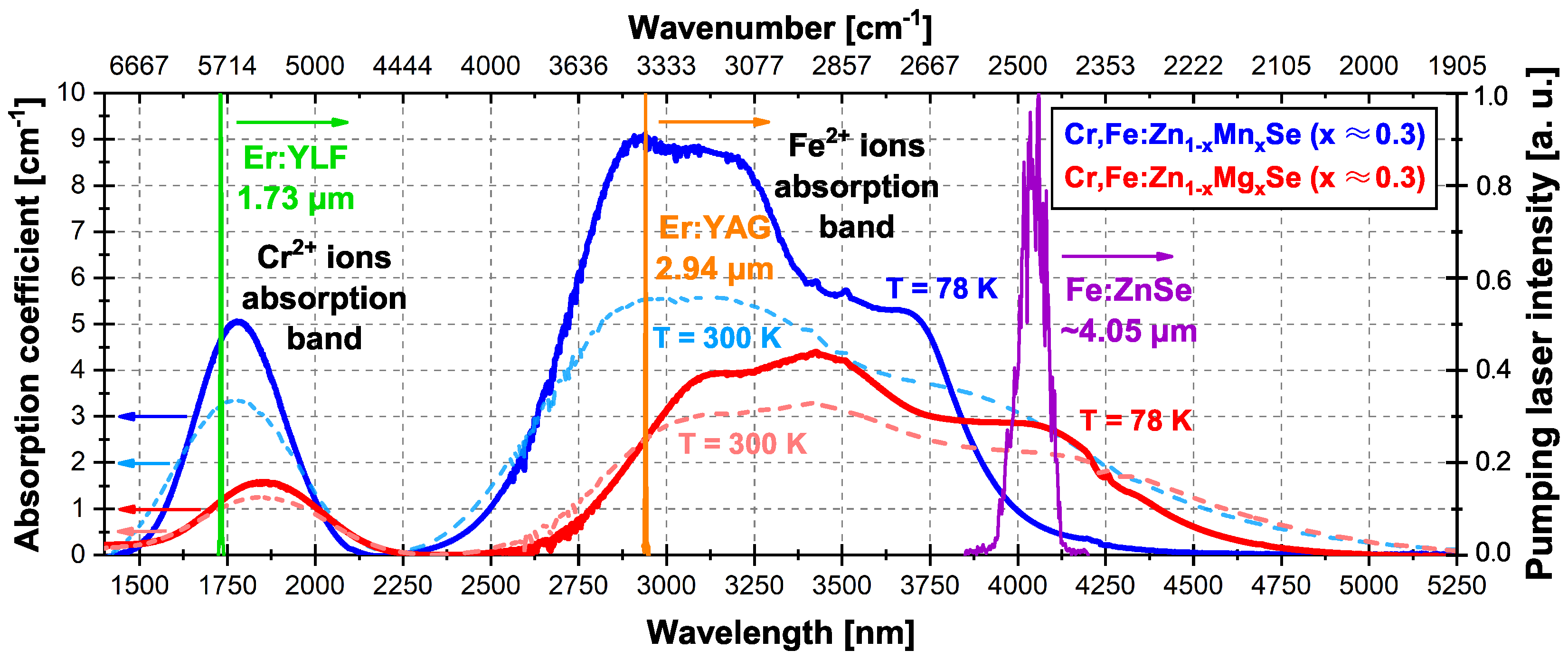
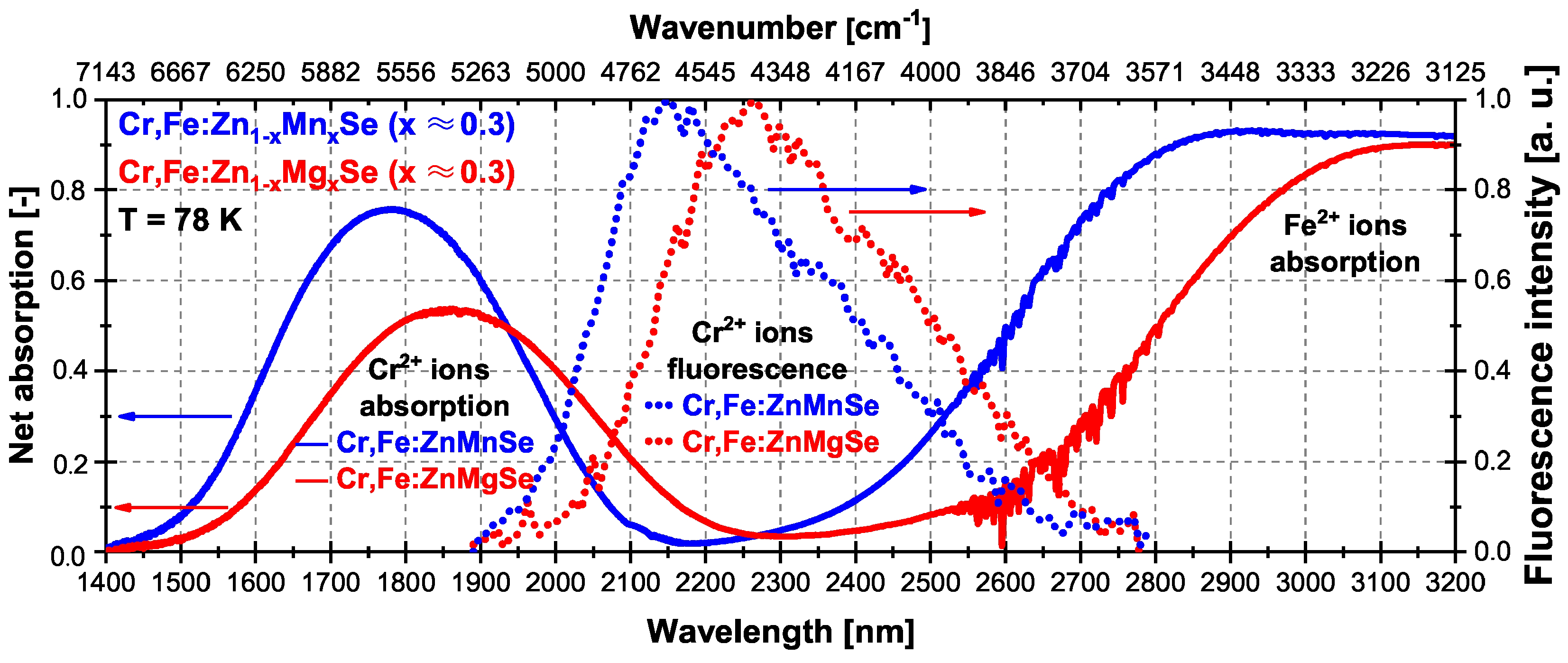
3.1.1. Absorption Spectra
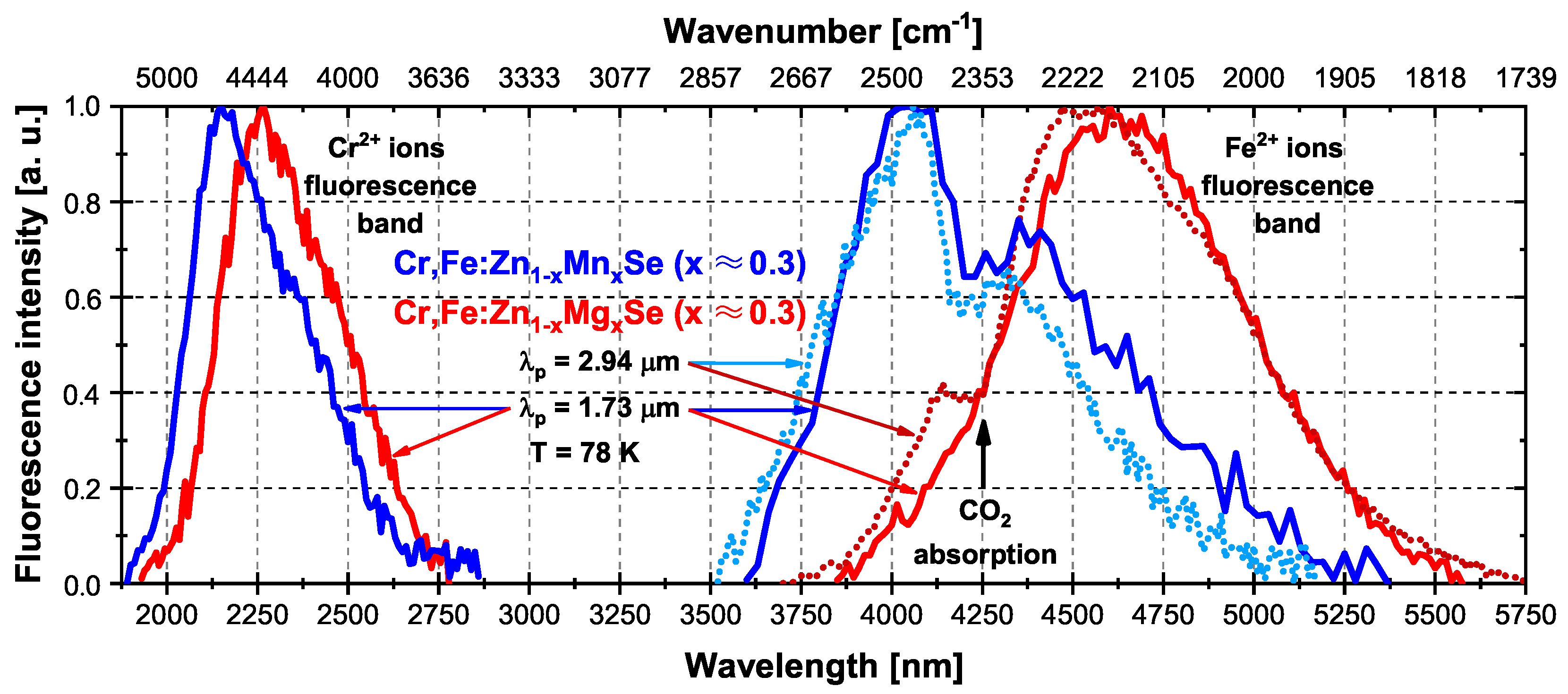
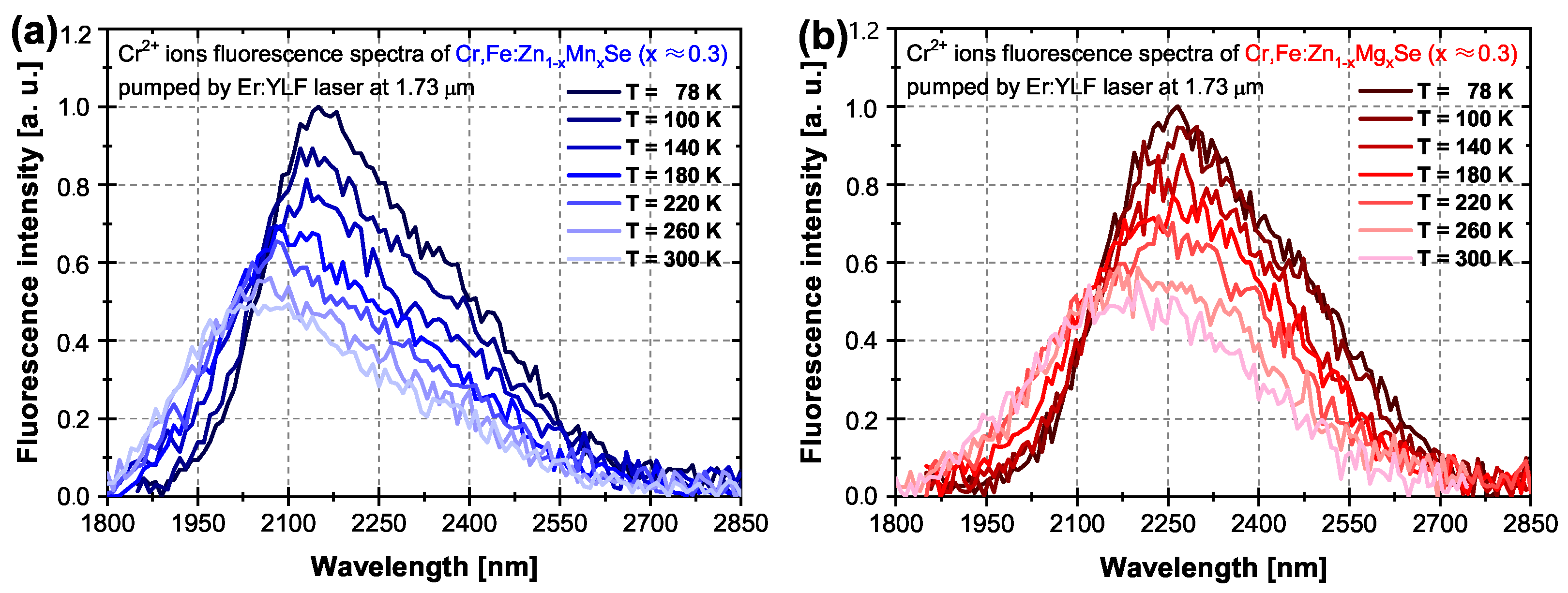
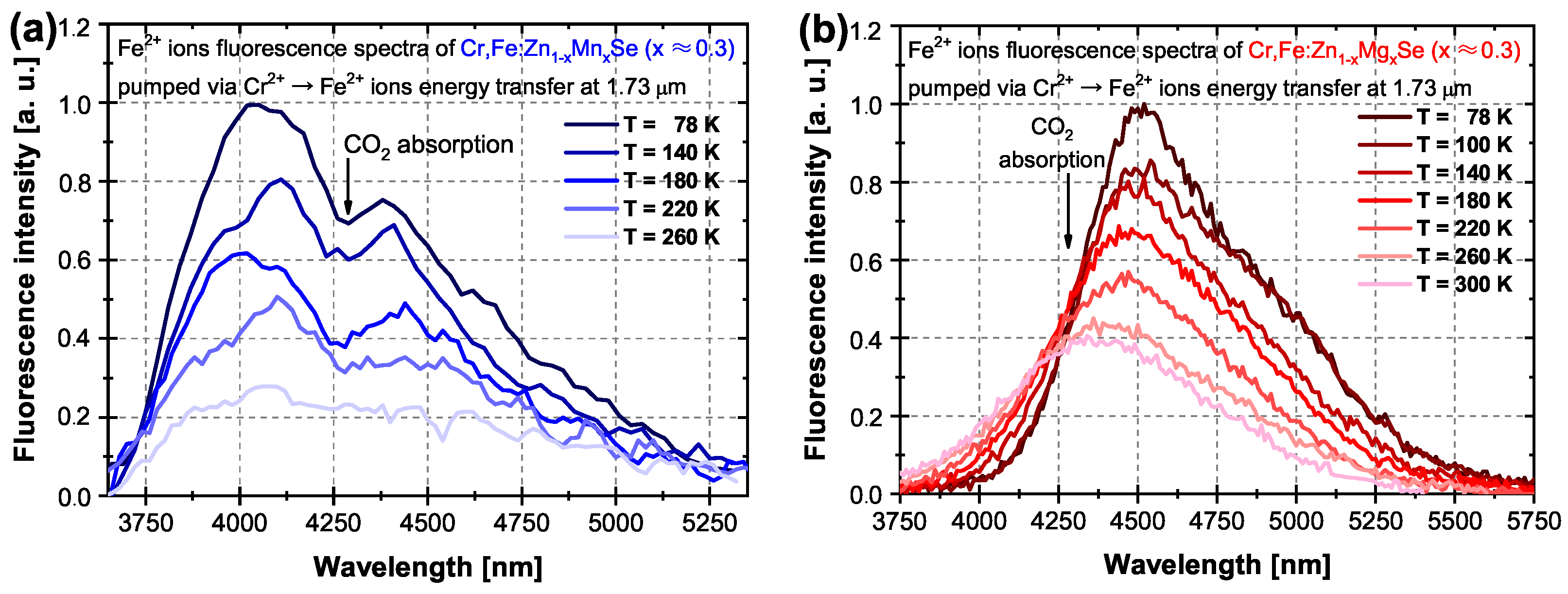
3.1.2. Fluorescence Spectra
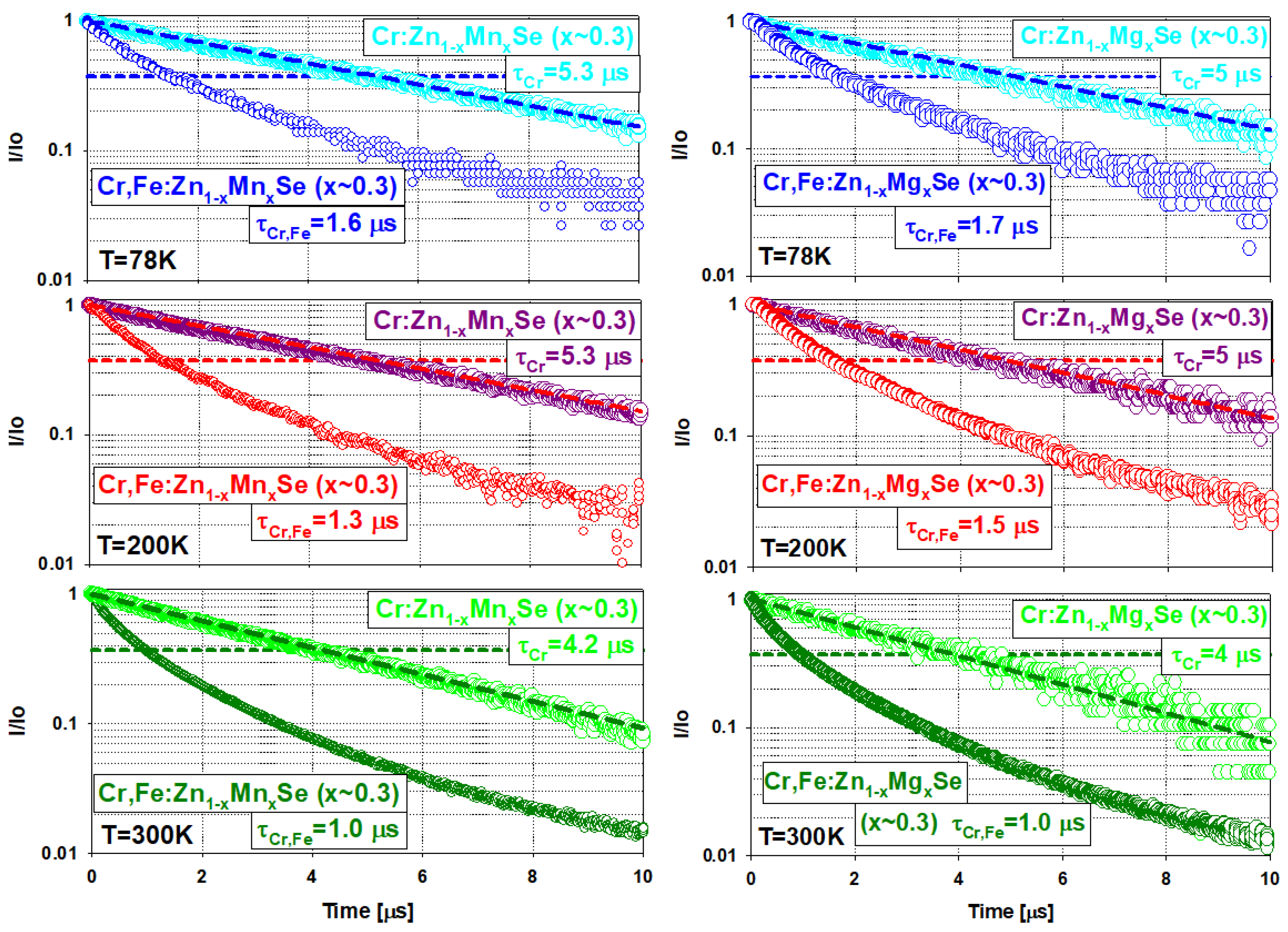
3.1.3. Fluorescence Decay Time and Energy Transfer Efficiency
3.2. Cr,Fe:ZnMnSe and Cr,Fe:ZnMgSe (x ≈ 0.3) Lasers’ Output Properties
3.2.1. Er:YLF Laser Excitation at 1.73 m
3.2.2. Er:YAG and Fe:ZnSe Laser Excitation at 2.94 and ∼4.05 m
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tittel, F.K.; Richter, D.; Fried, A. Mid-Infrared Laser Applications in Spectroscopy. In Solid-State Mid-Infrared Laser Sources; Sorokina, I.T., Vodopyanov, K.L., Eds.; Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 2003; pp. 458–529. [Google Scholar] [CrossRef]
- Grishin, M. Advances in Solid State Lasers Development and Applications; IntechOpen: London, UK, 2010. [Google Scholar] [CrossRef]
- Mirov, S.B.; Fedorov, V.V.; Martyshkin, D.; Moskalev, I.S.; Mirov, M.; Vasilyev, S. Progress in mid-IR lasers based on Cr and Fe-doped II–VI chalcogenides. IEEE J. Sel. Top. Quantum Electron. 2015, 21, 292–310. [Google Scholar] [CrossRef]
- Sennaroglu, A. Solid-State Lasers and Applications; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781315222059. [Google Scholar] [CrossRef]
- DeLoach, L.; Page, R.; Wilke, G.; Payne, S.; Krupke, W. Transition metal-doped zinc chalcogenides: Spectroscopy and laser demonstration of a new class of gain media. IEEE J. Quantum Electron. 1996, 32, 885–895. [Google Scholar] [CrossRef]
- Page, R.; Schaffers, K.; DeLoach, L.; Wilke, G.; Patel, F.; Tassano, J.; Payne, S.; Krupke, W.; Chen, K.T.; Burger, A. Cr2+-doped zinc chalcogenides as efficient, widely tunable mid-infrared lasers. IEEE J. Quantum Electron. 1997, 33, 609–619. [Google Scholar] [CrossRef]
- Adams, J.J.; Bibeau, C.; Page, R.H.; Krol, D.M.; Furu, L.H.; Payne, S.A. 4.0–4.5 µm lasing of Fe:ZnSe below 180 K, a new mid-infrared laser material. Opt. Lett. 1999, 24, 1720. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, S.; Mirov, M.; Gapontsev, V. Mid-IR Kerr-Lens Mode-Locked Polycrystalline Cr2+:ZnS Laser with 0.5 MW Peak Power. In Proceedings of the Advanced Solid State Lasers (2015), Berlin, Germany, 4–9 October 2015; Optica Publishing Group: Washington, DC, USA, 2015; p. AW4A.3. [Google Scholar] [CrossRef]
- Kozlovsky, V.I.; Korostelin, Y.V.; Podmar’kov, Y.P.; Skasyrsky, Y.K.; Frolov, M.P. Middle infrared Fe2+:ZnS, Fe2+:ZnSe and Cr2+:CdSe lasers: New results. J. Phys. Conf. Ser. 2016, 740, 012006. [Google Scholar] [CrossRef]
- Sorokin, E.; Sorokina, I.; Di Lieto, A.; Tonelli, M.; Page, R. Tunable diode-pumped continuous-wave single crystal and ceramic Cr2+:ZnSe lasers. In Proceedings of the Technical Digest. CLEO/Pacific Rim 2001, 4th Pacific Rim Conference on Lasers and Electro-Optics (Cat. No.01TH8557), Chiba, Japan, 15–19 July 2001; pp. 26–27. [Google Scholar] [CrossRef]
- Jelínková, H.; Doroshenko, M.E.; Jelínek, M.; Vyhlídal, D.; Šulc, J.; Němec, M.; Kubeček, V.; Zagoruiko, Y.A.; Kovalenko, N.O.; Gerasimenko, A.S.; et al. Fe:ZnSe laser oscillation under cryogenic and room temperature. In Proceedings of the Solid State Lasers XXII: Technology and Devices, San Jose, CA, USA, 3–15 February 2013; International Society for Optics and Photonics: Bellingham, WA, USA, 2013; Volume 8599, p. 85990E. [Google Scholar] [CrossRef]
- Migal, E.A.; Balabanov, S.S.; Savin, D.V.; Ikonnikov, V.B.; Gavrishchuk, E.M.; Potemkin, F.V. Amplification properties of polycrystalline Fe:ZnSe crystals for high power femtosecond mid-IR laser systems. Opt. Mater. 2020, 111, 110640. [Google Scholar] [CrossRef]
- Frolov, M.P.; Gordienko, V.M.; Korostelin, Y.V.; Kozlovsky, V.I.; Podmar’kov, Y.P.; Potemkin, F.V.; Skasyrsky, Y.K. Fe2+-doped CdSe single crystal: Growth, spectroscopic and laser properties, potential use as a 6 µm broadband amplifier. Laser Phys. Lett. 2016, 14, 025001. [Google Scholar] [CrossRef]
- Mond, M.; Albrecht, D.; Heumann, E.; Huber, G.; Kück, S.; Levchenko, V.I.; Yakimovich, V.N.; Shcherbitsky, V.G.; Kisel, V.E.; Kuleshov, N.V.; et al. 1.9-µm and 2.0-µm laser diode pumping of Cr2+:ZnSe and Cr2+:CdMnTe. Opt. Lett. 2002, 27, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.W.; Dolasinski, B.D.; Harris, T.R.; Cleary, J.W.; Berry, P.A. Demonstration and power scaling of an Fe:CdMnTe laser at 5.2 microns. Opt. Mater. Express 2017, 7, 860–867. [Google Scholar] [CrossRef]
- Doroshenko, M.E.; Jelínková, H.; Jelínek, M.; Šulc, J.; Vyhlídal, D.; Kovalenko, N.O.; Terzin, I.S. Room temperature Fe2+:Cd1−xMnxTe laser generating at 5.4–6 µm. Opt. Lett. 2018, 43, 5058–5061. [Google Scholar] [CrossRef]
- Doroshenko, M.; Jelínková, H.; Osiko, V.; Jelínek, M.; Vyhlídal, D.; Šulc, J.; Němec, M.; Kovalenko, N.; Gerasimenko, A. Fe:ZnMnSe laser active material at 78–300 K: Spectroscopic properties and laser generation at 4.2–5.0 µm. J. Lumin. 2017, 192, 1300–1307. [Google Scholar] [CrossRef]
- Jelínková, H.; Doroshenko, M.E.; Osiko, V.V.; Jelínek, M.; Šulc, J.; Vyhlídal, D.; Kovalenko, N.O.; Gerasimenko, A.S. Fe:Zn0.6Mn0.4Se spectroscopic properties and laser generation at 5.0–5.8 µm in the temperature range of 78–300 K. In Proceedings of the CLEO Pacific Rim Conference 2018, Hong Kong, China, 29 July–3 August 2018; Optical Society of America: Washington, DC, USA, 2018; p. W3A.56. [Google Scholar] [CrossRef]
- Švejkar, R.; Šulc, J.; Říha, A.; Jelínková, H.; Doroshenko, M.E.; Kovalenko, N.O.; Gerasimenko, A.S. Er:YAG pumped compact Fe:ZnMnSe laser tunable in spectral range 3950–4500 nm at 80 K. In Proceedings of the 2018 International Conference Laser Optics (ICLO), St. Petersburg, Russia, 4–8 June 2018; p. 56. [Google Scholar] [CrossRef]
- Jelínková, H.; Doroshenko, M.E.; Jelínek, M.; Šulc, J.; Vyhlídal, D.; Říha, A.; Kovalenko, N.O.; Terzin, I.S. Fe:ZnMnTe laser generating around 5 µm at 78 K. In Proceedings of the Solid State Lasers XXIX: Technology and Devices, San Francisco, CA, USA, 4–6 February 2020; International Society for Optics and Photonics: Bellingham, WA, USA, 2020; Volume 11259, p. 1125923. [Google Scholar] [CrossRef]
- Doroshenko, M.E.; Jelínková, H.; Jelínek, M.; Říha, A.; Šulc, J.; Kovalenko, N.O.; Terzin, I.S. Comparison of novel Fe2+:Zn1−xMnxTe (x = 0.3) laser crystal operating near 5 µm at 78 K with other known Mn co-doped AII-BVI solid solutions. Opt. Mater. 2020, 108, 110392. [Google Scholar] [CrossRef]
- Doroshenko, M.E.; Osiko, V.V.; Jelínková, H.; Jelínek, M.; Němec, M.; Šulc, J.; Kovalenko, N.O.; Gerasimenko, A.S.; Puzikov, V.M. Spectroscopic and laser properties of Cr2+ ions in Zn1−xMgxSe solid solutions. Opt. Mater. 2015, 47, 185–189. [Google Scholar] [CrossRef]
- Doroshenko, M.E.; Osiko, V.V.; Jelínková, H.; Jelínek, M.; Šulc, J.; Němec, M.; Vyhlídal, D.; Čech, M.; Kovalenko, N.O.; Gerasimenko, A.S. Spectroscopic and laser properties of bulk iron doped zinc magnesium selenide Fe:ZnMgSe generating at 4.5–5.1 µm. Opt. Express 2016, 24, 19824–19834. [Google Scholar] [CrossRef] [PubMed]
- Martyshkin, D.V.; Fedorov, V.V.; Mirov, M.; Moskalev, I.; Vasilyev, S.; Smolski, V.; Zakrevskiy, A.; Mirov, S.B. High Power (9.2 W) CW 4.15 µm Fe:ZnSe laser. In Proceedings of the Conference on Lasers and Electro-Optics (2017), San Jose, CA, USA, 14–19 May 2017; Optical Society of America: Washington, DC, USA, 2017; p. STh1L.6. [Google Scholar] [CrossRef]
- Pushkin, A.V.; Migal, E.A.; Uehara, H.; Goya, K.; Tokita, S.; Frolov, M.P.; Korostelin, Y.V.; Kozlovsky, V.I.; Skasyrsky, Y.K.; Potemkin, F.V. Compact, highly efficient, 2.1-W continuous-wave mid-infrared Fe:ZnSe coherent source, pumped by an Er:ZBLAN fiber laser. Opt. Lett. 2018, 43, 5941. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, I.S.; Fedorov, V.V.; Mirov, S.B. InP diode-pumped Cr:ZnS and Cr:ZnSe highly efficient, widely tunable, mid-IR lasers. In Proceedings of the Solid State Lasers XIX: Technology and Devices, San Francisco, CA, USA, 24–28 January 2010; International Society for Optics and Photonics: Bellingham, WA, USA, 2010; Volume 7578, p. 75781K. [Google Scholar] [CrossRef]
- Nagl, N.; Gröbmeyer, S.; Pervak, V.; Krausz, F.; Pronin, O.; Mak, K.F. Directly diode-pumped, Kerr-lens mode-locked, few-cycle Cr:ZnSe oscillator. Opt. Express 2019, 27, 24445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Říha, A.; Jelínková, H.; Doroshenko, M.E.; Jelínek, M.; Němec, M.; Kovalenko, N.O.; Terzin, I.S. Mid-IR lasing of Fe2+ ions via Cr2+ → Fe2+ energy transfer process with YLF:Er or laser diode pumping at 1.7 µm. Opt. Mater. Express 2020, 10, 662. [Google Scholar] [CrossRef]
- Wang, Y.; Fernandez, T.T.; Coluccelli, N.; Gambetta, A.; Laporta, P.; Galzerano, G. 47-fs Kerr-lens mode-locked Cr:ZnSe laser with high spectral purity. Opt. Express 2017, 25, 25193–25200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migal, E.; Pushkin, A.; Bravy, B.; Gordienko, V.; Minaev, N.; Sirotkin, A.; Potemkin, F. 3.5-mJ 150-fs Fe:ZnSe hybrid mid-IR femtosecond laser at 4.4 µm for driving extreme nonlinear optics. Opt. Lett. 2019, 44, 2550–2553. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, I.T. Cr2+-doped II–VI materials for lasers and nonlinear optics. Opt. Mater. 2004, 26, 395–412. [Google Scholar] [CrossRef]
- Fedorov, V.; Mirov, S.; Gallian, A.; Badikov, D.; Frolov, M.; Korostelin, Y.; Kozlovsky, V.; Landman, A.; Podmar’kov, Y.; Akimov, V.; et al. 3.77–5.05 µm tunable solid-state lasers based on Fe2+-doped ZnSe crystals operating at low and room temperatures. IEEE J. Quantum Electron. 2006, 42, 907–917. [Google Scholar] [CrossRef]
- Fedorov, V.; Carlson, T.; Mirov, S. Energy transfer in iron-chromium co-doped ZnSe middle-infrared laser crystals. Opt. Mater. Express 2019, 9, 2340–2347. [Google Scholar] [CrossRef]
- Doroshenko, M.E.; Jelínková, H.; Říha, A.; Jelínek, M.; Němec, M.; Kovalenko, N.O.; Gerasimenko, A.S. Mid-IR (4.4 µm) Zn1−xMnxSe:Cr2+,Fe2+ (x = 0.3) laser pumped by 1.7 µm laser using Cr2+ → Fe2+ energy transfer. Opt. Lett. 2019, 44, 2724–2727. [Google Scholar] [CrossRef]
- Doroshenko, M.; Jelínek, M.; Říha, A.; Šulc, J.; Jelínková, H.; Kubeček, V.; Kovalenko, N.O.; Gerasimenko, A.S. Long-pulse 4.4–4.6 µm laser oscillations of Fe2+ ions in a Zn1−xMnxSe (x = 0.3) crystal pumped by a 1940 nm Tm fiber laser through Cr2+ → Fe2+ energy transfer. Opt. Lett. 2019, 44, 5334–5337. [Google Scholar] [CrossRef] [PubMed]
- Říha, A.; Doroshenko, M.E.; Jelínková, H.; Němec, M.; Jelínek, M.; Šulc, J.; Vyhlídal, D.; Kovalenko, N.O.; Terzin, I.S. 2.3- and 4.4-µm Lasing in Cr,Fe:Zn1−xMnxSe (x = 0.3) Single Crystal Pumped by Q-Switched Er:YLF Laser at 1.73 µm. Phys. Wave Phenom. 2020, 28, 231–235. [Google Scholar] [CrossRef]
- Říha, A.; Jelínková, H.; Doroshenko, M.E.; Jelínek, M.; Šulc, J.; Vyhlidal, D.; Kovalenko, N.O.; Terzin, I.S. Comparison of gain-switched operated Cr2+ and Fe2+ ions co-doped Zn1−xMnxSe and Zn1−xMgxSe (x = 0.3) lasers in mid-IR under different excitation wavelengths. In Proceedings of the High Power Lasers and Applications, Online, 19–30 August 2021; Butcher, T.J., Hein, J., Bakule, P., Haefner, C.L., Korn, G., Silva, L.O., Eds.; SPIE: Bellingham, WA, USA, 2021; p. 25. [Google Scholar] [CrossRef]
- Říha, A.; Jelínková, H.; Doroshenko, M.E.; Jelínek, M.; Šulc, J.; Němec, M.; Čech, M.; Vyhlídal, D.; Kovalenko, N.O. Gain-switched Laser Operation of Cr2+,Fe2+:Zn1−xMgxSe (x = 0.2; x = 0.3) Single Crystals under Cr2+ → Fe2+ Energy Transfer at 1.73 µm and Direct Fe2+ Ions Excitation at 2.94 µm. J. Lumin. 2021, 240, 118375. [Google Scholar] [CrossRef]
- Doroshenko, M.E.; Jelínková, H.; Němec, M.; Šulc, J.; Jelínek, M.; Komar, V.K.; Gerasimenko, A.S.; Puzikov, V.M.; Badikov, V.V. Cr:ZnMgSe laser pumped by 1.7 µm Er:YLF radiation. In Proceedings of the Solid State Lasers XXII: Technology and Devices, San Francisco, CA, USA, 2–7 February 2013; International Society for Optics and Photonics: Bellingham, WA, USA, 2013; Volume 8599, p. 859921. [Google Scholar] [CrossRef]
- Dibartolo, B. Energy Transfer Processes in Condensed Matter; Nato Science Series B; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar] [CrossRef]
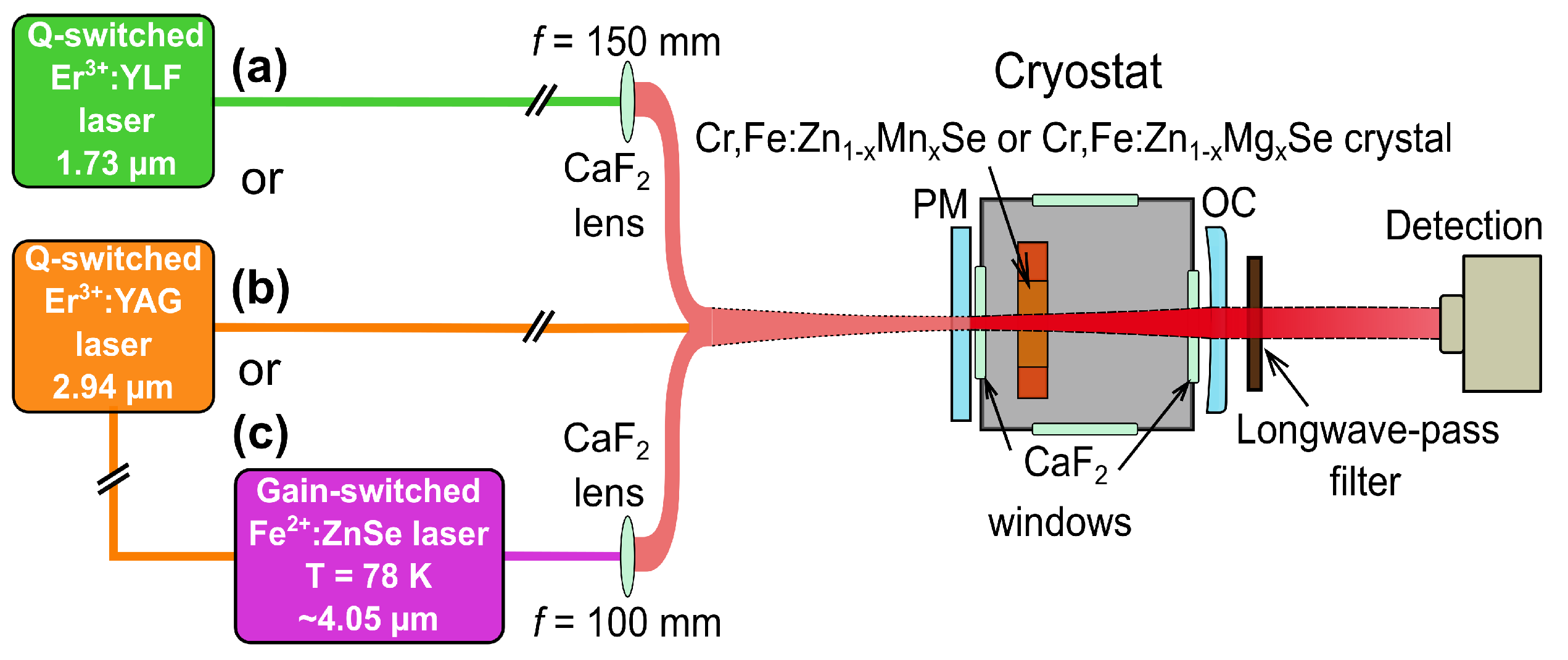
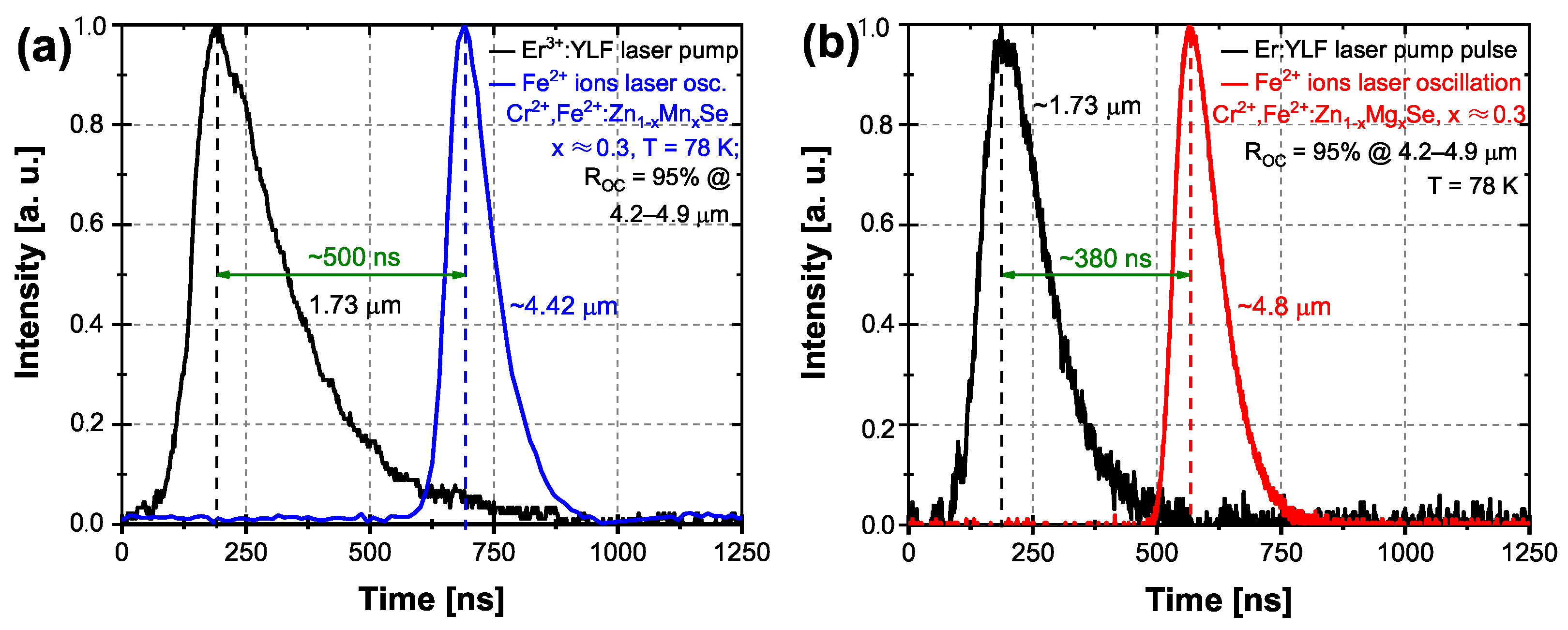
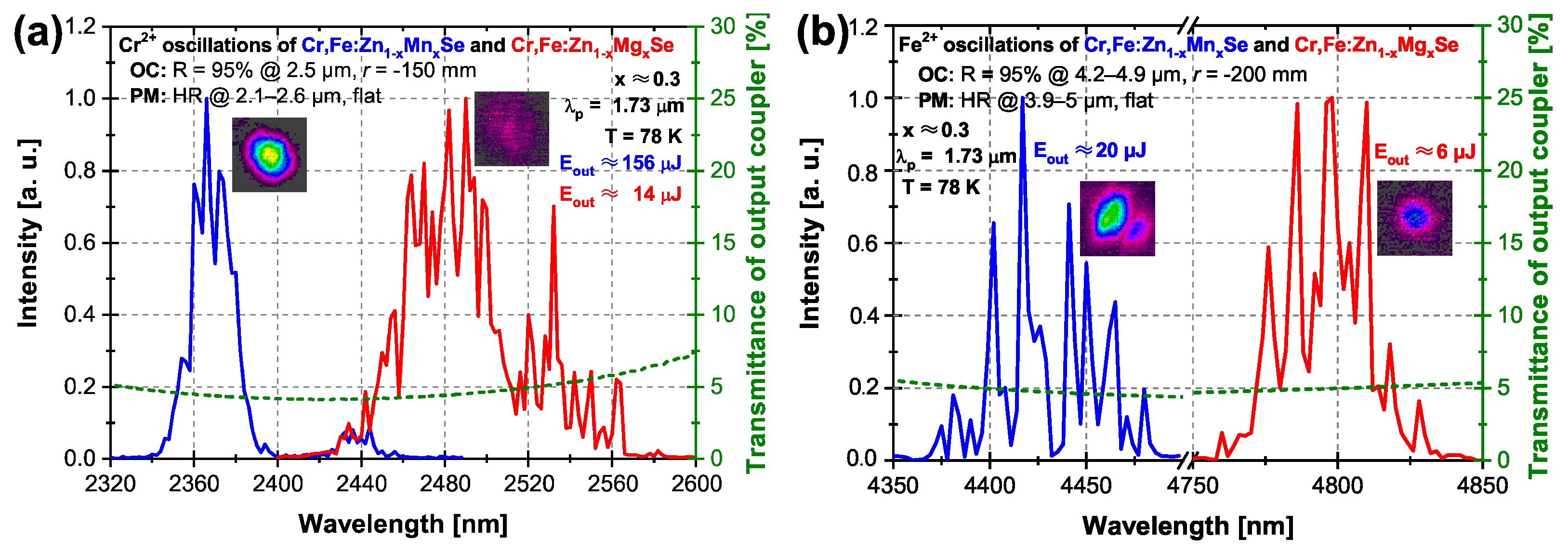
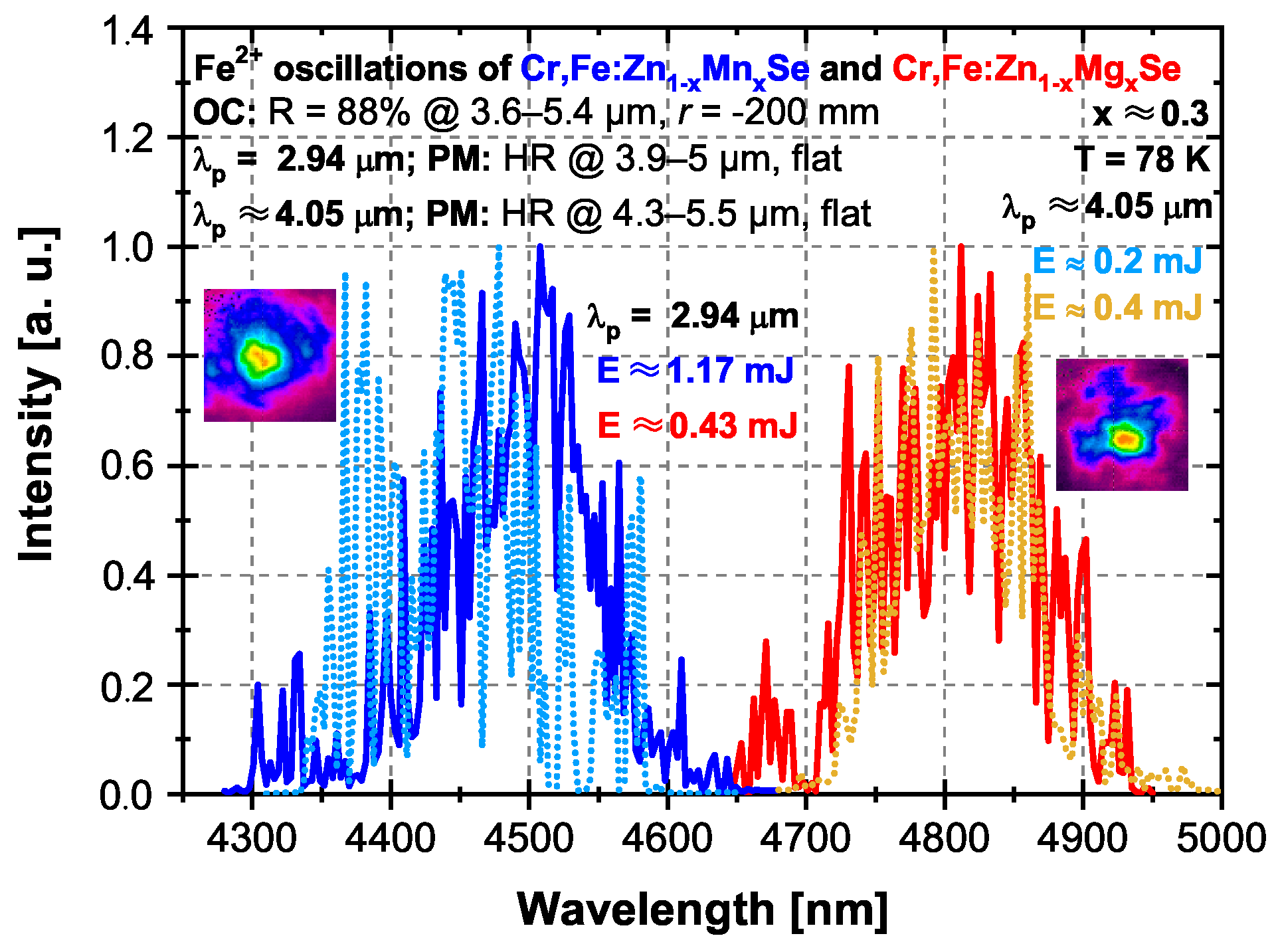
| Laser; Operation Temperature | Central Osc. Wavelength (m) | Pulse Duration (FWHM) (ns) | Pulse Energy (mJ) | Repetition Rate (Hz) |
|---|---|---|---|---|
| (a) Q-switched Er:YLF; RT | 1.73 | 175 | ∼13 | 1 |
| (b) Q-switched Er:YAG; RT | 2.94 | 160 | ∼14 | 2.5 |
| (c) Fe:ZnSe; 78 K | ∼4.05 | 150 | ∼9 | 1 |
| Crystal Sample | Cr Ions’ Absorption Band FWHM at 78/300 K | Cr Ions Max. Absorption Wavelength at 78/300 K | Fe Ions’ Absorption Band FWHM at 78/300 K | Fe Ions Max. Absorption Wavelength at 78/300 K |
|---|---|---|---|---|
| Cr,Fe:ZnMnSe (x ≈ 0.3) | 294/386 nm | 1774/1782 nm | 1060/1460 nm | 2930/2950–3200 nm |
| Cr,Fe:ZnMgSe (x ≈ 0.3) | 370/416 nm | 1850/1870 nm | 1290/1540 nm | 3425/3425 nm |
| Crystal Sample | Thickness (mm) | Absorption Coefficient at 1.73 m; 78/300 K | Absorption Coefficient at 2.94 m; 78/300 K | Absorption Coefficient at ∼4.05 m; 78/300 K |
|---|---|---|---|---|
| Cr,Fe:ZnMnSe (x ≈ 0.3) | 2.6 mm | 4.7/3.3 cm | 9.0/5.5 cm | 0.7/2.9 cm |
| Cr,Fe:ZnMgSe (x ≈ 0.3) | 5.0 mm | 1.2/1.0 cm | 2.5/2.5 cm | 2.8/2.2 cm |
Temperature (K) | Crystal Sample, x ≈ 0.3 | Fluorescence Decay Time (1/e Level) (s) | Cr Lifetime (Measured at the Tail) (s) | Energy Transfer Efficiency According to Equation (1) (%) | Energy Transfer Efficiency According to Equation (2) (%) |
|---|---|---|---|---|---|
| 78 K | Cr,Fe(Cr):ZnMnSe | 1.6 | 5.3 | 70 | 57 |
| Cr,Fe(Cr):ZnMgSe | 1.7 | 5.0 | 66 | 53 | |
| 200 K | Cr,Fe(Cr):ZnMnSe | 1.3 | 5.3 | 75 | 62 |
| Cr,Fe(Cr):ZnMgSe | 1.5 | 5.0 | 70 | 57 | |
| 300 K | Cr,Fe(Cr):ZnMnSe | 1.0 | 4.2 | 76 | 67 |
| Cr,Fe(Cr):ZnMgSe | 1.0 | 4.0 | 75 | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Říha, A.; Jelínková, H.; Doroshenko, M.E.; Jelínek, M.; Šulc, J.; Němec, M.; Vyhlídal, D.; Kovalenko, N.O. Mid-Infrared Laser Generation of Zn1−xMnxSe and Zn1−xMgxSe (x ≈ 0.3) Single Crystals Co-Doped by Cr2+ and Fe2+ Ions—Comparison of Different Excitation Wavelengths. Materials 2022, 15, 5277. https://doi.org/10.3390/ma15155277
Říha A, Jelínková H, Doroshenko ME, Jelínek M, Šulc J, Němec M, Vyhlídal D, Kovalenko NO. Mid-Infrared Laser Generation of Zn1−xMnxSe and Zn1−xMgxSe (x ≈ 0.3) Single Crystals Co-Doped by Cr2+ and Fe2+ Ions—Comparison of Different Excitation Wavelengths. Materials. 2022; 15(15):5277. https://doi.org/10.3390/ma15155277
Chicago/Turabian StyleŘíha, Adam, Helena Jelínková, Maxim E. Doroshenko, Michal Jelínek, Jan Šulc, Michal Němec, David Vyhlídal, and Nazar O. Kovalenko. 2022. "Mid-Infrared Laser Generation of Zn1−xMnxSe and Zn1−xMgxSe (x ≈ 0.3) Single Crystals Co-Doped by Cr2+ and Fe2+ Ions—Comparison of Different Excitation Wavelengths" Materials 15, no. 15: 5277. https://doi.org/10.3390/ma15155277
APA StyleŘíha, A., Jelínková, H., Doroshenko, M. E., Jelínek, M., Šulc, J., Němec, M., Vyhlídal, D., & Kovalenko, N. O. (2022). Mid-Infrared Laser Generation of Zn1−xMnxSe and Zn1−xMgxSe (x ≈ 0.3) Single Crystals Co-Doped by Cr2+ and Fe2+ Ions—Comparison of Different Excitation Wavelengths. Materials, 15(15), 5277. https://doi.org/10.3390/ma15155277







