Anti-Corrosion Performance of Migratory Corrosion Inhibitors on Reinforced Concrete Exposed to Varying Degrees of Chloride Erosion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Specimen Preparation
2.2. Chloride Erosion Test
2.3. Electrochemical Tests
2.4. SEM-EDS Tests
3. Results and Discussion
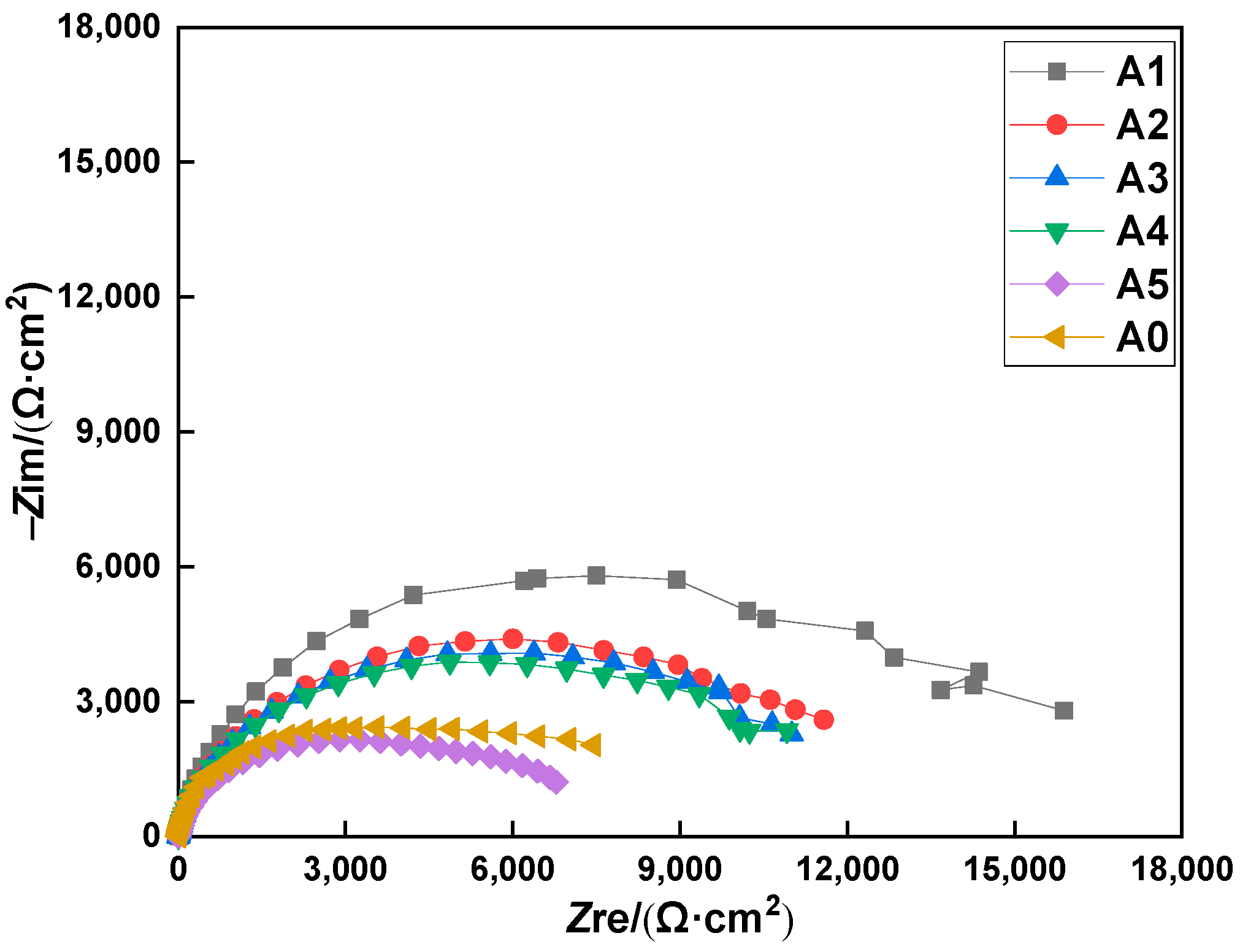
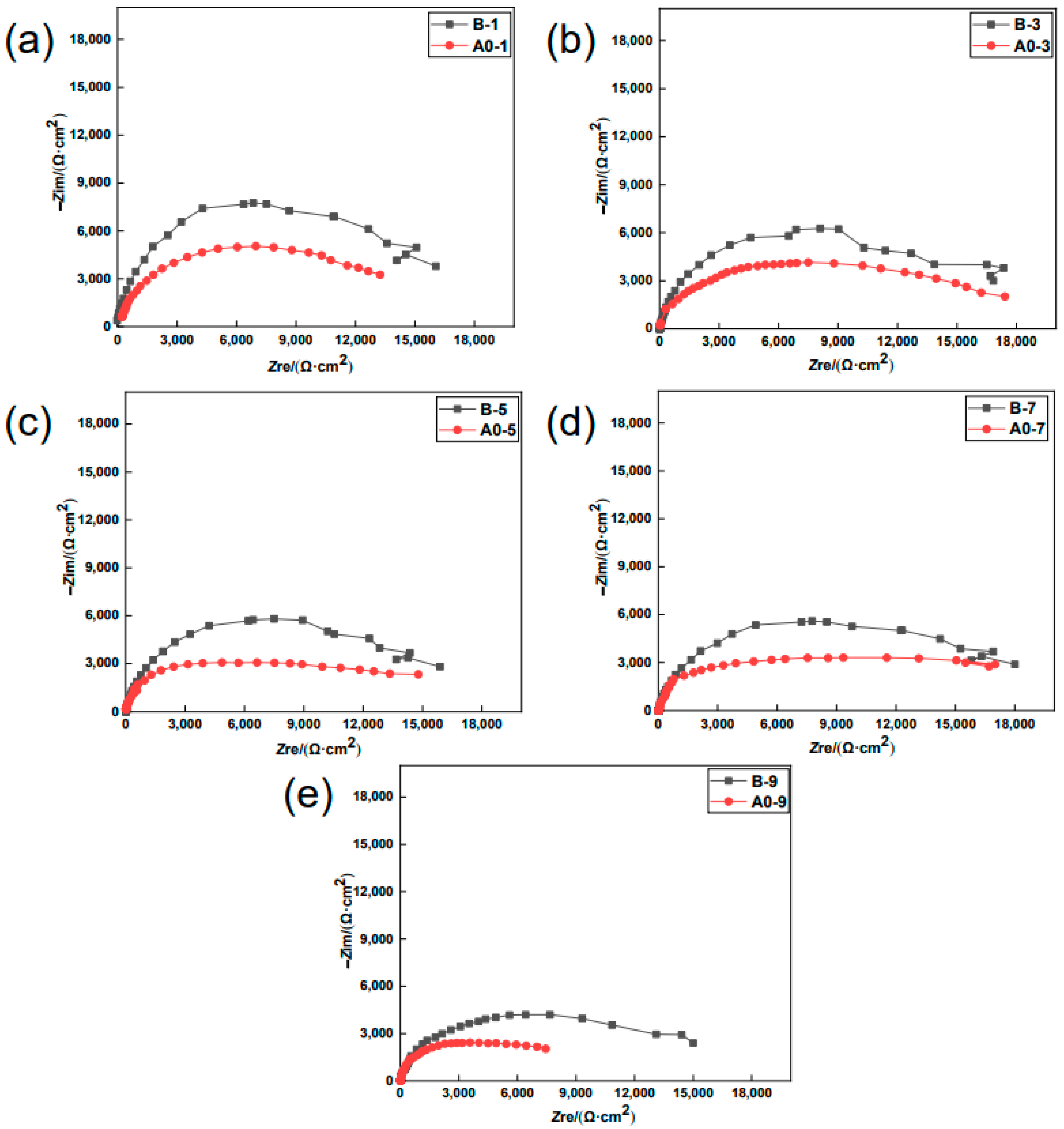
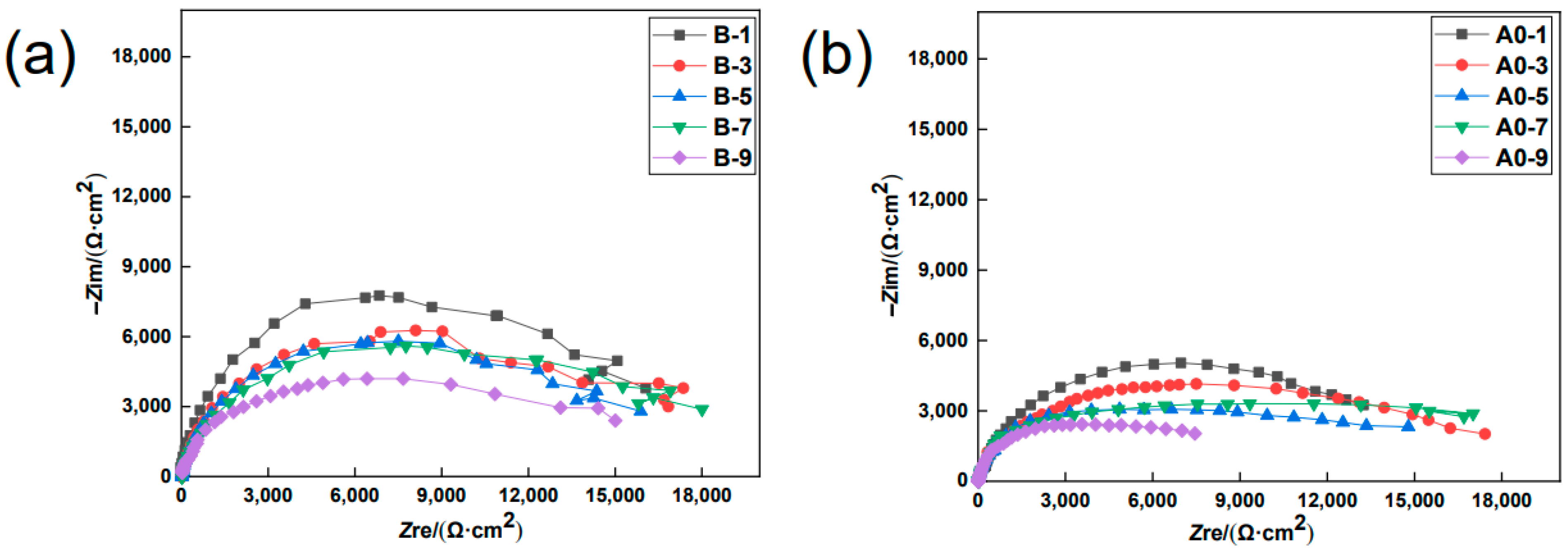
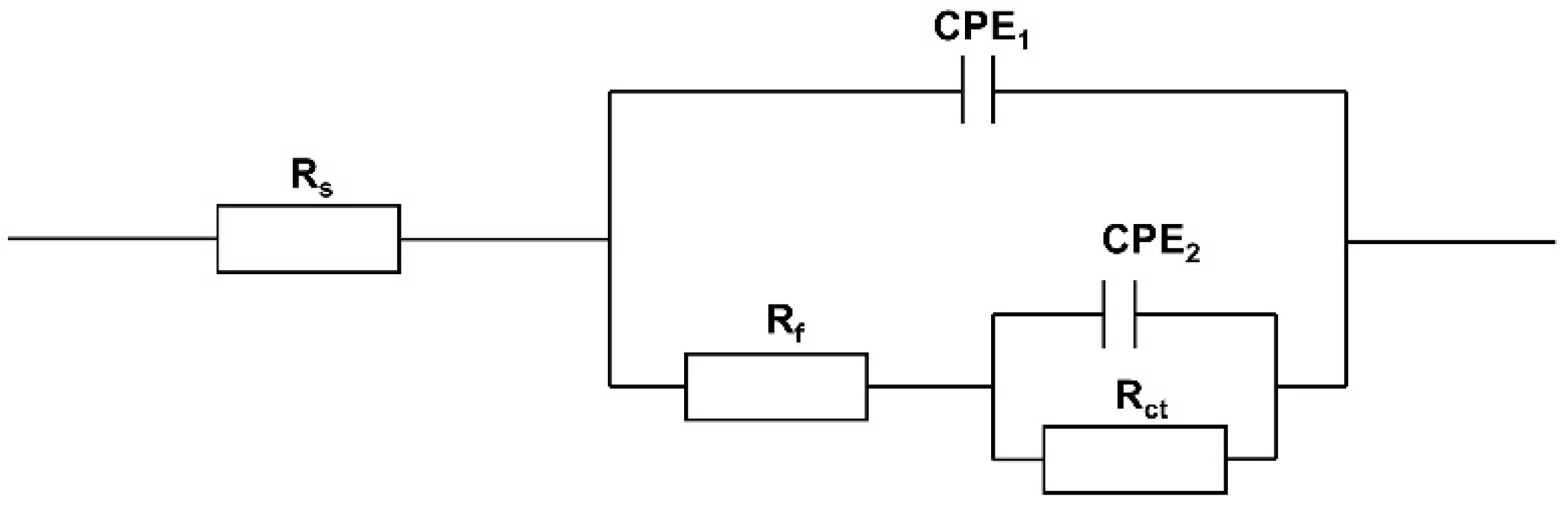
3.1. EIS Studies
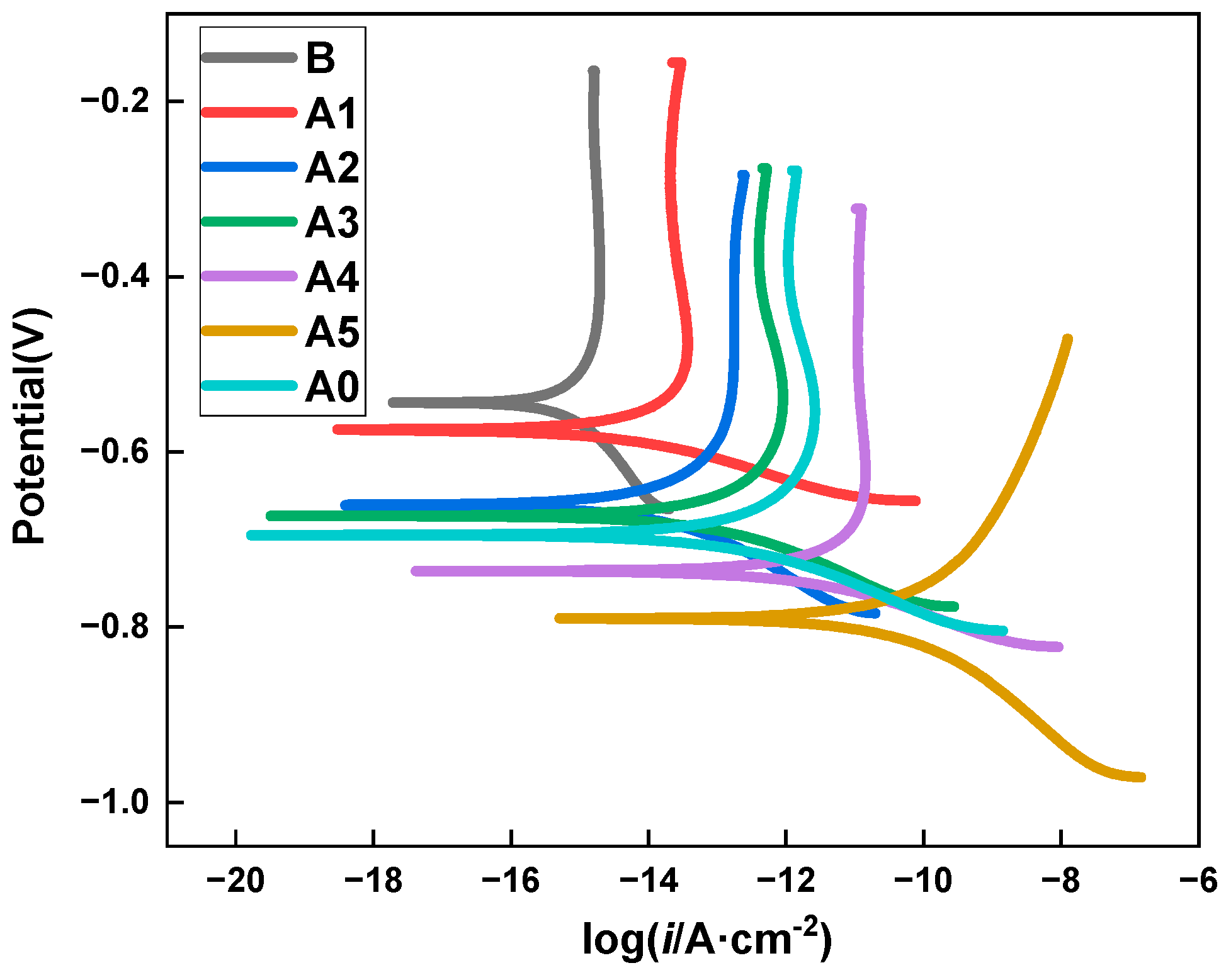
3.2. PD Studies
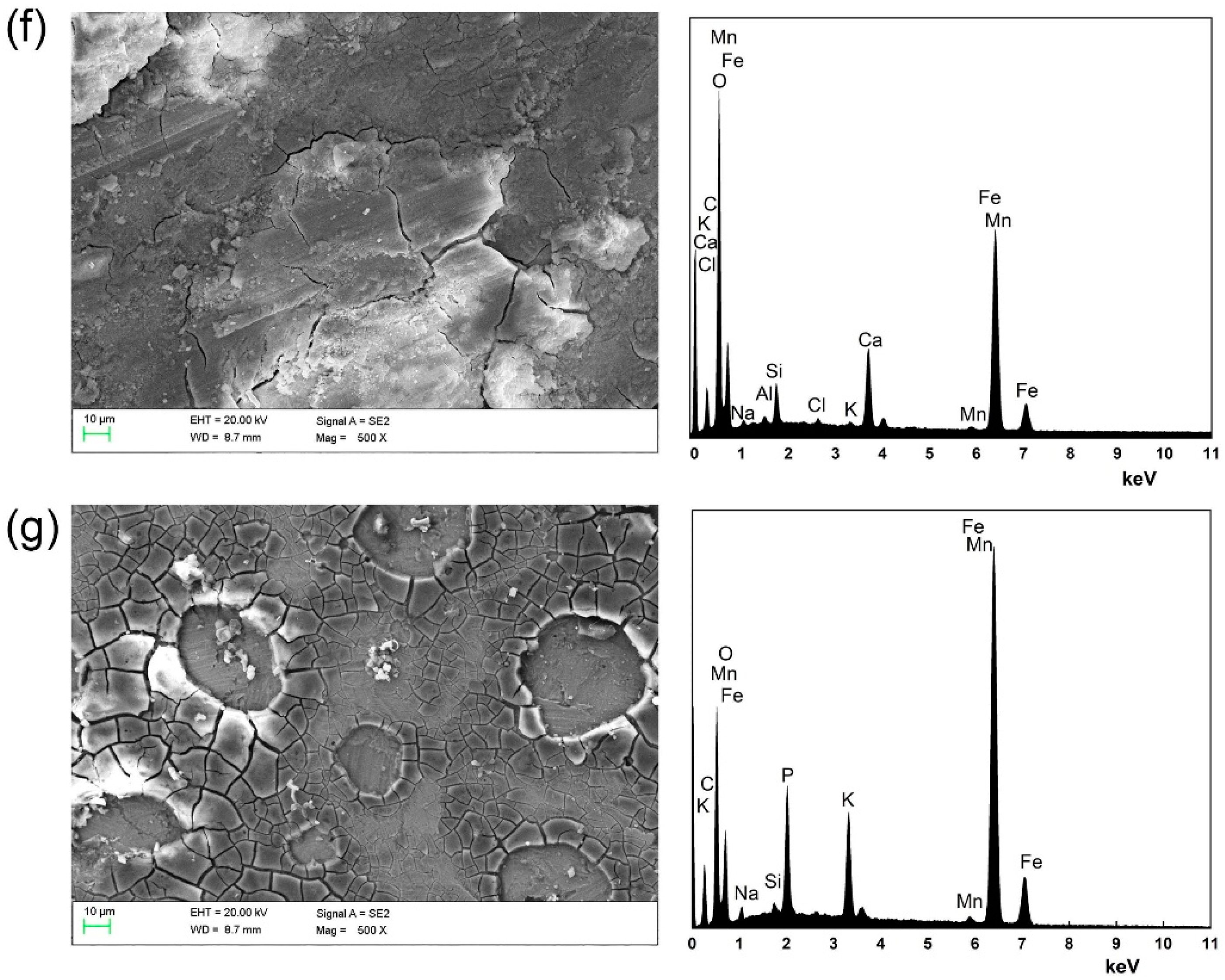
3.3. SEM-EDS Analysis
4. Conclusions
- The corrosion resistance of the steel bars in the concrete significantly improved after coating with the MCIs. The protective capacity performed well not only after coating with the MCIs before chloride erosion but also after the concrete underwent varying degrees of chloride erosion.
- The EIS results show that the earlier the specimen was coated with the MCIs, the higher the obtained anti-corrosion efficiency became. When the specimens were coated with the MCIs before chloride erosion, the charge transfer resistance value of the steel bar and the anti-corrosion efficiency were 7.01 kΩ/cm2 and 55.39%, respectively; however, the charge transfer resistance values and the anti-corrosion efficiency decreased to 3.24 kΩ/cm2 and 3.40% when the MCIs coating was applied after the ninth drying–wetting cycle.
- The PD results revealed that the oxidative dissolution of iron by the anode under chloride erosion was effectively inhibited due to a protective MCIs-molecule film that formed on the surface of the steel bar.
- The SEM-EDS results showed that there were very few corrosion products on the steel bar surface in specimens coated with the MCIs before chloride erosion, while the corrosion product gradually accumulated on the steel bar surface with the delayed MCIs coating time. The element Fe content values were 73.52% on the steel bar surfaces in specimens coated with the MCIs before chloride erosion, which gradually decreased with the delayed MCIs coating time.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.C.; Li, N. A review on concrete surface treatment Part I: Types and mechanisms. Constr. Build. Mater. 2017, 132, 578–590. [Google Scholar] [CrossRef]
- Zheng, H.; Li, W.; Ma, F.; Kong, Q. The performance of a surface-applied corrosion inhibitor for the carbon steel in saturated Ca(OH)2 solutions. Cem. Concr. Res. 2014, 55, 102–108. [Google Scholar] [CrossRef]
- Li, C.; Jiang, L.; Li, S. Effect of limestone powder addition on threshold chloride concentration for steel corrosion in reinforced concrete. Cem. Concr. Res. 2020, 131, 106018. [Google Scholar] [CrossRef]
- Sun, M.; Sun, C.; Zhang, P.; Liu, N.; Li, Y.; Duan, J.; Hou, B. Influence of carbonation on chloride binding of mortars made with simulated marine sand. Constr. Build. Mater. 2021, 303, 124455. [Google Scholar] [CrossRef]
- Sun, C.; Yuan, L.; Zhai, X.; Qu, F.; Li, Y.; Hou, B. Numerical and experimental study of moisture and chloride transport in unsaturated concrete. Constr. Build. Mater. 2018, 189, 1067–1075. [Google Scholar] [CrossRef]
- Söylev, T.A.; McNally, C.; Richardson, M. Effectiveness of amino alcohol-based surface-applied corrosion inhibitors in chloride-contaminated concrete. Cem. Concr. Res. 2007, 37, 972–977. [Google Scholar] [CrossRef]
- Söylev, T.A.; Richardson, M.G. Corrosion inhibitors for steel in concrete: State-of-the-art report. Constr. Build. Mater. 2008, 22, 609–622. [Google Scholar] [CrossRef]
- Hooton, R.D.; Titherington, M.P. Chloride resistance of high-performance concretes subjected to accelerated curing. Cem. Concr. Res. 2004, 34, 1561–1567. [Google Scholar] [CrossRef]
- Krishnan, N.; Kamde, D.K.; Veedu, Z.D.; Pillai, R.G.; Shah, D.; Velayudham, R. Long-term performance and life-cycle-cost benefits of cathodic protection of concrete structures using galvanic anodes. J. Build. Eng. 2021, 42, 102467. [Google Scholar] [CrossRef]
- Nguyen, T.H.Y.; Tran, V.M.; Pansuk, W.; Cao, N.T. Electrochemical chloride extraction on reinforced concrete contaminated external chloride: Efficiencies of intermittent applications and impacts on hydration products. Cem. Concr. Compos. 2021, 121, 104076. [Google Scholar] [CrossRef]
- Zheng, H.; Li, W.; Ma, F.; Kong, Q. The effect of a surface-applied corrosion inhibitor on the durability of concrete. Constr. Build. Mater. 2012, 37, 36–40. [Google Scholar] [CrossRef]
- Goyal, A.; Ganjian, E.; Pouya, H.S.; Tyrer, M. Inhibitor efficiency of migratory corrosion inhibitors to reduce corrosion in reinforced concrete exposed to high chloride environment. Constr. Build. Mater. 2021, 303, 124461. [Google Scholar] [CrossRef]
- Benzina Mechmeche, L.; Dhouibi, L.; Ouezdou, M.B.; Triki, E.; Zucchi, F. Investigation of the early effectiveness of an amino-alcohol based corrosion inhibitor using simulated pore solutions and mortar specimens. Cem. Concr. Compos. 2008, 30, 167–173. [Google Scholar] [CrossRef]
- Tritthart, J. Transport of a surface-applied corrosion inhibitor in cement paste and concrete. Cem. Concr. Res. 2003, 33, 829–834. [Google Scholar] [CrossRef]
- Holloway, L.; Nairn, K.; Forsyth, M. Concentration monitoring and performance of a migratory corrosion inhibitor in steel-reinforced concrete. Cem. Concr. Res. 2004, 34, 1435–1440. [Google Scholar] [CrossRef]
- Tiwari, A.; Goyal, S.; Luxami, V.; Chakraborty, M.K.; Prabhakar, G. Assessment of corrosion inhibition efficiency of generic compounds having different functional groups in carbonated pore solution with chlorides and migration ability in concrete. Constr. Build. Mater. 2021, 290, 123275. [Google Scholar] [CrossRef]
- Malik, A.U.; Andijani, I.; Al-Moaili, F.; Ozair, G. Studies on the performance of migratory corrosion inhibitors in protection of rebar concrete in Gulf seawater environment. Cem. Concr. Compos. 2004, 26, 235–242. [Google Scholar] [CrossRef]
- Bolzoni, F.; Brenna, A.; Ormellese, M. Recent advances in the use of inhibitors to prevent chloride-induced corrosion in reinforced concrete. Cem. Concr. Res. 2022, 154, 106719. [Google Scholar] [CrossRef]
- Jamil, H.E.; Shriri, A.; Boulif, R.; Montemor, M.F.; Ferreira, M.G.S. Corrosion behaviour of reinforcing steel exposed to an amino alcohol based corrosion inhibitor. Cem. Concr. Compos. 2005, 27, 671–678. [Google Scholar] [CrossRef]
- Ben Harb, M.; Abubshait, S.; Etteyeb, N.; Kamoun, M.; Dhouib, A. Olive leaf extract as a green corrosion inhibitor of reinforced concrete contaminated with seawater. Arab. J. Chem. 2020, 13, 4846–4856. [Google Scholar] [CrossRef]
- Anitha, R.; Chitra, S.; Hemapriya, V.; Chung, I.M.; Kim, S.H.; Prabakaran, M. Implications of eco-addition inhibitor to mitigate corrosion in reinforced steel embedded in concrete. Constr. Build. Mater. 2019, 213, 246–256. [Google Scholar] [CrossRef]
- Cao, F.T.; Wei, J.; Dong, J.; Ke, W. The corrosion inhibition effect of phytic acid on 20SiMn steel in simulated carbonated concrete pore solution. Corros. Sci. 2015, 100, 365–376. [Google Scholar] [CrossRef]
- Soylev, T.A.; McNally, C.; Richardson, M.G. The effect of a new generation surface-applied organic inhibitor on concrete properties. Cem. Concr. Compos. 2007, 29, 357–364. [Google Scholar] [CrossRef]
- Zhi, F.; Jiang, L.; Jin, M.; Xu, P.; Xiao, B.; Jiang, Q.; Chen, L.; Gu, Y. Inhibition effect and mechanism of polyacrylamide for steel corrosion in simulated concrete pore solution. Constr. Build. Mater. 2020, 259, 120425. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, J.; Li, G.; Wang, P.; Wang, P.; Li, F.; Zhang, B.; Chi, H. Corrosion inhibition efficiency of compound nitrite with D-sodium gluconate on carbon steel in simulated concrete pore solution. Constr. Build. Mater. 2021, 288, 123101. [Google Scholar] [CrossRef]
- Lee, H.S.; Yang, H.M.; Singh, J.K.; Prasad, S.K.; Yoo, B. Corrosion mitigation of steel rebars in chloride contaminated concrete pore solution using inhibitor: An electrochemical investigation. Constr. Build. Mater. 2018, 173, 443–451. [Google Scholar] [CrossRef]

| Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | TiO2 | P2O5 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 19.90 | 4.14 | 2.82 | 63.27 | 1.60 | 4.49 | 0.73 | - | - | 98.55 |
| FA | 43.90 | 43.80 | 2.71 | 3.31 | 0.54 | 0.75 | 0.82 | 1.63 | 0.74 | 98.2 |
| Cement | Fly Ash | Water | Sand | Gravel | Water Reducer |
|---|---|---|---|---|---|
| 290 | 65 | 160 | 750 | 1150 | 5.4 |
| Name | Rs (Ω·cm−2) | Cf (μF·cm−2) | n1 | Rf (kΩ·cm−2) | Cdl (μF·cm−2) | n2 | Rct (kΩ·cm−2) | η (%) |
|---|---|---|---|---|---|---|---|---|
| A0 | 11.23 | 4.281 | 0.50 | 2.82 | 6.988 | 0.48 | 3.13 | -- |
| B | 12.24 | 4.719 | 0.35 | 5.46 | 1.284 | 0.59 | 7.01 | 55.35 |
| A1 | 11.22 | 5.482 | 0.35 | 4.08 | 2.337 | 0.55 | 4.54 | 31.06 |
| A2 | 11.01 | 5.384 | 0.64 | 3.49 | 6.539 | 0.46 | 4.37 | 28.38 |
| A3 | 12.02 | 5.100 | 0.49 | 3.40 | 6.813 | 0.48 | 4.29 | 27.04 |
| A4 | 12.22 | 4.591 | 0.31 | 2.48 | 3.945 | 0.70 | 4.00 | 21.75 |
| A5 | 11.02 | 4.450 | 0.34 | 9.94 | 1.278 | 0.60 | 3.24 | 3.40 |
| Elements | Fe | O | C | Cl | Mn | Ca | Others |
|---|---|---|---|---|---|---|---|
| A0 | 34.65 | 61.51 | 2.52 | 0.32 | 0.57 | 0.00 | 0.43 |
| B | 73.52 | 12.78 | 9.52 | 0.00 | 1.03 | 0.00 | 3.15 |
| A1 | 68.97 | 24.20 | 3.50 | 0.00 | 1.00 | 0.93 | 1.40 |
| A2 | 51.14 | 40.90 | 2.32 | 0.00 | 0.67 | 1.45 | 3.52 |
| A3 | 37.44 | 53.19 | 2.64 | 0.35 | 0.49 | 2.63 | 3.26 |
| A4 | 20.39 | 69.16 | 2.44 | 0.25 | 0.27 | 3.88 | 3.61 |
| A5 | 35.31 | 46.49 | 3.68 | 6.88 | 0.46 | 0.00 | 7.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Sun, M.; Liu, J.; Dong, Z.; Fan, L.; Duan, J. Anti-Corrosion Performance of Migratory Corrosion Inhibitors on Reinforced Concrete Exposed to Varying Degrees of Chloride Erosion. Materials 2022, 15, 5138. https://doi.org/10.3390/ma15155138
Sun C, Sun M, Liu J, Dong Z, Fan L, Duan J. Anti-Corrosion Performance of Migratory Corrosion Inhibitors on Reinforced Concrete Exposed to Varying Degrees of Chloride Erosion. Materials. 2022; 15(15):5138. https://doi.org/10.3390/ma15155138
Chicago/Turabian StyleSun, Congtao, Ming Sun, Junde Liu, Zhenping Dong, Liang Fan, and Jizhou Duan. 2022. "Anti-Corrosion Performance of Migratory Corrosion Inhibitors on Reinforced Concrete Exposed to Varying Degrees of Chloride Erosion" Materials 15, no. 15: 5138. https://doi.org/10.3390/ma15155138
APA StyleSun, C., Sun, M., Liu, J., Dong, Z., Fan, L., & Duan, J. (2022). Anti-Corrosion Performance of Migratory Corrosion Inhibitors on Reinforced Concrete Exposed to Varying Degrees of Chloride Erosion. Materials, 15(15), 5138. https://doi.org/10.3390/ma15155138






