Development of Calcium Carbonate-Based Coatings by the Carbonation of Gamma-C2S (γ-C2S)
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Preparation
2.2. Test Methods
3. Results
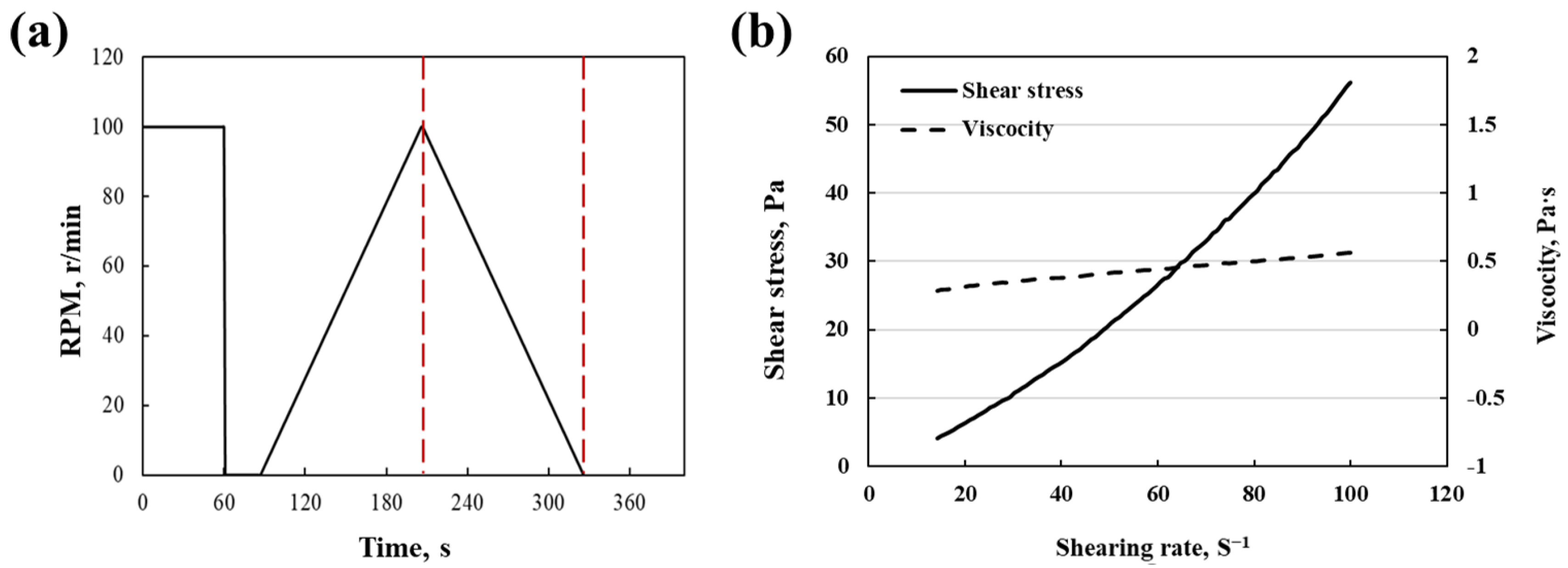
3.1. Characterization of Slurry
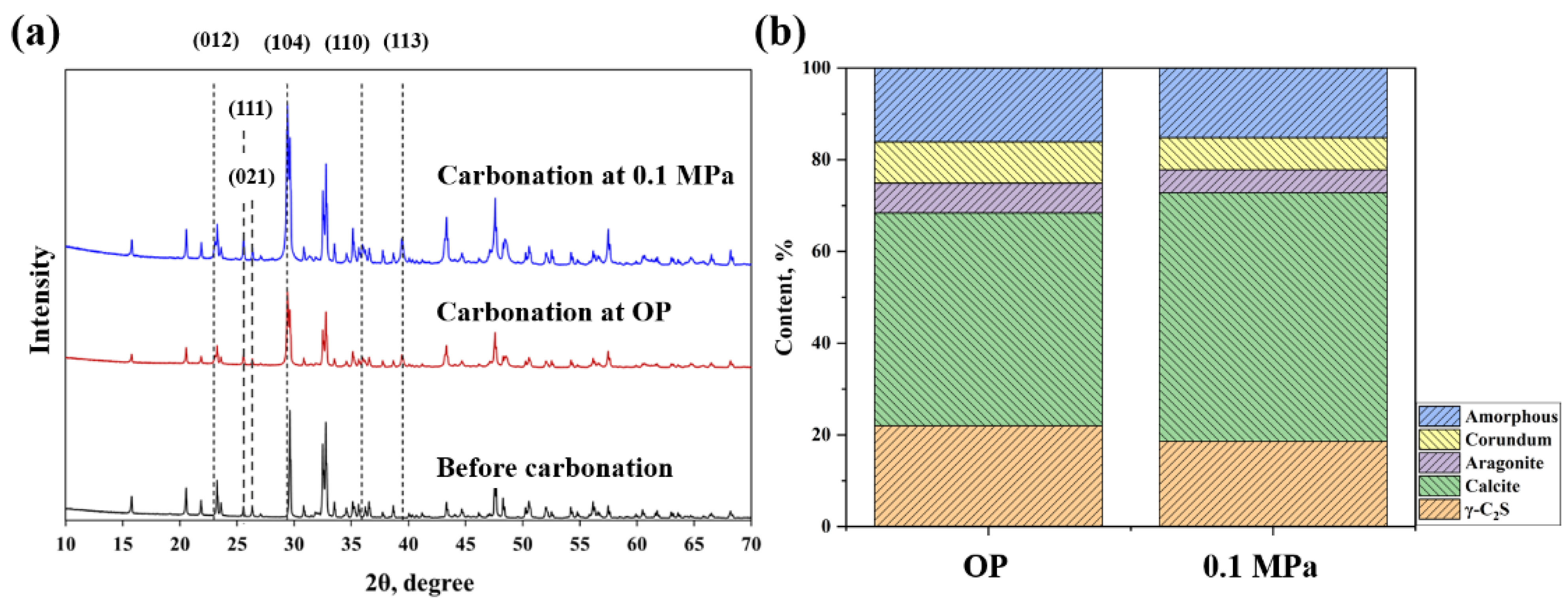
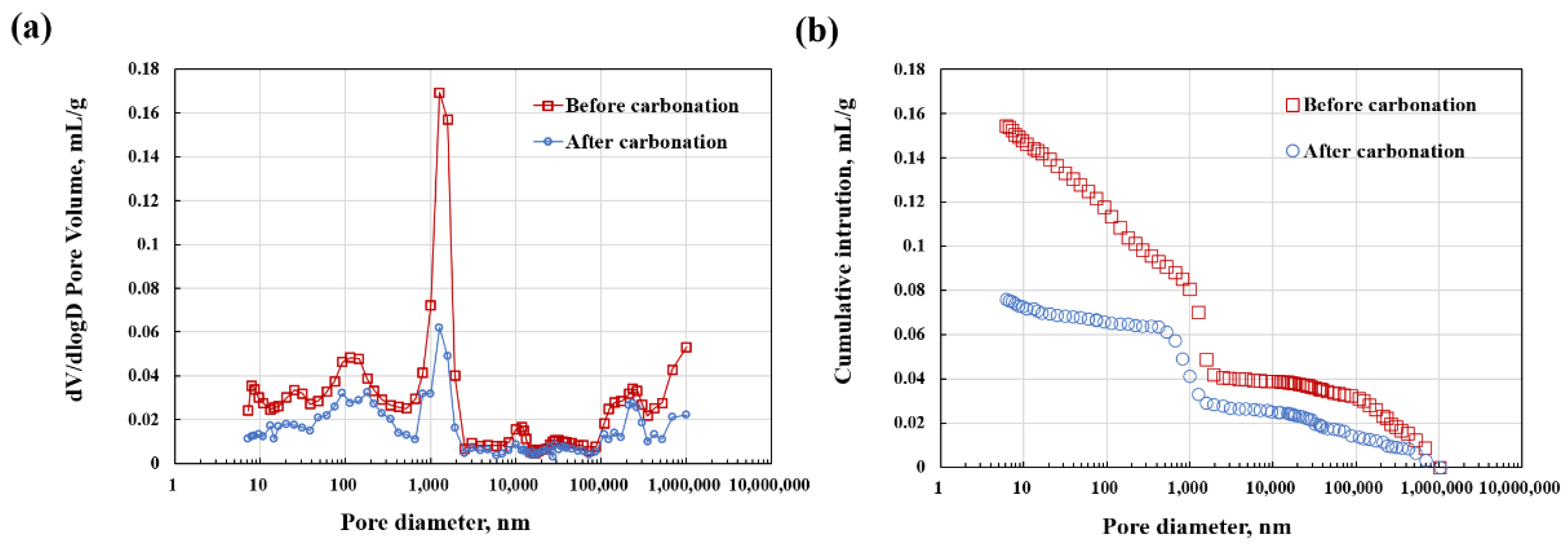
3.2. Phase Assemblage, Morphology, and Porosity
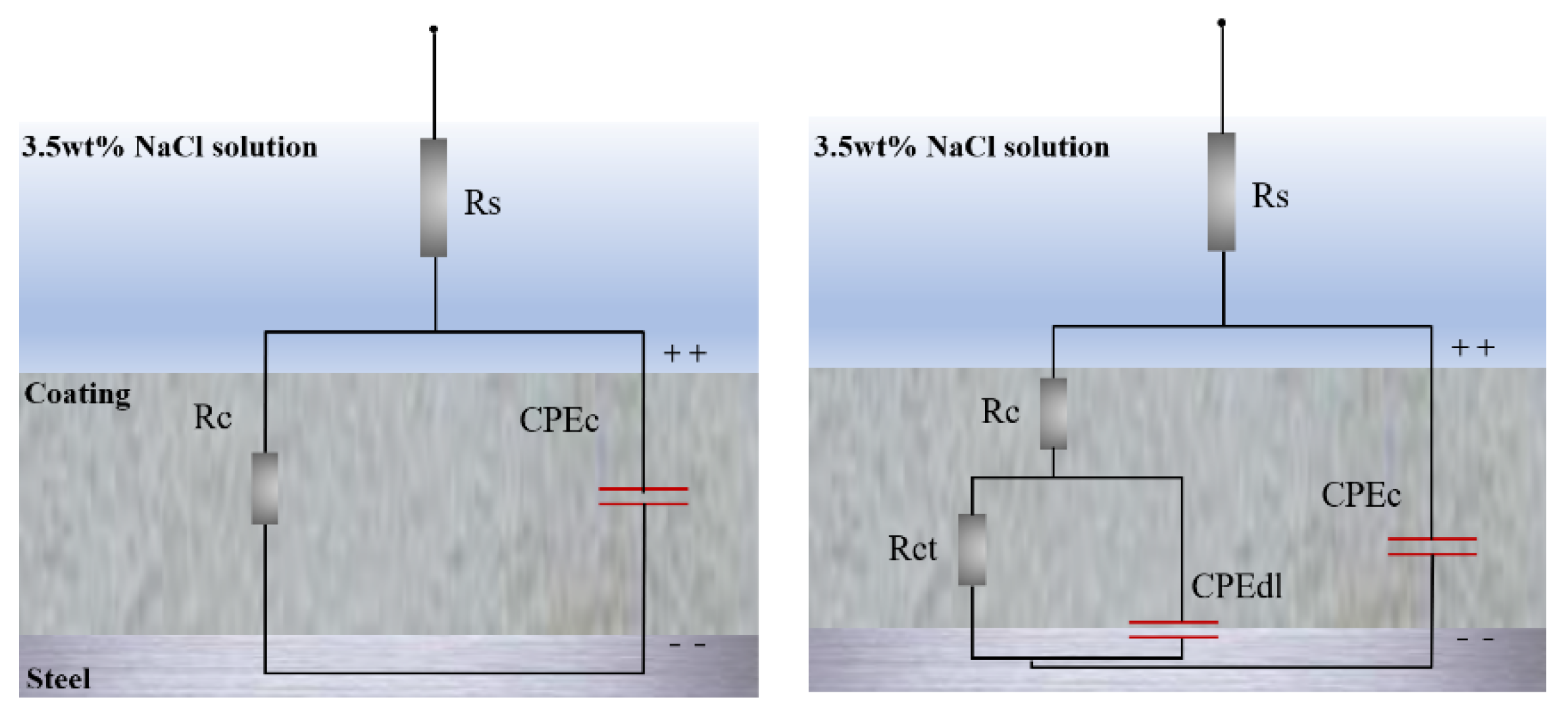
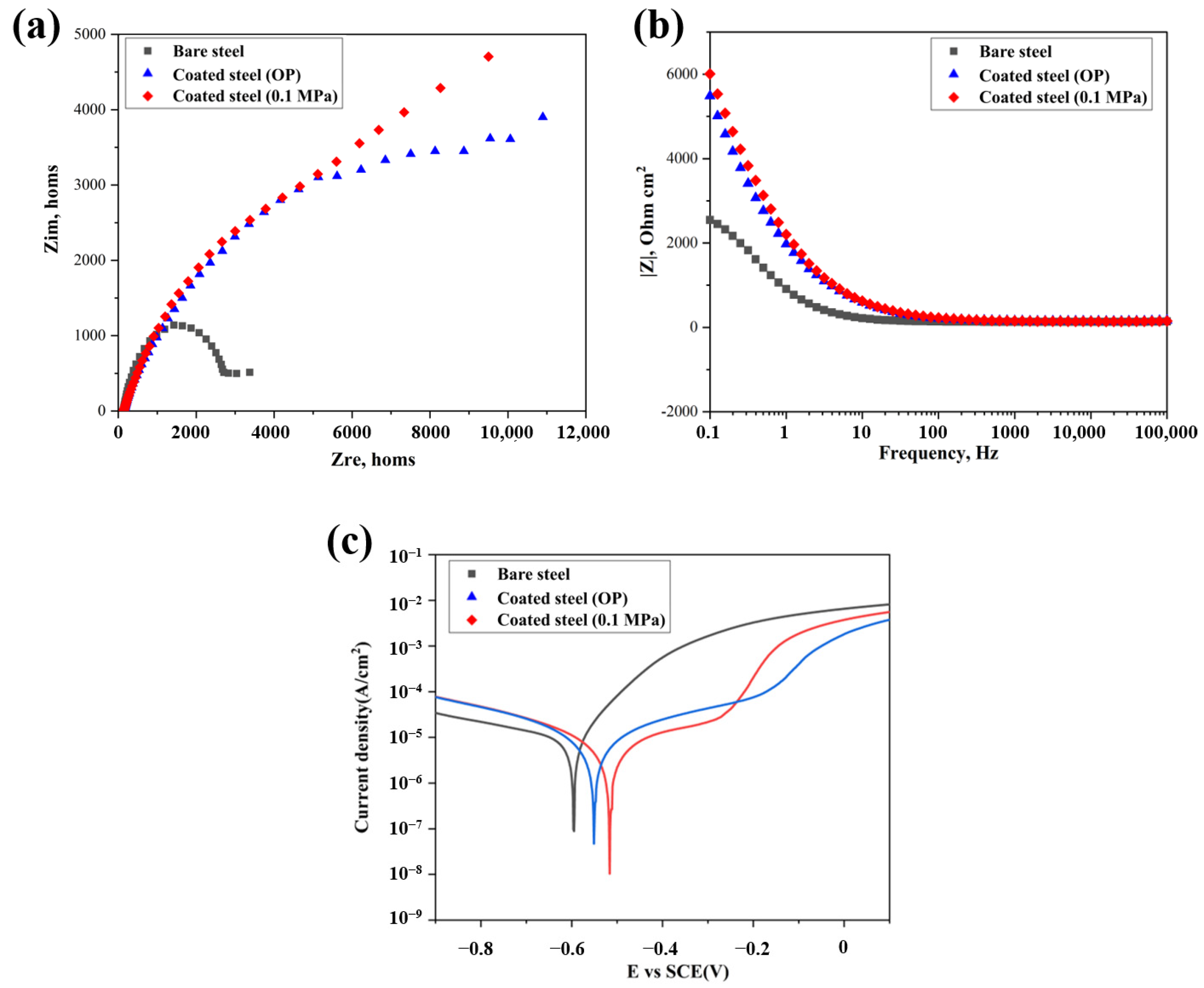
3.3. Corrosion Resistance of Coating
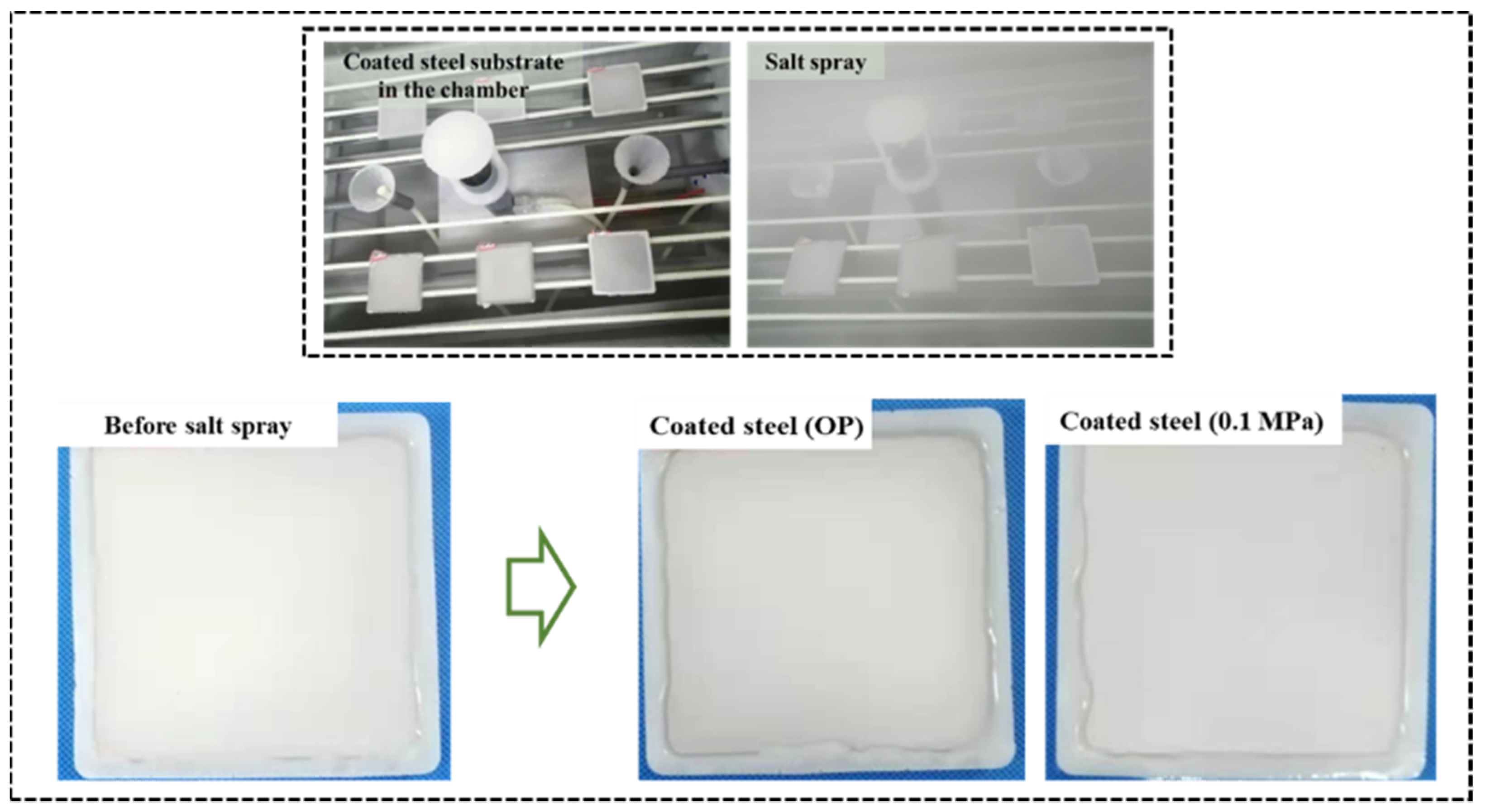
3.4. Application Prospect
4. Conclusions
- (1)
- Calcite and aragonite are the main products of γ-C2S-based coatings after carbonation.
- (2)
- The disconnection of pores is accompanied by a significant decrease in porosity after carbonation, making it difficult for corrosive substances to reach the interface between the coating and the steel substrate for erosion.
- (3)
- The corrosion resistance of the coating is significantly improved after carbonation. With the increase of carbonation pressure, the corrosion resistance of the coating is also gradually enhanced, which is mainly due to the fact that the higher CO2 pressure increases the production of calcite and aragonite to form a denser matrix, which can also be observed by XPS results.
- (4)
- The carbonated calcium carbonate-based coating has superior resistance of anti-UV aging degradation, and salt spray erosion due to the high cross linked calcium carbonate crystal and the filling effect of added silica fume.
- (5)
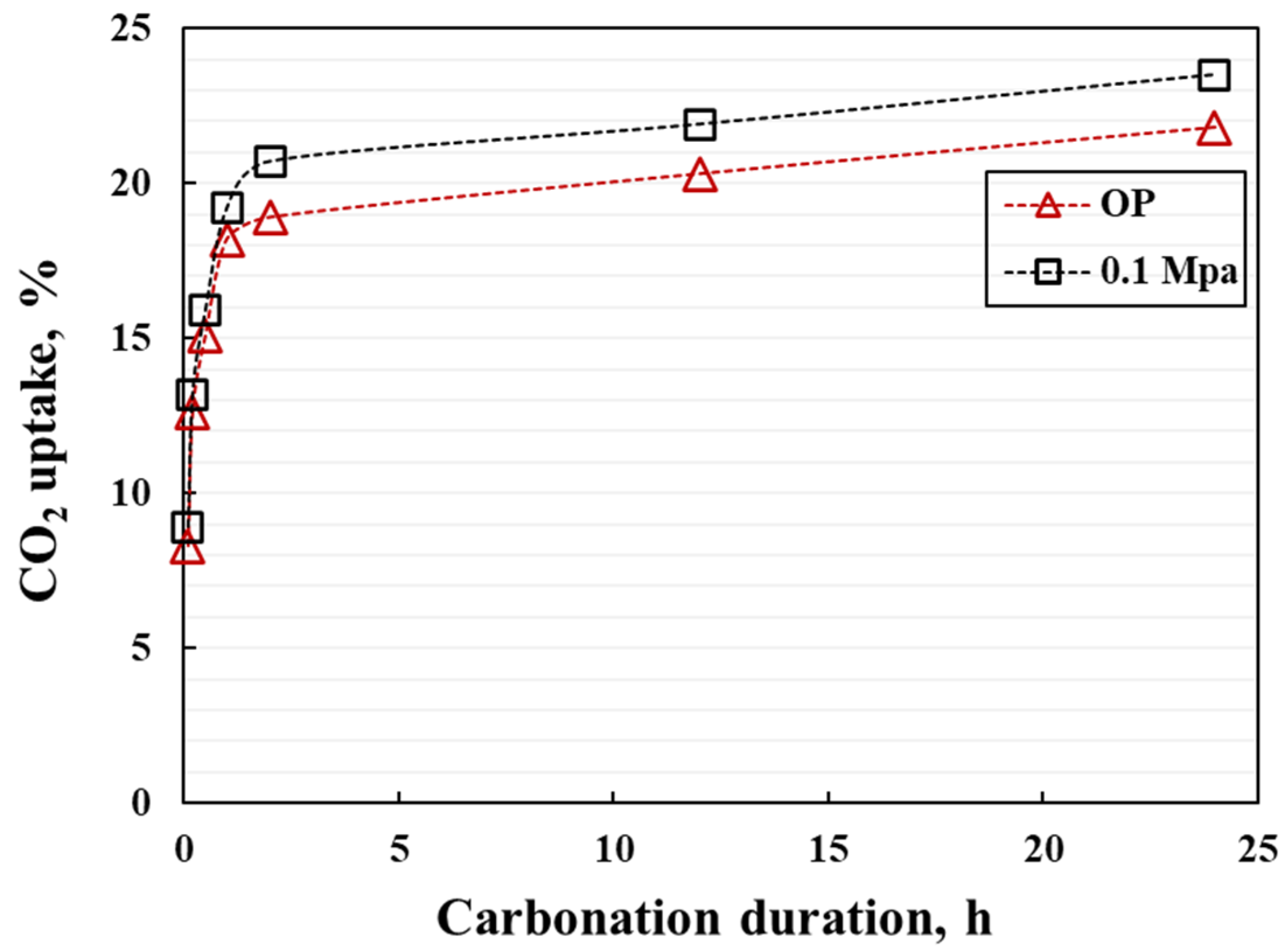
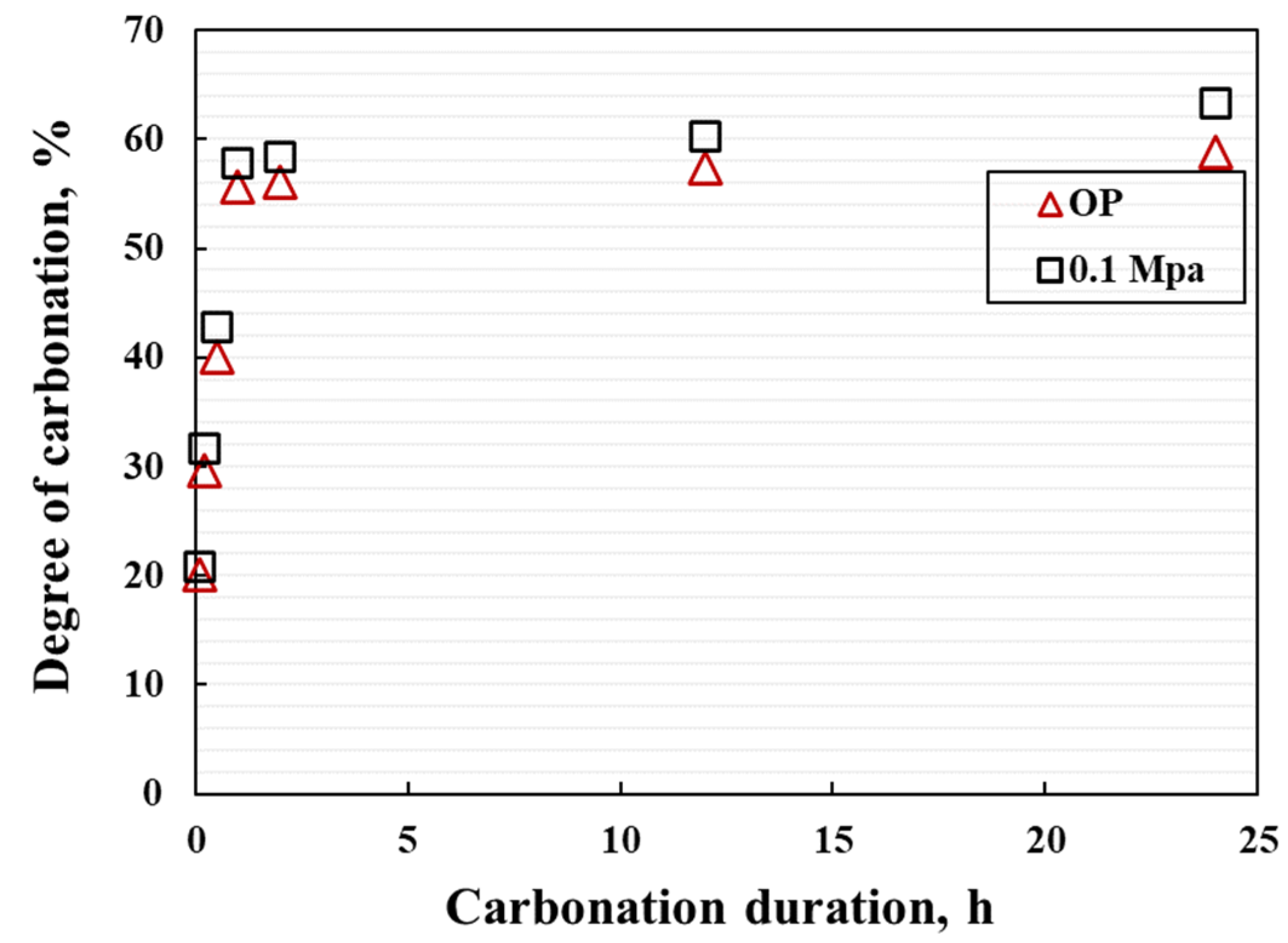
- The coatings exhibit rapid strength development, within the first 2 h of carbonation. Both the CO2 uptake efficiency and degree of carbonation (DOC) can reach more than 95% of the total CO2 uptake efficiency and final DOC values.
- (6)
- The calcium carbonate-based coating can also be compounded with organics (such as styrene–acrylic emulsion) to form a composite coating. The refined hybrid coating significantly improves its densification to further extend the application field of this inorganic coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Ye, F.; Zhang, G.; Yao, J.; Liu, Y.; Dong, S. Investigation of Erosion-Corrosion Behavior of Q235B Steel in Liquid-Solid Flows. Pet. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Lee, H.H.; Hiam, D. Corrosion Resistance of Galvannealed Steel. Corrosion 1989, 45, 852–856. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Bell, T. Structure and Corrosion Resistance of Plasma Nitrided Stainless Steel. Surf. Eng. 1985, 1, 131–136. [Google Scholar] [CrossRef]
- Stansbury, E.E.; Buchanan, R.A. Fundamentals of Electrochemical Corrosion; ASM international: Almere, The Netherlands, 2000. [Google Scholar]
- Uhlig, H.H.; Revie, R.W. Corrosion and Corrosion Control; John Wiley & Sons: Hoboken, NJ, USA, 1985. [Google Scholar]
- Hansen, D.C. Metal Corrosion in the Human Body: The Ultimate Bio-Corrosion Scenario. Electrochem. Soc. Interface 2008, 17, 31. [Google Scholar] [CrossRef]
- Iverson, W.P. Biological Corrosion. In Advances in Corrosion Science and Technology; Springer: Berlin/Heidelberg, Germany, 1972; pp. 1–42. [Google Scholar]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in Corrosion Protection by Organic Coatings: What We Know and What We Would like to Know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, O.Ø.; Forsgren, A. Corrosion Control through Organic Coatings; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- da Silva, L.R.R.; Carvalho, B.A.; Pereira, R.C.S.; Diogenes, O.B.F.; Pereira, U.C.; da Silva, K.T.; Araujo, W.S.; Mazzetto, S.E.; Lomonaco, D. Bio-Based One-Component Epoxy Resin: Novel High-Performance Anticorrosive Coating from Agro-Industrial Byproduct. Prog. Org. Coat. 2022, 167, 106861. [Google Scholar] [CrossRef]
- Zayat, M.; Garcia-Parejo, P.; Levy, D. Preventing UV-Light Damage of Light Sensitive Materials Using a Highly Protective UV-Absorbing Coating. Chem. Soc. Rev. 2007, 36, 1270–1281. [Google Scholar] [CrossRef]
- Funke, W. Problems and Progress in Organic Coatings Science and Technology. Prog. Org. Coat. 1997, 31, 5–9. [Google Scholar] [CrossRef]
- Koehler, E.L. The Mechanism of Cathodic Disbondment of Protective Organic Coatings—Aqueous Displacement at Elevated PH. Corrosion 1984, 40, 5–8. [Google Scholar] [CrossRef]
- Chen, X.; Wen, S.F.; Feng, T.; Yuan, X. High Solids Organic-Inorganic Hybrid Coatings Based on Silicone-Epoxy-Silica Coating with Improved Anticorrosion Performance for AA2024 Protection. Prog. Org. Coat. 2020, 139, 105374. [Google Scholar] [CrossRef]
- Ni, H.; Johnson, A.H.; Soucek, M.D.; Grant, J.T.; Vreugdenhil, A.J. Polyurethane/Polysiloxane Ceramer Coatings: Evaluation of Corrosion Protection. Macromol. Mater. Eng. 2002, 287, 470–479. [Google Scholar] [CrossRef]
- Broitman, E.; Gueorguiev, G.K.; Furlan, A.; Son, N.T.; Gellman, A.J.; Stafström, S.; Hultman, L. Water Adsorption on Fullerene-like Carbon Nitride Overcoats. Thin Solid Film. 2008, 517, 1106–1110. [Google Scholar] [CrossRef]
- Furlan, A.; Gueorguiev, G.K.; Czigány, Z.; Högberg, H.; Braun, S.; Stafström, S.; Hultman, L. Synthesis of Phosphorus-Carbide Thin Films by Magnetron Sputtering. Phys. Status Solidi-Rapid Res. Lett. 2008, 2, 191–193. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, D.; Liu, Z.; Li, X.; Jiang, S.; Zhang, Q. Formation Mechanisms of Environmentally Acceptable Chemical Conversion Coatings for Zinc: A Review. J. Coat. Technol. Res. 2019, 16, 1–13. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Vakili, H.; Amini, R. Improved Performance of Cerium Conversion Coatings on Steel with Zinc Phosphate Post-Treatment. J. Ind. Eng. Chem. 2015, 30, 225–233. [Google Scholar] [CrossRef]
- Bera, S.; Rout, T.K.; Udayabhanu, G.; Narayan, R. Water-Based & Eco-Friendly Epoxy-Silane Hybrid Coating for Enhanced Corrosion Protection & Adhesion on Galvanized Steel. Prog. Org. Coat. 2016, 101, 24–44. [Google Scholar]
- Liao, K.-Y.; Chang, P.-K.; Peng, Y.-N.; Yang, C.-C. A Study on Characteristics of Interfacial Transition Zone in Concrete. Cem. Concr. Res. 2004, 34, 977–989. [Google Scholar] [CrossRef]
- Wang, G.; Kong, Y.; Sun, T.; Shui, Z. Effect of Water–Binder Ratio and Fly Ash on the Homogeneity of Concrete. Constr. Build. Mater. 2013, 38, 1129–1134. [Google Scholar] [CrossRef]
- Al-Jaroudi, S.S.; Ul-Hamid, A.; Mohammed, A.-R.I.; Saner, S. Use of X-Ray Powder Diffraction for Quantitative Analysis of Carbonate Rock Reservoir Samples. Powder Technol. 2007, 175, 115–121. [Google Scholar] [CrossRef]
- Mu, Y.; Liu, Z.; Wang, F.; Huang, X. Effect of Barium Doping on Carbonation Behavior of γ-C2S. J. CO2 Util. 2018, 27, 405–413. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Z.; Mu, Y.; Wang, F.; He, Y. Effect of Chitosan on the Carbonation Behavior of γ-C2S. Cem. Concr. Compos. 2020, 111, 103637. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Z.; Wang, F.; Hu, S.; Liu, C. Effect of Extended Carbonation Curing on the Properties of γ-C2S Compacts and Its Implications on the Multi-Step Reaction Mechanism. ACS Sustain. Chem. Eng. 2021, 9, 6673–6684. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z.; Qiu, H.; Dai, Y.; Zheng, Q.; Li, J.; Yang, J. Self-Aligned Graphene as Anticorrosive Barrier in Waterborne Polyurethane Composite Coatings. J. Mater. Chem. A 2014, 2, 14139–14145. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe 2+ and Fe 3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of Multiplet Splitting of Fe 2p XPS Spectra and Bonding in Iron Compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Comparelli, R.; Fanizza, E.; Curri, M.L.; Cozzoli, P.D.; Mascolo, G.; Agostiano, A. UV-Induced Photocatalytic Degradation of Azo Dyes by Organic-Capped ZnO Nanocrystals Immobilized onto Substrates. Appl. Catal. B Environ. 2005, 60, 1–11. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S.; Fedrizzi, L.; Zanella, C. Comparison of Organic Coating Accelerated Tests and Natural Weathering Considering Meteorological Data. Prog. Org. Coat. 2007, 59, 244–250. [Google Scholar] [CrossRef]
- Mu, Y.; Liu, Z.; Wang, F. Study on Heat Resistance of Carbonated γ-C2S Binder: Strength, Phase and Microstructure Evolution. Constr. Build. Mater. 2022, 329, 127049. [Google Scholar] [CrossRef]





| Elements | Fe | C | Si | Mn | P | S | Al |
|---|---|---|---|---|---|---|---|
| wt% | 99.27 | 0.08 | 0.26 | 0.31 | 0.03 | 0.07 | 0.01 |
| Elements | SiO2 | CaO | Al2O3 | K2O | MgO | MnO | LOI |
|---|---|---|---|---|---|---|---|
| wt% | 94.58 | 0.40 | 0.52 | 1.06 | 0.65 | 0.65 | 1.70 |
| Composition | γ-C2S | SF | H2O | Chitosan | WR |
|---|---|---|---|---|---|
| Mass, g | 80 | 10 | 30 | 1.2 | 2 |
| Composition | NaCl | MgCl2·6H2O | CaCl2·2H2O | Na2SO4 | KCl | NaHCO3 |
|---|---|---|---|---|---|---|
| Concentration (g L−1) | 25.1 | 11.2 | 1.5 | 4.1 | 0.65 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, M.; Liu, Z.; Wang, F.; Hu, S. Development of Calcium Carbonate-Based Coatings by the Carbonation of Gamma-C2S (γ-C2S). Materials 2022, 15, 5088. https://doi.org/10.3390/ma15155088
Lei M, Liu Z, Wang F, Hu S. Development of Calcium Carbonate-Based Coatings by the Carbonation of Gamma-C2S (γ-C2S). Materials. 2022; 15(15):5088. https://doi.org/10.3390/ma15155088
Chicago/Turabian StyleLei, Ming, Zhichao Liu, Fazhou Wang, and Shuguang Hu. 2022. "Development of Calcium Carbonate-Based Coatings by the Carbonation of Gamma-C2S (γ-C2S)" Materials 15, no. 15: 5088. https://doi.org/10.3390/ma15155088





