Electrical Potentiometry with Intraoral Applications
Abstract
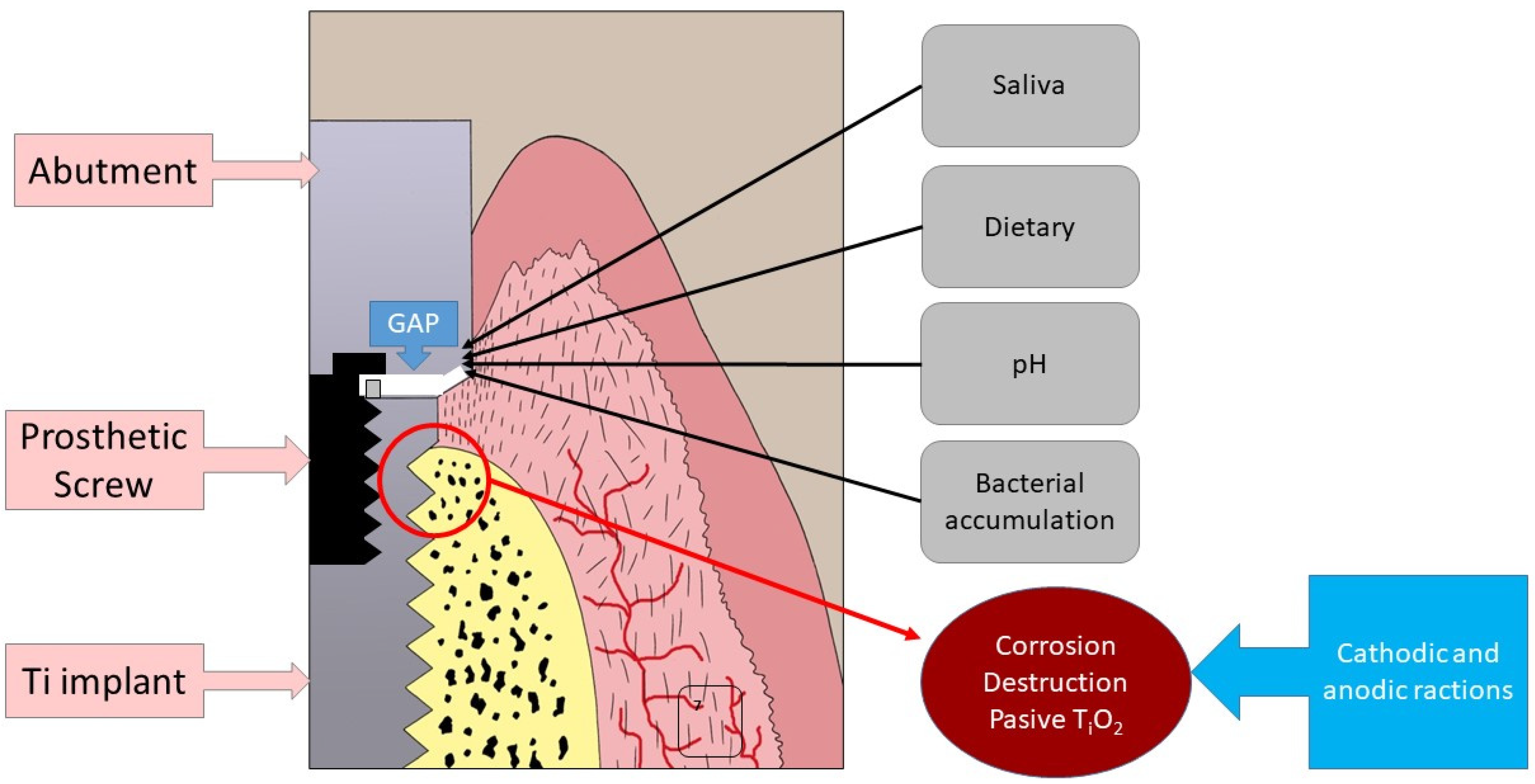
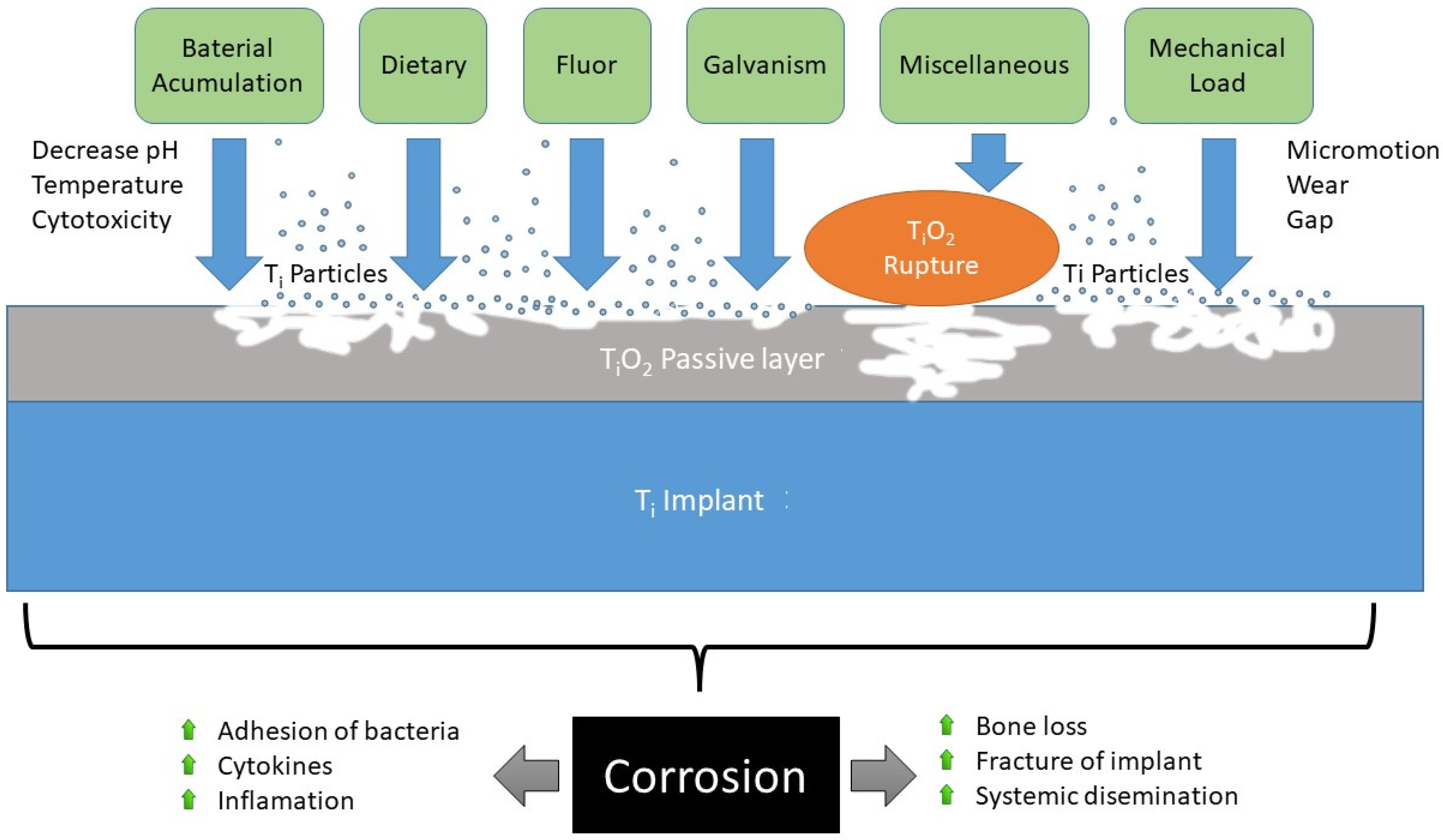
:1. Introduction
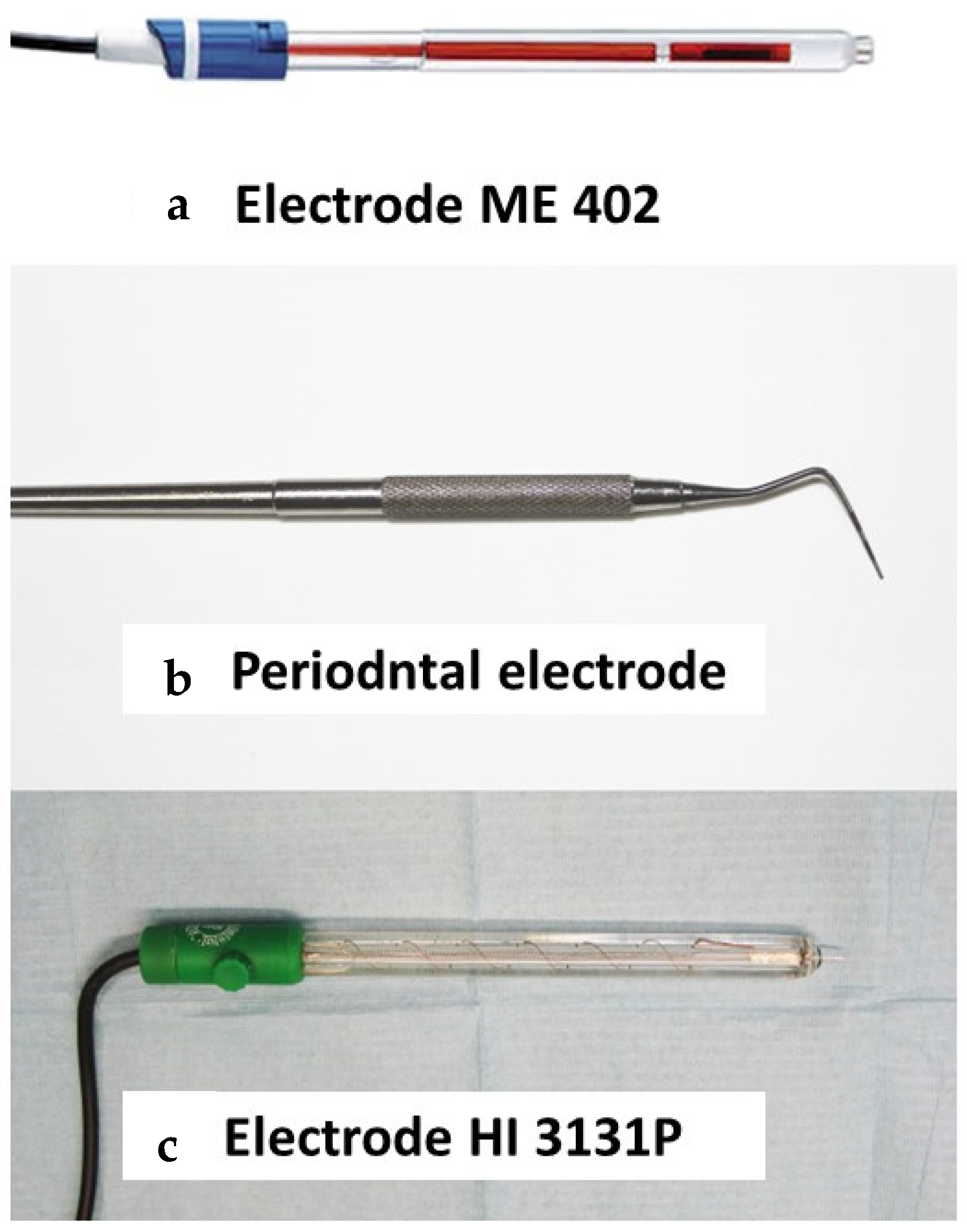
2. Materials and Methods
2.1. Materials
2.2. Method
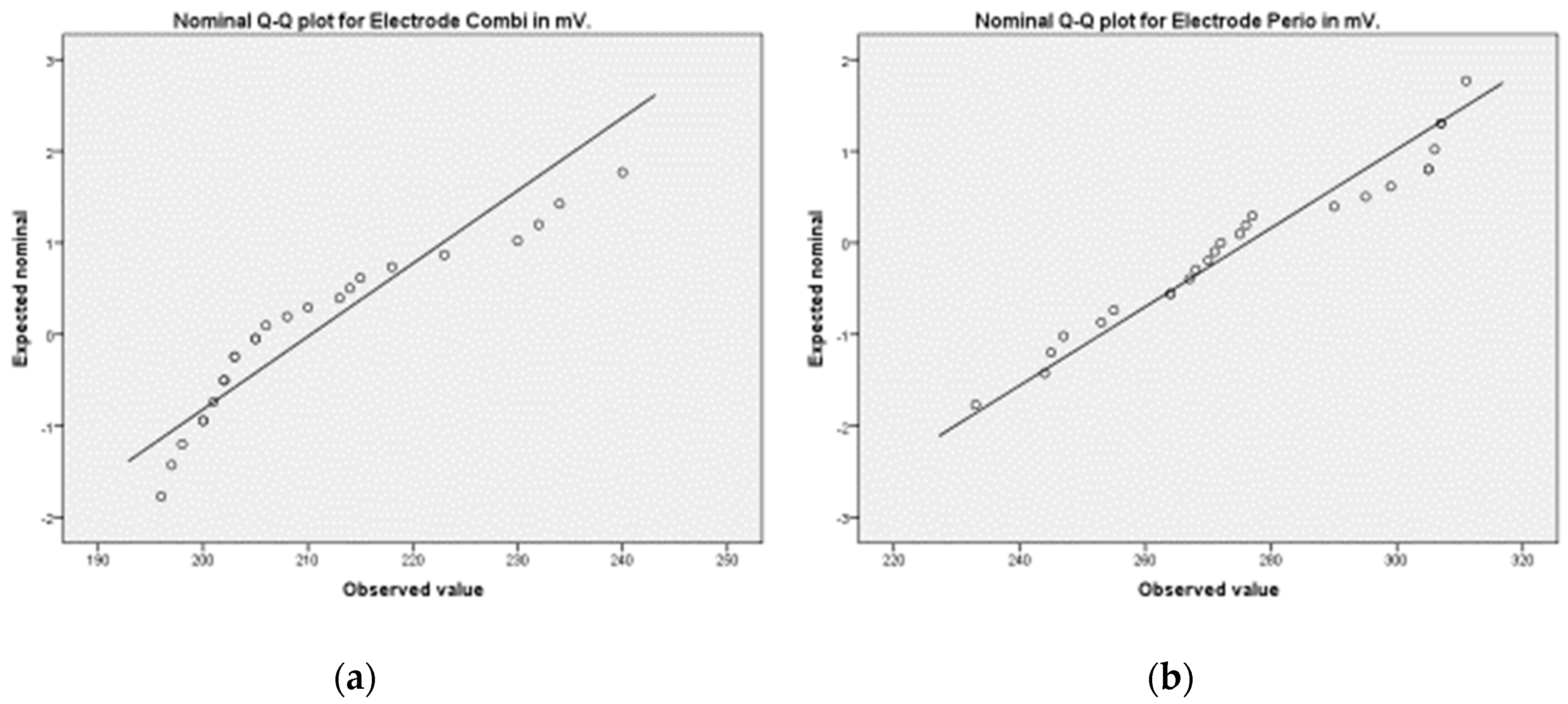
2.3. Statistical Analysis
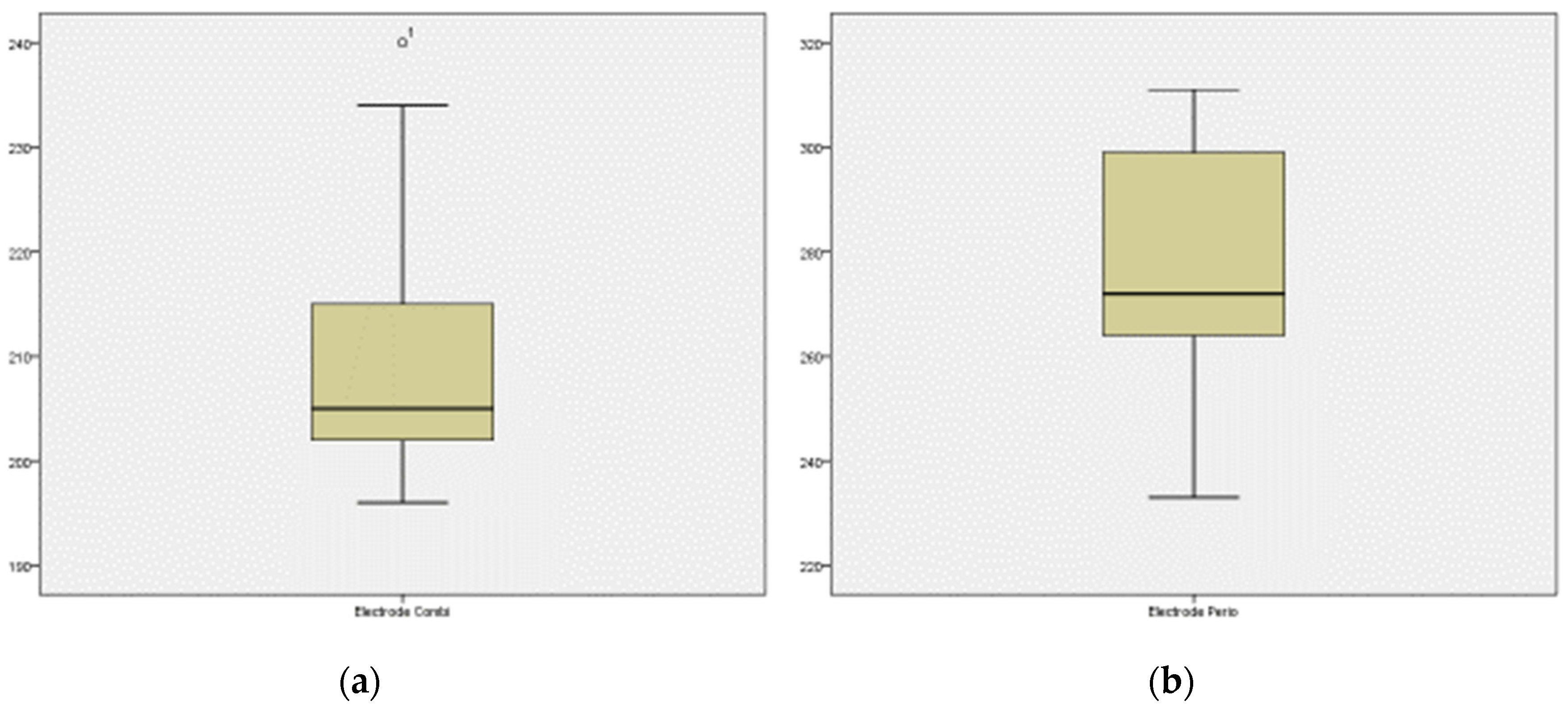
3. Results
4. Discussion
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gillispie, C. Complete Dictionary of Scientific Biography; Charles Scribner’s Sons: Detroit, MI, USA, 2008; Volume 9, ISBN 9780684315591. [Google Scholar]
- Axéll, T.; Nilner, K.; Nilsson, B. Clinical evaluation of patients referred with symptoms related to oral galvanism. Swed. Dent. J. 1983, 7, 169–178. [Google Scholar] [PubMed]
- Inovay, J.; Bánóczy, J. The role of electrical potential differences in the etiology of chronic diseases of the oral mucosa. J. Dent. Res. 1961, 40, 884–890. [Google Scholar] [CrossRef]
- Bánóczy, J.; Roed-Petersen, B.; Pindborg, J.J.; Inovay, J. Clinical and histologic studies on electrogalvanically induced oral white lesions. Oral Surg. Oral Med. Oral Pathol. 1979, 48, 319–323. [Google Scholar] [CrossRef]
- van Loon, L.A.; van Elsas, P.W.; van Joost, T.; Davidson, C.L. Contact stomatitis and dermatitis to nickel and palladium. Contact Dermat. 1984, 11, 294–297. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Valderrama, P.; Wilson, T.G.; Palmer, K.; Thomas, A.; Sridhar, S.; Adapalli, A.; Burbano, M.; Wadhwani, C. Titanium corrosion mechanisms in the oral environment: A retrieval study. Materials 2013, 6, 5258–5274. [Google Scholar] [CrossRef] [Green Version]
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Albrektsson, T.; Donos, N. Implant survival and complications. The Third EAO consensus conference 2012. Clin. Oral Implant. Res. 2012, 23, 63–65. [Google Scholar] [CrossRef]
- Lindhe, J.; Meyle, J. Peri-implant diseases: Consensus report of the sixth european workshop on periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef] [Green Version]
- Cochran, D.L.; Hermann, J.S.; Schenk, R.K.; Higginbottom, F.L.; Buser, D. Biologic width around titanium implants. A histometric analysis of the implanto-gingival junction around unloaded and loaded nonsubmerged implants in the canine mandible. J. Periodontol. 1997, 68, 186–198. [Google Scholar] [CrossRef]
- Berryman, Z.; Bridger, L.; Hussaini, H.M.; Rich, A.M.; Atieh, M.; Tawse-Smith, A. Titanium particles: An emerging risk factor for peri-implant bone loss. Saudi Dent. J. 2020, 32, 283–292. [Google Scholar] [CrossRef]
- Heringa, M.B.; Peters, R.J.B.; Bleys, R.; van der Lee, M.K.; Tromp, P.C.; van Kesteren, P.C.E.; van Eijkeren, J.C.H.; Undas, A.K.; Oomen, A.G.; Bouwmeester, H. Detection of titanium particles in human liver and spleen and possible health implications. Part. Fibre Toxicol. 2018, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Cruz, H.; Souza, J.; Henriques, M.; Rocha, L. Tribocorrosion and bio-tribocorrosion in the oral environment: The case of dental implants. In Biomedical Tribology; Davim, J.P., Ed.; Nova Science Publishers: New York, NY, USA, 2011; pp. 1–33. [Google Scholar]
- Souza, J.C.; Barbosa, S.L.; Ariza, E.A.; Henriques, M.; Teughels, W.; Ponthiaux, P.; Celis, J.P.; Rocha, L.A. How do titanium and Ti6Al4V corrode in fluoridated medium as found in the oral cavity? An in vitro study. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 47, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Juanito, G.M.; Morsch, C.S.; Benfatti, C.A.; Fredel, M.C.; Magini, R.S.; Souza, J.C. Effect of fluoride and bleaching agents on the degradation of titanium: Literature review. Dentistry 2015, 5, 273. [Google Scholar]
- Souza, J.C.; Ponthiaux, P.; Henriques, M.; Oliveira, R.; Teughels, W.; Celis, J.P.; Rocha, L.A. Corrosion behaviour of titanium in the presence of Streptococcus mutans. J. Dent. 2013, 41, 528–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, J.C.M.; Henriques, M.; Teughels, W.; Ponthiaux, P.; Celis, J.P.; Rocha, L.A. Wear and corrosion interactions on titanium in oral environment: Literature review. J. Bio-Tribo-Corros. 2015, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, F.; Sahm, N.; Mihatovic, I.; Golubovic, V.; Becker, J. Surgical therapy of advanced ligature-induced peri-implantitis defects: Cone-beam computed tomographic and histological analysis. J. Clin. Periodontol. 2011, 38, 939–949. [Google Scholar] [CrossRef]
- Goodman, S.B. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials 2007, 28, 5044–5048. [Google Scholar] [CrossRef] [Green Version]
- Urban, R.M.; Jacobs, J.J.; Tomlinson, M.J.; Gavrilovic, J.; Black, J.; Peoc’h, M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J. Bone Jt. Surg. 2000, 82, 457–476. [Google Scholar] [CrossRef]
- Sutow, E.J.; Maillet, W.A.; Taylor, J.C.; Hall, G.C.; Millar, M. Time-dependent corrosion potential of newly-placed admixed dental amalgam restorations. Dent. Mater. 2007, 23, 644–647. [Google Scholar] [CrossRef]
- Muller, A.W.; De Groot, D.A.; Davidson, C.L. The determination of the electrical potential of a metallic restoration in the oral cavity. J. Oral Rehabil. 1989, 16, 271–277. [Google Scholar] [CrossRef]
- Mathew, M.T.; Abbey, S.; Hallab, N.J.; Hall, D.J.; Sukotjo, C.; Wimmer, M.A. Influence of pH on the tribocorrosion behavior of CpTi in the oral environment: Synergistic interactions of wear and corrosion. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Reichl, F.X.; Wang, Y.; Michalke, B.; Milz, S.; Yang, Y.; Stolper, P.; Lindemaier, G.; Graw, M.; Hickel, R.; et al. Analysis of titanium and other metals in human jawbones with dental implants—A case series study. Dent. Mater. 2016, 32, 1042–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, M.M.; Wilson, B.M.; Ross, R.D.; Virdi, A.S.; Sumner, D.R. Arthrotomy-based preclinical models of particle-induced osteolysis: A systematic review. J. Orthop. Res. 2017, 35, 2595–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisken, K.W.; Dandie, G.W.; Lugowski, S.; Jordan, G. A study of titanium release into body organs following the insertion of single threaded screw implants into the mandibles of sheep. Aust. Dent. J. 2002, 47, 214–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Haddouti, E.M.; Welle, K.; Burger, C.; Wirtz, D.C.; Schildberg, F.A.; Kabir, K. The effects of biomaterial implant wear debris on osteoblasts. Front. Cell Dev. Biol. 2020, 8, 352. [Google Scholar] [CrossRef]
- Curtin, J.P.; Wang, M. Are clinical findings of systemic titanium dispersion following implantation explained by available in vitro evidence? An evidence-based analysis. J. Biol. Inorg. Chem. 2017, 22, 799–806. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.; Zhong, Y.; Wang, L. Multi-scale surface treatments of titanium implants for rapid osseointegration: A review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef]
- Suárez-López Del Amo, F.; Garaicoa-Pazmiño, C.; Fretwurst, T.; Castilho, R.M.; Squarize, C.H. Dental implants-associated release of titanium particles: A systematic review. Clin. Oral Implant. Res. 2018, 29, 1085–1100. [Google Scholar] [CrossRef]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal nanoparticles released from dental implant surfaces: Potential contribution to chronic inflammation and peri-implant bone loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Bai, J.; Shi, J.; Shen, J.; Guo, X.; Liu, Y.; Ge, G.; Lin, J.; Tao, Y.; Yang, H.; et al. MiR-106b inhibition suppresses inflammatory bone destruction of wear debris-induced periprosthetic osteolysis in rats. J. Cell. Mol. Med. 2020, 24, 7490–7503. [Google Scholar] [CrossRef]
- Hjalmarsson, L.; Smedberg, J.I.; Wennerberg, A. Material degradation in implant-retained cobalt-chrome and titanium frameworks. J. Oral Rehabil. 2011, 38, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Henry, P.J. Clinical experiences with dental implants. Adv. Dent. Res. 1999, 13, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berbel, L.O.; Banczek, E.D.P.; Karoussis, I.K.; Kotsakis, G.A.; Costa, I. Determinants of corrosion resistance of Ti-6Al-4V alloy dental implants in an In Vitro model of peri-implant inflammation. PLoS ONE 2019, 14, e0210530. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Shi, Q.; Wang, J.; Chen, X.; Hao, Y.; Zhang, Y.; Wang, X. The unfavorable role of titanium particles released from dental implants. Nanotheranostics 2021, 5, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ruiz, R.; Romanos, G. Potential causes of titanium particle and ion release in implant dentistry: A systematic review. Int. J. Mol. Sci. 2018, 19, 3585. [Google Scholar] [CrossRef] [Green Version]
- Penmetsa, S.L.D.; Shah, R.; Thomas, R.; Kumar, A.B.T.; Gayatri, P.S.D.; Mehta, D.S. Titanium particles in tissues from peri-implant mucositis: An exfoliative cytology-based pilot study. J. Indian Soc. Periodontol. 2017, 21, 192–194. [Google Scholar] [CrossRef]
- Chapter II 4 4 Degradative Effects of the Biological Environment on Metals and Ceramics 2013 Biomaterials Science Third Edition. Available online: https://es.scribd.com/document/352048599/Chapter-II-4-4-Degradative-Effects-of-the-Biological-Environment-on-Metals-and-Ceramics-2013-Biomaterials-Science-Third-Edition (accessed on 27 May 2022).
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Gil, F.J.; Canedo, R.; Padrós, A.; Bañeres, M.V.; Arano, J.M. Fretting corrosion behaviour of ball-and-socket joint on dental implants with different prosthodontic alloys. Bio-Med. Mater. Eng. 2003, 13, 27–34. [Google Scholar]
- Sánchez-Pérez, A.; Moya-Villaescusa, M.J.; Jornet-Garcia, A.; Gomez, S. Etiology, risk factors and management of implant fractures. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e504–e508. [Google Scholar] [CrossRef] [Green Version]
- Noguti, J.; de Oliveira, F.; Peres, R.C.; Renno, A.C.; Ribeiro, D.A. The role of fluoride on the process of titanium corrosion in oral cavity. Biometals 2012, 25, 859–862. [Google Scholar] [CrossRef]
- Licausi, M.P.; Igual Muñoz, A.; Amigó Borrás, V. Influence of the fabrication process and fluoride content on the tribocorrosion behaviour of Ti6Al4V biomedical alloy in artificial saliva. J. Mech. Behav. Biomed. Mater. 2013, 20, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Electrical implications of corrosion for osseointegration of titanium implants. J. Dent. Res. 2011, 90, 1389–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Shafer, T.; Watanabe, I.; Nunn, M.E.; Okabe, T. Electrochemical characterization of cast titanium alloys. Biomaterials 2003, 24, 213–218. [Google Scholar] [CrossRef]
- Venugopalan, R.; Lucas, L.C. Evaluation of restorative and implant alloys galvanically coupled to titanium. Dent. Mater. 1998, 14, 165–172. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Soares, F.M.S.; Elias, C.N.; Monteiro, E.S.; Coimbra, M.E.R.; Santana, A.I.C. Galvanic Corrosion of Ti Dental Implants Coupled to CoCrMo Prosthetic Component. Int. J. Biomater. 2021, 2021, 1313343. [Google Scholar] [CrossRef] [PubMed]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2021, 7, e10239. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; Peña, M.; Herrero-Climent, M.; Rios-Santos, J.V.; Rios-Carrasco, B.; Brizuela, A.; Gil, J. Corrosion Behavior of Titanium Dental Implants with Implantoplasty. Materials 2022, 15, 1563. [Google Scholar] [CrossRef]
- Verdeguer, P.; Gil, J.; Punset, M.; Manero, J.M.; Nart, J.; Vilarrasa, J.; Ruperez, E. Citric Acid in the Passivation of Titanium Dental Implants: Corrosion Resistance and Bactericide Behavior. Materials 2022, 15, 545. [Google Scholar] [CrossRef]
- Gürbüz-Urvasızoğlu, G.; Ataol, M.; Özgeriş, F.B. Trace elements released from dental implants with periimplantitis: A cohort study. Ir. J. Med. Sci. 2022. [Google Scholar] [CrossRef]
- Nagay, B.E.; Cordeiro, J.M.; Barao, V.A.R. Insight into Corrosion of Dental Implants: From Biochemical Mechanisms to Designing Corrosion-Resistant Materials. Curr. Oral Health Rep. 2022, 9, 7–21. [Google Scholar] [CrossRef]


| Chemical Components of Artificial Saliva | ||
|---|---|---|
| Sodium Biphosphate 200 mg | Potassium Chloride 1.2 gr | Potassium Thiocyanate 330 mg |
| Sodium Bisphosphate 260 mg | Sodium Chloride 700 mg | Sodium Bicarbonate 1.5 gr |
| Urea 1.5 gr | Purified water sqt 1000 ml | Lactic acid sqt pH 6–7 |
| Statistical | Combined Electrode | Periodontal Electrode |
|---|---|---|
| Mean | 276.24 | −210.28 |
| Lower limit of the CI * | 266.64 | −215.47 |
| Upper limit of the CI * | 285.84 | −205.09 |
| 5% trimmed mean | 276.63 | −209.48 |
| Median | 272 | −205 |
| Variance | 540.44 | 157.96 |
| Standard deviation | 23.24 | 12.56 |
| Minimum | 233 | −240 |
| Maximum | 311 | −196 |
| Range | 78 | 44 |
| Interquartile range | 43 | 15 |
| Asymmetry | −0.00 | −1.07 |
| Kurtosis | −1.09 | 0.12 |
| T-Test | T-Test Value | p |
|---|---|---|
| Values | −189.99 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jornet-García, A.; Sanchez-Perez, A.; Montoya-Carralero, J.M.; Moya-Villaescusa, M.J. Electrical Potentiometry with Intraoral Applications. Materials 2022, 15, 5100. https://doi.org/10.3390/ma15155100
Jornet-García A, Sanchez-Perez A, Montoya-Carralero JM, Moya-Villaescusa MJ. Electrical Potentiometry with Intraoral Applications. Materials. 2022; 15(15):5100. https://doi.org/10.3390/ma15155100
Chicago/Turabian StyleJornet-García, Alfonso, Arturo Sanchez-Perez, José María Montoya-Carralero, and María José Moya-Villaescusa. 2022. "Electrical Potentiometry with Intraoral Applications" Materials 15, no. 15: 5100. https://doi.org/10.3390/ma15155100
APA StyleJornet-García, A., Sanchez-Perez, A., Montoya-Carralero, J. M., & Moya-Villaescusa, M. J. (2022). Electrical Potentiometry with Intraoral Applications. Materials, 15(15), 5100. https://doi.org/10.3390/ma15155100






