Mechanical Reinforcement in Nylon 6 Nanocomposite Fiber Incorporated with Dopamine Reduced Graphene Oxide
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Synthesis of Functionalized GO, rGO, and PDA-rGO
2.3. Preparation of PA6/-rGO and PA6/PDA-rGO Composite Fibers
2.4. Characterization
3. Results and Discussion
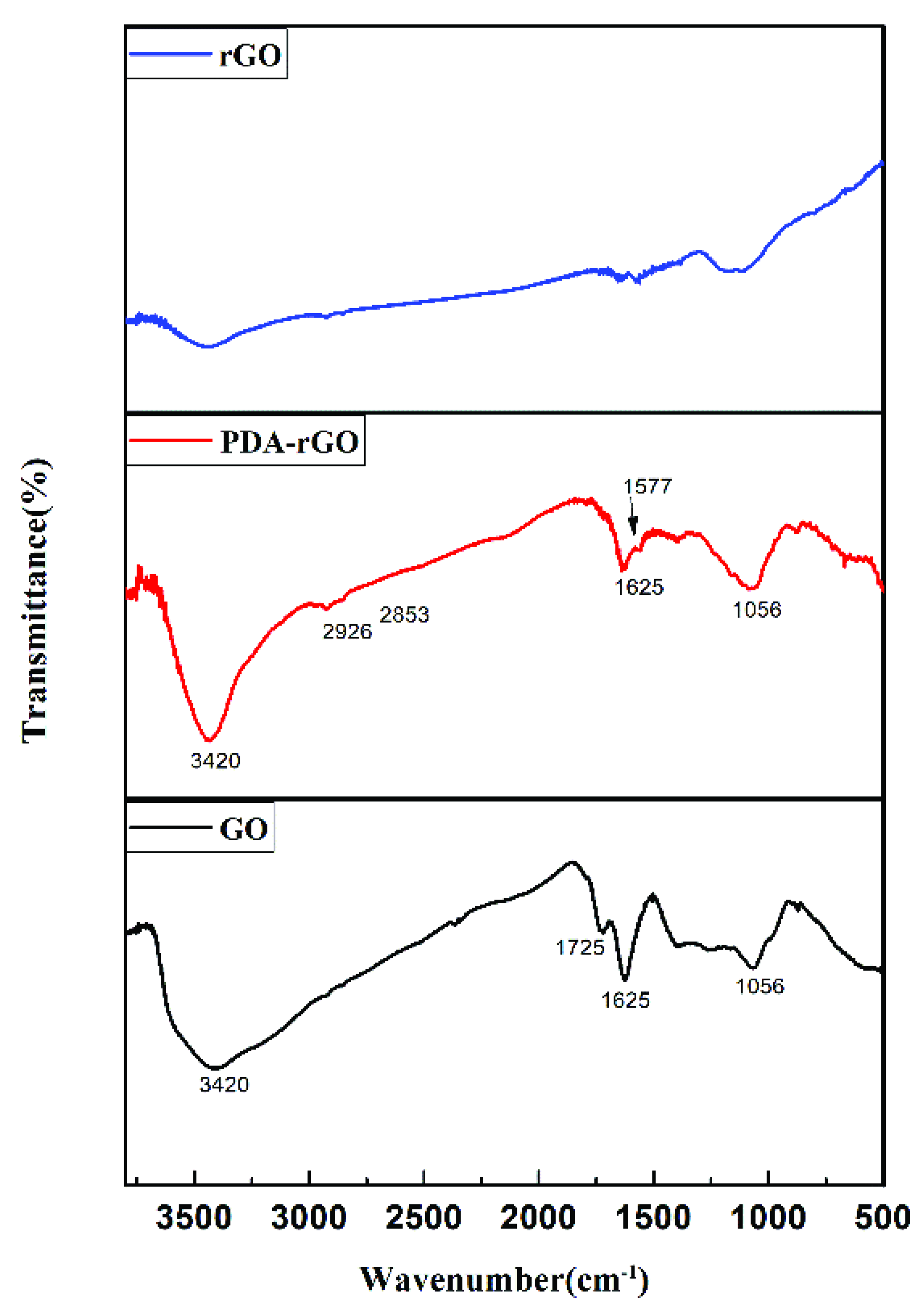
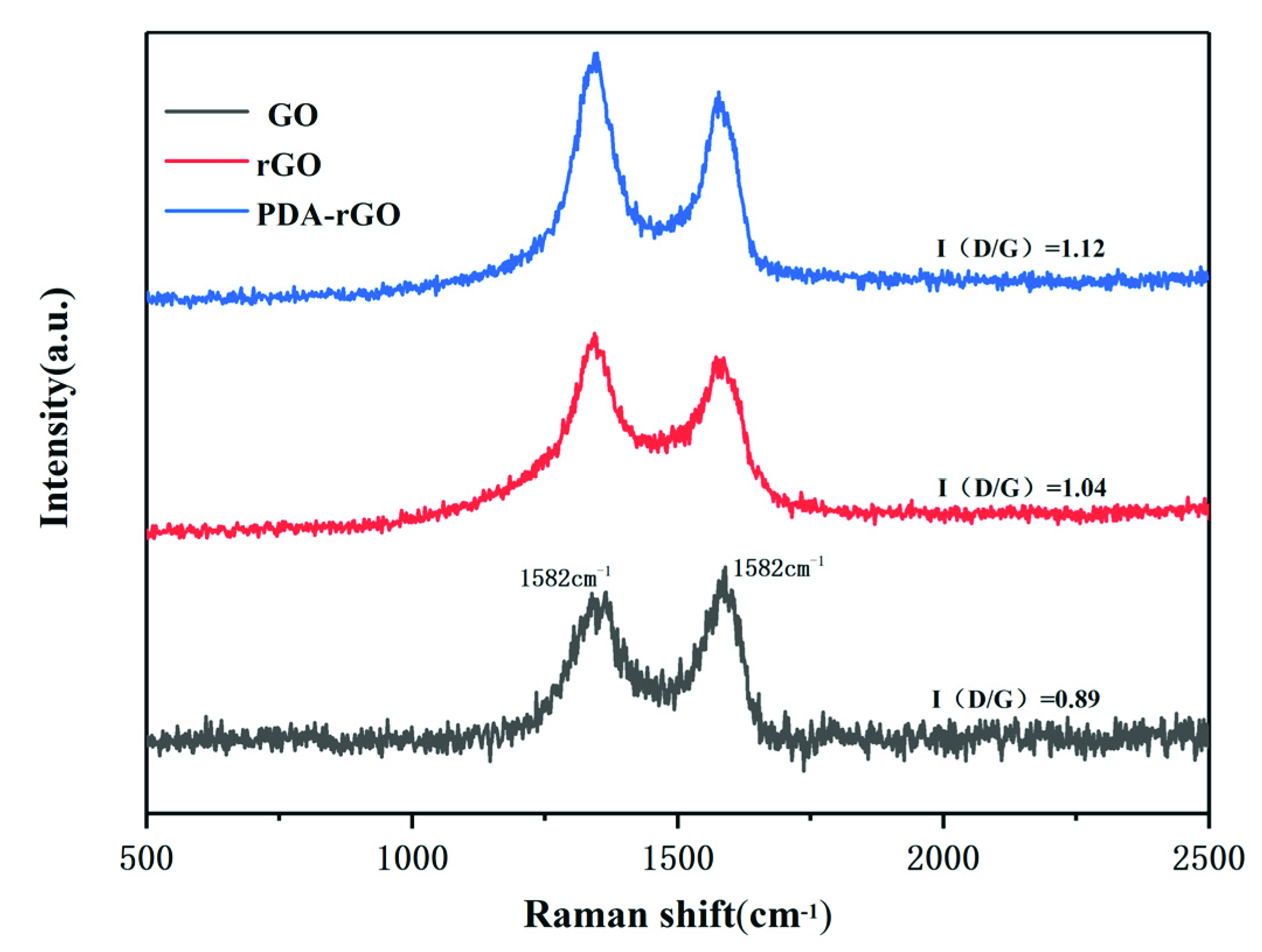
3.1. The Morphology, Composition and Thermal Properties of PDA-rGO
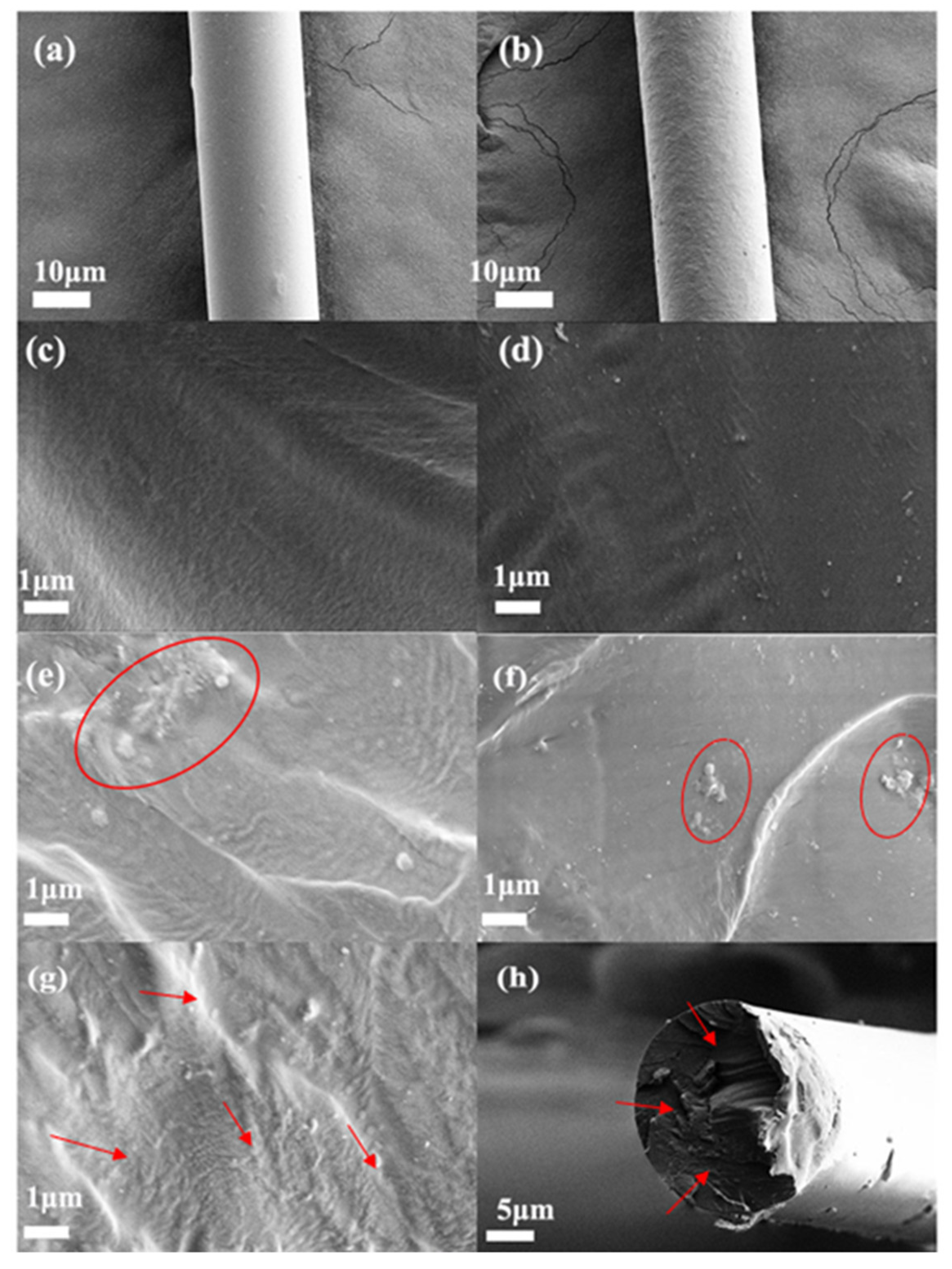
3.2. The Morphology of Composite Fibers
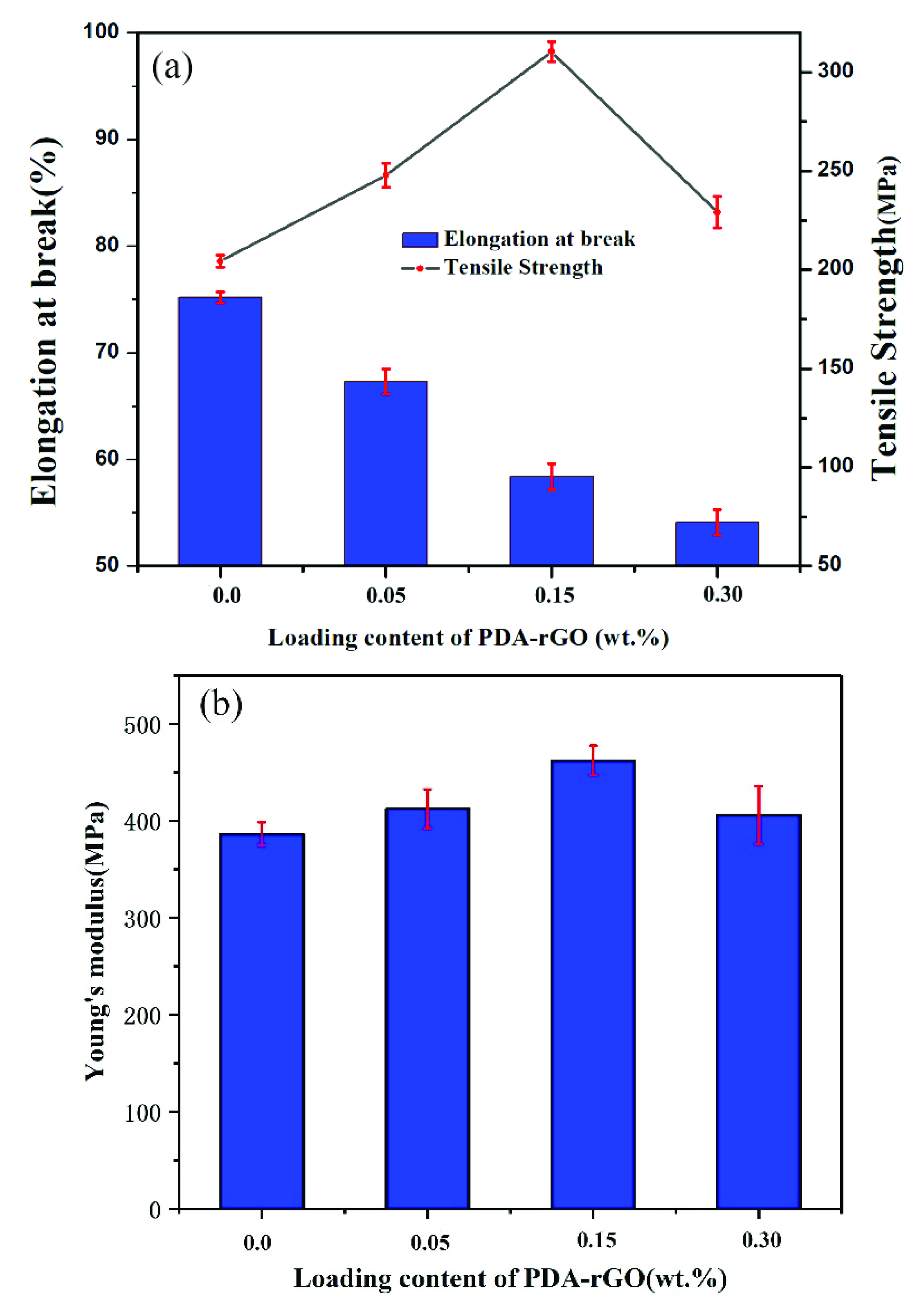
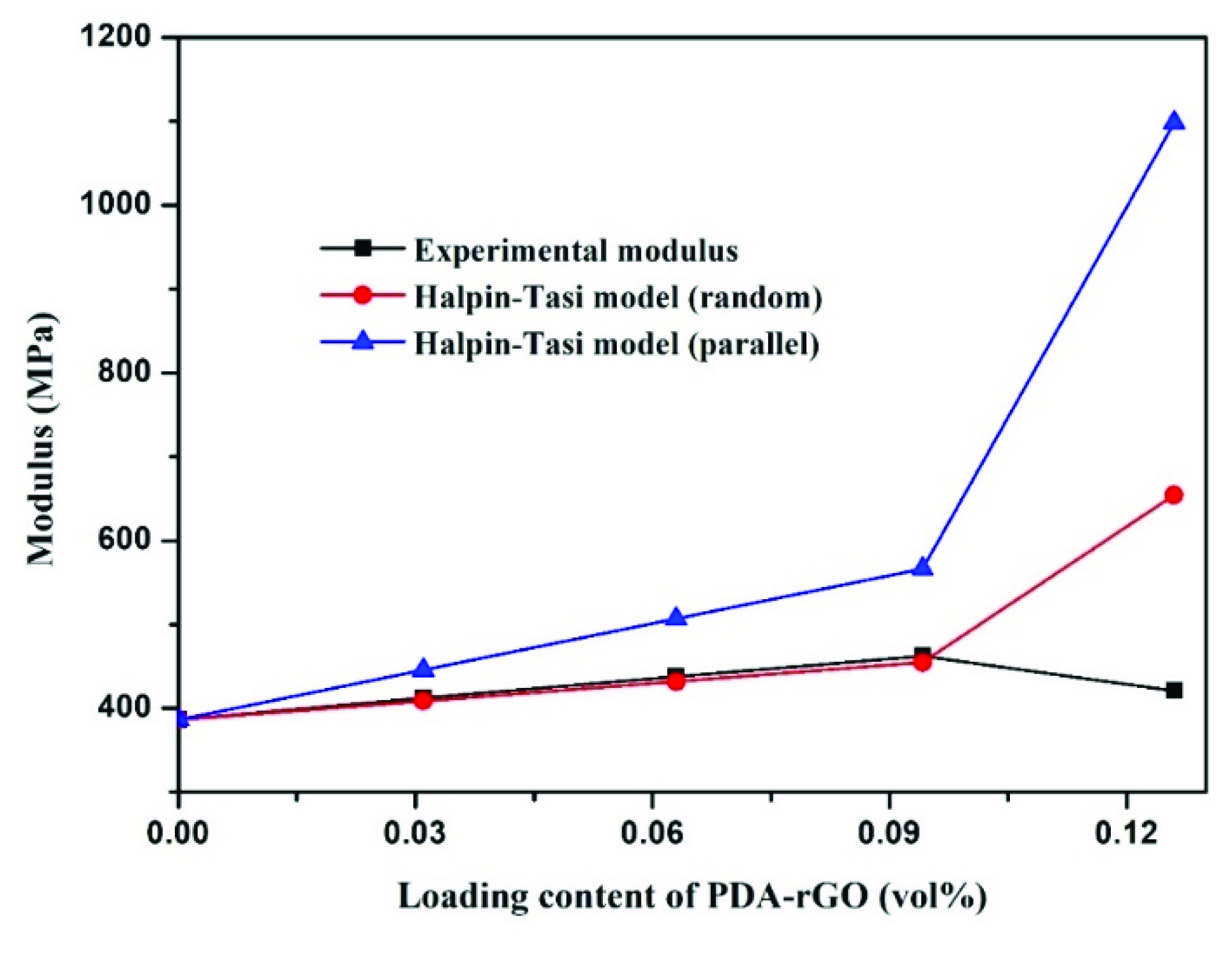
3.3. The Mechanical Properties of Composite Fibers
| Loading Content (wt%) | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| 0 | 204.3 | 75.2 | 386.4 |
| 0.05 PDA-rGO | 247.8 | 67.3 | 412.3 |
| 0.15 PDA-rGO | 310.4 | 58.4 | 462.3 |
| 0.3 PDA-rGO | 229.1 | 54.1 | 405.8 |
| 0.15 rGO | 213.5 | 46.5 | 394.6 |
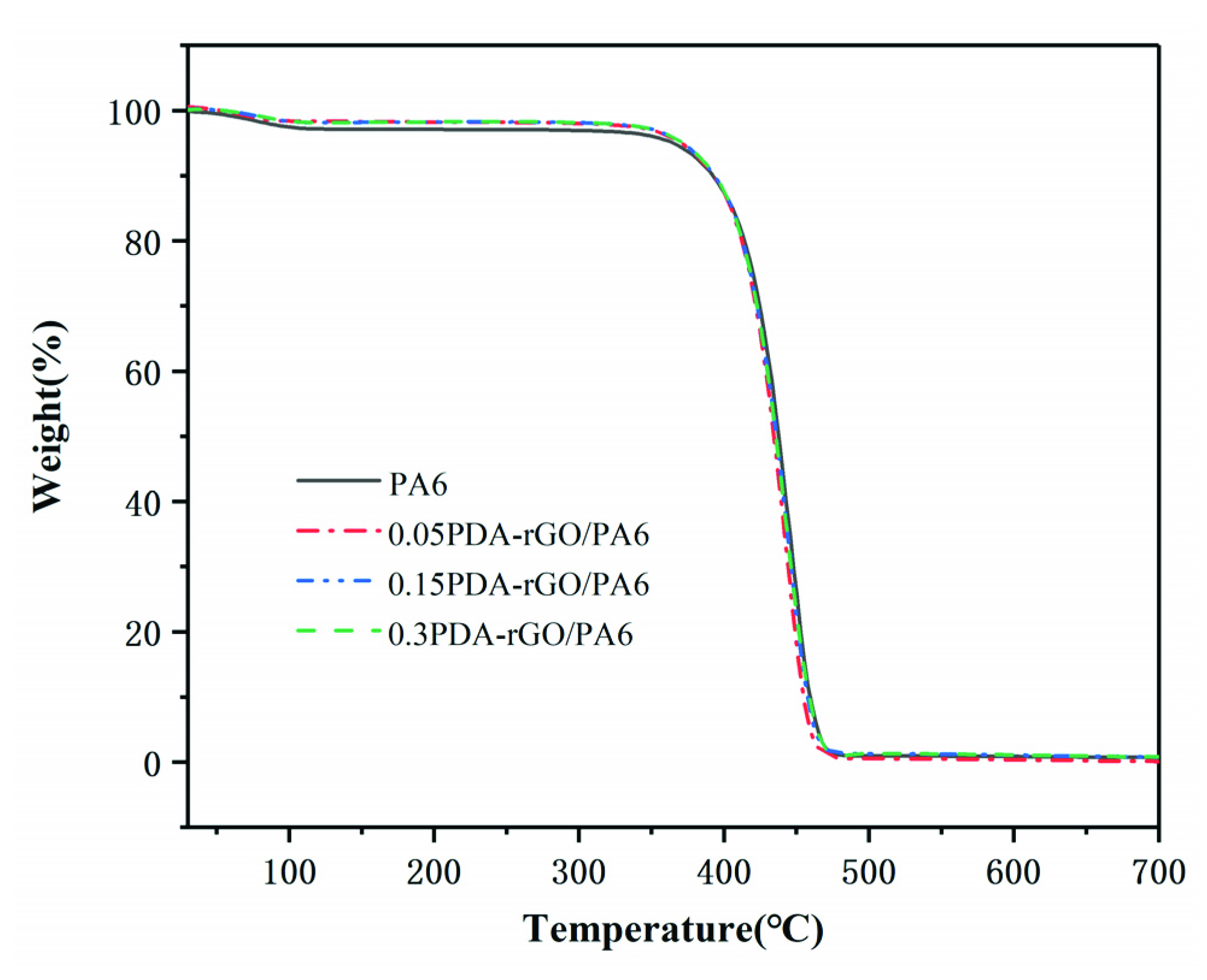
3.4. The Thermal Properties of Composite Fibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, X.; Wang, L.; Su, Q.; Zheng, J. Use of unmodified SiO2 as nanofiller to improve mechanical properties of polymer-based nanocomposites. Compos. Sci. Technol. 2013, 89, 52–60. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Agarwal, U.S.; Ma, Y.Y. Study on mechanical properties, thermal stability and crystallization behavior of PET/MMT nanocomposites. Compos. Part B Eng. 2006, 37, 399–407. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Zeng, L.; Li, R.; Zhong, W.H. A review of the electrical and mechanical properties of carbon nanofiller-reinforced polymer composites. J. Mater. Sci. 2019, 54, 1036–10762. [Google Scholar] [CrossRef]
- Mussell, J. Graphene: Status and Prospects. Science 2014, 324, 1530–1534. [Google Scholar]
- Dimiev, A.M.; Tour, J.M. Mechanism of graphene oxide formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef]
- Wang, J.; Jin, X.; Li, C.; Wang, W.; Wu, H.; Guo, S. Graphene and graphene derivatives toughening polymers: Toward high toughness and strength. Chem. Eng. J. 2019, 370, 831–854. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, C.; Wang, A.; Zhou, X.; Xing, X.; Liu, J.; Lu, Q. Research progress on self-healing polymer/graphene anticorrosion coatings. Prog. Org. Coat. 2021, 155, 106231. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Li, H.; Yan, C. Facile preparation route for graphene oxide reinforced polyamide 6 composites via in situ anionic ring-opening polymerization. J. Mater. Chem. 2012, 22, 24081–24091. [Google Scholar] [CrossRef]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent progress in graphene/polymer nanocomposites. Adv. Mater. 2021, 33, 2001105. [Google Scholar] [CrossRef]
- Song-Jie, Q.; Xiang-Nan, X.; Yang, Q.; He-Chong, X.; Yue-Feng, Z. Simultaneous reduction and functionalization of graphene oxide by 4-hydrazinobenzenesulfonic acid for polymer nanocomposites. Nanomaterials 2016, 6, 29. [Google Scholar]
- Zhang, Y.; Lu, Y.; Yan, X.J.; Gao, W.S.; Bai, Y.X. Functional & enhanced graphene/polyamide 6 composite fiber constructed by a facile and universal method. Compos. Part A Appl. 2019, 123, 149–157. [Google Scholar]
- Meng, X.; Wang, M.; Yang, L.; Ye, H.M.; Zhou, Q. Effects of amino-functionalized graphene oxide on the mechanical and thermal properties of polyoxymethylene. Ind. Eng. Chem. Res. 2017, 56, 15038–15048. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, G.; Wang, W.; Cui, X.; Dong, W.; Wang, C.; Liu, L. Trade-off between interface stiffening and Young’s modulus weakening in graphene/PMMA nanocomposites. Compos. Sci. Technol. 2022, 225, 109483. [Google Scholar] [CrossRef]
- Izzuddin, Z.; Hsu-Chiang, K.; Qingshi, M.; Andrew, M.; Nobuyuki, K.; Terry, P.; Liqun, Z.; Sherif, G.; Lee, L.; Jun, M. A facile approach to chemically modified graphene and its polymer nanocomposites. Adv. Funct. Mater. 2012, 22, 2735–2743. [Google Scholar]
- Ji, H.R.; Phillip, B.M.; Haeshin, L. Polydopamine surface chemistry: A decade of discovery. ACS. Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar]
- Delparastan, P.; Malollari, K.; Lee, H.; Messersmith, P. Direct evidence for the polymeric nature of polydopamine. Angew. Chem. 2019, 131, 1089–1094. [Google Scholar] [CrossRef]
- Lee, H.A.; Yanfei, M.; Feng, Z.; Hong, S.; Lee, H. Material-independent surface chemistry beyond polydopamine coating. Acc. Chem. Res. 2019, 52, 704–713. [Google Scholar] [CrossRef]
- Weichao, Z.; Hailong, F.; Le, W.; Zhaoxia, J. Oxidative self-polymerization of dopamine in an acidic environment. Langmuir 2015, 31, 11671–11677. [Google Scholar]
- Li, W.; Shang, T.; Yang, W.; Yang, H.; Lin, S.; Jia, X. Effectively exerting the reinforcement of dopamine reduced graphene oxide on epoxy-based composites via strengthened interfacial bonding. ACS Appl. Mater. Interfaces 2016, 8, 13037–13050. [Google Scholar] [CrossRef]
- Lu, Y.L.; Ma, J.; Xu, T.Y.; Wang, W.C.; Jiang, Y.; Zhang, L.Q. Preparation and properties of natural rubber reinforced with polydopamine-coating modified carbon nanotubes. Express Polym. Lett. 2017, 11, 21–34. [Google Scholar] [CrossRef]
- Fang, Y.; Dong, J.; Zhao, X.; Chen, T.; Zhang, Q. Covalently linked polydopamine-modified boron nitride nanosheets/polyimide composite fibers with enhanced heat diffusion and mechanical behaviors. Compos. Part B Eng. 2020, 199, 108281. [Google Scholar] [CrossRef]
- Keping, C.; Qiang, T.; Chunrong, T.; Guanyun, Y.; Fen, C.; Shuen, L.; Xiaolin, W. Mechanical reinforcement in thermoplastic polyurethane nanocomposite incorporated with polydopamine functionalized graphene nanoplatelet. Ind. Eng. Chem. Res. 2017, 56, 11827–11838. [Google Scholar]
- Tang, L.; Li, Y.; Chen, Y.; Ji, P.; Wang, C.; Wang, H. Preparation and characterization of graphene reinforced PA6 fiber. J. Appl. Polym. Sci. 2018, 135, 45834. [Google Scholar] [CrossRef]
- Rangari, V.K.; Yousuf, M.; Jeelani, S.; Pulikkathara, M.X.; Khabashesku, V.N. Alignment of carbon nanotubes and reinforcing effects in nylon-6 polymer composite fibers. Nanotechnology 2008, 19, 245703. [Google Scholar] [CrossRef]
- Jinqing, H.; Yonghuan, Z.; Yang, M.; Juanjuan, S.; Jian, H. Long-lasting antimicrobial activity achieved through the synergy of graphene oxide and cuprous oxide coating on PET fabrics. Synth. Met. 2022, 286, 117033. [Google Scholar]
- Zhao, Z.; Guo, L.; Feng, L.; Lu, H.; Zou, X. Polydopamine functionalized graphene oxide nanocomposites reinforced the corrosion protection and adhesion properties of waterborne polyurethane coatings. Eur. Polym. J. 2019, 120, 109249. [Google Scholar] [CrossRef]
- Hu, X.; Qi, R.; Zhu, J.; Lu, J.; Luo, Y.; Jin, J. Preparation and properties of dopamine reduced graphene oxide and its composites of epoxy. J. Appl. Polym. Sci. 2014, 131, 39754. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Cao, H.; Zhao, Z.; Su, C.; Wen, H. Improvement of the antifouling performance and stability of an anion exchange membrane by surface modification with graphene oxide (GO) and polydopamine (PDA). J. Membr. Sci. 2018, 566, 44–53. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Zhang, B.; Liu, J.; Zhang, H.; Song, C. Preparation and characterization of HPEI-GO/PES ultrafiltration membrane with antifouling and antibacterial properties. J. Membr. Sci. 2013, 447, 452–462. [Google Scholar] [CrossRef]
- Woong, B.P.; Parthasarathi, B.; Thanh, T.N.; Tapas, K.; Nam, H.K.; Joong, H.L. Effect of high molecular weight polyethyleneimine functionalized graphene oxide coated polyethylene terephthalate film on the hydrogen gas barrier properties. Compos. Part B Eng. 2016, 106, 316–323. [Google Scholar]
- Wenjun, H.; Baiqing, T.; Lingling, L.; Jun, S.; Lixing, D. Preparation and physico-mechanical properties of amine-functionalized graphene/polyamide 6 nanocomposite fiber as a high-performance material. RSC Adv. 2014, 4, 4848–4855. [Google Scholar]
- Yanjuan, R.; Yafei, Z.; Haichang, G.; Ruicong, L.; Shu-Lin, B. A double mixing process to greatly enhance thermal conductivity of graphene filled polyamide 6 composites. Compos. Part A Appl. Sci. Manuf. 2014, 126, 105578. [Google Scholar]
- Bortz, D.R.; Heras, E.G.; Martin-Gullon, I. Impressive fatigue life and fracture toughness improvements in graphene oxide/epoxy composites. Macromolecules 2012, 45, 238–245. [Google Scholar] [CrossRef]
- Roy, S.; Tang, X.; Das, T.; Zhang, L.; Li, Y.; Ting, S. Enhanced molecular level dispersion and interface bonding at low loading of modified graphene oxide to fabricate super nylon 12 composites. ACS Appl. Mater. Interfaces 2015, 7, 3142–3151. [Google Scholar] [CrossRef]
- Liu, H.H.; Peng, W.W.; Tan, Y.; Hou, L.C.; Zhang, X.X. Preparation and properties of polyamide 6-functionalized nanometer-Sized graphene composite fiber. In Key Engineering Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2012; Volume 519, pp. 20–23. [Google Scholar]
- Roberto, S.; Andrea, M. Optimization of two-step techniques engineered for the preparation of polyamide 6 graphene oxide nanocomposites. Compos. Part B Eng. 2019, 65, 55–64. [Google Scholar]
- Liu, H.; Hou, L.; Peng, W.; Zhang, Q.; Zhang, X. Fabrication and characterization of polyamide 6-functionalized graphene nanocomposite fiber. J. Mater. Sci. 2012, 47, 8052–8060. [Google Scholar] [CrossRef]
- Park, M.; Lee, H.; Jang, J.U.; Park, J.H.; Kim, C.H.; Kim, S.Y. Phenyl glycidyl ether as an effective noncovalent functionalization agent for multiwalled carbon nanotube reinforced polyamide 6 nanocomposite fibers. Compos. Sci. Technol. 2019, 177, 96–102. [Google Scholar] [CrossRef]




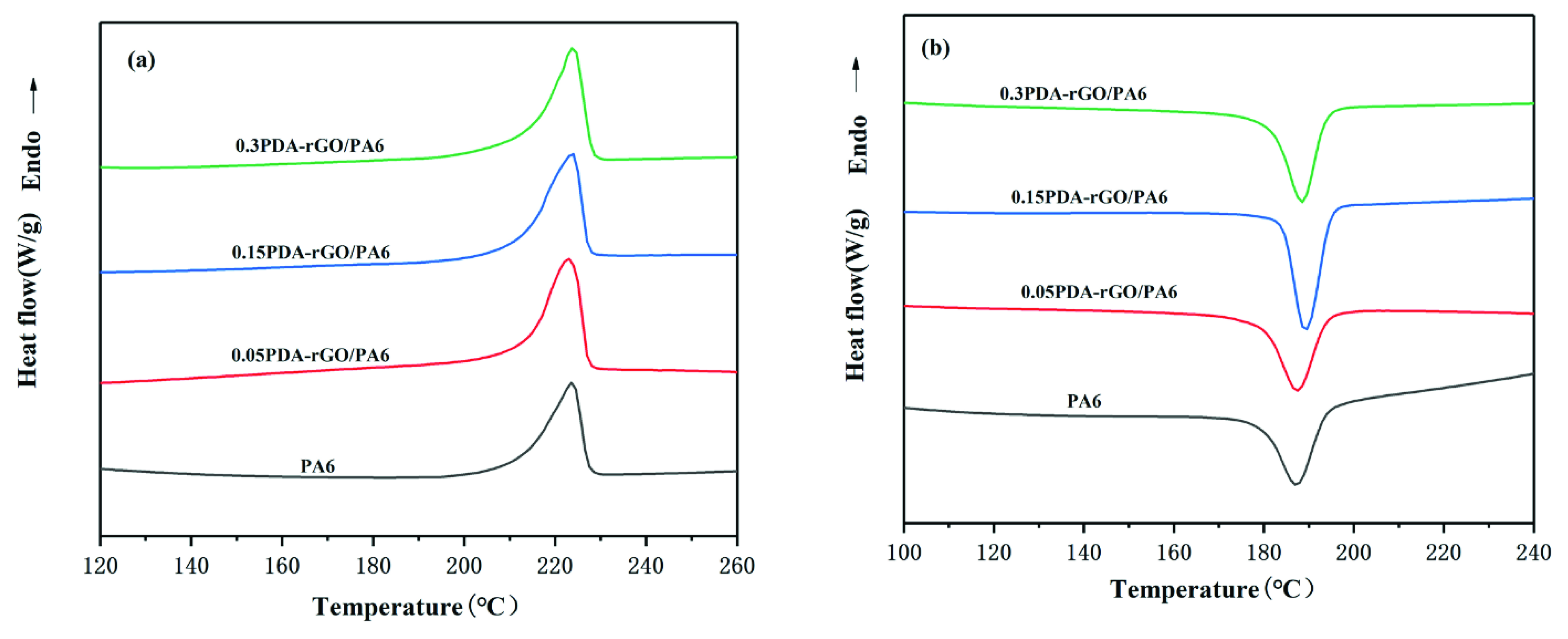
| Loading Content (wt%) | Tm (°C) | ΔHf (J/g) | Tc (°C) | Xc (%) |
|---|---|---|---|---|
| 0 | 223.6 | 54.3 | 187.4 | 28.6 |
| 0.05 PDA-rGO | 223.0 | 58.6 | 187.5 | 31 |
| 0.15 PDA-rGO | 223.8 | 63.2 | 189.2 | 33.3 |
| 0.3 PDA-rGO | 223.4 | 59.8 | 188.6 | 31.6 |
| Loading Content (wt%) | T5 (°C) | Tmax (°C) |
|---|---|---|
| 0 | 351.3 | 440.5 |
| 0.05 PDA-rGO | 355.8 | 443.8 |
| 0.15 PDA-rGO | 364.3 | 445.7 |
| 0.3 PDA-rGO | 363.9 | 442.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Meng, Y.; Zhu, F.; Su, J.; Han, J. Mechanical Reinforcement in Nylon 6 Nanocomposite Fiber Incorporated with Dopamine Reduced Graphene Oxide. Materials 2022, 15, 5095. https://doi.org/10.3390/ma15155095
Zhao Y, Meng Y, Zhu F, Su J, Han J. Mechanical Reinforcement in Nylon 6 Nanocomposite Fiber Incorporated with Dopamine Reduced Graphene Oxide. Materials. 2022; 15(15):5095. https://doi.org/10.3390/ma15155095
Chicago/Turabian StyleZhao, Yonghuan, Yang Meng, Feichao Zhu, Juanjuan Su, and Jian Han. 2022. "Mechanical Reinforcement in Nylon 6 Nanocomposite Fiber Incorporated with Dopamine Reduced Graphene Oxide" Materials 15, no. 15: 5095. https://doi.org/10.3390/ma15155095
APA StyleZhao, Y., Meng, Y., Zhu, F., Su, J., & Han, J. (2022). Mechanical Reinforcement in Nylon 6 Nanocomposite Fiber Incorporated with Dopamine Reduced Graphene Oxide. Materials, 15(15), 5095. https://doi.org/10.3390/ma15155095







