Tunable Luminescence and Energy Transfer of Sr3B2O6:Ce3+, Sm3+ Phosphors with Potential Anti-Counterfeiting Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Crystal Structure, Morphology and Phase Purity
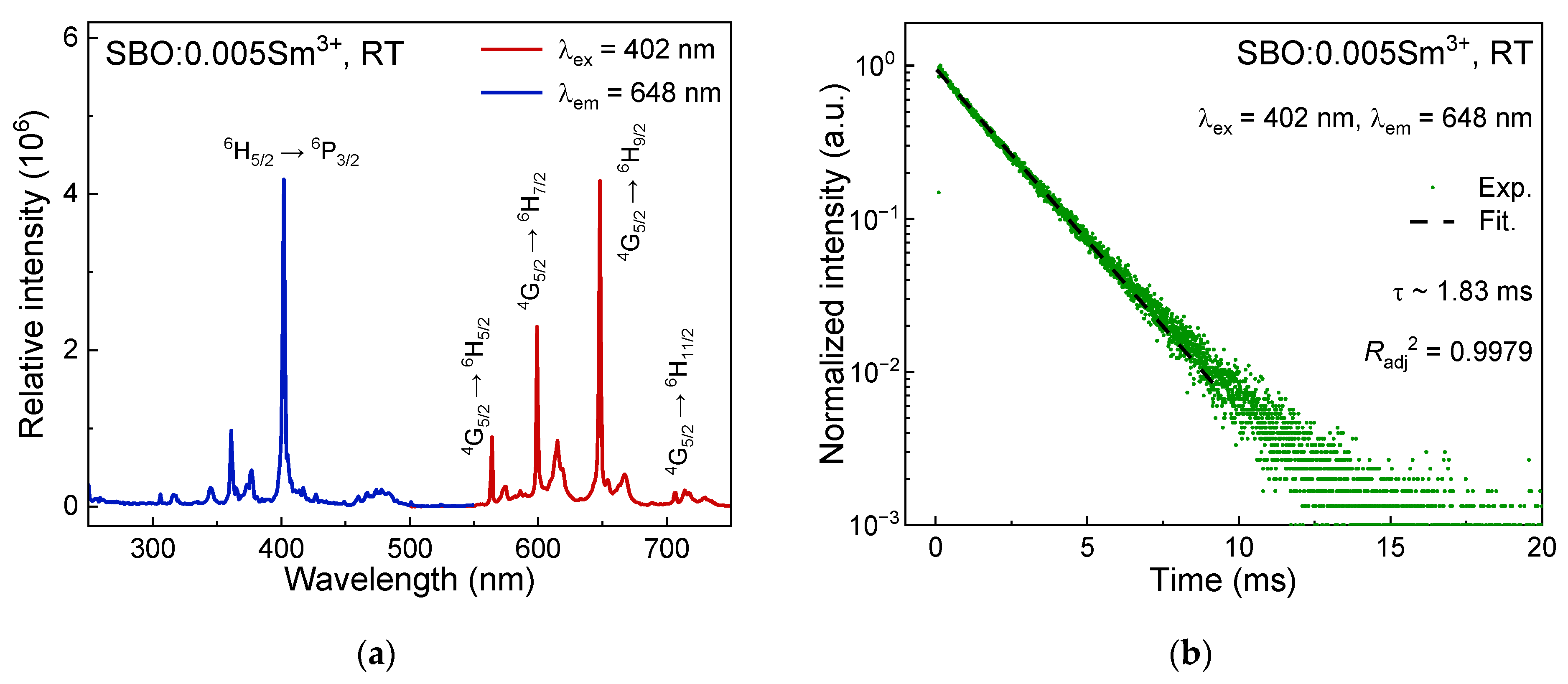
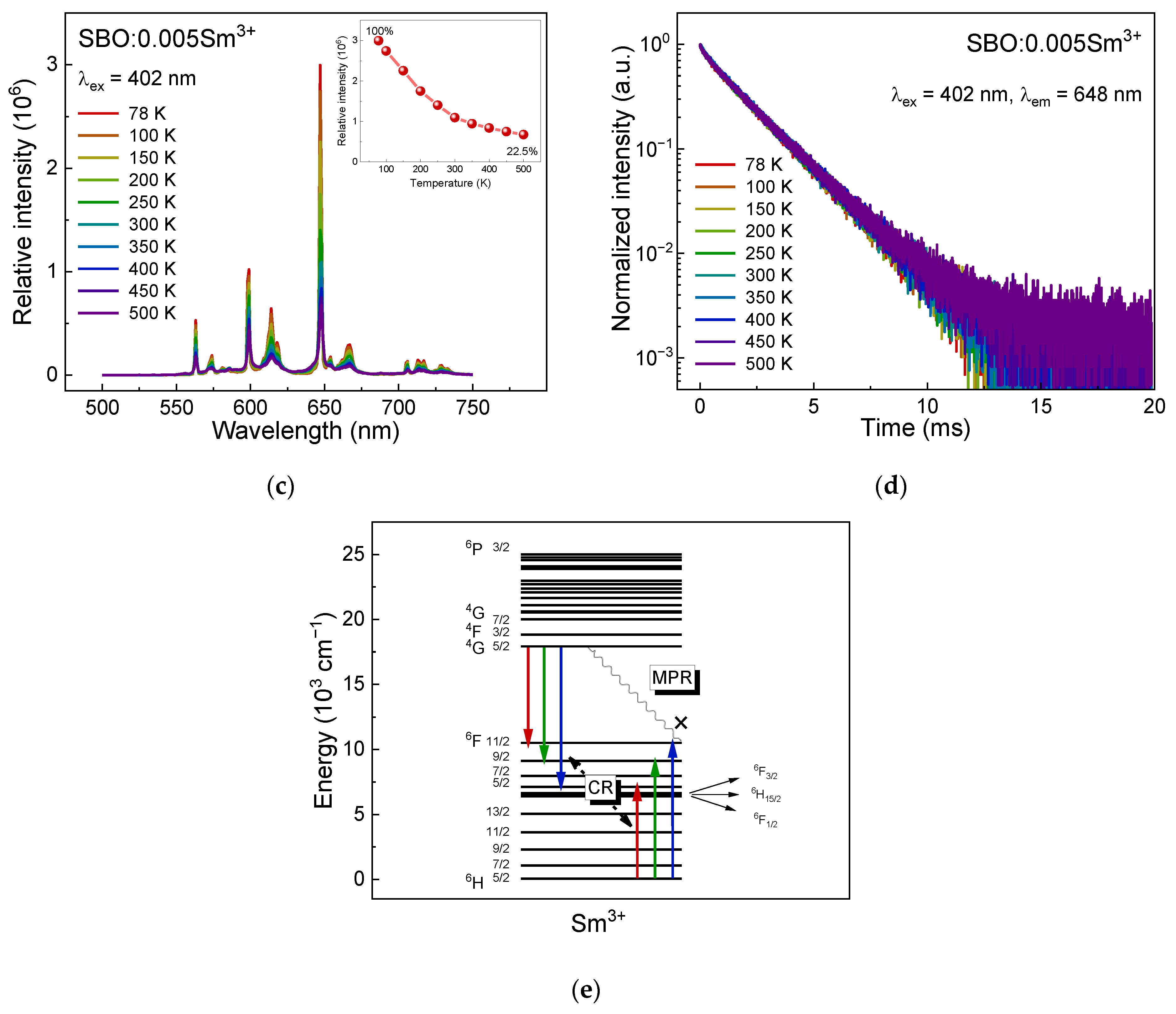
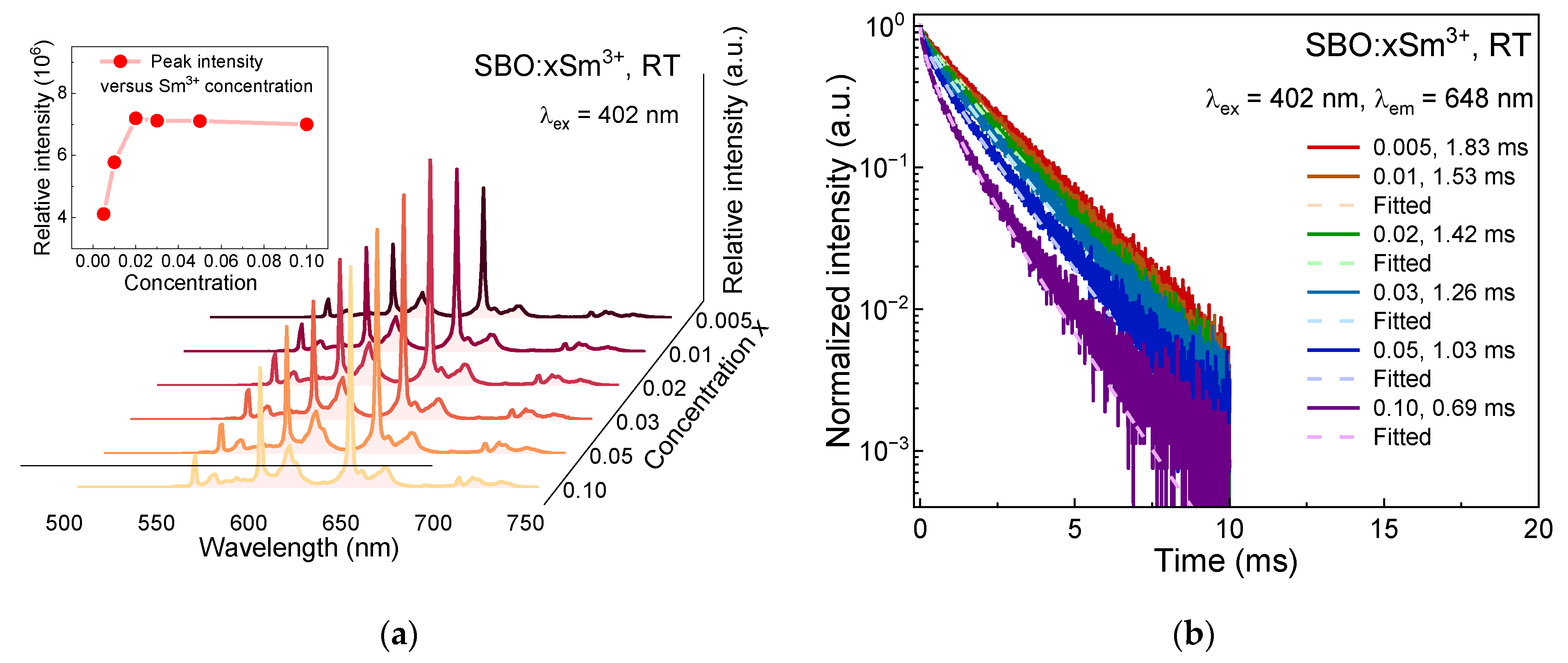
3.2. Luminescence Properties of Sm3+ in SBO Host
3.3. Luminescence Properties of SBO:0.01Ce3+ Sample
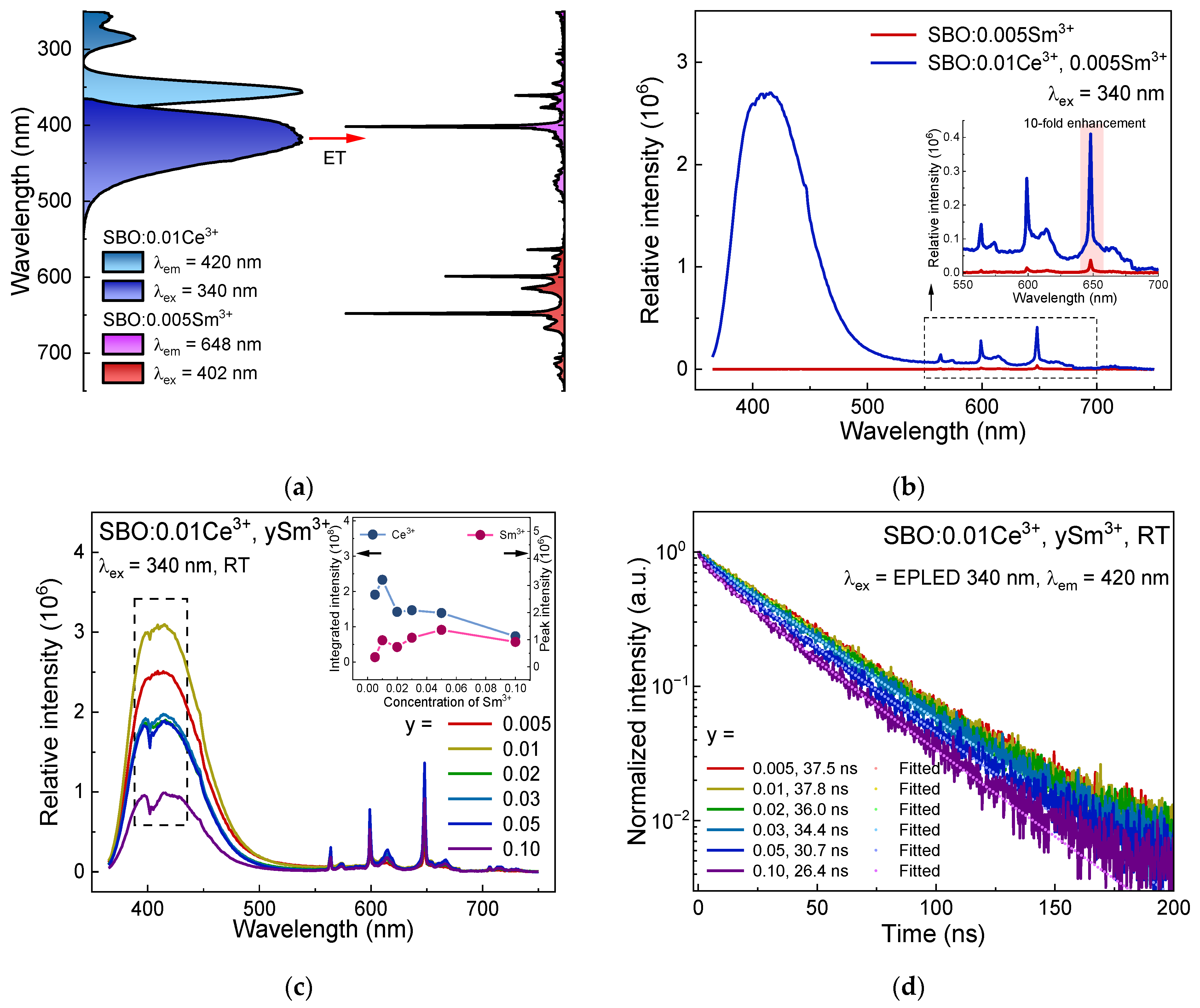
3.4. Luminescence of Sm3+, Ce3+ Co-Doped Samples and Ce3+-Sm3+ ET Dynamics
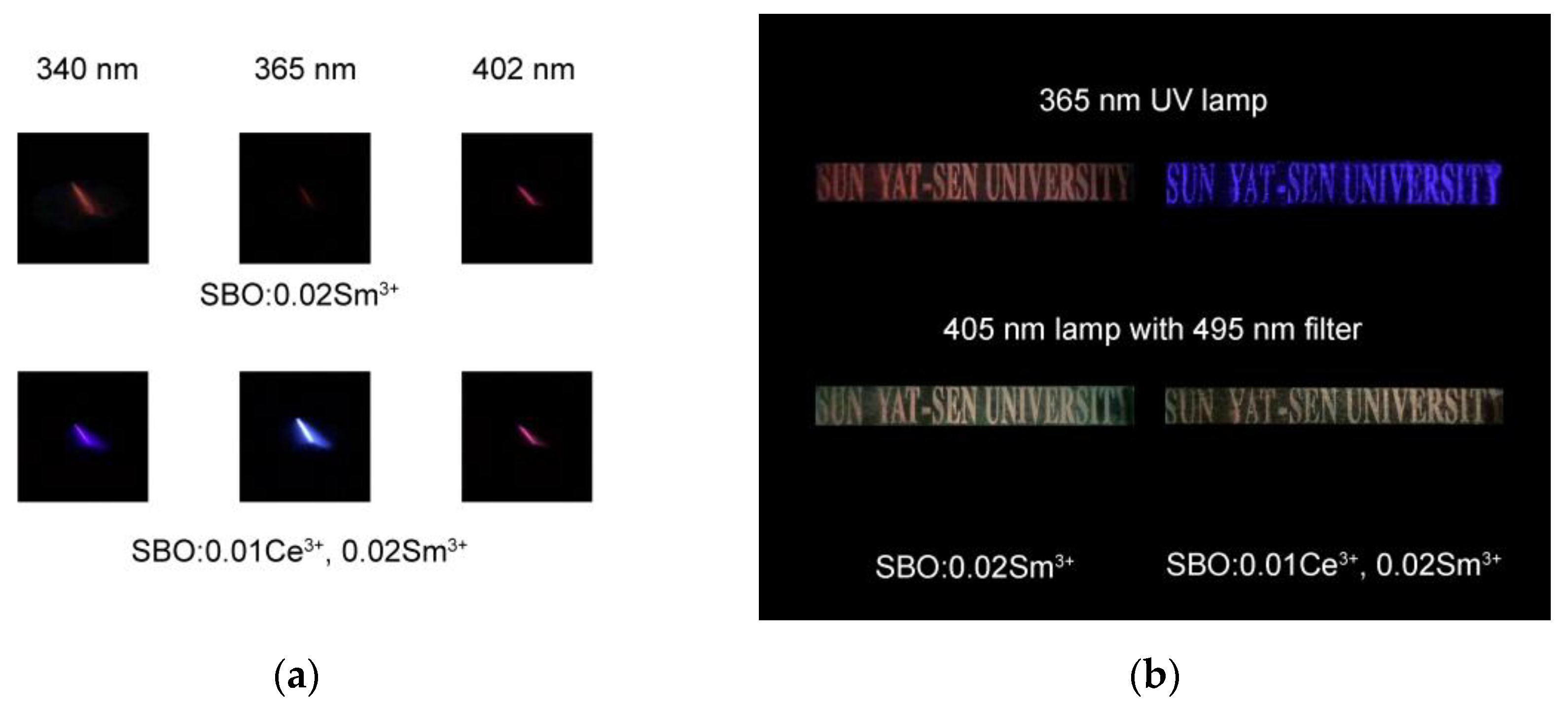
3.5. Color Variation under Different Excitation Conditions of Samples and Their Potential Anti-Counterfeiting Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Zhao, J.; Zhang, R.; Liu, Y.; Liu, D.; Goldys, E.M.; Yang, X.; Xi, P.; Sunna, A.; Lu, J.; et al. Tunable lifetime multiplexing using luminescent nanocrystals. Nat. Photon. 2014, 8, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-C.; Pan, C.; Zhu, Y.-F.; Zhao, L.-Z.; He, H.-W.; Liu, X.; Qiu, J. Achieving thermo-mechano-opto-responsive bitemporal colorful luminescence via multiplexing of dual lanthanides in piezoelectric particles and its multidimensional anticounterfeiting. Adv. Mater. 2018, 30, 1804644. [Google Scholar] [CrossRef]
- Feng, A.; Smet, P.F. A review of mechanoluminescence in inorganic solids: Compounds, mechanisms, models and applications. Materials 2018, 11, 484. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, A.; Freslon, S.; Fan, X.; Rojo, A.; Daiguebonne, C.; Suffren, Y.; Bernot, K.; Calvez, G.; Roisnel, T.; Guillou, O. Lanthanide-based coordination polymers with 1,4-carboxyphenylboronic ligand: Multiemissive compounds for multisensitive luminescent thermometric probes. Inorg. Chem. 2019, 58, 462–475. [Google Scholar] [CrossRef]
- Wang, J.; Suffren, Y.; Daiguebonne, C.; Freslon, S.; Bernot, K.; Calvez, G.; Le Pollès, L.; Roiland, C.; Guillou, O. Multi-emissive lanthanide-based coordination polymers for potential application as luminescent bar-codes. Inorg. Chem. 2019, 58, 2659–2668. [Google Scholar] [CrossRef] [PubMed]
- de Rohello, E.L.; Suffren, Y.; Merdrignac-Conanec, O.; Guillou, O.; Cheviré, F. Effect of cationic substitutions on the photoluminescence properties of Eu2+ doped SrCN2 prepared by a facile C3N4 based synthetic approach. J. Eur. Ceram. Soc. 2020, 40, 6316–6321. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, H.; Yang, X.; Yang, L.; Min, Q.; Ma, H.; Yu, X.; Qiu, J.; Xu, X. A dynamic three-path authenticating model for anti-counterfeiting in a single host of CaAl2Si2O8. Chem. Eng. J. 2021, 412, 128695. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, X.; Zhang, Z.; Xiang, J.; Suo, H.; Guo, C. Dual-mode dichromatic SrBi4Ti4O15: Er3+ emitting phosphor for anti-counterfeiting application. Cerac. Int. 2021, 47, 15067–15072. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, L.; Ye, R.; Jia, G.; Hua, Y.; Deng, D.; Xu, S. Integrating positive and negative thermal quenching effect for ultrasensitive ratiometric temperature sensing and anti-counterfeiting. ACS Appl. Mater. Interfaces 2021, 13, 23951–23959. [Google Scholar] [CrossRef]
- Lv, S.; Shanmugavelu, B.; Wang, Y.; Mao, Q.; Zhao, Y.; Yu, Y.; Hao, J.; Zhang, Q.; Qiu, J.; Zhou, S. Transition metal doped smart glass with pressure and temperature sensitive luminescence. Adv. Opt. Mater. 2018, 6, 1800881. [Google Scholar] [CrossRef]
- Liang, H.; Lin, H.; Zhang, G.; Dorenbos, P.; Su, Q. Luminescence of Ce3+ and Pr3+ doped Sr2Mg(BO3)2 under VUV–UV and X-ray excitation. J. Lumin. 2011, 131, 194–198. [Google Scholar] [CrossRef]
- Zhu, Q.-Q.; Wang, L.; Hirosaki, N.; Hao, L.Y.; Xu, X.; Xie, R.-J. Extra-broad band orange-emitting Ce3+-doped Y3Si5N9O phosphor for solid-state lighting: Electronic, crystal structures and luminescence properties. Chem. Mater. 2016, 28, 4829–4839. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhuang, W.; Hu, Y.; Liu, R.; Xu, H.; Chen, M.; Liu, Y.; Li, Y.; Zheng, Y.; Chen, G. Cyan-green phosphor (Lu2M)(Al4Si)O12:Ce3+ for high-quality LED lamp: Tunable photoluminescence properties and enhanced thermal stability. Inorg. Chem. 2019, 58, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Filatov, S.K.; Krzhizhanovskaya, M.G.; Bubnova, R.S.; Shablinskii, A.P.; Belousova, O.L.; Firsova, V.A. Thermal expansion and structural complexity of strontium borates. Struct. Chem. 2016, 27, 1663–1671. [Google Scholar] [CrossRef]
- Coelho, A.A. Coelho Software, Version 4; TOPAS Academic: Brisbane, Australia, 2005.
- Wei, Z.F.; Chen, X.L.; Wang, F.M.; Li, W.C.; He, M.; Zhang, Y. Phase relations in the ternary system SrO-TiO2-B2O3. J. Alloys Compd. 2001, 327, L10–L13. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Shi, R.; Xu, J.; Liu, G.; Zhang, X.; Zhou, W.; Pan, F.; Huang, Y.; Tao, Y.; Liang, H. Spectroscopy and luminescence dynamics of Ce3+ and Sm3+ in LiYSiO4. J. Phys. Chem. C 2016, 120, 4529–4537. [Google Scholar] [CrossRef]
- Pekgözlü, İ. A novel reddish orange luminescent material Sr3B2O6:Sm3+. J. Appl. Spectrosc. 2019, 85, 1136–1139. [Google Scholar] [CrossRef]
- Cavalli, E.; Angiuli, F.; Belletti, A.; Boutinaud, P. Luminescence spectroscopy of YVO4:Ln3+, Bi3+ (Ln3+ = Eu3+, Sm3+, Dy3+). Opt. Mater. 2014, 36, 1642–1648. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Golyeva, E.V.; Kurochkin, M.A.; Kolesnikov, E.Y.; Lähderanta, E. Concentration series of Sm3+-doped YVO4 nanoparticles: Structural, luminescence and thermal properties. J. Lumin. 2020, 219, 116946. [Google Scholar] [CrossRef]
- Wang, L.; Noh, H.M.; Moon, B.K.; Park, S.H.; Kim, K.H.; Shi, J.; Jeong, J.H. Dual-mode luminescence with broad near UV and blue excitation band from Sr2CaMoO6:Sm3+ phosphor for white LEDs. J. Phys. Chem. C 2015, 119, 15512–15525. [Google Scholar] [CrossRef]
- Hua, Y.; Yu, J.S. Synthesis and luminescence properties of reddish-orange-emitting Ca2GdNbO6:Sm3+ phosphors with good thermal stability for high CRI white applications. Ceram. Int. 2021, 47, 6059–6067. [Google Scholar] [CrossRef]
- Han, B.; Zhang, J.; Li, P.; Shi, H. Photoluminescence properties of novel yellowish orange emitting phosphor KBaBP2O8:Sm3+. Mater. Lett. 2014, 126, 113–115. [Google Scholar] [CrossRef]
- Cao, S.; Chen, Q.; Li, Y.; Wu, C.; Xu, J.; Cheng, G.; Gao, F. Novel strategy for the enhancement of anti-counterfeiting ability of photochromic ceramics: Sm3+ doped KSr2Nb5O15 textured ceramics with anisotropic luminescence modulation behavior. J. Eur. Ceram. Soc. 2021, 41, 4924–4933. [Google Scholar] [CrossRef]
- Ningombam, G.S.; Singh, N.R.; Ningthoujam, R.S. Controlled synthesis of CaWO4:Sm3+ microsphere particles by a reverse-micelle method and their energy transfer rate in luminescence. Colloid. Surface A 2017, 518, 249–262. [Google Scholar] [CrossRef]
- Bachmann, V.; Ronda, C.; Meijerink, A. Temperature quenching of yellow Ce3+ luminescence in YAG:Ce. Chem. Mater. 2009, 21, 2077–2084. [Google Scholar] [CrossRef]
- Riseberg, L.A.; Moos, H.W. Multiphonon orbit-lattice relaxation of excited states of rare-earth ions in crystals. Phys. Rev. 1968, 174, 429–438. [Google Scholar] [CrossRef]
- Wang, X.; Qi, H.; Li, Y.; Yu, F.; Wang, H.; Chen, F.; Liu, Y.; Wang, Z.; Xu, X.; Zhao, X. Synthesis and characterization of new Sr3(BO3)2 crystal for stimulated Raman scattering applications. Crystals 2017, 7, 125. [Google Scholar] [CrossRef] [Green Version]
- Zatryb, G.; Klak, M.M. On the choice of proper average lifetime formula for an ensemble of emitters showing non-single exponential photoluminescence decay. J. Phys. Condens. Matter 2020, 32, 415902. [Google Scholar] [CrossRef]
- Zhang, X.; Seo, H.J. Luminescence properties of novel Sm3+, Dy3+ doped LaMoBO6 phosphors. J. Alloys Compd. 2011, 509, 2007–2010. [Google Scholar] [CrossRef]
- Suhasini, T.; Kumar, J.S.; Sasikala, T.; Jang, K.; Lee, H.S.; Jayasimhadri, M.; Jeong, J.H.; Yi, S.S.; Moorthy, L.R. Absorption and fluorescence properties of Sm3+ ions in fluoride containing phosphate glasses. Opt. Mater. 2009, 31, 1167–1172. [Google Scholar] [CrossRef]
- Inokuti, M.; Hirayama, F. Influence of energy transfer by the exchange mechanism on donor luminescence. J. Chem. Phys. 1965, 43, 1978–1989. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, X.; Li, Z.; Wu, P.; Xu, S. Interaction of Sm3+ ions in borate glass. J. Lumin. 1988, 40–41, 657–658. [Google Scholar] [CrossRef]
- Zhou, W.; Gu, M.; Ou, Y.; Zhang, C.; Zhang, X.; Zhou, L.; Liang, H. Concentration-driven selectivity of energy transfer channels and color tunability in Ba3La(PO4)3:Tb3+, Sm3+ for warm white LEDs. Inorg. Chem. 2017, 56, 7433–7442. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Arunkumar, P.; Jeyakumar, D.; Lakshminarasimhan, N. White light emission in alkali metal ion co-doped single host lattice phosphor Sr3B2O6:Ce3+, Eu2+, A+ [A = Li, Na and K]. Ceram. Int. 2015, 41, 497–3501. [Google Scholar] [CrossRef]
- Ou, Y.; Zhou, W.; Yang, Y.; Ma, F.; Qi, Z.; Brik, M.G.; Liang, H. Concentration palette enabling temperature-responsive luminescence co-modulations of inorganic phosphors for thermally-triggered security tag. to be submitted.
- Wan, Y.; Abudouwufu, T.; Yusufu, T.; He, J.; Sidike, A. Photoluminescence properties and energy transfer of a single-phased white-emitting NaAlSiO4:Ce3+, Sm3+ phosphor. J. Rare Earths 2017, 35, 850–856. [Google Scholar] [CrossRef]
- Benrejeb, H.; Soler-Carracedo, K.; Hraiech, S.; Martin, I.R. Photoluminescence and energy transfer studies in Ce3+-Sm3+ co-doped phosphate glasses. J. Lumin. 2022, 241, 118471. [Google Scholar] [CrossRef]
- Su, F.; Zhou, W.; Yang, Y.; Ou, Y.; Qi, Z.; Duan, C.-K.; Brik, M.G.; Dorenbos, P.; Liang, H. Structure, luminescence of Eu2+ and Eu3+ in CaMgSi2O6 and their co-existence for the excitation-wavelength/temperature driven colour evolution. Dalton. Trans. 2021, 50, 10050–10058. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Yang, Y.; Ou, Y.; Zhou, W.; Ma, F.; Zhang, J.; Brik, M.G.; Liang, H. Site occupancies, electron-vibration interaction and energy transfer of CaMgSi2O6:Eu2+, Mn2+ phosphors for potential temperature-sensing and anti-counterfeiting applications. Chem. Eur. J. 2022, 28, e202200381. [Google Scholar] [CrossRef] [PubMed]


| Atom | x | y | z | Occ. | Biso |
|---|---|---|---|---|---|
| Sr | 0.3554(1) | 0 | 0.2500 | 0.9966 | 1.1227(13) |
| Sm | 0.3554(1) | 0 | 0.2500 | 0.0017 | 1.3435(13) |
| Na | 0.3554(1) | 0 | 0.2500 | 0.0017 | 0.9375(21) |
| B | 0 | 0 | 0.1185(8) | 1 | 1.3979(20) |
| O | 0.1601(4) | 0.0118(5) | 0.1156(2) | 1 | 1.1704(42) |
| Samples | SBO:0.01Ce3+ | SBO:0.10Sm3+ | SBO:0.01Ce3+, 0.10Sm3+ |
|---|---|---|---|
| a (Å) | 9.042(1) | 9.039(2) | 9.038(2) |
| c (Å) | 12.570(3) | 12.567(3) | 12.565(3) |
| V (Å3) | 890.3(2) | 889.5(1) | 889.2(3) |
| Sm3+ Concentration | CA (×1025 m−3) | CDA (×10−53 m6/s) | Radj2 |
|---|---|---|---|
| 0.01 | 6.742 | 2.740 | 0.9975 |
| 0.02 | 13.48 | 3.338 | 0.9978 |
| 0.03 | 20.23 | 3.856 | 0.9974 |
| 0.05 | 33.71 | 4.542 | 0.9973 |
| 0.10 | 67.42 | 4.175 | 0.9973 |
| average | - | 3.730 | - |
| Sm3+ Concentration | CA (×1025 m−3) | CDA (×10−49 m6/s) | Radj2 |
|---|---|---|---|
| 0.005 | 3.371 | 0.8791 | 0.9978 |
| 0.01 | 6.742 | 2.424 | 0.9983 |
| 0.02 | 13.48 | 1.393 | 0.9983 |
| 0.03 | 20.23 | 2.359 | 0.9978 |
| 0.05 | 33.71 | 4.089 | 0.9984 |
| 0.10 | 67.42 | 2.464 | 0.9982 |
| average | - | 2.268 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, Y.; Wei, J.; Liang, H. Tunable Luminescence and Energy Transfer of Sr3B2O6:Ce3+, Sm3+ Phosphors with Potential Anti-Counterfeiting Applications. Materials 2022, 15, 5189. https://doi.org/10.3390/ma15155189
Ou Y, Wei J, Liang H. Tunable Luminescence and Energy Transfer of Sr3B2O6:Ce3+, Sm3+ Phosphors with Potential Anti-Counterfeiting Applications. Materials. 2022; 15(15):5189. https://doi.org/10.3390/ma15155189
Chicago/Turabian StyleOu, Yiyi, Junyu Wei, and Hongbin Liang. 2022. "Tunable Luminescence and Energy Transfer of Sr3B2O6:Ce3+, Sm3+ Phosphors with Potential Anti-Counterfeiting Applications" Materials 15, no. 15: 5189. https://doi.org/10.3390/ma15155189
APA StyleOu, Y., Wei, J., & Liang, H. (2022). Tunable Luminescence and Energy Transfer of Sr3B2O6:Ce3+, Sm3+ Phosphors with Potential Anti-Counterfeiting Applications. Materials, 15(15), 5189. https://doi.org/10.3390/ma15155189






