A New Process of Direct Zinc Oxide Production by Carbothermal Reduction of Zinc Ash
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Thermodynamic Basis for Reduction
2.3. Experimental Method
3. Results
3.1. Zinc Ash Dechlorination Experiment
3.2. Effect of Temperature on Reduction Rate of Zinc Oxide
3.3. Effect of Time on Reduction Rate of Zinc Oxide
3.4. Effect of Reducing Agent Dosage on Reduction Rate of Zinc Oxide
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.Y.; Yu, L.H.; Fu, T.; Wang, J.; Shen, F.Q.; Cui, K.K. Microstructure evolution and growth mechanism of Si-MoSi2 composite coatings on TZM (Mo-0.5Ti-0.1Zr-0.02 C) alloy. J. Alloys Compd. 2022, 894, 162403. [Google Scholar] [CrossRef]
- Cui, K.K.; Mao, H.B.; Zhang, Y.Y.; Wang, J.; Wang, H.; Tan, T.B.; Fu, T. Microstructure, mechanical properties, and reinforcement mechanism of carbide toughened ZrC-based ultra-high temperature ceramics: A review. Compos. Interfaces 2022, 29, 729–748. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Fu, T.; Yu, L.H.; Shen, F.Q.; Wang, J.; Cui, K.K. Improving oxidation resistance of TZM alloy by deposited Si-MoSi2 composite coating with high silicon concentration. Ceram. Int. 2022, 48, 20895–20904. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yu, L.H.; Fu, T.; Wang, J.; Shen, F.Q.; Cui, K.K.; Wang, H. Microstructure and oxidation resistance of Si-MoSi2 ceramic coating on TZM (Mo-0.5Ti-0.1Zr-0.02C) alloy at 1500 °C. Surf. Coat. Technol. 2022, 431, 128037. [Google Scholar] [CrossRef]
- Fu, T.; Shen, F.; Zhang, Y.; Yu, L.; Cui, K.; Wang, J.; Zhang, X. Oxidation protection of high-temperature coatings on the surface of Mo-based alloys—A Review. Coatings 2022, 12, 141. [Google Scholar] [CrossRef]
- Mao, H.B.; Shen, F.Q.; Zhang, Y.Y.; Wang, J.; Cui, K.K.; Wang, H.; Lv, T.; Fu, T.; Tan, T.B. Microstructure and mechanical properties of carbide reinforced TiC-based ultra-high temperature ceramics: A Review. Coatings 2021, 11, 1444. [Google Scholar] [CrossRef]
- Doustkhah, E.; Esmat, M.; Fukata, N.; Ide, Y.; Hanaor, A.H.; Assadi, M.N. MOF-derived nanocrystalline ZnO with controlled orientation and photocatalytic activity. Chemosphere 2022, 303, 134932. [Google Scholar] [CrossRef]
- Shafi, M.A.; Bouich, A.; Fradi, K.; Guaita, J.M.; Khan, L.; Mari, B. Effect of deposition cycles on the properties of ZnO thin films deposited by spin coating method for CZTS-based solar cells. Optik 2022, 258, 168854. [Google Scholar] [CrossRef]
- Guaita, J.M.; Bouich, A.; Mari, B. Shedding light on phase stability and surface engineering of formamidinium lead iodide (FaPbI3) thin films for solar cells. Eng. Proc. 2021, 12, 1. [Google Scholar]
- Wang, J.; Zhang, Y.Y.; Yu, L.H.; Cui, K.K.; Fu, T.; Mao, H.B. Effective separation and recovery of valuable metals from waste Ni-based batteries: A comprehensive review. Chem. Eng. J. 2022, 439, 135767. [Google Scholar] [CrossRef]
- Sharma, P.; Hasan, M.R.; Mehto, N.K.; Deepak; Bishoyi, A.; Narang, J. 92 years of zinc oxide: Has been studied by the scientific community since the 1930s-An overview. Sens. Int. 2022, 3, 100182. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [Green Version]
- Hirase, R.; Nagatani, A.; Yuguchi, Y. Development of powdering method for cellulose nanofibers assisted by zinc oxide for compounding reinforced natural rubber composite. Curr. Res. Green Sustain. Chem. 2020, 3, 100005. [Google Scholar] [CrossRef]
- Ahmed, N.M.; Nashar, D.E.E.I. The effect of zinc oxide-phosphate core-shell pigments on the properties of blend rubber composites. Mater. Des. 2013, 44, 1–11. [Google Scholar] [CrossRef]
- Moezzi, A.; McDonagh, A.M.; Cortie, M.B. Zinc oxide particles: Synthesis, properties and applications. Chem. Eng. J. 2012, 185–186, 1–22. [Google Scholar] [CrossRef]
- Zuo, X.; Yoon, S.D.; Yang, A. Ferromagnetism in pure wurtzite zinc oxide. J. Appl. Phys. 2009, 105, 07C508. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Y.Y.; Cui, K.K.; Gao, T.F.J.J.; Hussain, S.; AlGarni, T.S. Pyrometallurgical recovery of zinc and valuable metals from electric arc furnace dust-A review. J. Clean. Prod. 2021, 298, 126788. [Google Scholar] [CrossRef]
- Anandaraj, S.; Karthik, S.; Vijaymohan, S.; Rampradheep, G.S.; Indhiradevi, P.; Anusha, G. Effects of using white flour, zinc oxide and zinc ash as an admixture in mortar and concrete. Mater. Today: Proc. 2022, 52, 1788–1793. [Google Scholar] [CrossRef]
- Garg, R.; Garg, R. Effect of zinc oxide nanoparticles on mechanical properties of silica fume-based cement composites. Mater. Today: Proc. 2021, 43, 778–783. [Google Scholar] [CrossRef]
- Kumar, M.; Bansal, M.; Garg, R. An overview of beneficiary aspects of zinc oxide nanoparticles on performance of cement composites. Mater. Today: Proc. 2021, 43, 892–898. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.H.; Chen, L.; Li, Y.C. Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Shamsipur, M.A.; Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Facile synthesis of zinc carbonate and zinc oxide nanoparticles via direct carbonation and thermal decomposition. Ceram. Int. 2013, 39, 819–827. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Wang, D.Z.; Pan, J. A study on the zinc removal kinetics and mechanism of zinc-bearing dust pellets in direct reduction. Powder Technol. 2021, 380, 273–281. [Google Scholar] [CrossRef]
- Wang, H.Q.; Li, C.H.; Zhao, H.G. Preparation of nano-sized flower-like ZnO bunches by a direct precipitation method. Adv. Powder Technol. 2013, 24, 599–604. [Google Scholar] [CrossRef]
- Rudnik, E. Hydrometallurgical recovery of zinc from industrial hot dipping top ash. Trans. Nonferrous Met. Soc. China 2020, 30, 2239–2255. [Google Scholar] [CrossRef]
- Rudnik, E. Recovery of zinc from zinc ash by leaching in sulphuric acid and electrowinning. Hydrometallurgy 2019, 188, 256–263. [Google Scholar] [CrossRef]
- Wang, S.X.; Xu, C.Y.; Lei, Z.; Li, J.R.; Lu, J.L.; Xiang, Q.Q.; Chen, X.; Hua, Y.X.; Li, Y. Recycling of zinc oxide dust using ChCl-urea deep eutectic solvent with nitrilotriacetic acid as complexing agents. Miner. Eng. 2022, 175, 107295. [Google Scholar] [CrossRef]


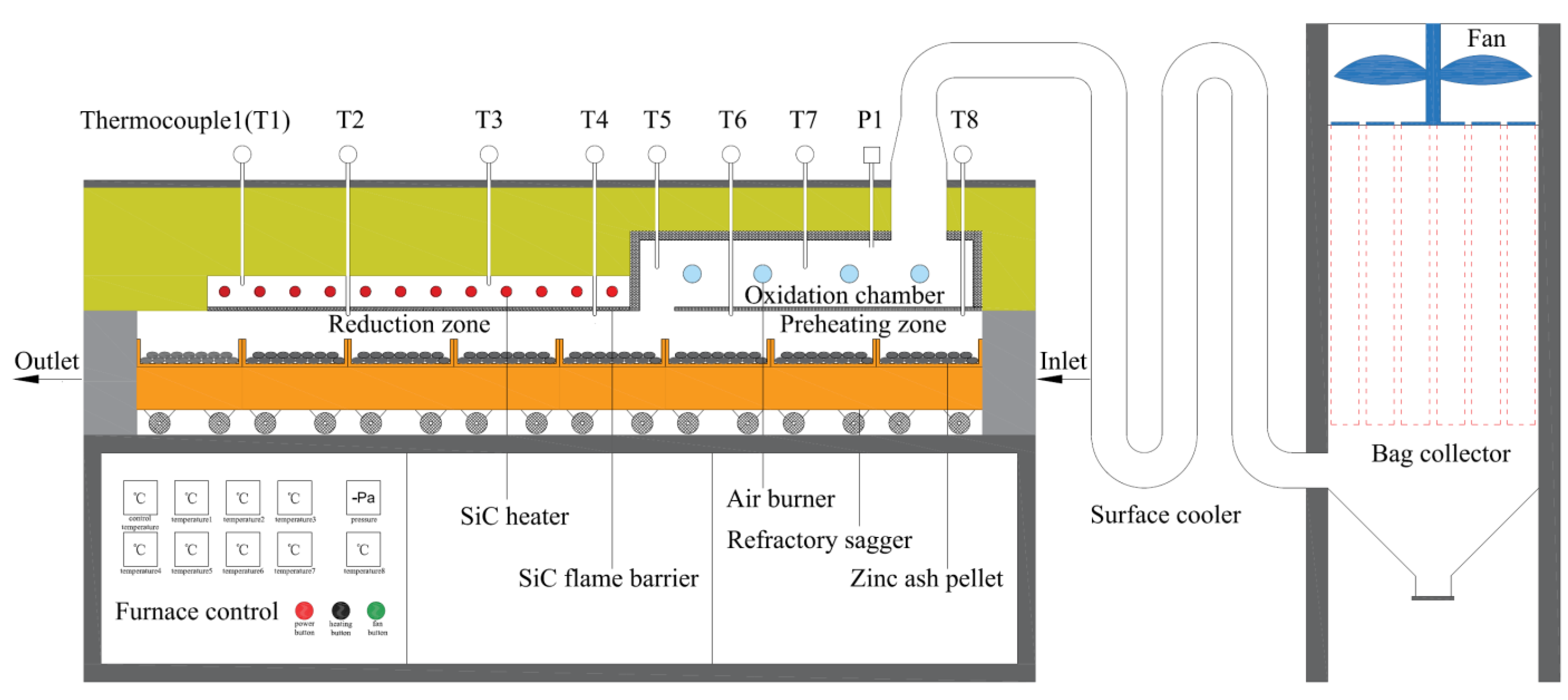





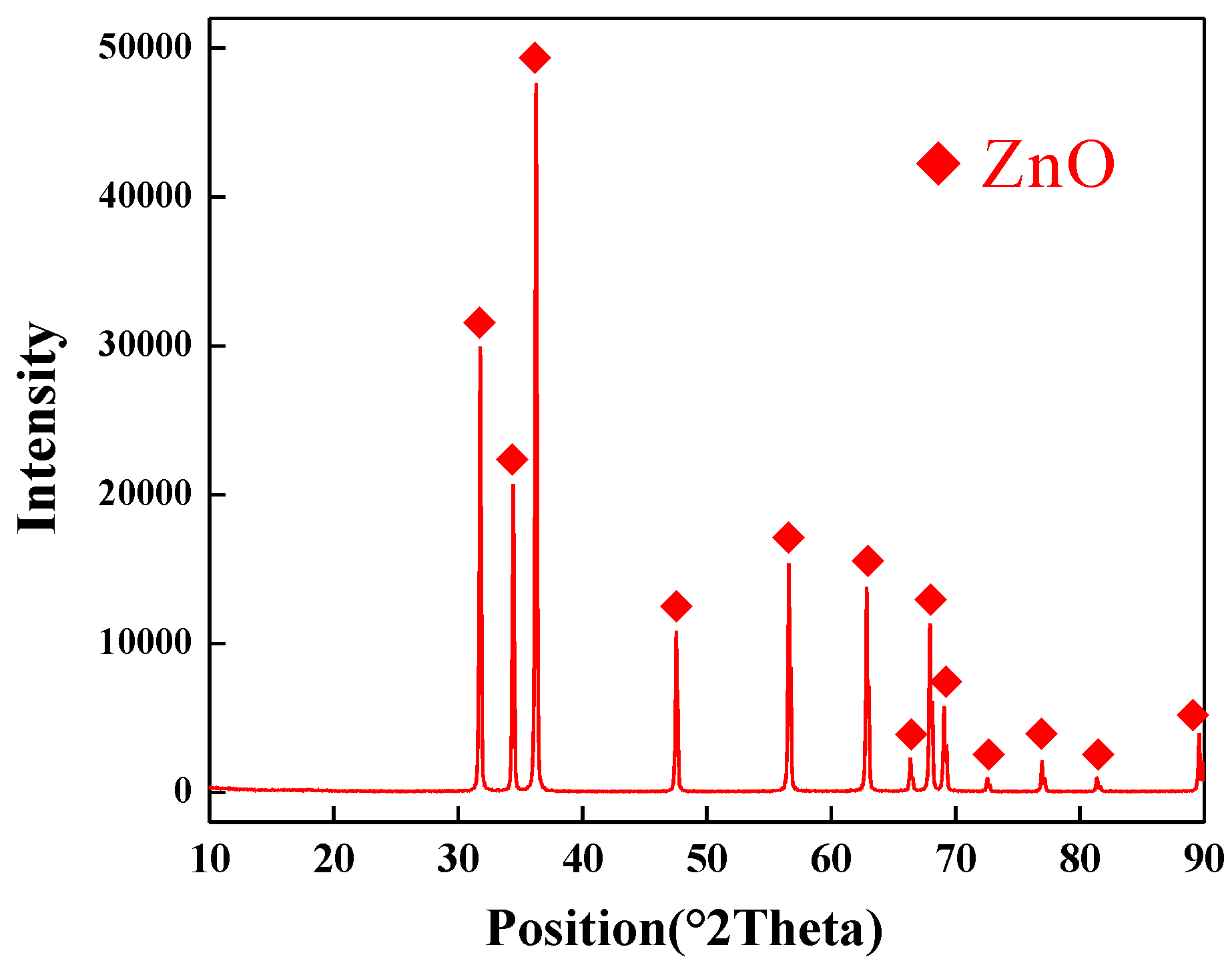

| Component | ZnO | Cl | Fe2O3 | SiO2 | CaO | MgO | Al2O3 |
|---|---|---|---|---|---|---|---|
| Content | 87.91 | 4.44 | 0.26 | 1.27 | 0.49 | 0.23 | 0.50 |
| Component | C | Ash | Volatiles | S | P | H2O |
|---|---|---|---|---|---|---|
| Content | 78.73 | 10.16 | 8.55 | 0.28 | 0.023 | 2.56 |
| Component | ZnO | Cl | Fe2O3 | SiO2 | CaO | MgO | Al2O3 |
|---|---|---|---|---|---|---|---|
| Content | 95.05 | 0.021 | 0.35 | 1.68 | 0.63 | 0.42 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Wang, H.; Wang, J.; Zhang, Y.; Wang, F.; Yang, S.; Li, S. A New Process of Direct Zinc Oxide Production by Carbothermal Reduction of Zinc Ash. Materials 2022, 15, 5246. https://doi.org/10.3390/ma15155246
Gao J, Wang H, Wang J, Zhang Y, Wang F, Yang S, Li S. A New Process of Direct Zinc Oxide Production by Carbothermal Reduction of Zinc Ash. Materials. 2022; 15(15):5246. https://doi.org/10.3390/ma15155246
Chicago/Turabian StyleGao, Jianjun, Hong Wang, Jie Wang, Yingyi Zhang, Feng Wang, Shuang Yang, and Shinan Li. 2022. "A New Process of Direct Zinc Oxide Production by Carbothermal Reduction of Zinc Ash" Materials 15, no. 15: 5246. https://doi.org/10.3390/ma15155246
APA StyleGao, J., Wang, H., Wang, J., Zhang, Y., Wang, F., Yang, S., & Li, S. (2022). A New Process of Direct Zinc Oxide Production by Carbothermal Reduction of Zinc Ash. Materials, 15(15), 5246. https://doi.org/10.3390/ma15155246







