Synergetic Antimicrobial Effect of Silver Nanoparticles Conjugated with Iprodione against Valsa mali
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungicides and Isolate
2.2. Determination of Fungicides Sensitivity against V. mali
2.3. Biosynthesis and Characterization of Nanoparticles
2.4. Fungus Growth Influence
2.5. Inhibition Zone Measurement
2.6. Leakage of DNA and Protein
2.7. Synergistic Inhibition Effect of Silver Nanoparticles and Iprodione
3. Results
3.1. Sensitivity of Fungicides against V. mali
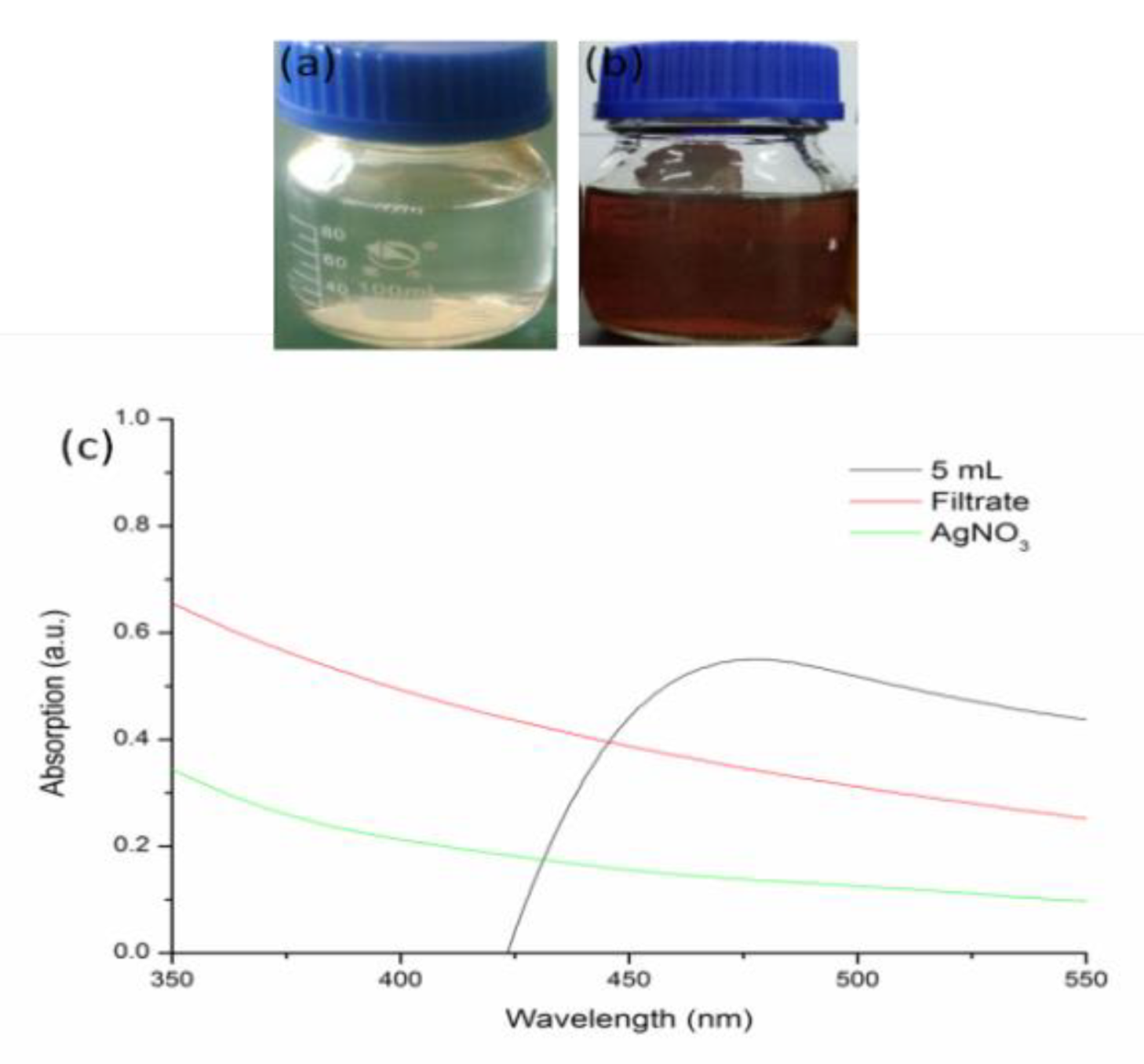
3.2. Biosynthesis of Silver Nanoparticles
3.3. Characterization
3.3.1. TEM Analysis
3.3.2. XRD Measurement
3.3.3. AFM Analysis
3.4. Antifungal Activity of Silver Nanoparticles
3.4.1. Colony Growth Inhibition
3.4.2. Inhibition Zone Diameter
3.4.3. Determination of the Leakage of DNA and Protein
3.4.4. Synergistic Antimicrobial Effect of Silver Nanoparticles Conjugated with Iprodione
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.L.; Wei, J.L.; Huang, L.L.; Kang, Z.S. Re-evaluation of pathogens causing Valsa canker on apple in China. Mycologia 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Wang, X.L.; Zang, R.; Yin, Z.Y.; Kang, Z.S.; Huang, L.L. Delimiting cryptic pathogen species causing apple Valsa canker with multilocus data. Ecol. Evol. 2014, 4, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Guo, L.; Li, B.; Sun, G.; Chen, H. Investigations on the occurrence and control of apple canker in China. Plant Prot. 2009, 35, 114–116. [Google Scholar]
- Lei, P.; Xu, Y.; Du, J.; Yang, X.L.; Yuan, H.Z.; Xu, G.F.; Ling, Y. Design, synthesis and fungicidal activity of N-substituted benzoyl-1,2,3,4-tetrahydroquinolyl-1-carboxamide. Bioorg. Med. Chem. 2016, 26, 2544–2546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Lei, P.; Sun, T.D.; Jin, X.Y.; Yang, X.L.; Ling, Y. Design, synthesis, and fungicidal activity of novel thiosemicarbazide derivatives containing piperidine fragments. Molecules 2017, 22, 2085. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Wei, P.; Wang, D.; Hao, S.H.; Li, W.W.; Ding, F. Design, synthesis, antifungal activity, and 3D-QSAR of coumarin derivatives. J. Pestic. Sci. 2018, 43, 88–95. [Google Scholar]
- Kim, B.S.; Hwang, B.K. Microbial fungicides in the control of plant diseases. J. Phytopathol. 2007, 155, 641–653. [Google Scholar] [CrossRef]
- Yin, Z.Y.; Liu, H.Q.; Li, Z.P.; Ke, X.W.; Dou, D.L.; Gao, X.N.; Song, N.; Dai, Q.Q.; Wu, Y.X.; Xu, J.R. Genome sequence of Valsa canker pathogens uncovers a potential adaptation of colonization of woody bark. New Phytol. 2015, 208, 1202–1216. [Google Scholar] [CrossRef]
- Duan, S.Z.; Du, Y.M.; Hou, X.D.; Yan, N.; Dong, W.J.; Mao, X.X.; Zhang, Z.F. Chemical Basis of the Fungicidal Activity of Tobacco Extracts against Valsa mali. Molecules 2016, 21, 1743. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.W.; Xu, B.L.; Zhang, J.H.; Gan, Y.T. Identification of the antifungal activity of Trichoderma longibrachiatum T6 and assessment of bioactive substances in controlling phytopathgens. Pestic. Biochem. Phys. 2018, 147, 59–66. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Zhang, F.; Zheng, D.; Chang, Y.; Xu, L.; Huang, L. Biocontrol activity of Bacillus velezensis D4 against apple Valsa canker. Biol. Control 2021, 163, 104760. [Google Scholar] [CrossRef]
- Lai, W.F. Non-conjugated polymers with intrinsic luminescence for drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101916. [Google Scholar] [CrossRef]
- Lai, W.F.; Tang, R.; Wong, W.T. Ionically Crosslinked Complex Gels Loaded with Oleic Acid-Containing Vesicles for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.; Singh, H.B.; Abhilash, P.C. The unseen impact of nanoparticles: More or less. Curr. Sci. 2014, 106, 350–352. [Google Scholar]
- Khademhosseini, A.; Parak, W.J.; Weiss, P.S. Nanoscience and Nanotechnology around the World. ACS Nano 2016, 10, 4883–4884. [Google Scholar] [CrossRef] [Green Version]
- Yi, Y.M.; Wang, C.J.; Cheng, X.X.; Yi, K.C.; Huang, W.D.; Yu, H.B. Biosynthesis of Silver Nanoparticles by Conyza canadensis and Their Antifungal Activity against Bipolaris maydis. Crystals 2021, 11, 1443. [Google Scholar] [CrossRef]
- Khatami, M.; Heli, H.; Jahani, P.M. Copper/copper oxide nanoparticles synthesis using Stachys lavandulifolia and its antibacterial activity. IET Nanobiotechnol. 2017, 11, 709–713. [Google Scholar] [CrossRef]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release 2016, 224, 86–102. [Google Scholar] [CrossRef]
- Rajwade, J.M.; Chikte, R.G.; Paknikar, K.M. Nanomaterials: New weapons in a crusade against phytopathogens. Appl. Microbiol. Biot. 2020, 104, 1437–1461. [Google Scholar] [CrossRef]
- Khalil, N.M.; El-Ghany, M.N.A.; Rodríguez-Couto, S. Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by Fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere 2019, 218, 477–486. [Google Scholar] [CrossRef]
- Huang, W.D.; Fang, H.; Zhang, S.Y.; Yu, H.B. Optimised green synthesis of copper oxide nanoparticles and their antifungal activity. MicroNano Lett. 2021, 16, 374–380. [Google Scholar] [CrossRef]
- Miri, A.; Najafzadeh, H.; Darroudi, M.; Miri, M.J.; Kouhbanani, M.A.J.; Sarani, M. Iron Oxide Nanoparticles: Biosynthesis, Magnetic Behavior, Cytotoxic Effect. ChemistryOpen 2021, 10, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Tripathi, A. Green synthesis of nanoparticles and its key applications in various sectors. Mater. Today Proc. 2022, 48, 1626–1632. [Google Scholar] [CrossRef]
- Samuel, M.S.; Ravikumar, M.; John, A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A Review on Green Synthesis of Nanoparticles and Their Diverse Biomedical and Environmental Applications. Catalysts 2022, 12, 459. [Google Scholar] [CrossRef]
- Shokoofeh, N.; Moradi-Shoeili, Z.; Naeemi, A.S. Biosynthesis of Fe3O4@Ag nanocomposite and evaluation of its performance on expression of norA and norB efflux pump genes in ciprofloxacin-resistant Staphylococcus aureus. Biol. Trace Elem. Res. 2019, 191, 522–530. [Google Scholar] [CrossRef]
- Qasim, M.; Singh, B.R.; Naqvi, A.H.; Paik, P.; Das, D. Silver nanoparticles embedded mesoporous SiO2 nanosphere: An effective anticandidal agent against Candida albicans 077. Nanotechnology 2015, 26, 285102. [Google Scholar] [CrossRef]
- Sarani, M.; Tosan, F.; Hasani, S.A.; Barani, M.; Adeli-sardou, M.; Khosravani, M.; Niknam, S.; Kouhbanani, M.A.J. Study of in vitro cytotoxic performance of biosynthesized α-Bi2O3 NPs, Mn-doped and Zn-doped Bi2O3 NPs against MCF-7 and HUVEC cell lines. J. Mater. Res. Technol. 2022, 19, 140–150. [Google Scholar] [CrossRef]
- Waghmare, S.R.; Mulla, M.N.; Marathe, S.R.; Sonawane, K.D. Ecofriendly production of silver nanoparticles using Candida utilis and its mechanistic action against pathogenic microorganisms. Biotechnology 2015, 5, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Sarsar, V.; Selwal, M.K.; Selwal, K.K. Biogenic synthesis, optimisation and antibacterial efficacy of extracellular silver nanoparticles using novel fungal isolate Aspergillus fumigates MA. IET Nanobiotechnol. 2016, 10, 215–221. [Google Scholar] [CrossRef]
- Anandalakshmi, K. Review on biosynthesis of silver nanoparticles and its characterization. Plant Arch. 2021, 21, 2393–2400. [Google Scholar]
- Sayyid, N.H.; Zghair, Z.R. Biosynthesis of silver nanoparticles produced by Klebsiella pneumonia. Mater. Today 2021, 42, 2045–2049. [Google Scholar]
- Krishnan, S.; Srisrimal, D.; Srisrimal, A.K. Antimicrobial Effectiveness of Silver Nanoparticles enriched Tea Leaves. Int. J. Pharm. Qual. Assur. 2020, 11, 395–398. [Google Scholar]
- Chaudhari, P.; Chaudhari, P.M.; PatilP, H. Antimicrobial effects of silver nanoparticle using various Indian traditional herbs. Int. J. Adv. Res. 2020, 8, 797–802. [Google Scholar] [CrossRef] [Green Version]
- Dhara, B.; Roy, I.; Maity, A. Comparative Account of the Genotoxic and Antimicrobial Effects of Silver Nanoparticles Synthesized from Extract of Pleurotus ostreatus and Chemically Synthesized Nanoparticles. Cell Tissue Biol. 2021, 15, 77–89. [Google Scholar] [CrossRef]
- Abdul, S.; Kadhem, S.; Salman, K. Antimicrobial effect of silver nanoparticles with Kluyvera cryocrescens and biofilm cultures. Life Sci. Arch. 2022, 7, 2130–2138. [Google Scholar]
- Hwang, I.S.; Hwang, J.H.; Choi, H.; Kim, K.J.; Lee, D.G. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J. Med. Microbiol. 2012, 61, 1719–1726. [Google Scholar] [CrossRef] [Green Version]
- Hasson, S.O.; Al-Awady, M.J.; Al-Hamadani, A.H.; Al-Azawi, I.H. Boosting antimicrobial activity of imipenem in combination with silver nanoparticles towards S. fonticola and Pantoeasp. Nano Biomed. Eng. 2019, 11, 200–214. [Google Scholar] [CrossRef]
- Huang, W.D.; Wang, J.; Wang, Z.X.; Yu, H.B. Synergistic antimicrobial activity of silver nanoparticles combined with streptomycin sulfate against gram-negative and gram-positive bacteria. Mol. Cryst. Liq. Cryst. 2021, 714, 80–88. [Google Scholar] [CrossRef]
- Da Frota, S.M.; Cunha, F.A.; Cunha, M.D.C.D.S.O.; Martins, R.T.; Menezes, E.A.; Fechine, P.B.A. Synergistic Eeffect of Polyene Antifungals and Silver Nanoparticles Against Candida parapsilosis. J. Antibiot. Res. 2018, 2, 104–108. [Google Scholar]
- Li, H.; Wang, L.H.; Chai, Y.F.; Cao, Y.B.; Lu, F. Synergistic effect between silver nanoparticles and antifungal agents on Candida albicans revealed by dynamic surface-enhanced Raman spectroscopy. Nanotoxicology 2018, 10, 1230–1240. [Google Scholar] [CrossRef]
- Huang, W.D.; Yan, M.H.; Duan, H.M.; Bi, Y.L.; Cheng, X.X.; Yu, H.B. Synergistic Antifungal Activity of Green Synthesized Silver Nanoparticles and Epoxiconazole against Setosphaeria turcica. J. Nanomater. 2020, 2020, 9535432. [Google Scholar] [CrossRef] [Green Version]
- McShan, D.; Zhang, Y.; Deng, H.; Ray, P.C.; Yu, H.T. Synergistic Antibacterial Effect of Silver Nanoparticles Combined with Ineffective Antibiotics on Drug Resistant Salmonella typhimurium DT104. J. Environ. Sci. Health C 2015, 33, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Longhi, C.; Santos, J.P.; Morey, A.T.; Marcato, P.D.; Duran, N.; Pinge-Filho, P.; Nakazato, G.; Yamada-Ogatta, S.F.; Yamauchi, L.M. Combination of fluconazole with silver nanoparticles produced by Fusarium oxysporum improves antifungal effect against planktonic cells and biofilm of drug-resistant Candida albicans. Med. Mycol. 2015, 54, 428–432. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.; Noman, M.; Luo, J.Y.; Muhammad, S.; Shahid, M.; Arshad, A.M.; Zhang, M.C.; Li, B. Bioengineered chitosan-magnesium nanocomposite: A novel agricultural antimicrobial agent against Acidovorax oryzae and Rhizoctonia solani for sustainable rice production. Int. J. Biol. Macromol. 2021, 168, 834–845. [Google Scholar] [CrossRef]
- Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-Synthesization of Silver Nanoparticles Using Endophytic Bacteria Isolated from Garlic and Its Antifungal Activity against Wheat Fusarium Head Blight Pathogen Fusarium graminearum. Nanomaterials 2020, 10, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatami, M.; Mehnipor, R.; Poor, M.H.S.; Jouzani, G.S. Facile Biosynthesis of Silver Nanoparticles Using Descurainia sophia and Evaluation of Their Antibacterial and Antifungal Properties. J. Cluster Sci. 2016, 27, 1601–1612. [Google Scholar] [CrossRef]
- Sathiya, C.K.; Akilandeswari, S. Fabrication and characterization of silver nanoparticles using Delonixelata leaf broth. Spectrochim. Acta A 2014, 128, 337–341. [Google Scholar] [CrossRef]
- Philip, D. Green synthesis of gold and silver nanoparticles using Hibiscus rosasinensis. Phys. E Low Dimens. Syst. Nanostruct. 2010, 42, 1417–1424. [Google Scholar] [CrossRef]





| Fungicide | Concentration Gradient (μg·mL−1) | Manufacturer |
|---|---|---|
| mancozeb 96% TC | 5.0, 10.0, 20.0, 50.0, 100.0 | Limin Chemical Co. LTD, Xinyi, China |
| metalaxyl 97% TC | 0.05, 0.2, 0.5, 2.0, 5.0 | Yifan Biotechnology Group Co. LTD, Wenzhou, China |
| iprodione 96% TC | 0.05, 0.2, 0.5, 2.0, 5.0 | Jiangxi Heyi Chemical Co., LTD, Jiujiang, China |
| prochloraz 97% TC | 0.05, 0.2, 0.5, 2.0, 5.0 | Jiangsu Yunfan Chemical Co., LTD, Qidong, China |
| difenoconazole 95% TC | 10.0, 20.0, 50.0, 100.0, 200.0 | Limin Chemical Co. LTD, Xinyi, China |
| Fungicide | Toxicity Regression | IC50 (μg·mL−1) | 95% Confidence Limit (μg·mL−1) | R2 |
|---|---|---|---|---|
| mancozeb | y = 1.26880x + 2.10396 | 45.52 | 34.09–66.62 | 0.708 |
| iprodione | y = 0.75206 − 0.15605 | 0.62 | 0.39–0.99 | 0.856 |
| prochloraz | y = 0.92575x − 0.00364 | 0.99 | 0.69–1.50 | 0.937 |
| metalaxyl | y = 0.67065x + 1.16562 | 54.71 | 34.56–85.83 | 0.984 |
| difenoconazole | y = 0.66464x − 0.18641 | 1.90 | 1.13–4.09 | 0.775 |
| Fungistat | Concentration (μg·mL−1) | Inhibition Zone Diameter (mm) |
|---|---|---|
| sterile water | / | 0.00 ± 0.00 |
| iprodione | 2 | 18.50 ± 1.81 |
| iprodione | 5 | 22.30 ± 2.02 |
| silvernanoparticles | 100 | 10.80 ± 1.13 |
| silvernanoparticles | 200 | 12.50 ± 1.22 |
| Volume Ratio | Actual Inhibition Rate (%) | Theoretical Inhibition Rate (%) | Toxicity Ratio |
|---|---|---|---|
| 10:0 | 50.83 | 50.83 | 1.00 |
| 9:1 | 53.33 | 51.25 | 1.04 |
| 8:2 | 58.33 | 51.67 | 1.13 |
| 7:3 | 52.67 | 52.08 | 1.01 |
| 6:4 | 51.67 | 52.50 | 0.98 |
| 5:5 | 50.00 | 52.92 | 0.94 |
| 4:6 | 46.67 | 53.33 | 0.88 |
| 3:7 | 43.83 | 53.75 | 0.82 |
| 2:8 | 40.50 | 54.17 | 0.75 |
| 1:9 | 50.00 | 54.58 | 0.92 |
| 0:1 | 55.00 | 55.00 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Huang, W.; Yu, H. Synergetic Antimicrobial Effect of Silver Nanoparticles Conjugated with Iprodione against Valsa mali. Materials 2022, 15, 5147. https://doi.org/10.3390/ma15155147
Li T, Huang W, Yu H. Synergetic Antimicrobial Effect of Silver Nanoparticles Conjugated with Iprodione against Valsa mali. Materials. 2022; 15(15):5147. https://doi.org/10.3390/ma15155147
Chicago/Turabian StyleLi, Tao, Weidong Huang, and Haibing Yu. 2022. "Synergetic Antimicrobial Effect of Silver Nanoparticles Conjugated with Iprodione against Valsa mali" Materials 15, no. 15: 5147. https://doi.org/10.3390/ma15155147
APA StyleLi, T., Huang, W., & Yu, H. (2022). Synergetic Antimicrobial Effect of Silver Nanoparticles Conjugated with Iprodione against Valsa mali. Materials, 15(15), 5147. https://doi.org/10.3390/ma15155147





