Forecasting the Post-Pandemic Effects of the SARS-CoV-2 Virus Using the Bullwhip Phenomenon Alongside Use of Nanosensors for Disease Containment and Cure
Abstract
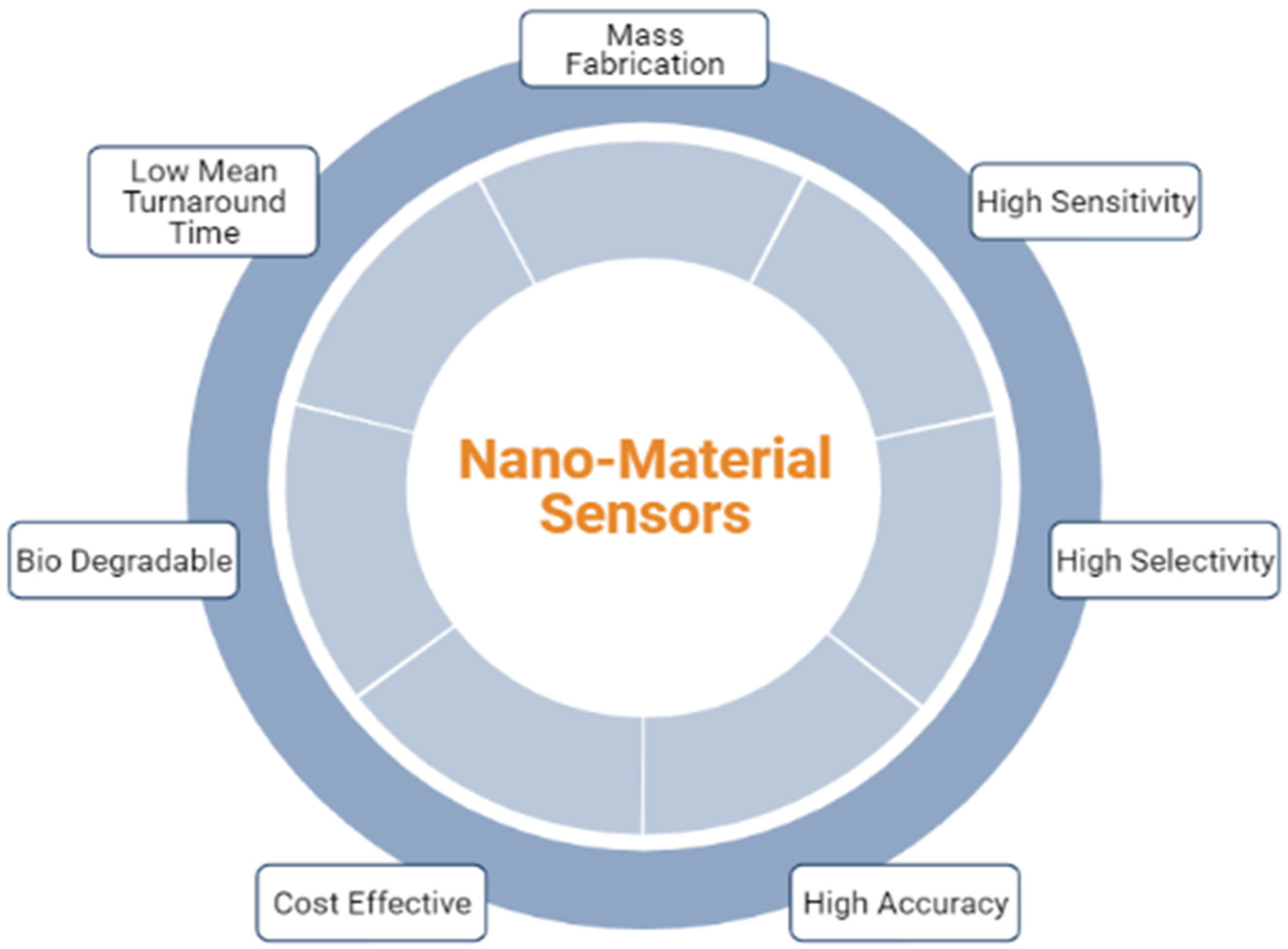
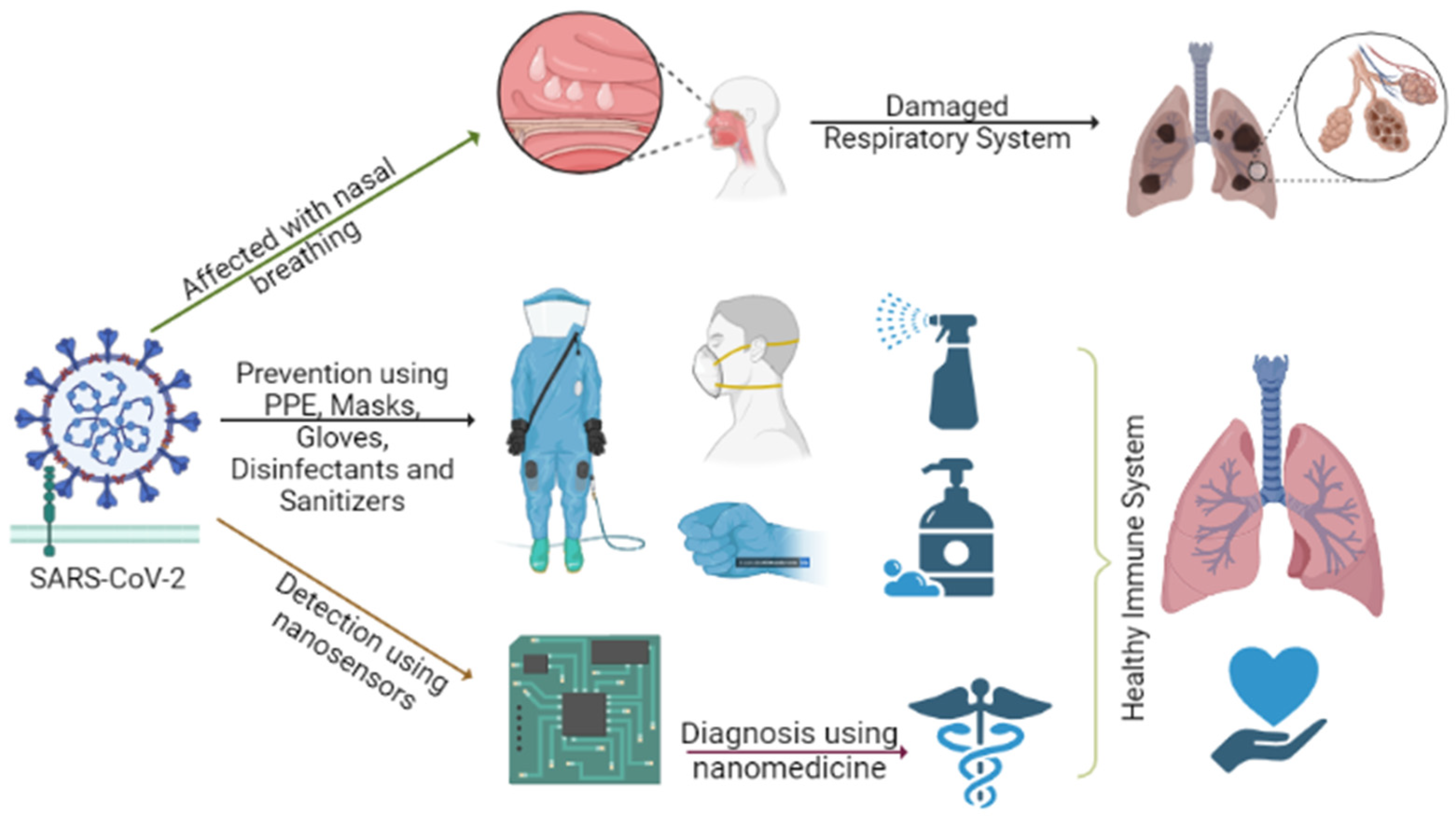
:1. Introduction
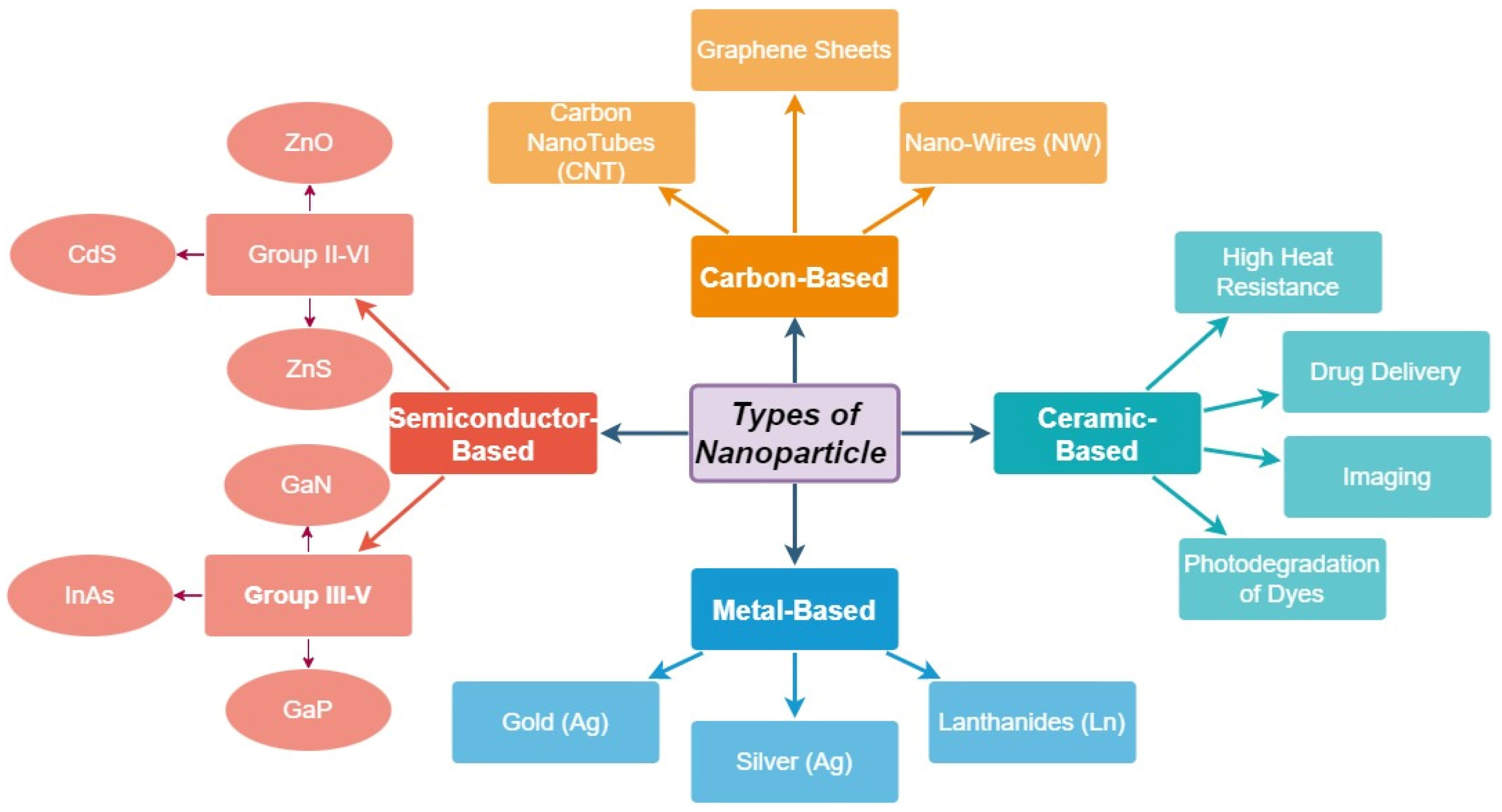
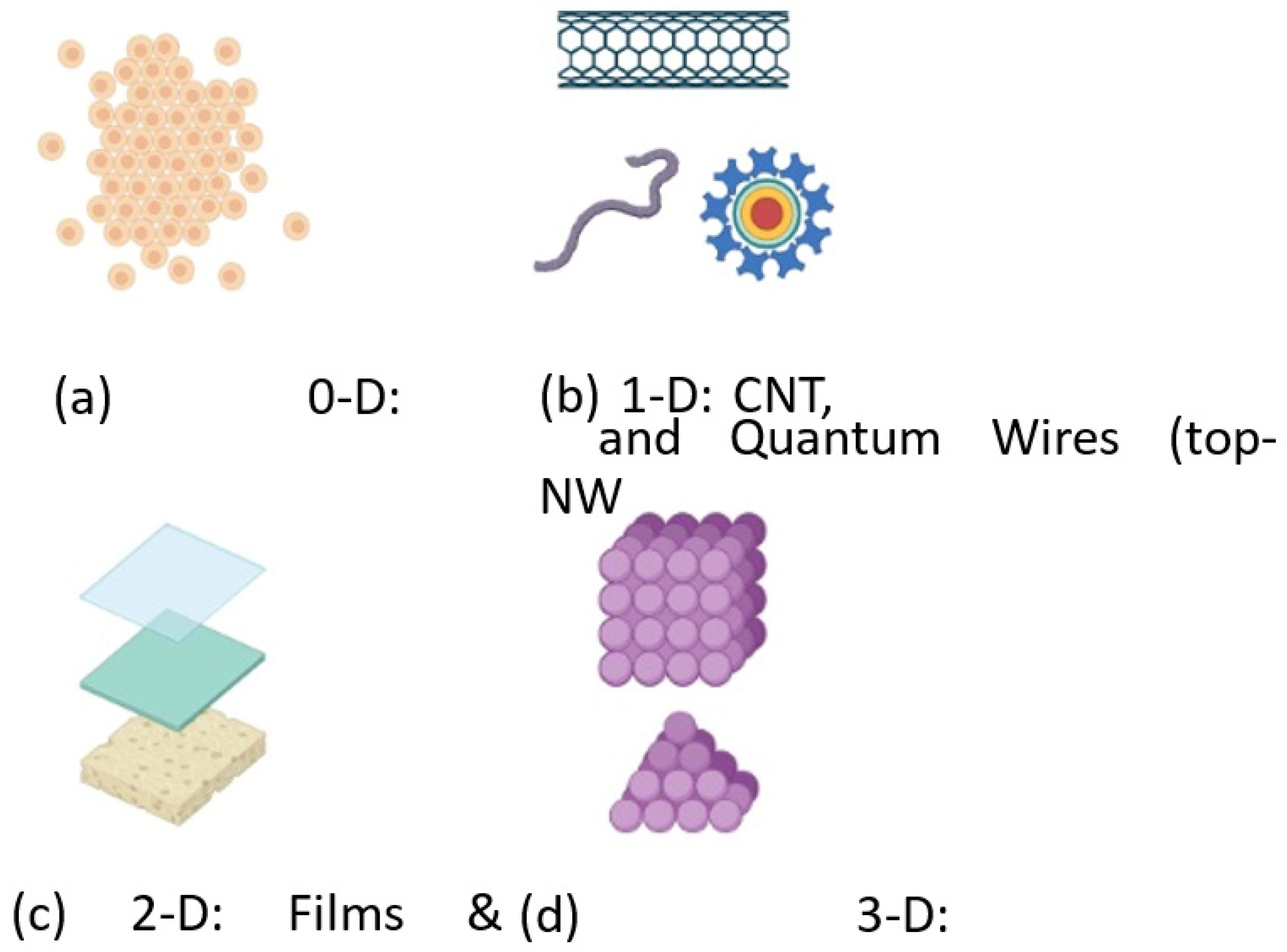
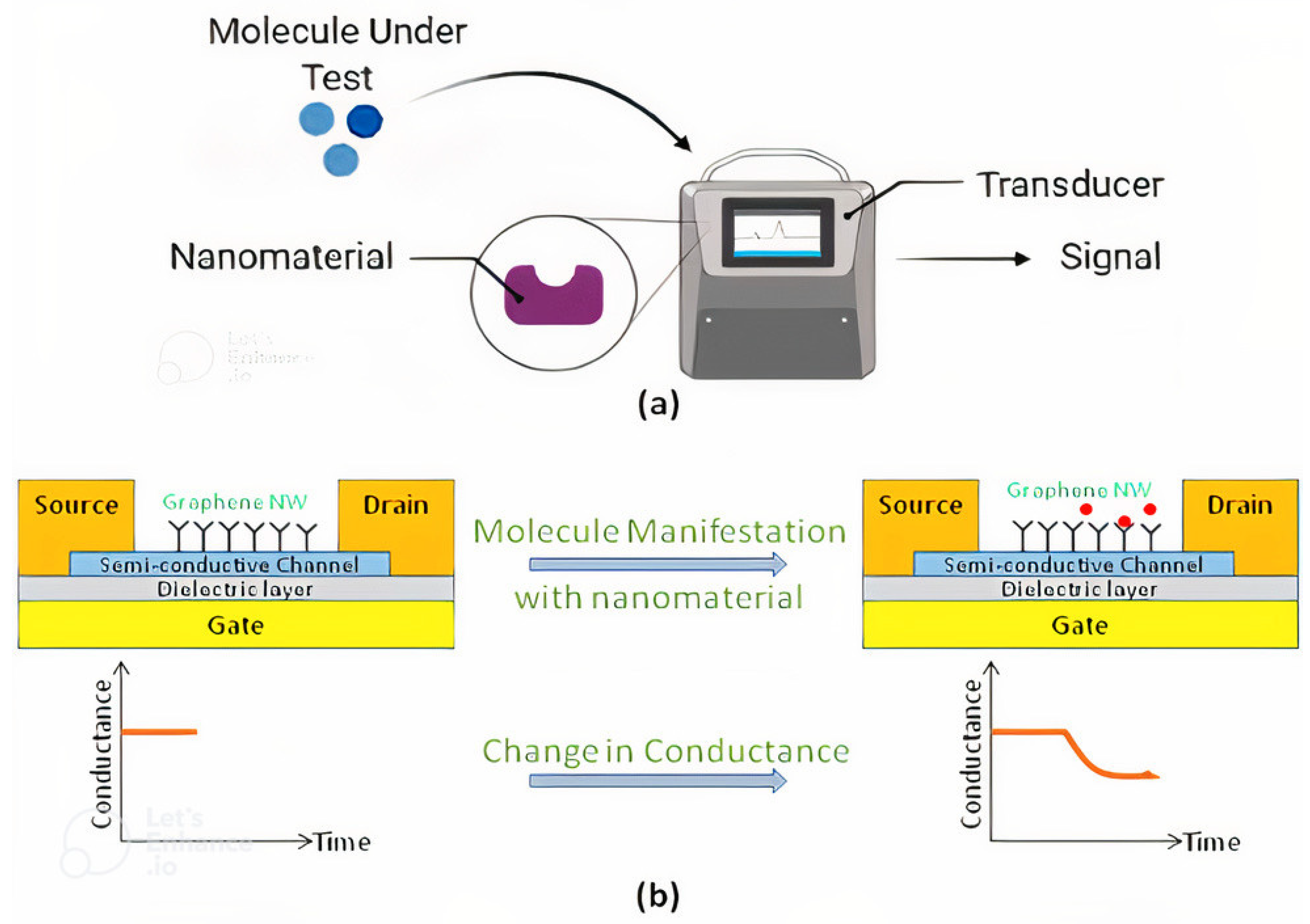
2. Fabrication of Nanomaterial Structures
2.1. Top down Approach
2.2. Bottom up Approach
3. Synergy between COVID-19 Prevention, Detection and Diagnosis Using Nanosensors
3.1. Chemical Sensor
3.2. Gas Sensor
3.3. DNA Sensor
3.4. Electro-Chemical Sensor
3.5. Optical Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, H.; Stratton, C.W.; Tang, Y.-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020, 92, 401–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coronavirus Worldometers. 2022. Available online: https://www.worldometers.info/coronavirus/ (accessed on 16 February 2022).
- Carbonell, R.; Urgelés, S.; Rodríguez, A.; Bodí, M.; Martín-Loeches, I.; Solé-Violán, J.; Díaz, E.; Gómez, J.; Trefler, S.; Vallverdú, M.; et al. Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: A multicentre retrospective cohort study. Lancet Reg. Health Eur. 2021, 11, 100243. [Google Scholar] [CrossRef] [PubMed]
- Loeffelholz, M.J.; Tang, Y.-W. Laboratory diagnosis of emerging human coronavirus infections—The state of the art. Emerg. Microbes Infect. 2020, 9, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, taaa021. [Google Scholar] [CrossRef] [Green Version]
- Love, J.; Wimmer, M.T.; Toth, D.J.A.; Chandran, A.; Makhija, D.; Cooper, C.K.; Samore, M.H.; Keegan, L.T. Comparison of antigen- and RT-PCR-based testing strategies for detection of SARS-CoV-2 in two high-exposure settings. PLoS ONE 2021, 16, e0253407. [Google Scholar] [CrossRef]
- Reich, N.; Lowe, C.F.; Puddicombe, D.; Matic, N.; Greiner, J.; Simons, J.; Leung, V.; Chu, T.; Naik, H.; Myles, N.; et al. Diagnostic accuracy of RT-PCR for detection of SARS-CoV-2 compared to a ‘composite reference standard’ in hospitalized patients. medRxiv 2021. [Google Scholar] [CrossRef]
- Brihn, A.; Chang, J.; Oyong, K.; Balter, S.; Terashita, D.; Rubin, Z.; Yeganeh, N. Diagnostic Performance of an Antigen Test with RT-PCR for the Detection of SARS-CoV-2 in a Hospital Setting—Los Angeles County, California, June–August 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 702–706. [Google Scholar] [CrossRef]
- Asif, M.; Ajmal, M.; Ashraf, G.; Muhammad, N.; Aziz, A.; Iftikhar, T.; Wang, J.; Liu, H. The role of biosensors in coronavirus disease-2019 outbreak. Curr. Opin. Electrochem. 2020, 23, 174–184. [Google Scholar] [CrossRef]
- Cai, X.; Chen, M.; Prominski, A.; Lin, Y.; Ankenbruck, N.; Rosenberg, J.; Nguyen, M.; Shi, J.; Tomatsidou, A.; Randall, G.; et al. A Multifunctional Neutralizing Antibody-Conjugated Nanoparticle Inhibits and Inactivates SARS-CoV-2. Adv. Sci. 2021, 9, 2103240. [Google Scholar] [CrossRef]
- Yayehrad, A.T.; Siraj, E.A.; Wondie, G.B.; Alemie, A.A.; Derseh, M.T.; Ambaye, A.S. Could Nanotechnology Help to End the Fight Against COVID-19? Review of Current Findings, Challenges and Future Perspectives. Int. J. Nanomed. 2021, 16, 5713–5743. [Google Scholar] [CrossRef]
- Zamzami, M.A.; Rabbani, G.; Ahmad, A.; Basalah, A.A.; Al-Sabban, W.H.; Ahn, S.N.; Choudhry, H. Carbon nanotube field-effect transistor (CNT-FET)-based biosensor for rapid detection of SARS-CoV-2 (COVID-19) surface spike protein S1. Bioelectrochemistry 2021, 143, 107982. [Google Scholar] [CrossRef] [PubMed]
- Mujawar, M.A.; Gohel, H.; Bhardwaj, S.K.; Srinivasan, S.; Hickman, N. Nano-Enabled Biosensing Systems for Intelligent Healthcare: Nano-Enabled Biosensing Systems for Intelligent Healthcare: Towards COVID-19 Management towards COVID-19 Management. Available online: https://digitalcommons.fiu.edu/covid-19_research (accessed on 4 March 2022).
- Rai, M.; Bonde, S.; Yadav, A.; Bhowmik, A.; Rathod, S.; Ingle, P.; Gade, A. Nanotechnology as a Shield against COVID-19: Current Advancement and Limitations. Viruses 2021, 13, 1224. [Google Scholar] [CrossRef] [PubMed]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draz, M.S.; Shafiee, H. Applications of gold nanoparticles in virus detection. Theranostics 2018, 8, 1985–2017. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Hosseinali, S.H.; Alizadeh, R.H.; Hasan, A.; Attar, F.; Salihi, A.; Shekha, M.S.; Amen, K.M.; Aziz, F.M.; Saboury, A.A.; et al. Plasmonic and chiroplasmonic nanobiosensors based on gold nanoparticles. Talanta 2020, 212, 120782. [Google Scholar] [CrossRef]
- Pilaquinga, F.; Morey, J.; Torres, M.; Seqqat, R.; de las Nieves Piña, M. Silver nanoparticles as a potential treatment against SARS-CoV-2: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, e1707. [Google Scholar] [CrossRef]
- Nikaeen, G.; Yousefinejad, S.; Rahmdel, S.; Samari, F.; Mahdavinia, S. Central Composite Design for Optimizing the Biosynthesis of Silver Nanoparticles using Plantago major Extract and Investigating Antibacterial, Antifungal and Antioxidant Activity. Sci. Rep. 2020, 10, 9642. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Zhai, X.; Li, Y.; Lin, L.; Zhao, H.; Bian, L.; Li, P.; Yu, L.; Wu, Y.; et al. Rapid and Sensitive Detection of anti-SARS-CoV-2 IgG, Using Lanthanide-Doped Nanoparticles-Based Lateral Flow Immunoassay. Anal. Chem. 2020, 92, 7226–7231. [Google Scholar] [CrossRef]
- Lee, T.; Ahn, J.-H.; Park, S.Y.; Kim, G.-H.; Kim, J.; Kim, T.-H.; Nam, I.; Park, C.; Lee, M.-H. Recent Advances in AIV Biosensors Composed of Nanobio Hybrid Material. Micromachines 2018, 9, 651. [Google Scholar] [CrossRef] [Green Version]
- Broza, Y.Y.; Haick, H. Nanomaterial-based sensors for detection of disease by volatile organic compounds. Nanomedicine 2013, 8, 785–806. [Google Scholar] [CrossRef]
- Bhandari, M.P.; Veliks, V.; Stonāns, I.; Padilla, M.; Šuba, O.; Svare, A.; Krupnova, I.; Ivanovs, Ņ.; Bēma, D.; Mitrovics, J.; et al. Breath Sensor Technology for the Use in Mechanical Lung Ventilation Equipment for Monitoring Critically Ill Patients. Diagnostics 2022, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-Y.; Chen, W.-C.; Tsai, R.-C. Accuracy of the Electronic Nose Breath Tests in Clinical Application: A Systematic Review and Meta-Analysis. Biosensors 2021, 11, 469. [Google Scholar] [CrossRef]
- A, B.V.; Subramoniam, M.; Mathew, L. Noninvasive detection of COPD and Lung Cancer through breath analysis using MOS Sensor array based e-nose. Expert Rev. Mol. Diagn. 2021, 21, 1223–1233. [Google Scholar] [CrossRef]
- Cruz, C.; Matatagui, D.; Ramírez, C.; Badillo-Ramirez, I.; de la O-Cuevas, E.; Saniger, J.M.; Horrillo, M.C. Carbon SH-SAW-Based Electronic Nose to Discriminate and Classify Sub-ppm NO2. Sensors 2022, 22, 1261. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kim, I.-D.; Park, H.J. 2D layered Mn and Ru oxide nanosheets for real-time breath humidity monitoring. Appl. Surf. Sci. 2021, 573, 151481. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Carbon nanomaterials to combat virus: A perspective in view of COVID-19. Carbon Trends 2020, 2, 100019. [Google Scholar] [CrossRef]
- Shan, B.; Broza, Y.Y.; Li, W.; Wang, Y.; Wu, S.; Liu, Z.; Wang, J.; Gui, S.; Wang, L.; Zhang, Z.; et al. Multiplexed Nanomaterial-Based Sensor Array for Detection of COVID-19 in Exhaled Breath. ACS Nano 2020, 14, 12125–12132. [Google Scholar] [CrossRef]
- Al-Dayyeni, W.S.; Al-Yousif, S.; Taher, M.M.; Al-Faouri, A.W.; Tahir, N.M.; Jaber, M.M.; Ghabban, F.; Najm, I.A.; Alfadli, I.M.; Ameerbakhsh, O.Z.; et al. A Review on Electronic Nose: Coherent Taxonomy, Classification, Motivations, Challenges, Recommendations and Datasets. IEEE Access 2021, 9, 88535–88551. [Google Scholar] [CrossRef]
- Nikaeen, G.; Abbaszadeh, S.; Yousefinejad, S. Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine 2020, 15, 1501–1512. [Google Scholar] [CrossRef]
- NASA’s E-Nose Device Advanced to ‘Sniff’ COVID-19 from Human Breath|NASA. Available online: https://www.nasa.gov/feature/ames/e-nose (accessed on 4 March 2022).
- Wojnowski, W.; Kalinowska, K. Machine learning and electronic noses for medical diagnostics. In Artificial Intelligence in Medicine; Springer International Publishing: New York, NY, USA, 2022; pp. 1203–1218. [Google Scholar]
- Lamote, K.; Janssens, E.; Schillebeeckx, E.; Lapperre, T.S.; De Winter, B.Y.; Van Meerbeeck, J.P. The scent of COVID-19: Viral (semi-)volatiles as fast diagnostic biomarkers? J. Breath Res. 2020, 14, 042001. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, M.; de León-Martínez, L.D.; Zamora-Mendoza, B.N.; Comas-García, A.; Palomares, S.E.G.; García-Sepúlveda, C.A.; Alcántara-Quintana, L.E.; Díaz-Barriga, F.; Flores-Ramírez, R. Comparative analysis of chemical breath-prints through olfactory technology for the discrimination between SARS-CoV-2 infected patients and controls. Clin. Chim. Acta 2021, 519, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Soto, F.; Ozen, M.O.; Guimarães, C.F.; Wang, J.; Hokanson, K.; Ahmed, R.; Reis, R.L.; Paulmurugan, R.; Demirci, U. Wearable Collector for Noninvasive Sampling of SARS-CoV-2 from Exhaled Breath for Rapid Detection. ACS Appl. Mater. Interfaces 2021, 13, 41445–41453. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef] [PubMed]
- Ilkhani, H.; Hedayat, N.; Farhad, S. Novel approaches for rapid detection of COVID-19 during the pandemic: A review. Anal. Biochem. 2021, 634, 114362. [Google Scholar] [CrossRef] [PubMed]
- Lew, T.T.S.; Aung, K.M.M.; Ow, S.Y.; Amrun, S.N.; Sutarlie, L.; Ng, L.F.P.; Su, X. Epitope-Functionalized Gold Nanoparticles for Rapid and Selective Detection of SARS-CoV-2 IgG Antibodies. ACS Nano 2021, 15, 12286–12297. [Google Scholar] [CrossRef]
- Mavrikou, S.; Moschopoulou, G.; Tsekouras, V.; Kintzios, S. Development of a Portable, Ultra-Rapid and Ultra-Sensitive Cell-Based Biosensor for the Direct Detection of the SARS-CoV-2 S1 Spike Protein Antigen. Sensors 2020, 20, 3121. [Google Scholar] [CrossRef]
- Islam, A.; Ahsan, Z. Plausible Approach for Rapid Detection of SARS-CoV-2 Virus by Magnetic Nanoparticle Based Biosensors. Am. J. Nanosci. 2020, 6, 6. [Google Scholar] [CrossRef]
- Tymm, C.; Zhou, J.; Tadimety, A.; Burklund, A.; Zhang, J.X.J. Scalable COVID-19 Detection Enabled by Lab-on-Chip Biosensors. Cell. Mol. Bioeng. 2020, 13, 313–329. [Google Scholar] [CrossRef]
- Rabiee, N.; Bagherzadeh, M.; Ghasemi, A.; Zare, H.; Ahmadi, S.; Fatahi, Y.; Dinarvand, R.; Rabiee, M.; Ramakrishna, S.; Shokouhimehr, M.; et al. Point-of-Use Rapid Detection of SARS-CoV-2: Nanotechnology-Enabled Solutions for the COVID-19 Pandemic. Int. J. Mol. Sci. 2020, 21, 5126. [Google Scholar] [CrossRef]
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef]
- Samson, R.; Navale, G.R.; Dharne, M.S. Biosensors: Frontiers in rapid detection of COVID-19. 3 Biotech 2020, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, Y.; Liu, J.; Guo, L.; Wang, Z.; Xu, X.; Song, S.; Hao, C.; Liu, L.; Xin, M.; et al. Rapid, ultrasensitive and highly specific biosensor for the diagnosis of SARS-CoV-2 in clinical blood samples. Mater. Chem. Front. 2020, 4, 2000–2005. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, H.S. Diagnostic methods and potential portable biosensors for coronavirus disease 2019. Biosens. Bioelectron. 2020, 165, 112349. [Google Scholar] [CrossRef] [PubMed]
- Drobysh, M.; Ramanaviciene, A.; Viter, R.; Chen, C.-F.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Biosensors for the Determination of SARS-CoV-2 Virus and Diagnosis of COVID-19 Infection. Int. J. Mol. Sci. 2022, 23, 666. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Vishinkin, R.; Barash, O.; Nakhleh, M.K.; Haick, H. Synergy between nanomaterials and volatile organic compounds for non-invasive medical evaluation. Chem. Soc. Rev. 2018, 47, 4781–4859. [Google Scholar] [CrossRef]
- Sharma, S.; Saini, S.; Khangembam, M.; Singh, V. Nanomaterials-Based Biosensors for COVID-19 Detection—A Review. IEEE Sens. J. 2020, 21, 5598–5611. [Google Scholar] [CrossRef]
- Rahimpour, E.; Lotfipour, F.; Jouyban, A. A minireview on nanoparticle-based sensors for the detection of coronaviruses. Bioanalysis 2021, 13, 1837–1850. [Google Scholar] [CrossRef]
- Krishnan, S.; Dusane, A.; Morajkar, R.; Venkat, A.; Vernekar, A.A. Deciphering the role of nanostructured materials in the point-of-care diagnostics for COVID-19: A comprehensive review. J. Mater. Chem. B 2021, 9, 5967–5981. [Google Scholar] [CrossRef]
- Suleman, S.; Shukla, S.K.; Malhotra, N.; Bukkitgar, S.D.; Shetti, N.P.; Pilloton, R.; Narang, J.; Tan, Y.N.; Aminabhavi, T.M. Point of care detection of COVID-19: Advancement in biosensing and diagnostic methods. Chem. Eng. J. 2021, 414, 128759. [Google Scholar] [CrossRef]
- Pradhan, A.; Lahare, P.; Sinha, P.; Singh, N.; Gupta, B.; Kuca, K.; Ghosh, K.K.; Krejcar, O. Biosensors as Nano-Analytical Tools for COVID-19 Detection. Sensors 2021, 21, 7823. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, N.; Mishra, P.; Malhotra, B.D. Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci. Total Environ. 2020, 754, 142363. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Ranjan, P.; Sanghi, S.K.; Srivastava, A.K.; Khan, R. Point-of-Care Biosensor-Based Diagnosis of COVID-19 Holds Promise to Combat Current and Future Pandemics. ACS Appl. Bio Mater. 2020, 3, 7326–7343. [Google Scholar] [CrossRef]
- Zeng, C.; Hou, X.; Bohmer, M.; Dong, Y. Advances of nanomaterials-based strategies for fighting against COVID-19. VIEW 2021, 2, 20200180. [Google Scholar] [CrossRef]
- Bhalla, N.; Pan, Y.; Yang, Z.; Payam, A.F. Opportunities and Challenges for Biosensors and Nanoscale Analytical Tools for Pandemics: COVID-19. ACS Nano 2020, 14, 7783–7807. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Soufi, G.J.; Iravani, S.; Varma, R.S. Nanomaterials and Nanotechnology-Associated Innovations against Viral Infections with a Focus on Coronaviruses. Nanomaterials 2020, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; Pereira, A.E.S.; De Oliveira, J.L.; Carvalho, L.B.; Guilger-Casagrande, M.; De Lima, R.; Fraceto, L.F. How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J. Nanobiotechnol. 2020, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.M.D.O.; Moreira, B.J.; Comparetti, E.J.; Sampaio, I.; Ferreira, L.M.B.; Lins, P.M.P.; Zucolotto, V. Is Nanotechnology Helping in the Fight Against COVID-19? Front. Nanotechnol. 2020, 2, 588915. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Kukushkina, E.A.; Hossain, S.I.; Picca, R.A.; DiTaranto, N.; Cioffi, N. Can Nanotechnology and Materials Science Help the Fight against SARS-CoV-2? Nanomaterials 2020, 10, 802. [Google Scholar] [CrossRef] [Green Version]
- Talebian, S.; Conde, J. Why Go NANO on COVID-19 Pandemic? Matter 2020, 3, 598–601. [Google Scholar] [CrossRef]
- Lete, C.; López-Iglesias, D.; García-Guzmán, J.J.; Leau, S.-A.; Stanciu, A.E.; Marin, M.; Palacios-Santander, J.M.; Lupu, S.; Cubillana-Aguilera, L. A Sensitive Electrochemical Sensor Based on Sonogel-Carbon Material Enriched with Gold Nanoparticles for Melatonin Determination. Sensors 2021, 22, 120. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Han, L.; Chen, T.; Wang, L.; Li, H.; Li, S.; He, L.; Fu, X.; Chen, S.; et al. Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosens. Bioelectron. 2020, 166, 112437. [Google Scholar] [CrossRef] [PubMed]
- Graphene QD Help Block SARS-CoV-2 Variant from Entering Cells. Available online: https://www.azonano.com/news.aspx?newsID=38758 (accessed on 27 March 2022).
- Pramanik, A.; Sharma, P.C.; Patibandla, S.; Gao, Y.; Ruppa-Kasani, V.; Goli, J.; Kumar, A.; Chatterjee, A.; Sinha, S.S.; Bates, J.T.; et al. Blocking SARS-CoV-2 Delta Variant (B.1.617.2) Spike Protein Receptor-Binding Domain Binding with the ACE2 Receptor of the Host Cell and Inhibiting Virus Infections Using Human Host Defense Peptide-Conjugated Graphene Quantum Dots. ACS Omega 2022, 7, 8150–8157. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, M.; Roy, A.R.; Wang, H.; Möckl, L.; Zeng, L.; Moerner, W.; Qi, L.S. Multi-color super-resolution imaging to study human coronavirus RNA during cellular infection. Cell Rep. Methods 2022, 2, 100170. [Google Scholar] [CrossRef] [PubMed]
- A Nanoscale Look at Coronavirus Infection. Available online: https://phys.org/news/2022-03-nanoscale-coronavirus-infection.html?fbclid=IwAR1rRHMTJl4kJ3cu8bJChY3fBi1zfSEZcTwjImx2SG_ExZFE_v0adtfObPs (accessed on 27 March 2022).
- Rosati, G.; Idili, A.; Parolo, C.; Fuentes-Chust, C.; Calucho, E.; Hu, L.; Silva, C.D.C.C.E.; Rivas, L.; Nguyen, E.P.; Bergua, J.F.; et al. Nanodiagnostics to Face SARS-CoV-2 and Future Pandemics: From an Idea to the Market and Beyond. ACS Nano 2021, 15, 17137–17149. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, S.; Davis, R.W.; Saha, A.K. Microfluidic Point-of-Care Testing: Commercial Landscape and Future Directions. Front. Bioeng. Biotechnol. 2021, 8, 602659. [Google Scholar] [CrossRef]
- Vadlamani, B.S.; Uppal, T.; Verma, S.C.; Misra, M. Functionalized TiO2 Nanotube-Based Electrochemical Biosensor for Rapid Detection of SARS-CoV-2. Sensors 2020, 20, 5871. [Google Scholar] [CrossRef]
- Bogue, R. Nanosensors: A review of recent research. Sens. Rev. 2009, 29, 310–315. [Google Scholar] [CrossRef]
- Honda, H.; Takahashi, T.; Shiiki, Y.; Zeng, H.; Nakamura, K.; Nagata, S.; Hosomi, T.; Tanaka, W.; Zhang, G.; Kanai, M.; et al. Impact of Lateral SnO2 Nanofilm Channel Geometry on a 1024 Crossbar Chemical Sensor Array. ACS Sens. 2022, 7, 460–468. [Google Scholar] [CrossRef]
- Schlicke, H.; Bittinger, S.C.; Vossmeyer, T. Lithographic Patterning and Selective Functionalization of Metal Nanoparticle Composite Films. ACS Appl. Electron. Mater. 2020, 2, 3741–3748. [Google Scholar] [CrossRef]
- Sur, U.K.; Santra, C. Spectroscopy: A versatile sensing tool for cost-effective and rapid detection of novel coronavirus (COVID-19). Emergent Mater. 2022, 5, 249–260. [Google Scholar] [CrossRef]
- Liangou, A.; Tasoglou, A.; Huber, H.J.; Wistrom, C.; Brody, K.; Menon, P.G.; Bebekoski, T.; Menschel, K.; Davidson-Fiedler, M.; DeMarco, K.; et al. A method for the identification of COVID-19 biomarkers in human breath using Proton Transfer Reaction Time-of-Flight Mass Spectrometry. eClinicalMedicine 2021, 42, 101207. [Google Scholar] [CrossRef] [PubMed]
- R., N.V.; Mohapatra, A.K.; K., U.V.; Sinha, R.K.; Nayak, R.; Kartha, V.B.; Chidangil, S. Breath analysis for the screening and diagnosis of diseases. Appl. Spectrosc. Rev. 2020, 56, 702–732. [Google Scholar] [CrossRef]
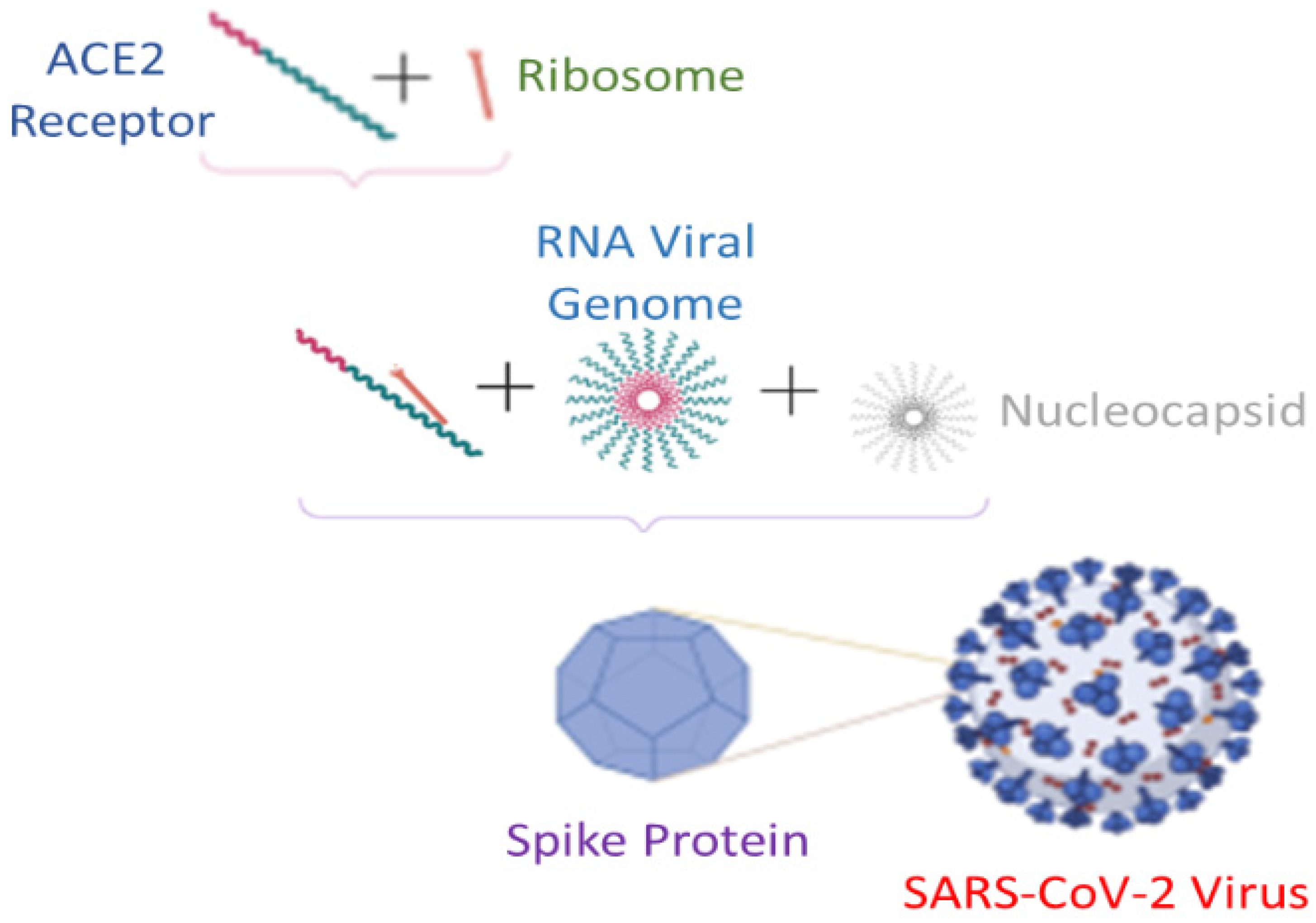
- Ye, Q.; West, A.M.V.; Silletti, S.; Corbett, K.D. Architecture and self-assembly of the SARS-CoV-2 nucleocapsid protein. bioRxiv 2020, 29, 1890–1901. [Google Scholar] [CrossRef] [PubMed]
- Understanding Variants|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/understanding-variants.html (accessed on 15 March 2022).
- Vanaparthy, R.; Mohan, G.; Vasireddy, D.; Atluri, P. Review of COVID-19 viral vector-based vaccines and COVID-19 variants. Infez. Med. 2021, 29, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Govindarajulu, M.; Parise, R.S.; Neel, L.; Shankar, T.; Patel, S.; Lowery, P.; Smith, F.; Dhanasekaran, M.; Moore, T. Emerging SARS-CoV-2 Variants: A Review of Its Mutations, Its Implications and Vaccine Efficacy. Vaccines 2021, 9, 1195. [Google Scholar] [CrossRef]
- Thye, A.Y.-K.; Law, J.W.-F.; Pusparajah, P.; Letchumanan, V.; Chan, K.-G.; Lee, L.-H. Emerging SARS-CoV-2 Variants of Concern (VOCs): An Impending Global Crisis. Biomedicines 2021, 9, 1303. [Google Scholar] [CrossRef]
- Yang, W.; Shaman, J. COVID-19 pandemic dynamics in India, the SARS-CoV-2 Delta variant and implications for vaccination. J. R. Soc. Interface 2022, 19. [Google Scholar] [CrossRef]
- Conti, P.; Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; Frydas, I.; Younes, A.; Di Emidio, P.; Tetè, G.; Pregliasco, F.; Ronconi, G. The British variant of the new coronavirus-19 (Sars-Cov-2) should not create a vaccine problem. J. Biol. Regul. Homeost. Agents 2021, 35, 1–4. [Google Scholar] [CrossRef]
- Vasireddy, D.; Vanaparthy, R.; Mohan, G.; Malayala, S.V.; Atluri, P. Review of COVID-19 Variants and COVID-19 Vaccine Efficacy: What the Clinician Should Know? J. Clin. Med. Res. 2021, 13, 317–325. [Google Scholar] [CrossRef]
- Choi, J.Y.; Smith, D.M. SARS-CoV-2 Variants of Concern. Yonsei Med J. 2021, 62, 961–968. [Google Scholar] [CrossRef]
- Liu, Y.; Shukla, D.; Newman, H.; Zhu, Y. Soft wearable sensors for monitoring symptoms of COVID-19 and other respiratory diseases: A review. Prog. Biomed. Eng. 2021, 4, 012001. [Google Scholar] [CrossRef]
- Fani, M.; Zandi, M.; Soltani, S.; Abbasi, S. Future developments in biosensors for field-ready SARS-CoV-2 virus diagnostics. Biotechnol. Appl. Biochem. 2020, 68, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Kalkal, A.; Jindal, S.; Packirisamy, G.; Manhas, S. Four electrode-based impedimetric biosensors for evaluating cytotoxicity of tamoxifen on cervical cancer cells. RSC Adv. 2021, 11, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Tisch, U.; Haick, H. Nanomaterials for cross-reactive sensor arrays. MRS Bull. 2010, 35, 797–803. [Google Scholar] [CrossRef]
- Ozer, T.; Geiss, B.J.; Henry, C.S. Review—Chemical and Biological Sensors for Viral Detection. J. Electrochem. Soc. 2019, 167, 037523. [Google Scholar] [CrossRef] [Green Version]
- Rasmi, Y.; Saloua, K.; Nemati, M.; Choi, J. Recent Progress in Nanotechnology for COVID-19 Prevention, Diagnostics and Treatment. Nanomaterials 2021, 11, 1788. [Google Scholar] [CrossRef]
- Vaquer, A.; Alba-Patiño, A.; Adrover-Jaume, C.; Russell, S.M.; Aranda, M.; Borges, M.; Mena, J.; del Castillo, A.; Socias, A.; Martín, L.; et al. Nanoparticle Transfer Biosensors for the Non-Invasive Detection of SARS-CoV-2 Antigens Trapped in Surgical Face Masks. Sens. Actuators B Chem. 2021, 345, 130347. [Google Scholar] [CrossRef]
- Talebian, S.; Wallace, G.G.; Schroeder, A.; Stellacci, F.; Conde, J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020, 15, 618–621. [Google Scholar] [CrossRef]
- New Face Mask Prototype Can Detect Covid-19 Infection. MITNews. 28 June 2021. Available online: https://news.mit.edu/2021/face-mask-covid-19-detection-0628 (accessed on 23 June 2022).
- Vibhuti; Jindal, N.; Singh, H.; Rana, P.S. Face mask detection in COVID-19: A strategic review. Multimed. Tools Appl. 2022, 1–30. [Google Scholar] [CrossRef]
- Rodelo, C.G.; Salinas, R.A.; Jaime, E.A.; Armenta, S.; Galdámez-Martínez, A.; Castillo-Blum, S.E.; la Vega, H.A.-D.; Grace, A.N.; Aguilar-Salinas, C.A.; Rodelo, J.G.; et al. Zinc associated nanomaterials and their intervention in emerging respiratory viruses: Journey to the field of biomedicine and biomaterials. Co-ord. Chem. Rev. 2022, 457, 214402. [Google Scholar] [CrossRef]
- Lukas, H.; Xu, C.; Yu, Y.; Gao, W. Emerging Telemedicine Tools for Remote COVID-19 Diagnosis, Monitoring, and Management. ACS Nano 2020, 14, 16180–16193. [Google Scholar] [CrossRef]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K.; et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Jindal, S.; Gopinath, P. Nanotechnology based approaches for combatting COVID-19 viral infection. Nano Express 2020, 1, 022003. [Google Scholar] [CrossRef]
- Toropov, N.; Osborne, E.; Joshi, L.T.; Davidson, J.; Morgan, C.; Page, J.; Pepperell, J.; Vollmer, F. SARS-CoV-2 Tests: Bridging the Gap between Laboratory Sensors and Clinical Applications. ACS Sens. 2021, 6, 2815–2837. [Google Scholar] [CrossRef] [PubMed]
- Antiochia, R. Nanobiosensors as new diagnostic tools for SARS, MERS and COVID-19: From past to perspectives. Mikrochim. Acta 2020, 187, 639. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-L.; Shang, Y.; Yan, K.-C.; Sedgwick, A.C.; Gan, H.-Q.; Chen, G.-R.; He, X.-P.; James, T.D.; Chen, D. Low-dimensional nanomaterials for antibacterial applications. J. Mater. Chem. B 2021, 9, 3640–3661. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; Gharaatifar, N.; Hasanzadeh, M.; Baradaran, B.; de la Guardia, M. Nanomaterial-based biosensors for detection of pathogenic virus. TrAC Trends Anal. Chem. 2017, 97, 445–457. [Google Scholar] [CrossRef]
- Wu, K.; Saha, R.; Su, D.; Krishna, V.D.; Liu, J.; Cheeran, M.C.-J.; Wang, J.-P. Magnetic-Nanosensor-Based Virus and Pathogen Detection Strategies before and during COVID-19. ACS Appl. Nano Mater. 2020, 3, 9560–9580. [Google Scholar] [CrossRef]
- Vaculovicova, M.; Michalek, P.; Krizkova, S.; Macka, M.; Adam, V. Nanotechnology-based analytical approaches for detection of viruses. Anal. Methods 2017, 9, 2375–2391. [Google Scholar] [CrossRef]
- Zafar, S.; D’Emic, C.; Jagtiani, A.; Kratschmer, E.; Miao, X.; Zhu, Y.; Mo, R.; Sosa, N.; Hamann, H.; Shahidi, G.; et al. Silicon Nanowire Field Effect Transistor Sensors with Minimal Sensor-to-Sensor Variations and Enhanced Sensing Characteristics. ACS Nano 2018, 12, 6577–6587. [Google Scholar] [CrossRef]
- Wu, G.; Meyyappan, M.; Lai, K.W.C. Simulation of Graphene Field-Effect Transistor Biosensors for Bacterial Detection. Sensors 2018, 18, 1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanihaichelvan, M.; Surendran, S.; Kumanan, T.; Sutharsini, U.; Ravirajan, P.; Valluvan, R.; Tharsika, T. Selective and electronic detection of COVID-19 (Coronavirus) using carbon nanotube field effect transistor-based biosensor: A proof-of-concept study. Mater. Today Proc. 2021, 49, 2546–2549. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, V.; Papi, M. Can graphene take part in the fight against COVID-19? Nano Today 2020, 33, 100883. [Google Scholar] [CrossRef]
- Ambhorkar, P.; Wang, Z.; Ko, H.; Lee, S.; Koo, K.-I.; Kim, K.; Cho, D.-I.D. Nanowire-Based Biosensors: From Growth to Applications. Micromachines 2018, 9, 679. [Google Scholar] [CrossRef] [Green Version]
- Beduk, T.; Beduk, D.; Filho, J.I.D.O.; Zihnioglu, F.; Cicek, C.; Sertoz, R.; Arda, B.; Goksel, T.; Turhan, K.; Salama, K.N.; et al. Rapid Point-of-Care COVID-19 Diagnosis with a Gold-Nanoarchitecture-Assisted Laser-Scribed Graphene Biosensor. Anal. Chem. 2021, 93, 8585–8594. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Kang, S.W.; Oh, S.; Lee, J.; Neethirajan, S. Chiral zirconium quantum dots: A new class of nanocrystals for optical detection of coronavirus. Heliyon 2018, 4, e00766. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Hao, Z.; Yu, S.; De Moraes, C.G.; Suh, L.H.; Zhao, X.; Lin, Q. An Ultraflexible and Stretchable Aptameric Graphene Nanosensor for Biomarker Detection and Monitoring. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Hash, S.; Martinez-Viedma, M.P.; Fung, F.; Han, J.E.; Yang, P.; Wong, C.; Doraisamy, L.; Menon, S.; Lightner, D. Nuclear magnetic resonance biosensor for rapid detection of Vibrio parahaemolyticus. Biomed. J. 2019, 42, 187–192. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Z.; Lei, C.; Lei, J.; Zhou, Y. An integrated giant magnetoimpedance biosensor for detection of biomarker. Biosens. Bioelectron. 2014, 58, 338–344. [Google Scholar] [CrossRef]
- Erdem, Ö.; Derin, E.; Sagdic, K.; Yilmaz, E.G.; Inci, F. Smart materials-integrated sensor technologies for COVID-19 diagnosis. Emergent Mater. 2021, 4, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, S.; Nikaeen, G.; Yousefinejad, S. Carbon nanomaterials as promising substrates in the design of sensors for SARS-CoV-2 and new emerging viral infections. Nanomedicine 2021, 16, 2033–2037. [Google Scholar] [CrossRef] [PubMed]
- Nekoeinia, M.; Salehriahi, F.; Moradlou, O.; Kazemi, H.; Yousefinejad, S. Enhanced Fenton-like catalytic performance of N-doped graphene quantum dot incorporated CuCo2O4. New J. Chem. 2018, 42, 9209–9220. [Google Scholar] [CrossRef]
- Skotadis, E.; Kanaris, A.; Aslanidis, E.; Kalatzis, N.; Chatzipapadopoulos, F.; Marianos, N.; Tsoukalas, D. Identification of Two Commercial Pesticides by a Nanoparticle Gas-Sensing Array. Sensors 2021, 21, 5803. [Google Scholar] [CrossRef]
- Drmosh, Q.; Al-Muhaish, N.; Al Wajih, Y.A.; Alam, M.W.; Yamani, Z. Surface composite and morphology tuning of tungsten oxide thin films for acetone gas sensing. Chem. Phys. Lett. 2021, 776, 138659. [Google Scholar] [CrossRef]
- Kwiatkowski, A.; Drozdowska, K.; Smulko, J. Embedded gas sensing setup for air samples analysis. Rev. Sci. Instrum. 2021, 92, 074102. [Google Scholar] [CrossRef] [PubMed]
- Rascha, F.; Posticab, V.; Schütta, F.; Mishra, Y.K.; Nia, A.S.; Lohe, M.R.; Fengd, X.; Adelunga, R.; Lupanab, O. Highly selective and ultra-low power consumption metal oxide based hydrogen gas sensor employing graphene oxide as molecular sieve. Sens. Actuators B Chem. 2020, 320, 128363. [Google Scholar] [CrossRef]
- Park, S.-H.; An, S.-Y.; Ko, H.-S.; Jin, C.-H.; Lee, C.-M. Enhanced Gas Sensing Properties of Bi2O3-Core/In2O3-Shell Nanorod Gas Sensors. Bull. Korean Chem. Soc. 2012, 33, 3368–3372. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Tabata, H. Gas Sensor Array Using a Hybrid Structure Based on Zeolite and Oxide Semiconductors for Multiple Bio-Gas Detection. ACS Omega 2021, 6, 21284–21293. [Google Scholar] [CrossRef]
- Huffman, J.A.; Perring, A.E.; Savage, N.J.; Clot, B.; Crouzy, B.; Tummon, F.; Shoshanim, O.; Damit, B.; Schneider, J.; Sivaprakasam, V.; et al. Real-time sensing of bioaerosols: Review and current perspectives. Aerosol Sci. Technol. 2019, 54, 465–495. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.H.; Kim, K.; An, T.; Choi, W.; Lim, G. Reliable Diameter Control of Carbon Nanotube Nanobundles Using Withdrawal Velocity. Nanoscale Res. Lett. 2016, 11, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokkinos, C. Electrochemical DNA Biosensors Based on Labeling with Nanoparticles. Nanomaterials 2019, 9, 1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soler, M.; Huertas, C.S.; Lechuga, L.M. Label-free plasmonic biosensors for point-of-care diagnostics: A review. Expert Rev. Mol. Diagn. 2019, 19, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kampeera, J.; Pasakon, P.; Karuwan, C.; Arunrut, N.; Sappat, A.; Sirithammajak, S.; Dechokiattawan, N.; Sumranwanich, T.; Chaivisuthangkura, P.; Ounjai, P.; et al. Point-of-care rapid detection of Vibrio parahaemolyticus in seafood using loop-mediated isothermal amplification and graphene-based screen-printed electrochemical sensor. Biosens. Bioelectron. 2019, 132, 271–278. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef] [Green Version]
- Tamersit, K.; Djeffal, F. Carbon Nanotube Field-Effect Transistor with Vacuum Gate Dielectric for Label-Free Detection of DNA Molecules: A Computational Investigation. IEEE Sens. J. 2019, 19, 9263–9270. [Google Scholar] [CrossRef]
- Shim, B.S.; Chen, W.; Doty, C.; Xu, C.; Kotov, N.A. Smart Electronic Yarns and Wearable Fabrics for Human Biomonitoring made by Carbon Nanotube Coating with Polyelectrolytes. Nano Lett. 2008, 8, 4151–4157. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, L.; Xu, C. Carbon nanotubes in biomedicine and biosensing. In Carbon Nanotubes; Naraghi, M., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, S.A.; Behbahan, N.G.G.; Bahrani, S.; Mousavi, S.M.; Gholami, A.; Ramakrishna, S.; Firoozsani, M.; Moghadami, M.; Lankarani, K.B.; Omidifar, N. Ultra-sensitive viral glycoprotein detection NanoSystem toward accurate tracing SARS-CoV-2 in biological/non-biological media. Biosens. Bioelectron. 2020, 171, 112731. [Google Scholar] [CrossRef]
- Gong, F.; Wei, H.-X.; Qi, J.; Ma, H.; Liu, L.; Weng, J.; Zheng, X.; Li, Q.; Zhao, D.; Fang, H.; et al. Pulling-Force Spinning Top for Serum Separation Combined with Paper-Based Microfluidic Devices in COVID-19 ELISA Diagnosis. ACS Sensors 2021, 6, 2709–2719. [Google Scholar] [CrossRef]
- Chandra, P. Miniaturized label-free smartphone assisted electrochemical sensing approach for personalized COVID-19 diagnosis. Sens. Int. 2020, 1, 100019. [Google Scholar] [CrossRef]
- Gupta, R.; Poonam, S.; Nitesh, P.; Sunaina, K.; Rajat, S.; Vikas, R.; Nitin, K. Nanotechnology-Based Approaches for the De-tection of SARS-CoV-2. Front. Nanotechnol. 2020, 2, 6. [Google Scholar] [CrossRef]
- Saylan, Y.; Erdem, Ö.; Ünal, S.; Denizli, A. An Alternative Medical Diagnosis Method: Biosensors for Virus Detection. Biosensors 2019, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Sharma, N.; Kumari, A.; Lee, H.-J.; Kim, T.; Tripathi, K.M. Nano-carbon based sensors for bacterial detection and discrimination in clinical diagnosis: A junction between material science and biology. Appl. Mater. Today 2019, 18, 100467. [Google Scholar] [CrossRef]
- Iravani, S. Nano- and biosensors for the detection of SARS-CoV-2: Challenges and opportunities. Mater. Adv. 2020, 1, 3092–3103. [Google Scholar] [CrossRef]
- Russell, S.M.; Alba-Patiño, A.; Barón, E.; Borges, M.; Gonzalez-Freire, M.; De La Rica, R. Biosensors for Managing the COVID-19 Cytokine Storm: Challenges Ahead. ACS Sens. 2020, 5, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Sagor, R.H.; Tathfif, I.; Rashid, K.S.; Radoan, M. An Optimized Dielectric-Metal-Dielectric Refractive Index Nanosensor. IEEE Sens. J. 2020, 21, 1461–1469. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, M.S.; Abbas, M.; Abdulmuqeet, M.; Alqahtani, A.S.; Alshahrani, M.Y.; Alsabaani, A.; Ramalingam, M. Forecasting the Post-Pandemic Effects of the SARS-CoV-2 Virus Using the Bullwhip Phenomenon Alongside Use of Nanosensors for Disease Containment and Cure. Materials 2022, 15, 5078. https://doi.org/10.3390/ma15145078
Alqahtani MS, Abbas M, Abdulmuqeet M, Alqahtani AS, Alshahrani MY, Alsabaani A, Ramalingam M. Forecasting the Post-Pandemic Effects of the SARS-CoV-2 Virus Using the Bullwhip Phenomenon Alongside Use of Nanosensors for Disease Containment and Cure. Materials. 2022; 15(14):5078. https://doi.org/10.3390/ma15145078
Chicago/Turabian StyleAlqahtani, Mohammed S., Mohamed Abbas, Mohammed Abdulmuqeet, Abdullah S. Alqahtani, Mohammad Y. Alshahrani, Abdullah Alsabaani, and Murugan Ramalingam. 2022. "Forecasting the Post-Pandemic Effects of the SARS-CoV-2 Virus Using the Bullwhip Phenomenon Alongside Use of Nanosensors for Disease Containment and Cure" Materials 15, no. 14: 5078. https://doi.org/10.3390/ma15145078
APA StyleAlqahtani, M. S., Abbas, M., Abdulmuqeet, M., Alqahtani, A. S., Alshahrani, M. Y., Alsabaani, A., & Ramalingam, M. (2022). Forecasting the Post-Pandemic Effects of the SARS-CoV-2 Virus Using the Bullwhip Phenomenon Alongside Use of Nanosensors for Disease Containment and Cure. Materials, 15(14), 5078. https://doi.org/10.3390/ma15145078







