High-Temperature Oxidation Behavior of Cr-Ni-Mo Hot-Work Die Steels
Abstract
:1. Introduction
2. Material and Methods
- Heat Treatment 1 (3Cr3Mo2NiW): annealed at 870 °C for 2 h, quenched at 980 °C for 1 h, water-cooled, and tempered at 680 °C for 2 h.
- Heat Treatment 2 (3CrNi3Mo): annealed at 880 °C for 1 h, quenched at 870 °C for 2 h, water-cooled, and tempered at 680 °C for 2 h.
| Steel | C | Si | Mn | Cr | Ni | Mo | W | P | S | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 3Cr3Mo2NiW | 0.30 | 0.02 | 0.15 | 3.16 | 0.50 | 1.89 | 0.30 | <0.015 | <0.015 | Bal. |
| 3CrNi3Mo | 0.30 | 0.20 | 0.20 | 0.90 | 2.84 | 0.20 | - | <0.015 | <0.015 | Bal. |
3. Results and Discussion
3.1. Initial Microstructure
3.2. Weight Gain
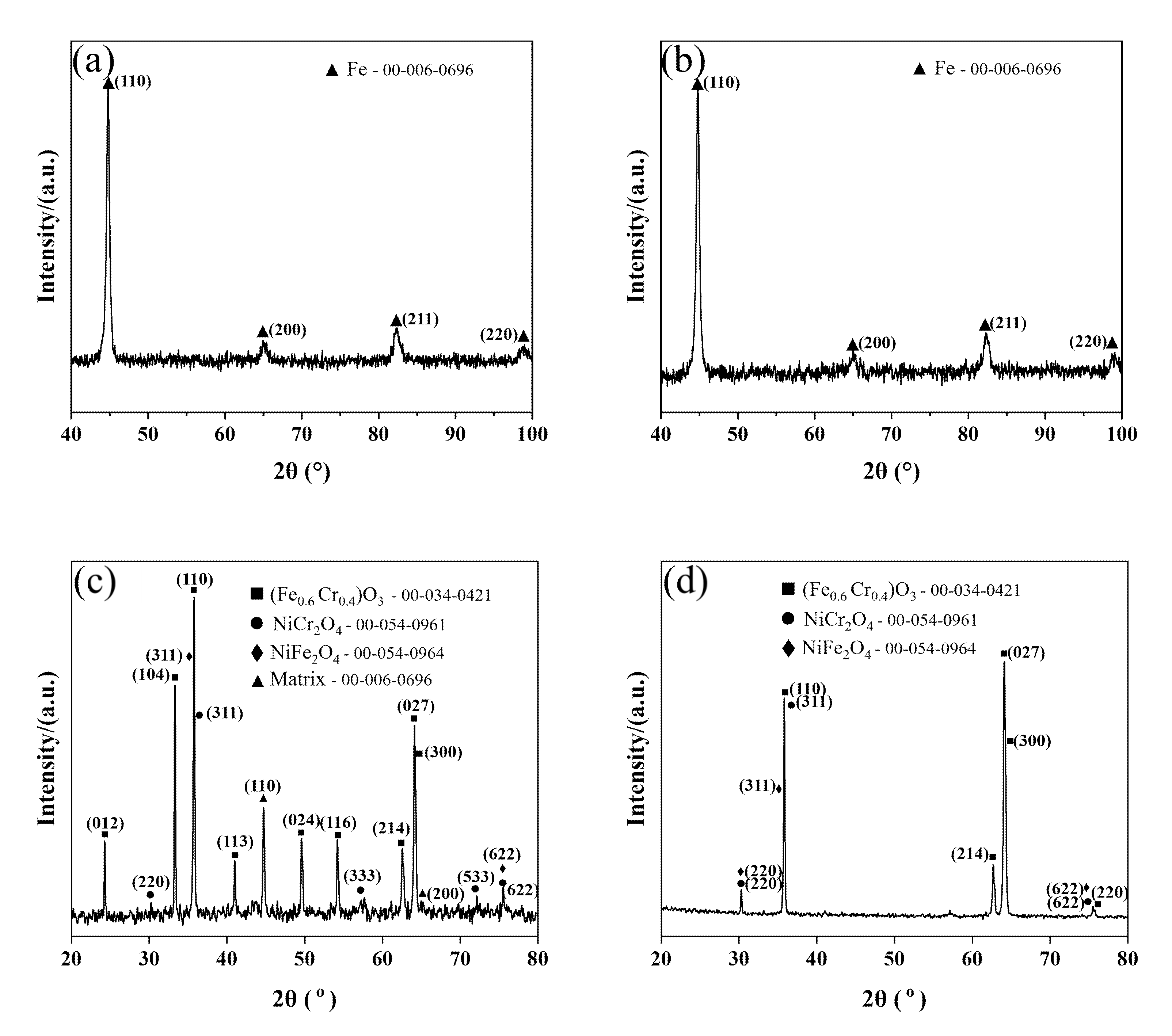
3.3. Phase Characterization of Oxide Film
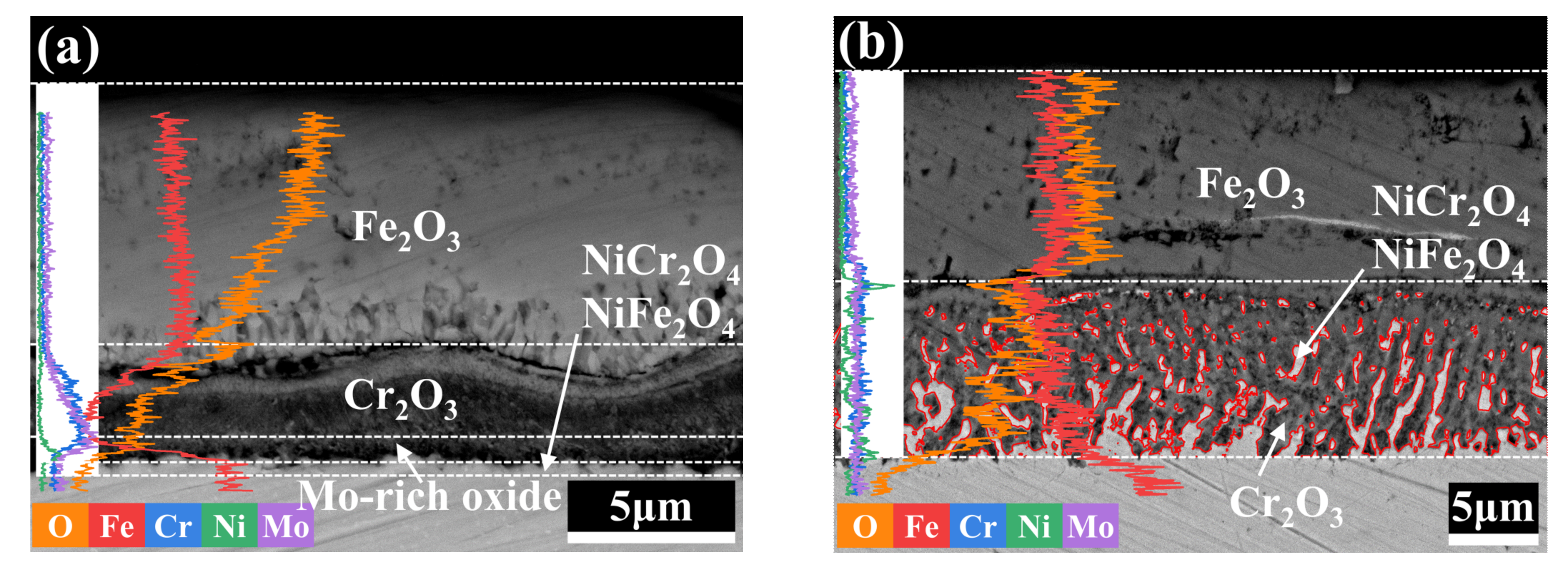
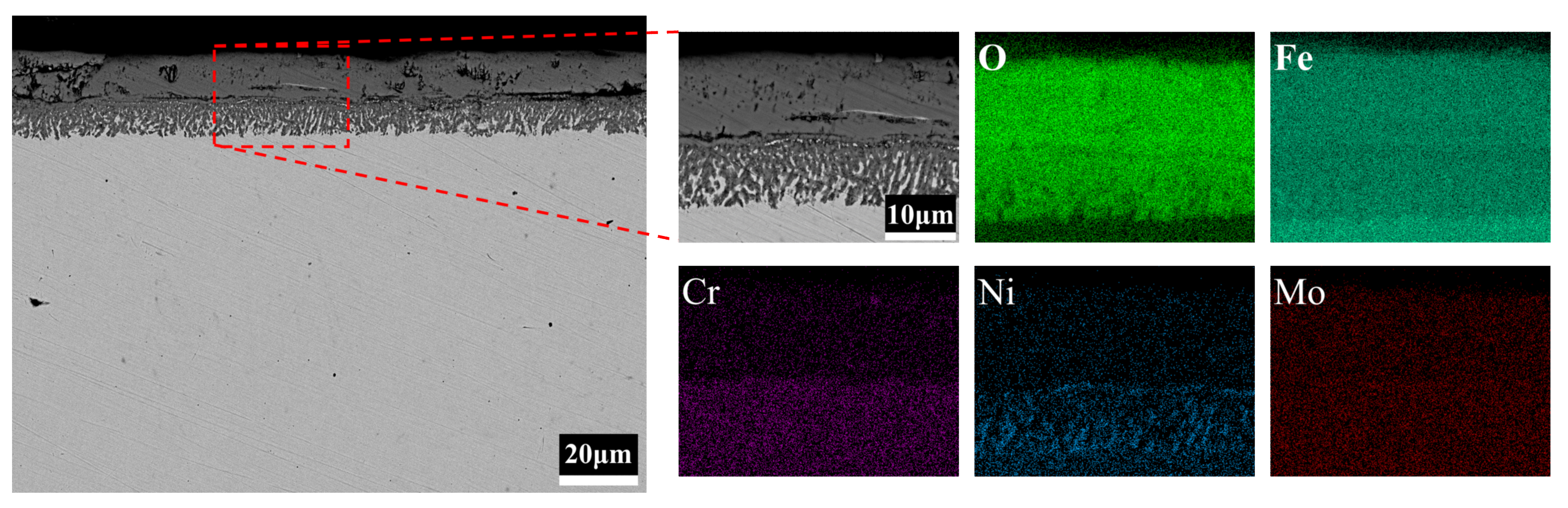
3.4. Cross-Sectional Morphology of the Oxide Film
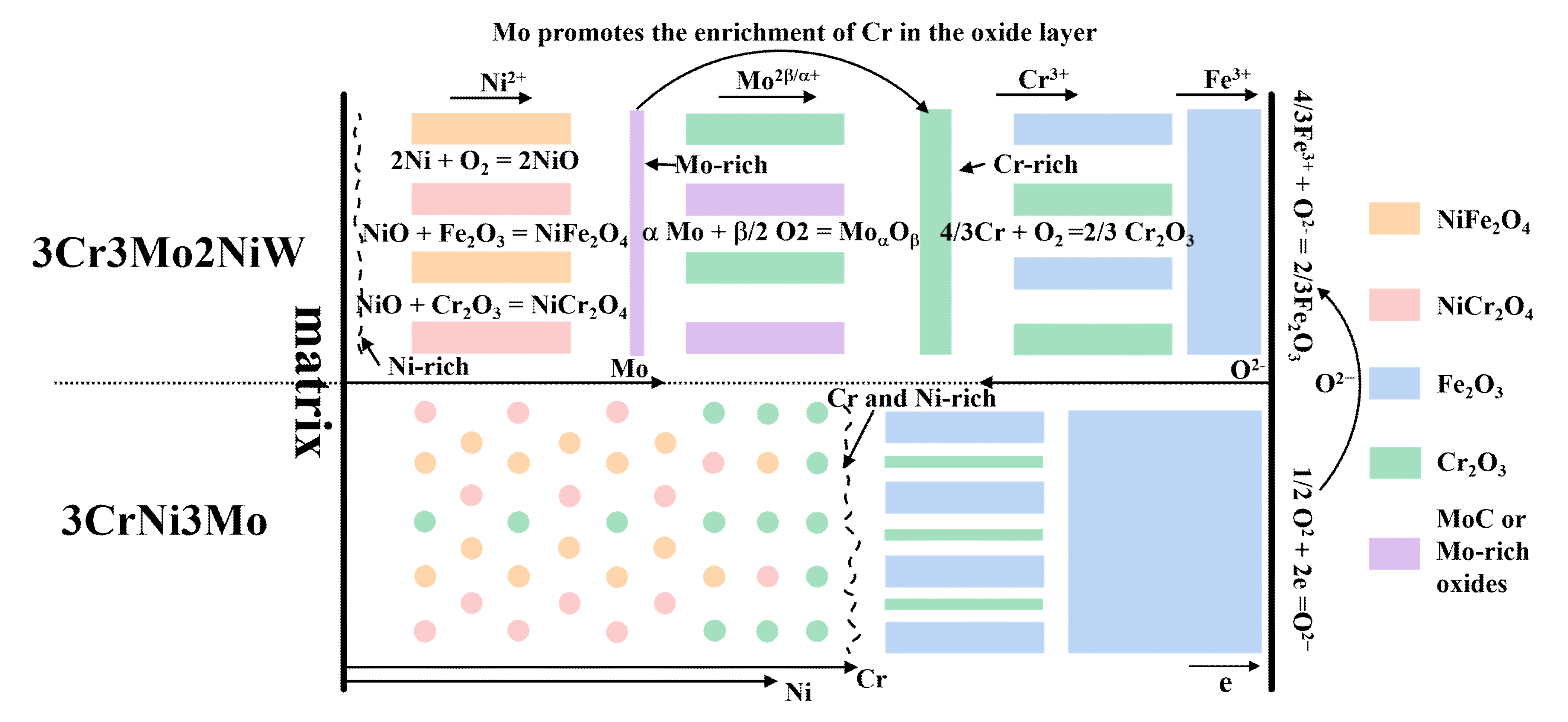
3.5. Oxidation Mechanism
4. Conclusions
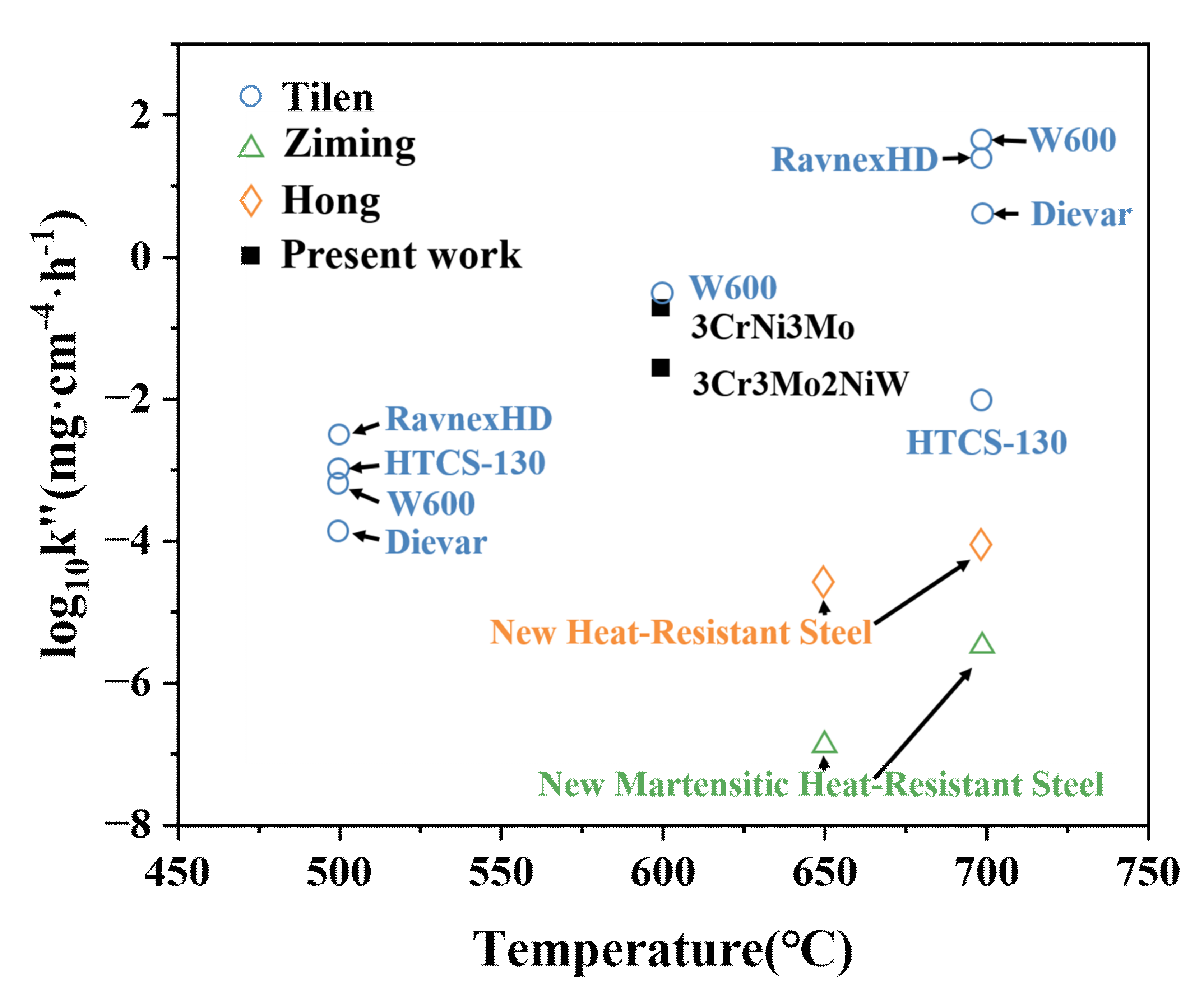
- At 600 °C, the two Cr-Ni-Mo hot-work die steels exhibit sub-oxidation resistance levels and conform to the ΔW2 = k″t oxidation mode. The oxidation resistance of the test steels at high temperature is closely related to the Cr and Mo contents.
- The reason for the better oxidation resistance of 3Cr3Mo2NiW steel is mainly its higher content of Cr and Mo. The Cr-rich oxide layers generated during oxidation act as barriers against further oxidation and inhibit the continued oxidation of the substrate. Mo can promote the formation of Cr-rich oxide layers. In addition, the higher twin density and grain refinement may also be reasons for its good oxidation resistance.
- Although the oxide layer of 3CrNi3Mo steel is thick, the inner part of its oxide layer forms a large number of Ni-containing spinel structural oxides, and the larger thickness of the inner layer and the mesh structure improve the adhesion of the oxide layer.
- In the process of high-temperature oxidation of the two Cr-Ni-Mo hot-work die steels, the interface reaction initially dominates, and the diffusion process gradually becomes the dominant oxidation factor with the thickening of the oxide film.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boher, C.; Le Roux, S.; Penazzi, L.; Dessain, C. Experimental investigation of the tribological behavior and wear mechanisms of tool steel grades in hot stamping of a high-strength boron steel. Wear 2012, 294–295, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Terek, P.; Kovačević, L.; Miletić, A.; Panjan, P.; Baloš, S.; Škorić, B.; Kakas, D. Effects of die core treatments and surface finishes on the sticking and galling tendency of Al–Si alloy casting during ejection. Wear 2016, 356–357, 122–134. [Google Scholar] [CrossRef]
- Wei, M.; Wang, F.; Wang, S.; Cui, X. Comparative research on the elevated-temperature wear resistance of a cast hot-working die steel. Mater. Des. 2009, 30, 3608–3614. [Google Scholar] [CrossRef]
- Cui, X.; Wang, S.; Wang, F.; Chen, K. Research on oxidation wear mechanism of the cast steels. Wear 2008, 265, 468–476. [Google Scholar] [CrossRef]
- Pellizzari, M.; Cescato, D.; Flora, M.G.D. Hot friction and wear behaviour of high speed steel and high chromium iron for rolls. Wear 2009, 267, 467–475. [Google Scholar] [CrossRef]
- Hernandez, S.; Hardell, J.; Winkelmann, H.; Ripoll, M.R.; Prakash, B. Influence of temperature on abrasive wear of boron steel and hot forming tool steels. Wear 2015, 338–339, 27–35. [Google Scholar] [CrossRef]
- Stott, F.; Lin, D.; Wood, G. The structure and mechanism of formation of the ‘GLAZE’ oxide layers produced on nickel-based alloys during wear at high temperatures. Corros. Sci. 1973, 13, 449–454. [Google Scholar] [CrossRef]
- Davies, M.H.; Simnad, M.T.; Birchenall, C.E. On the Mechanism and Kinetics of the Scaling of Iron. JOM J. Miner. Met. Mater. Soc. 1951, 3, 889–896. [Google Scholar] [CrossRef]
- Huang, J.; Fang, H.; Fu, X.; Huang, F.; Wan, H.; Zhang, Q.; Deng, S.; Zu, J. High-Temperature Oxidation Behavior and Mechanism of a New Type of Wrought Ni–Fe–Cr–Al Superalloy up to 1300 °C. Oxid. Met. 2000, 53, 273–287. [Google Scholar] [CrossRef]
- Col, A.; Parry, V.; Pascal, C. Oxidation of a Fe–18Cr–8Ni austenitic stainless steel at 850 °C in O2: Microstructure evolution during breakaway oxidation. Corros. Sci. 2017, 114, 17–27. [Google Scholar] [CrossRef]
- Li, K.; Zeng, Y.; Luo, J.-L. Corrosion of SS310 and Alloy 740 in high temperature supercritical CO2 with impurities H2O and O2. Corros. Sci. 2021, 184, 109350. [Google Scholar] [CrossRef]
- Pint, B.A.; Distefano, J.R.; Wright, I.G. Oxidation resistance: One barrier to moving beyond Ni-base superalloys. Mater. Sci. Eng. A 2006, 415, 255–263. [Google Scholar] [CrossRef]
- Huntz, A.M.; Reckmann, A.; Haut, C.; Sévérac, C.; Herbst, M.; Resende, F.C.T.; Sabioni, A.C.S. Oxidation of AISI 304 and AISI 439 stainless steels. Mater. Sci. Eng. A 2007, 447, 266–276. [Google Scholar] [CrossRef]
- Karimi, N.; Riffard, F.; Rabaste, F.; Perrier, S.; Cueff, R.; Issartel, C.; Buscail, H. Characterization of the oxides formed at 1000°C on the AISI 304 stainless steel by X-ray diffraction and infrared spectroscopy. Appl. Surf. Sci. 2008, 254, 2292–2299. [Google Scholar] [CrossRef]
- Jin, X.; Chen, S.; Rong, L. Effects of Mn on the mechanical properties and high temperature oxidation of 9Cr2WVTa steel. J. Nucl. Mater. 2017, 494, 103–113. [Google Scholar] [CrossRef]
- Li, H.; Zhang, B.; Jiang, Z.; Zhang, S.; Feng, H.; Han, P.; Dong, N.; Zhang, W.; Li, G.; Fan, G.; et al. A new insight into high-temperature oxidation mechanism of super-austenitic stainless steel S32654 in air. J. Alloys Compd. 2016, 686, 326–338. [Google Scholar] [CrossRef]
- Doh, S.J.; Je, J.H.; Kim, J.S.; Kim, K.Y.; Kim, H.S.; Lee, Y.D.; Lee, J.M.; Hwu, Y. Influence of Cr and Mo on the passivation of stainless steel 430 (18Cr) and 444 (18Cr-2Mo): In situ xanes study. Nucl. Instrum. Methods Phys. Res. 2003, 199, 211–215. [Google Scholar] [CrossRef]
- Li, D.G.; Wang, J.D.; Chen, D.R.; Liang, P. Influence of Molybdenum on Tribo-Corrosion Behavior of 316L Stainless Steel in Artificial Saliva. J. Bio- Tribo-Corros. 2015, 1, 14. [Google Scholar] [CrossRef]
- Schultze, J.W.; Lohrengel, M.M.; Ross, D. Nucleation and growth of anodic oxide films. Electrochim. Acta 1983, 28, 973–984. [Google Scholar] [CrossRef]
- Yun, D.W.; Seo, H.S.; Jun, J.H.; Lee, J.M.; Kim, K.Y. Molybdenum effect on oxidation resistance and electric conduction of ferritic stainless steel for SOFC interconnect. Int. J. Hydrogen Energy 2012, 37, 10328–10336. [Google Scholar] [CrossRef]
- Buscail, H.; El Messki, S.; Riffard, F.; Perrier, S.; Cueff, R.; Issartel, C. Role of molybdenum on the AISI 316L oxidation at 900 °C. J. Mater. Sci. 2008, 43, 6960–6966. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, Y.; Zhang, Z.; Qi, Y.; Zhang, C.; Zheng, H.; Xu, Y. Oxidation behavior and high-temperature tensile properties of Fe-9Cr-(Mo, Mo/Ni) alloys. Corros. Sci. 2021, 181, 109243. [Google Scholar] [CrossRef]
- Teramoto, K.H.A. An X-ray photo-electron spectroscopic study on the role of molybdenum in increasing the corrosion resistance of ferritic stainless steels in HCl. Corros. Sci. 1979, 19, 3–14. [Google Scholar]
- Liu, Y.-Z.; Yang, C.-F.; Chai, F.; Pan, T.; Su, H. High Temperature Oxidation Resistance of 9Ni Steel. J. Iron Steel Res. Int. 2014, 21, 956–963. [Google Scholar] [CrossRef]
- Trindade, V.; Christ, H.-J.; Krupp, U. Grain-Size Effects on the High-Temperature Oxidation Behaviour of Chromium Steels. Oxid. Met. 2010, 73, 551–563. [Google Scholar] [CrossRef]
- Ma, W.; Luo, H.; Yang, X. The Effects of Grain Size and Twins Density on High Temperature Oxidation Behavior of Nickel-Based Superalloy GH738. Materials 2020, 13, 4166. [Google Scholar] [CrossRef]
- Zhang, L.L.; Yan, S.; Jiang, S. Hard X-ray micro-focusing beamline at SSRF. Nucl. Sci. Tech. 2015, 26, 060101. [Google Scholar]
- Balaško, T.; Burja, J.; Medved, J. High-temperature oxidation of four hot-work tool steels. Mater. Tehnol. 2018, 52, 775–780. [Google Scholar] [CrossRef]
- Balaško, T.; Vončina, M.; Burja, J.; Batič, B.Š.; Medved, J. High-Temperature Oxidation Behavior of Tool Steel with Increased Thermal Conductivity. Oxid. Met. 2022, 98, 135–161. [Google Scholar] [CrossRef]
- Bao, Z.; Han, R.; Zhu, Y.; Li, H.; Li, N.; Tang, M.; Zhang, H.; Zhao, C. High Temperature Oxidation Behavior of New Martensitic Heat-Resistant Steel. Mater. Sci. 2022, 28, 164–170. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Yan, T.; Ding, C.; Zhang, H.; Jiang, F. Properties of High Temperature Oxidation of Heat-resistant Steel with Aluminium and Copper. Mater. Sci. 2019, 25, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Pang, H.T.; Reed, P.A.S. Microstructure effects on high temperature fatigue crack initiation and short crack growth in turbine disc nickel-base superalloy Udimet 720Li. Mater. Sci. Eng. A 2007, 448, 67–79. [Google Scholar] [CrossRef]
- Taniguchi, S.; Yamamoto, K.; Megumi, D.; Shibata, T. Characteristics of scale/substrate interface area of Si-containing low-carbon steels at high temperatures. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2001, 308, 250–257. [Google Scholar] [CrossRef]
- Li, W.; Fan, J.; Fan, Y.; Xiao, L.; Cheng, H. MoSi2/(Mo, Ti)Si-2 dual-phase composite coating for oxidation protection of molybdenum alloy. J. Alloys Compd. 2018, 740, 711–718. [Google Scholar] [CrossRef]
- Hirbod, M. The Influence of Fine Structure, Morphology and Composition of Alloy and Oxide on the Growth of Cr2O3 Scales; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Wang, J.; He, Q.; Liu, G.; Zhang, Q.; Liu, G.; Huang, Z.; Zhu, X.; Fu, Y. High-Temperature Oxidation Behavior of AlTiNiCuCox High-Entropy Alloys. Materials 2021, 14, 5319. [Google Scholar] [CrossRef]



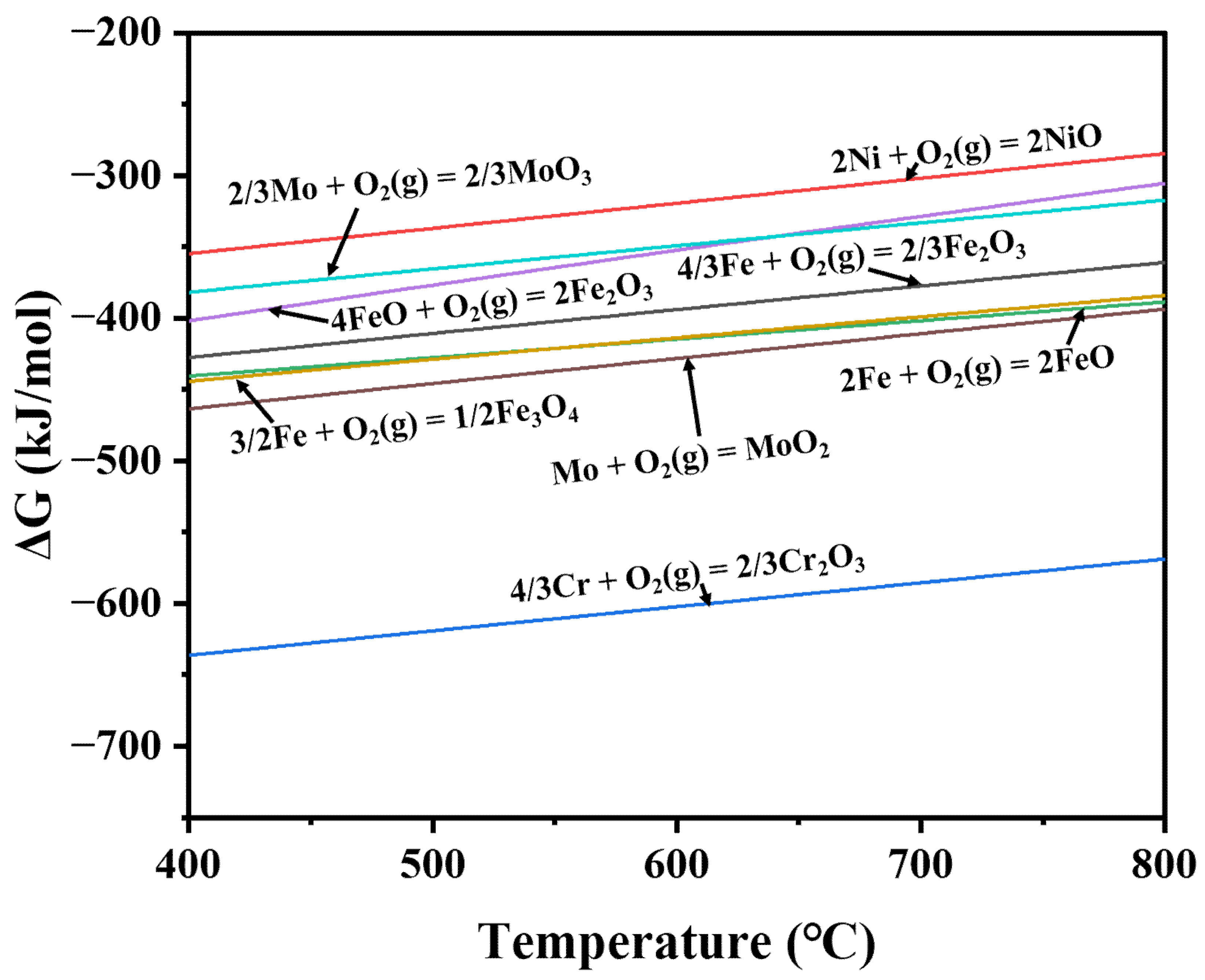
| Steel | Oxidation Constant (k″) | Oxidation Kinetic Equation | Correlation Coefficient (R2) |
|---|---|---|---|
| 3Cr3Mo2NiW | 0.20 ± 0.01 | ΔW2 = 0.20 t | 0.99 |
| 3CrNi3Mo | 0.47 ± 0.03 | ΔW2 = 0.47 t | 0.99 |
| Steel | Average Thickness of Oxide Layer (μm) |
|---|---|
| 3Cr3Mo2NiW | 12.55 |
| 3CrNi3Mo | 19.89 |
| Metals | Actions | ΔrG° (kJ/mol) |
|---|---|---|
| Fe | 4/3 Fe + O2 (g) = 2/3 Fe2O3 (1) | −393.846 # |
| Ni | 2Ni (s) + O2 (g) = 2NiO (s) (2) | −319.296 # |
| Cr | 4/3 Cr + O2 (g) = 2/3 Cr2O3 (3) | −602.199 # |
| NiO (s) + Fe2O3 (s) = NiFe2O4 (s) (4) | −23.569 # | |
| NiO (s) + Cr2O3 (s) = NiCr2O4 (s) (5) | −6.635 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, C.; Li, F.; Wang, Z.; Wang, X.; Wang, C.; Zhang, C.; Huang, J.; Mao, F.; Chen, C.; et al. High-Temperature Oxidation Behavior of Cr-Ni-Mo Hot-Work Die Steels. Materials 2022, 15, 5145. https://doi.org/10.3390/ma15155145
Zhang Y, Zhang C, Li F, Wang Z, Wang X, Wang C, Zhang C, Huang J, Mao F, Chen C, et al. High-Temperature Oxidation Behavior of Cr-Ni-Mo Hot-Work Die Steels. Materials. 2022; 15(15):5145. https://doi.org/10.3390/ma15155145
Chicago/Turabian StyleZhang, Yuqi, Cheng Zhang, Fei Li, Zhou Wang, Xiaodong Wang, Changji Wang, Cheng Zhang, Jinfeng Huang, Feng Mao, Chong Chen, and et al. 2022. "High-Temperature Oxidation Behavior of Cr-Ni-Mo Hot-Work Die Steels" Materials 15, no. 15: 5145. https://doi.org/10.3390/ma15155145
APA StyleZhang, Y., Zhang, C., Li, F., Wang, Z., Wang, X., Wang, C., Zhang, C., Huang, J., Mao, F., Chen, C., Jiang, T., Wei, S., Xiong, M., & Hu, J. (2022). High-Temperature Oxidation Behavior of Cr-Ni-Mo Hot-Work Die Steels. Materials, 15(15), 5145. https://doi.org/10.3390/ma15155145







