Selective Oxidation of Cellulose—A Multitask Platform with Significant Environmental Impact
Abstract
1. Introduction
2. Selective Oxidation of Cellulose by Different Methods
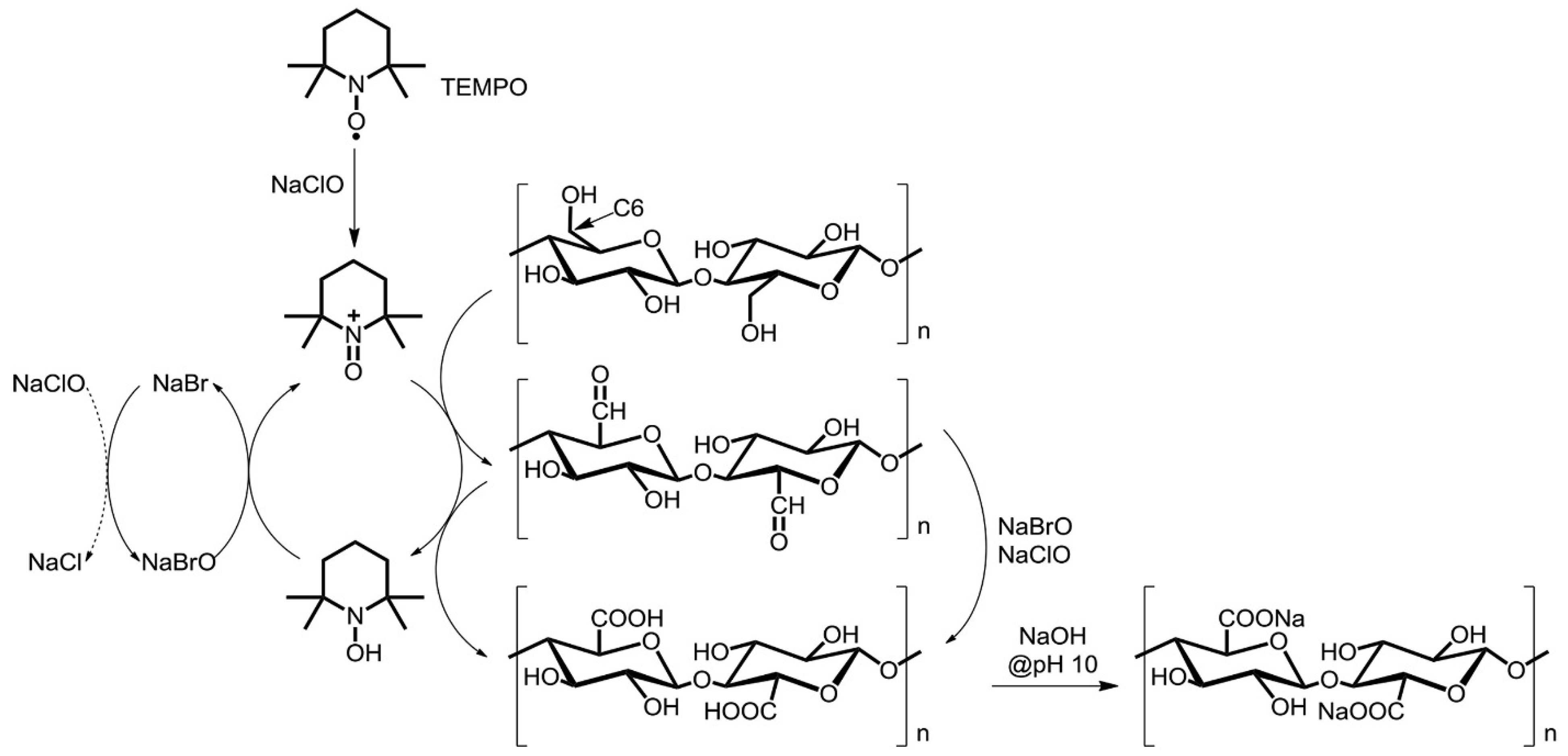
2.1. TEMPO-Assisted Oxidation (Stable Nitroxyl Radicals)
2.2. Non-Persistent (Transient) Nitroxyl Radicals, Reactive Intermediaries for the Selective Oxidation of Cellulose
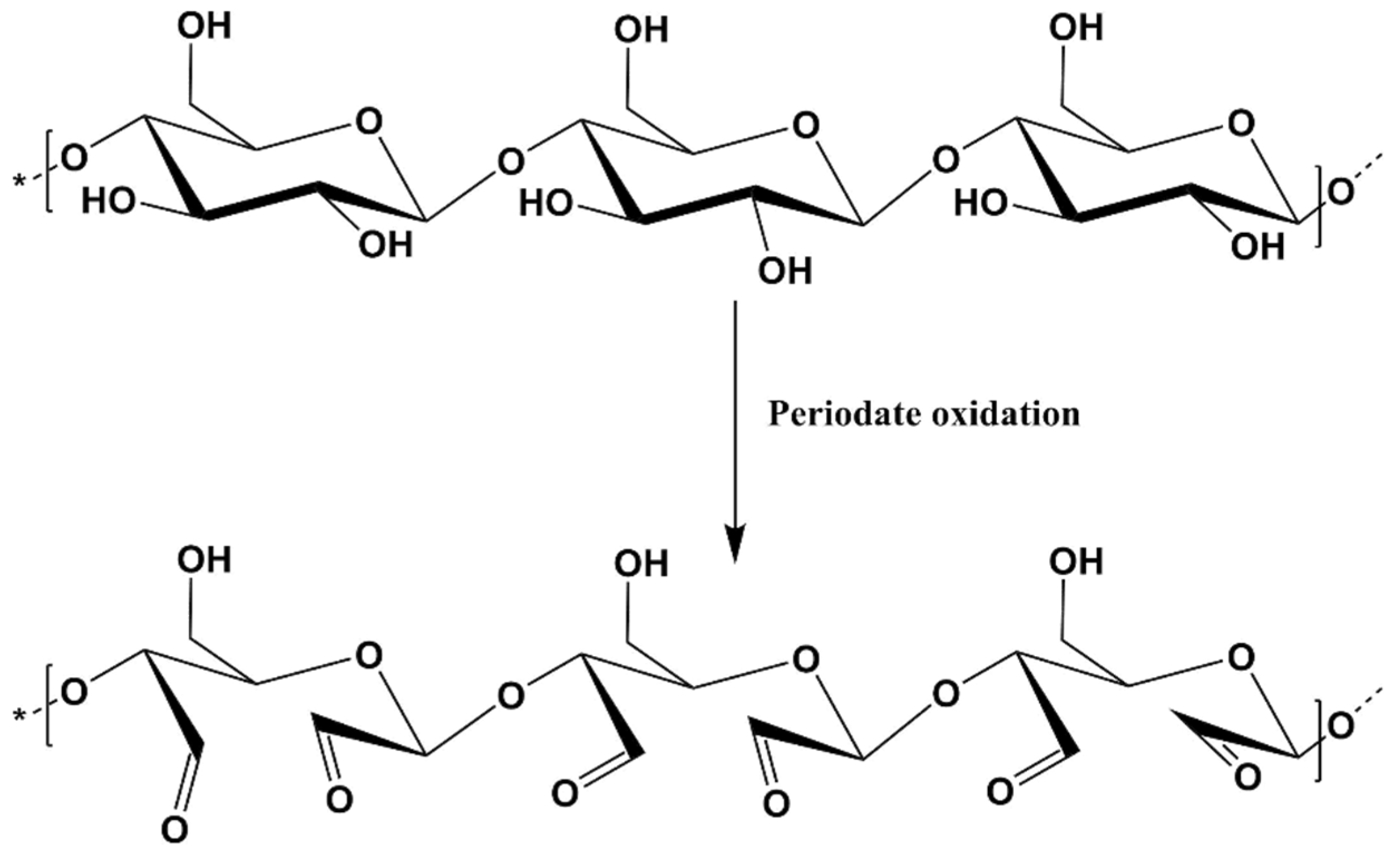
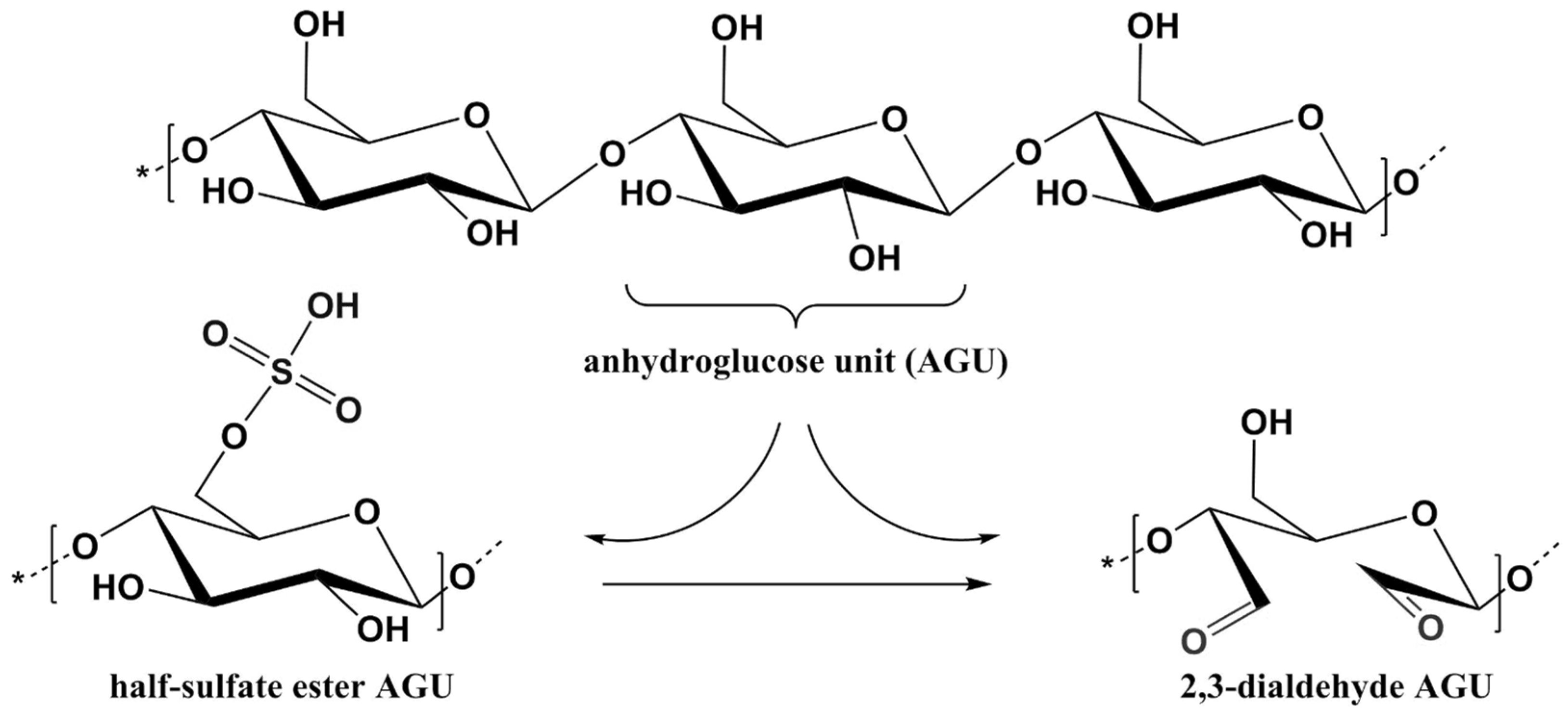
2.3. Cellulose Selective Oxidation in the Presence of Sodium Periodate
2.4. Miscellaneous
2.4.1. The TEMPO-Periodate Approach
2.4.2. The Periodate-Chlorite Method
2.4.3. Other Methods
3. Environmental Applications of Materials Based on Selectively Oxidized Cellulose
3.1. General Considerations
3.2. Main Characteristics of Selectively Oxidized Cellulose
3.3. Sorbents for the Removal of Heavy Metals
3.4. Adsorbent Materials for Dyes
3.5. Other Organic Pollutants
3.6. Other Applications
4. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Corsi, I.; Winther-Nielsen, M.; Sethi, R.; Punta, C.; Della Torre, C.; Libralato, G.; Lofrano, G.; Sabatini, L.; Aiello, M.; Fiordi, L.; et al. Ecofriendly nanotechnologies and nanomaterials for environmental applications: Key issue and consensus recommendations for sustainable and ecosafe nanoremediation. Ecotoxicol. Environ. Saf. 2018, 154, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Guidi, P.; Bernardeschi, M.; Palumbo, M.; Genovese, M.; Scarcelli, V.; Fiorati, A.; Riva, L.; Punta, C.; Corsi, I.; Frenzilli, G. Suitability of a Cellulose-Based Nanomaterial for the Remediation of Heavy Metal Contaminated Freshwaters: A Case-Study Showing the Recovery of Cadmium Induced DNA Integrity Loss, Cell Proliferation Increase, Nuclear Morphology and Chromosomal Alterations on Dreissena polymorpha. Nanomaterials 2020, 10, 1837. [Google Scholar] [CrossRef]
- Punta, C.; Fiorati, A.; Riva, L.; Grassi, G.; Liberatori, G.; Corsi, I. Sustainable and Eco-safe Nanocellulose-based Materials for Water Nano-treatment. Sustain. Nanotechnol. 2022, 143–158. [Google Scholar] [CrossRef]
- Farhaoui, M.; Derraz, M.; Farhaoui, M.; Derraz, M. Review on Optimization of Drinking Water Treatment Process. J. Water Resour. Prot. 2016, 8, 777–786. [Google Scholar] [CrossRef]
- Teodosiu, C.; Gilca, A.F.; Barjoveanu, G.; Fiore, S. Emerging pollutants removal through advanced drinking water treatment: A review on processes and environmental performances assessment. J. Clean. Prod. 2018, 197, 1210–1221. [Google Scholar] [CrossRef]
- Abu Hasan, H.; Muhammad, M.H.; Ismail, N.I. A review of biological drinking water treatment technologies for contaminants removal from polluted water resources. J. Water Process Eng. 2020, 33, 101035. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zhu, Y.; Zeng, G.; Zhang, Y.; Yang, Y.; Wen, X.; Chen, M.; Yi, H. Removal of microplastics via drinking water treatment: Current knowledge and future directions. Chemosphere 2020, 251, 126612. [Google Scholar] [CrossRef]
- Gerba, C.P.; Pepper, I.L. Drinking Water Treatment. In Environmental and Pollution Science; Academic Press: London, UK, 2019; pp. 435–454. [Google Scholar]
- Fetyan, N.A.H.; Salem Attia, T.M. Water purification using ultrasound waves: Application and challenges. Arab. J. Basic Appl. Sci. 2020, 27, 194–207. [Google Scholar] [CrossRef]
- Azzouz, I.; Habba, Y.G.; Capochichi-Gnambodoe, M.; Marty, F.; Vial, J.; Leprince-Wang, Y.; Bourouina, T. Zinc oxide nano-enabled microfluidic reactor for water purification and its applicability to volatile organic compounds. Microsyst. Nanoeng. 2018, 4, 17093. [Google Scholar] [CrossRef]
- Wang, C.F.; Wu, C.L.; Kuo, S.W.; Hung, W.S.; Lee, K.J.; Tsai, H.C.; Chang, C.J.; Lai, J.Y. Preparation of efficient photothermal materials from waste coffee grounds for solar evaporation and water purification. Sci. Rep. 2020, 10, 12769. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Cao, H.; Ma, J.; Huang, L.; Yu, S.; Ma, X.; Song, G.; Qiu, M.; Wang, X. Water purification and environmental remediation applications of carbonaceous nanofiber-based materials. J. Clean. Prod. 2022, 331, 130023. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development|Department of Economic and Social Affairs. Available online: https://sdgs.un.org/2030agenda (accessed on 30 June 2022).
- Van der Bruggen, B. Sustainable implementation of innovative technologies for water purification. Nat. Rev. Chem. 2021, 5, 217–218. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Uddin, F. Environmental hazard in textile dyeing wastewater from local textile industry. Cellulose 2021, 28, 10715–10739. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Bharagava, R.N. Environmental Hazards and Toxicity Profile of Organic and Inorganic Pollutants of Tannery Wastewater and Bioremediation Approaches. In Bioremediation of Industrial Waste for Environmental Safety; Springer: Singapore, 2020; pp. 381–398. [Google Scholar]
- Grumezescu, A.M. (Ed.) Water Purification. Nanotechnology in the Agri-Food Industry, 1st ed.; Elsevier Inc.: London, UK, 2017; ISBN 9780128043004. [Google Scholar]
- Usman, M.; Zeb, Z.; Ullah, H.; Suliman, M.H.; Humayun, M.; Ullah, L.; Shah, S.N.A.; Ahmed, U.; Saeed, M. A review of metal-organic frameworks/graphitic carbon nitride composites for solar-driven green H2 production, CO2 reduction, and water purification. J. Environ. Chem. Eng. 2022, 10, 107548. [Google Scholar] [CrossRef]
- Shin, S.; Shardt, O.; Warren, P.B.; Stone, H.A. Membraneless water filtration using CO2. Nat. Commun. 2017, 8, 15181. [Google Scholar] [CrossRef]
- Hadid, M.; Noukrati, H.; Ben youcef, H.; Barroug, A.; Sehaqui, H. Phosphorylated cellulose for water purification: A promising material with outstanding adsorption capacity towards methylene blue. Cellulose 2021, 28, 7893–7908. [Google Scholar] [CrossRef]
- Dongre, R.S.; Sadasivuni, K.K.; Deshmukh, K.; Mehta, A.; Basu, S.; Meshram, J.S.; Al-Maadeed, M.A.A.; Karim, A. Natural polymer based composite membranes for water purification: A review. Polym. Plast. Technol. Mater. 2019, 58, 1295–1310. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mezzenga, R. Protein nanofibrils for next generation sustainable water purification. Nat. Commun. 2021, 12, 3248. [Google Scholar] [CrossRef]
- Maity, S.; Mishra, B.; Nayak, K.; Dubey, N.C.; Tripathi, B.P. Zwitterionic microgel based anti(-bio)fouling smart membranes for tunable water filtration and molecular separation. Mater. Today Chem. 2022, 24, 100779. [Google Scholar] [CrossRef]
- Qadir, D.; Mukhtar, H.; Keong, L.K. Mixed Matrix Membranes for Water Purification Applications. Sep. Purif. Rev. 2016, 46, 62–80. [Google Scholar] [CrossRef]
- Dou, Z.; Wang, T.; Chen, W.; Lin, B.; Dong, H.; Sun, W.; Xie, X. Self-driven membrane filtration by core–shell polymer composites. J. Mater. Chem. A 2020, 8, 15942–15950. [Google Scholar] [CrossRef]
- Cantarella, M.; Impellizzeri, G.; Privitera, V. Functional nanomaterials for water purification. La Rivista del Nuovo Cimento 2017, 40, 595–632. [Google Scholar] [CrossRef]
- Berber, M.R. Current Advances of Polymer Composites for Water Treatment and Desalination. J. Chem. 2020, 2020, 7608423. [Google Scholar] [CrossRef]
- Namsaeng, J.; Punyodom, W.; Worajittiphon, P. Synergistic effect of welding electrospun fibers and MWCNT reinforcement on strength enhancement of PAN–PVC non-woven mats for water filtration. Chem. Eng. Sci. 2019, 193, 230–242. [Google Scholar] [CrossRef]
- Alam, I.; Chowdhury, I. Applications of two-dimensional nanostructures for water filtration. In Separation Science and Technology; Satinder Ahuja, Ed.; Academic Press: New York, NY, USA, 2022; Volume 15, pp. 281–286. [Google Scholar]
- You, X.; Wu, H.; Zhang, R.; Su, Y.; Cao, L.; Yu, Q.; Yuan, J.; Xiao, K.; He, M.; Jiang, Z. Metal-coordinated sub-10 nm membranes for water purification. Nat. Commun. 2019, 10, 4160. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Zhang, N.; Tang, Z.R.; Jiang, J.; Xu, Y.J. Microstructure and surface control of MXene films for water purification. Nat. Sustain. 2019, 2, 856–862. [Google Scholar] [CrossRef]
- Simeonidis, K.; Martinez-Boubeta, C.; Zamora-Pérez, P.; Rivera-Gil, P.; Kaprara, E.; Kokkinos, E.; Mitrakas, M. Implementing nanoparticles for competitive drinking water purification. Environ. Chem. Lett. 2019, 17, 705–719. [Google Scholar] [CrossRef]
- Fadillah, G.; Hidayat, R.; Saleh, T.A. Hydrothermal assisted synthesis of titanium dioxide nanoparticles modified graphene with enhanced photocatalytic performance. J. Ind. Eng. Chem. 2022. [Google Scholar] [CrossRef]
- Falyouna, O.; Maamoun, I.; Bensaida, K.; Tahara, A.; Sugihara, Y.; Eljamal, O. Encapsulation of iron nanoparticles with magnesium hydroxide shell for remarkable removal of ciprofloxacin from contaminated water. J. Colloid Interface Sci. 2022, 605, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Tanasă, F.; Teacă, C.A.; Nechifor, M.; Zănoagă, M. Multicomponent Polymer Systems Based on Agro-Industrial Waste. In Bioplastics for Sustainable Development; Kuddus, M., Roohi, Eds.; Springer: Singapore, 2021; pp. 467–513. [Google Scholar] [CrossRef]
- Tanasă, F.; Teacă, C.A.; Nechifor, M. Lignocellulosic Waste Materials for Industrial Water Purification. In Sustainable Green Chemical Processes and Their Allied Applications; Springer: Cham, Switzerland, 2020; pp. 381–407. [Google Scholar]
- Tran, V.S.; Ngo, H.H.; Guo, W.; Zhang, J.; Liang, S.; Ton-That, C.; Zhang, X. Typical low cost biosorbents for adsorptive removal of specific organic pollutants from water. Bioresour. Technol. 2015, 182, 353–363. [Google Scholar] [CrossRef]
- Dhabhai, R.; Niu, C.H.; Dalai, A.K. Agricultural byproducts-based biosorbents for purification of bioalcohols: A review. Bioresour. Bioprocess. 2018, 5, 37. [Google Scholar] [CrossRef]
- Khadir, A.; Motamedi, M.; Pakzad, E.; Sillanpää, M.; Mahajan, S. The prospective utilization of Luffa fibres as a lignocellulosic bio-material for environmental remediation of aqueous media: A review. J. Environ. Chem. Eng. 2021, 9, 104691. [Google Scholar] [CrossRef]
- Isogai, A.; Bergström, L. Preparation of cellulose nanofibers using green and sustainable chemistry. Curr. Opin. Green Sustain. Chem. 2018, 12, 15–21. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Chen, S.; Zhang, X.; Ma, J.; He, J. Oxidized cellulose-based hemostatic materials. Carbohydr. Polym. 2020, 230, 115585. [Google Scholar] [CrossRef]
- Pal, N.; Banerjee, S.; Roy, P.; Pal, K. Reduced graphene oxide and PEG-grafted TEMPO-oxidized cellulose nanocrystal reinforced poly-lactic acid nanocomposite film for biomedical application. Mater. Sci. Eng. C 2019, 104, 109956. [Google Scholar] [CrossRef]
- Lü, W.D.; Liu, Y.Z.; Yang, Y.Q.; Liu, Z.G.; Zhao, K.; Lu, J.R.; Lei, G.Y.; Wang, Y.Y.; Cai, L.; Sun, R.F. Effect of naturally derived surgical hemostatic materials on the proliferation of A549 human lung adenocarcinoma cells. Mater. Today Bio 2022, 14, 100233. [Google Scholar] [CrossRef]
- Caschera, D.; Toro, R.G.; Federici, F.; Montanari, R.; de Caro, T.; Al-Shemy, M.T.; Adel, A.M. Green approach for the fabrication of silver-oxidized cellulose nanocomposite with antibacterial properties. Cellulose 2020, 27, 8059–8073. [Google Scholar] [CrossRef]
- Liu, S.; Low, Z.X.; Xie, Z.; Wang, H. TEMPO-Oxidized Cellulose Nanofibers: A Renewable Nanomaterial for Environmental and Energy Applications. Adv. Mater. Technol. 2021, 6, 2001180. [Google Scholar] [CrossRef]
- Lv, P.; Lu, X.; Wang, L.; Feng, W. Nanocellulose-Based Functional Materials: From Chiral Photonics to Soft Actuator and Energy Storage. Adv. Funct. Mater. 2021, 31, 2104991. [Google Scholar] [CrossRef]
- Kim, S.S.; Jeon, J.H.; Kim, H.I.; Kee, C.D.; Oh, I.K. High-Fidelity Bioelectronic Muscular Actuator Based on Graphene-Mediated and TEMPO-Oxidized Bacterial Cellulose. Adv. Funct. Mater. 2015, 25, 3560–3570. [Google Scholar] [CrossRef]
- Huang, C.; Ji, H.; Yang, Y.; Guo, B.; Luo, L.; Meng, Z.; Fan, L.; Xu, J. TEMPO-oxidized bacterial cellulose nanofiber membranes as high-performance separators for lithium-ion batteries. Carbohydr. Polym. 2020, 230, 115570. [Google Scholar] [CrossRef]
- Lu, Y.; Han, J.; Ding, Q.; Yue, Y.; Xia, C.; Ge, S.; Van Le, Q.; Dou, X.; Sonne, C.; Lam, S.S. TEMPO-oxidized cellulose nanofibers/polyacrylamide hybrid hydrogel with intrinsic self-recovery and shape memory properties. Cellulose 2021, 28, 1469–1488. [Google Scholar] [CrossRef]
- Kwon, G.; Lee, K.; Kim, D.; Jeon, Y.; Kim, U.J.; You, J. Cellulose nanocrystal-coated TEMPO-oxidized cellulose nanofiber films for high performance all-cellulose nanocomposites. J. Hazard. Mater. 2020, 398, 123100. [Google Scholar] [CrossRef]
- Wu, B.; Geng, B.; Chen, Y.; Liu, H.; Li, G.; Wu, Q. Preparation and characteristics of TEMPO-oxidized cellulose nanofibrils from bamboo pulp and their oxygen-barrier application in PLA films. Front. Chem. Sci. Eng. 2017, 11, 554–563. [Google Scholar] [CrossRef]
- Rodionova, G.; Saito, T.; Lenes, M.; Eriksen, Ø.; Gregersen, Ø.; Fukuzumi, H.; Isogai, A. Mechanical and oxygen barrier properties of films prepared from fibrillated dispersions of TEMPO-oxidized Norway spruce and Eucalyptus pulps. Cellulose 2012, 19, 705–711. [Google Scholar] [CrossRef]
- Gao, P.; Cha, R.; Luo, H.; Xu, Y.; Zhang, P.; Han, L.; Wang, X.; Zhang, Z.; Jiang, X. Development of antimicrobial oxidized cellulose film for active food packaging. Carbohydr. Polym. 2022, 278, 118922. [Google Scholar] [CrossRef]
- Zhuang, C.; Tao, F.; Cui, Y. Eco-friendly biorefractory films of gelatin and TEMPO-oxidized cellulose ester for food packaging application. J. Sci. Food Agric. 2017, 97, 3384–3395. [Google Scholar] [CrossRef]
- De Castro, D.O.; Tabary, N.; Martel, B.; Gandini, A.; Belgacem, N.; Bras, J. Controlled release of carvacrol and curcumin: Bio-based food packaging by synergism action of TEMPO-oxidized cellulose nanocrystals and cyclodextrin. Cellulose 2018, 25, 1249–1263. [Google Scholar] [CrossRef]
- Saedi, S.; Garcia, C.V.; Kim, J.T.; Shin, G.H. Physical and chemical modifications of cellulose fibers for food packaging applications. Cellulose 2021, 28, 8877–8897. [Google Scholar] [CrossRef]
- Bragd, P.L.; Van Bekkum, H.; Besemer, A.C. TEMPO-mediated oxidation of polysaccharides: Survey of methods and applications. Top. Catal. 2004, 27, 49–66. [Google Scholar] [CrossRef]
- Pierre, G.; Punta, C.; Delattre, C.; Melone, L.; Dubessay, P.; Fiorati, A.; Pastori, N.; Galante, Y.M.; Michaud, P. TEMPO-mediated oxidation of polysaccharides: An ongoing story. Carbohydr. Polym. 2017, 165, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Camy, S.; Montanari, S.; Rattaz, A.; Vignon, M.; Condoret, J.S. Oxidation of cellulose in pressurized carbon dioxide. J. Supercrit. Fluids 2009, 51, 188–196. [Google Scholar] [CrossRef][Green Version]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Kim, U.J.; Kuga, S.; Wada, M.; Okano, T.; Kondo, T. Periodate oxidation of crystalline cellulose. Biomacromolecules 2000, 1, 488–492. [Google Scholar] [CrossRef]
- Mendoza, D.J.; Browne, C.; Raghuwanshi, V.S.; Simon, G.P.; Garnier, G. One-shot TEMPO-periodate oxidation of native cellulose. Carbohydr. Polym. 2019, 226, 115292. [Google Scholar] [CrossRef]
- Uribe, B.E.B.; Chiromito, E.M.S.; Carvalho, A.J.F.; Arenal, R.; Tarpani, J.R. TEMPO-oxidized cellulose nanofibers as interfacial strengthener in continuous-fiber reinforced polymer composites. Mater. Des. 2017, 133, 340–348. [Google Scholar] [CrossRef]
- Yang, W.; Bian, H.; Jiao, L.; Wu, W.; Deng, Y.; Dai, H. High wet-strength, thermally stable and transparent TEMPO-oxidized cellulose nanofibril film via cross-linking with poly-amide epichlorohydrin resin. RSC Adv. 2017, 7, 31567–31573. [Google Scholar] [CrossRef]
- Biliuta, G.; Sacarescu, L.; Socoliuc, V.; Iacob, M.; Gheorghe, L.; Negru, D.; Coseri, S. Carboxylated Polysaccharides Decorated with Ultrasmall Magnetic Nanoparticles with Antibacterial and MRI Properties. Macromol. Chem. Phys. 2017, 218, 1700062. [Google Scholar] [CrossRef]
- Coseri, S.; Biliuta, G.; Simionescu, B.C. Selective oxidation of cellulose, mediated by N-hydroxyphthalimide, under a metal-free environment. Polym. Chem. 2018, 9, 961–967. [Google Scholar] [CrossRef]
- Ong, J.H.; Liang, Y.N.; Hu, X.; Xu, R. TEMPO-Oxidized Microcrystalline Cellulose for Rapid Adsorption of Ammonium. Ind. Eng. Chem. Res. 2021, 61, 7665–7673. [Google Scholar] [CrossRef]
- Ciopec, M.; Biliuta, G.; Negrea, A.; Duțeanu, N.; Coseri, S.; Negrea, P.; Ghangrekar, M. Testing of chemically activated cellulose fibers as adsorbents for treatment of arsenic contaminated water. Materials 2021, 14, 3731. [Google Scholar] [CrossRef]
- Biliuta, G.; Coseri, S. Cellulose: A ubiquitous platform for ecofriendly metal nanoparticles preparation. Coord. Chem. Rev. 2019, 383, 155–173. [Google Scholar] [CrossRef]
- Curvello, R.; Mendoza, L.; McLiesh, H.; Manolios, J.; Tabor, R.F.; Garnier, G. Nanocellulose Hydrogel for Blood Typing Tests. ACS Appl. Bio Mater. 2019, 2, 2355–2364. [Google Scholar] [CrossRef]
- Nica, I.; Biliuta, G.; Coseri, S.; Zaharia, C.; Suteu, D. Microcrystalline cellulose as adsorbent for removal of dyes from wastewaters. Bul. Institutului Politeh. Iaşi 2017, 63, 35–44. [Google Scholar]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Miscellaneous Cellulose Derivatives and Reactions. In Cellulose Derivatives Synthesis, Structure, and Properties; Heinze, T., El Seoud, O.A., Koschella, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 479–531. [Google Scholar]
- McGee, P.A.; Fowler, W.F.; Taylor, E.W.; Unruh, C.C.; Kenyon, W.O. Investigation of the Properties of Cellulose Oxidized by Nitrogen Dioxide. V. Study of Mechanism of Oxidation in Presence of Carbon Tetrachloride. J. Am. Chem. Soc. 1947, 69, 355–361. [Google Scholar] [CrossRef]
- Pigman, W.W.; Browning, B.L.; McPherson, W.H.; Calkins, C.R.; Leaf, R.L. Oxidation of d-Galactose and Cellulose with Nitric Acid, Nitrous Acid and Nitrogen Oxides. J. Am. Chem. Soc. 1949, 71, 2200–2204. [Google Scholar] [CrossRef]
- Coseri, S. Cellulose: To depolymerize… or not to? Biotechnol. Adv. 2017, 35, 251–266. [Google Scholar] [CrossRef]
- Borisov, I.M.; Shirokova, E.N.; Mudarisova, R.K.; Muslukhov, R.R.; Zimin, Y.S.; Medvedeva, S.A.; Tolstikov, G.A.; Monakov, Y.B. Kinetics of oxidation of an arabinogalactan from larch (Larix sibirica L.) in an aqueous medium in the presence of hydrogen peroxide. Russ. Chem. Bull. 2004, 53, 318–324. [Google Scholar] [CrossRef]
- Kantouch, A.; Hebeish, A.; El-Rafie, M.H. Action of Sodium Chlorite on Cellulose and Cellulose Derivatives. Text. Res. J. 2016, 40, 178–184. [Google Scholar] [CrossRef]
- Manhas, M.S.; Mohammed, F.; Khan, Z. A kinetic study of oxidation of β-cyclodextrin by permanganate in aqueous media. Colloids Surf. A Physicochem. Eng. Asp. 2007, 295, 165–171. [Google Scholar] [CrossRef]
- Chen, C.; Ding, W.; Zhang, H.; Zhang, L.; Huang, Y.; Fan, M.; Yang, J.; Sun, D. Bacterial cellulose-based biomaterials: From fabrication to application. Carbohydr. Polym. 2022, 278, 118995. [Google Scholar] [CrossRef]
- Johansson, E.E.; Lind, J. Free Radical Mediated Cellulose Degradation during High Consistency Ozonation. J. Wood Chem. Technol. 2005, 25, 171–186. [Google Scholar] [CrossRef]
- Vijay, P.; Batchelor, W.; Saito, K. One-pot treatment of cellulose using iron oxide catalysts to produce nanocellulose and water-soluble oxidised cellulose. Carbohydr. Polym. 2022, 282, 119060. [Google Scholar] [CrossRef]
- Biliuta, G.; Fras, L.; Drobota, M.; Persin, Z.; Kreze, T.; Stana-Kleinschek, K.; Ribitsch, V.; Harabagiu, V.; Coseri, S. Comparison study of TEMPO and phthalimide-N-oxyl (PINO) radicals on oxidation efficiency toward cellulose. Carbohydr. Polym. 2013, 91, 502–507. [Google Scholar] [CrossRef]
- Kato, Y.; Kaminaga, J.; Matsuo, R.; Isogai, A. TEMPO-mediated oxidation of chitin, regenerated chitin and N-acetylated chitosan. Carbohydr. Polym. 2004, 58, 421–426. [Google Scholar] [CrossRef]
- Isogai, A.; Hänninen, T.; Fujisawa, S.; Saito, T. Review: Catalytic oxidation of cellulose with nitroxyl radicals under aqueous conditions. Prog. Polym. Sci. 2018, 86, 122–148. [Google Scholar] [CrossRef]
- Coseri, S.; Biliuta, G.; Simionescu, B.C.; Stana-Kleinschek, K.; Ribitsch, V.; Harabagiu, V. Oxidized cellulose—Survey of the most recent achievements. Carbohydr. Polym. 2013, 93, 207–215. [Google Scholar] [CrossRef]
- Sirvio, J.; Hyvakko, U.; Liimatainen, H.; Niinimaki, J.; Hormi, O. Periodate oxidation of cellulose at elevated temperatures using metal salts as cellulose activators. Carbohydr. Polym. 2011, 83, 1293–1297. [Google Scholar] [CrossRef]
- De Nooy, A.E.J.; Besemer, A.C.; van Bekkum, H. Highly selective tempo mediated oxidation of primary alcohol groups in polysaccharides. Recueil des Travaux Chimiques des Pays-Bas 1994, 113, 165–166. [Google Scholar] [CrossRef]
- Bordenave, N.; Grelier, S.; Coma, V. Advances on selective C-6 oxidation of chitosan by TEMPO. Biomacromolecules 2008, 9, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Bujedo, S.; Fleury, E.; Vignon, M.R. Preparation of Cellouronic Acids and Partially Acetylated Cellouronic Acids by TEMPO/NaClO Oxidation of Water-Soluble Cellulose Acetate. Biomacromolecules 2003, 5, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Tu, C.W.; Lee, R.H.; Yang, C.H.; Hung, W.C.; Andrew Lin, K.Y. Study of various diameter and functionality of TEMPO-oxidized cellulose nanofibers on paraquat adsorptions. Polym. Degrad. Stab. 2019, 161, 206–212. [Google Scholar] [CrossRef]
- Kato, Y.; Matsuo, R.; Isogai, A. Oxidation process of water-soluble starch in TEMPO-mediated system. Carbohydr. Polym. 2003, 51, 69–75. [Google Scholar] [CrossRef]
- Bailey, W.F.; Bobbitt, J.M.; Wiberg, K.B. Mechanism of the oxidation of alcohols by oxoammonium cations. J. Org. Chem. 2007, 72, 4504–4509. [Google Scholar] [CrossRef]
- Tahiri, C.; Vignon, M.R. TEMPO-oxidation of cellulose: Synthesis and characterisation of polyglucuronans. Cellulose 2000, 7, 177–188. [Google Scholar] [CrossRef]
- Isogai, A.; Kato, Y. Preparation of polyuronic acid from cellulose by TEMPO-mediated oxidation. Cellulose 1998, 5, 153–164. [Google Scholar] [CrossRef]
- Calvini, P.; Conio, G.; Lorenzoni, M.; Pedemonte, E. Viscometric determination of dialdehyde content in periodate oxycellulose. Part I. Methodology. Cellulose 2004, 11, 99–107. [Google Scholar] [CrossRef]
- Shibata, I.; Isogai, A. Depolymerization of cellouronic acid during TEMPO-mediated oxidation. Cellulose 2003, 10, 151–158. [Google Scholar] [CrossRef]
- Hirota, M.; Tamura, N.; Saito, T.; Isogai, A. Oxidation of regenerated cellulose with NaClO2 catalyzed by TEMPO and NaClO under acid-neutral conditions. Carbohydr. Polym. 2009, 78, 330–335. [Google Scholar] [CrossRef]
- Heinze, T.; Koschella, A.; Liebert, T.; Harabagiu, V.; Coseri, S. Cellulose: Chemistry of cellulose derivatization. In The European Polysaccharide Network of Excellence (EPNOE); Navard, P., Ed.; Springer: Vienna, Austria, 2013; pp. 283–327. [Google Scholar] [CrossRef]
- Isogai, T.; Saito, T.; Isogai, A. TEMPO electromediated oxidation of some polysaccharides including regenerated cellulose fiber. Biomacromolecules 2010, 11, 1593–1599. [Google Scholar] [CrossRef]
- Kuramae, R.; Saito, T.; Isogai, A. TEMPO-oxidized cellulose nanofibrils prepared from various plant holocelluloses. React. Funct. Polym. 2014, 85, 126–133. [Google Scholar] [CrossRef]
- Fan, F.; Zhu, M.; Fang, K.; Cao, E.; Yang, Y.; Xie, J.; Deng, Z.; Chen, Y.; Cao, X. Extraction and characterization of cellulose nanowhiskers from TEMPO oxidized sisal fibers. Cellulose 2022, 29, 213–222. [Google Scholar] [CrossRef]
- Madivoli, E.S.; Kareru, P.G.; Gachanja, A.N.; Mugo, S.M.; Sujee, D.M.; Fromm, K.M. Isolation of Cellulose Nanofibers from Oryza sativa Residues via TEMPO Mediated Oxidation. J. Nat. Fibers 2020, 19, 1310–1322. [Google Scholar] [CrossRef]
- Ono, Y.; Takeuchi, M.; Zhou, Y.; Isogai, A. Characterization of cellulose and TEMPO-oxidized celluloses prepared from Eucalyptus globulus. Holzforschung 2022, 76, 169–178. [Google Scholar] [CrossRef]
- Ono, Y.; Takeuchi, M.; Zhou, Y.; Isogai, A. TEMPO/NaBr/NaClO and NaBr/NaClO oxidations of cotton linters and ramie cellulose samples. Cellulose 2021, 28, 6035–6049. [Google Scholar] [CrossRef]
- Calderón-Vergara, L.A.; Ovalle-Serrano, S.A.; Blanco-Tirado, C.; Combariza, M.Y. Influence of post-oxidation reactions on the physicochemical properties of TEMPO-oxidized cellulose nanofibers before and after amidation. Cellulose 2020, 27, 1273–1288. [Google Scholar] [CrossRef]
- Takaichi, S.; Hiraoki, R.; Inamochi, T.; Isogai, A. One-step preparation of 2,3,6-tricarboxy cellulose. Carbohydr. Polym. 2014, 110, 499–504. [Google Scholar] [CrossRef]
- Shibuya, M.; Tomizawa, M.; Suzuki, I.; Iwabuchi, Y. 2-Azaadamantane N-oxyl (AZADO) and 1-Me-AZADO: Highly efficient organocatalysts for oxidation of alcohols. J. Am. Chem. Soc. 2006, 128, 8412–8413. [Google Scholar] [CrossRef]
- Takaichi, S.; Isogai, A. Oxidation of wood cellulose using 2-azaadamantane N-oxyl (AZADO) or 1-methyl-AZADO catalyst in NaBr/NaClO system. Cellulose 2013, 20, 1979–1988. [Google Scholar] [CrossRef]
- Coseri, S.; Nistor, G.; Fras, L.; Strnad, S.; Harabagiu, V.; Simionescu, B.C. Mild and selective oxidation of cellulose fibers in the presence of N-hydroxyphthalimide. Biomacromolecules 2009, 10, 2294–2299. [Google Scholar] [CrossRef]
- Coseri, S. A New and Efficient Heterogeneous System for the Phthalimide N-Oxyl (PINO) Radical Generation. European J. Org. Chem. 2007, 2007, 1725–1729. [Google Scholar] [CrossRef]
- Coseri, S. N-Hydroxyphthalimide (NHPI)/Lead Tetraacetate, a Peculiar System for the Phthalimide-N-Oxyl (PINO) Radical Generation. Mini. Rev. Org. Chem. 2008, 5, 222–227. [Google Scholar] [CrossRef]
- Coseri, S. Phthalimide-N-oxyl (PINO) Radical, a Powerful Catalytic Agent: Its Generation and Versatility Towards Various Organic Substrates. Catal. Rev. 2009, 51, 218–292. [Google Scholar] [CrossRef]
- Coseri, S. N-Hydroxyphthalimide (NHPI)/lead tetraacetate reactions with cyclic and acyclic alkenes. J. Phys. Org. Chem. 2009, 22, 397–402. [Google Scholar] [CrossRef]
- Coseri, S.; Biliuta, G. Bromide-free oxidizing system for carboxylic moiety formation in cellulose chain. Carbohydr. Polym. 2012, 90, 1415–1419. [Google Scholar] [CrossRef]
- Bragd, P.L.; Besemer, A.C.; Van Bekkum, H. Bromide-free TEMPO-mediated oxidation of primary alcohol groups in starch and methyl α-d-glucopyranoside. Carbohydr. Res. 2000, 328, 355–363. [Google Scholar] [CrossRef]
- Borden, W.T.; Hoffmann, R.; Stuyver, T.; Chen, B. Dioxygen: What Makes This Triplet Diradical Kinetically Persistent? J. Am. Chem. Soc. 2017, 139, 9010–9018. [Google Scholar] [CrossRef]
- Biliuta, G.; Fras, L.; Harabagiu, V.; Coseri, S. Mild oxidation of cellulose fibers using dioxygen as ultimate oxidizing agent. Dig. J. Nanomater. Biostructures 2011, 6, 293–299. [Google Scholar]
- Xu, S.; Huo, D.; Wang, K.; Yang, Q.; Hou, Q.; Zhang, F. Facile preparation of cellulose nanofibrils (CNFs) with a high yield and excellent dispersibility via succinic acid hydrolysis and NaClO2 oxidation. Carbohydr. Polym. 2021, 266, 118118. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, E.; Koshijima, T. Preparation and structural consideration of nitrogen-containing derivatives obtained from dialdehyde celluloses. J. Appl. Polym. Sci. 1991, 42, 169–178. [Google Scholar] [CrossRef]
- Crescenzi, V.; Dentini, M.; Meoli, C.; Casu, B.; Naggi, A.; Torri, G. Dicarboxyamylose and dicarboxycellulose, stereoregular polyelectrolytes: Binding of calcium and magnesium ions. Int. J. Biol. Macromol. 1984, 6, 142–144. [Google Scholar] [CrossRef]
- Varma, A.J.; Chavan, V.B.; Rajmohanan, P.R.; Ganapathy, S. Some observations on the high-resolution solid-state CP-MAS 13C-NMR spectra of periodate-oxidised cellulose. Polym. Degrad. Stab. 1997, 58, 257–260. [Google Scholar] [CrossRef]
- Casu, B.; Naggi, A.; Torri, G.; Allegra, G.; Meille, S.V.; Cosani, A.; Terbojevich, M. Stereoregular Acyclic Polyalcohols and Polyacetates from Cellulose and Amylose. Macromolecules 1985, 18, 2762–2767. [Google Scholar] [CrossRef]
- Kobayashi, M.; Suzawa, I.; Ichishima, E. Highly Reactive Dialdehydes of Cellulose and α-Cyclodextrin. Agric. Biol. Chem. 1990, 54, 1705–1709. [Google Scholar] [CrossRef]
- Koshijima, T.; Tanaka, R.; Muraki, E.; Yamada, A.; Yaku, F. Chelating polymers derived from cellulose and chitin. I. Formation of polymer complexes with metal ions. Cellul. Chem. Technol. 1973, 7, 197. [Google Scholar]
- Potthast, A.; Schiehser, S.; Rosenau, T.; Kostic, M. Oxidative modifications of cellulose in the periodate system—Reduction and beta-elimination reactions: 2nd ICC 2007, Tokyo, Japan, October 25–29, 2007. Holzforschung 2009, 63, 12–17. [Google Scholar] [CrossRef]
- Lucia, A.; van Herwijnen, H.W.G.; Oberlerchner, J.T.; Rosenau, T.; Beaumont, M. Resource-Saving Production of Dialdehyde Cellulose: Optimization of the Process at High Pulp Consistency. ChemSusChem 2019, 12, 4679–4684. [Google Scholar] [CrossRef]
- Siller, M.; Amer, H.; Bacher, M.; Roggenstein, W.; Rosenau, T.; Potthast, A. Effects of periodate oxidation on cellulose polymorphs. Cellulose 2015, 22, 2245–2261. [Google Scholar] [CrossRef]
- Calvini, P.; Conio, G.; Princi, E.; Vicini, S.; Pedemonte, E. Viscometric determination of dialdehyde content in periodate oxycellulose Part II. Topochemistry of oxidation. Cellulose 2006, 13, 571–579. [Google Scholar] [CrossRef]
- Calvini, P.; Gorassini, A. Surface and bulk reactions of cellulose oxidation by periodate. A simple kinetic model. Cellulose 2012, 19, 1107–1114. [Google Scholar] [CrossRef]
- Potthast, A.; Kostic, M.; Schiehser, S.; Kosma, P.; Rosenau, T. Studies on oxidative modifications of cellulose in the periodate system: Molecular weight distribution and carbonyl group profiles. Holzforschung 2007, 61, 662–667. [Google Scholar] [CrossRef]
- Potthast, A.; Rosenau, T.; Kosma, P.; Saariaho, A.M.; Vuorinen, T. On the Nature of Carbonyl Groups in Cellulosic Pulps. Cellulose 2005, 12, 43–50. [Google Scholar] [CrossRef]
- Potthast, A.; Rosenau, T.; Kosma, P. Analysis of Oxidized Functionalities in Cellulose. Adv. Polym. Sci. 2006, 205, 1–48. [Google Scholar] [CrossRef]
- Leguy, J.; Nishiyama, Y.; Jean, B.; Heux, L. Ultrastructural Characterization of the Core-Shell Structure of a Wide Range of Periodate-Oxidized Cellulose from Different Native Sources by Solid-State 13 C CP-MAS NMR. ACS Sustain. Chem. Eng. 2019, 7, 412–420. [Google Scholar] [CrossRef]
- Simon, J.; Tsetsgee, O.; Iqbal, N.A.; Sapkota, J.; Ristolainen, M.; Rosenau, T.; Potthast, A. A fast method to measure the degree of oxidation of dialdehyde celluloses using multivariate calibration and infrared spectroscopy. Carbohydr. Polym. 2022, 278, 118887. [Google Scholar] [CrossRef]
- Chen, D.; van de Ven, T.G.M. Morphological changes of sterically stabilized nanocrystalline cellulose after periodate oxidation. Cellulose 2016, 23, 1051–1059. [Google Scholar] [CrossRef]
- Yang, H.; Chen, D.; van de Ven, T.G.M. Preparation and characterization of sterically stabilized nanocrystalline cellulose obtained by periodate oxidation of cellulose fibers. Cellulose 2015, 22, 1743–1752. [Google Scholar] [CrossRef]
- Llàcer Navarro, S.; Nakayama, K.; Idström, A.; Evenäs, L.; Ström, A.; Nypelö, T. The effect of sulfate half-ester groups on cellulose nanocrystal periodate oxidation. Cellulose 2021, 28, 9633–9644. [Google Scholar] [CrossRef]
- Coseri, S.; Biliuta, G.; Zemljič, L.F.; Srndovic, J.S.; Larsson, P.T.; Strnad, S.; Kreže, T.; Naderi, A.; Lindström, T. One-shot carboxylation of microcrystalline cellulose in the presence of nitroxyl radicals and sodium periodate. RSC Adv. 2015, 5, 85889–85897. [Google Scholar] [CrossRef]
- Mendoza, D.J.; Hossain, L.; Browne, C.; Raghuwanshi, V.S.; Simon, G.P.; Garnier, G. Controlling the transparency and rheology of nanocellulose gels with the extent of carboxylation. Carbohydr. Polym. 2020, 245, 116566. [Google Scholar] [CrossRef]
- Qu, R.; Wang, Y.; Li, D.; Wang, L. The study of rheological properties and microstructure of carboxylated nanocellulose as influenced by level of carboxylation. Food Hydrocoll. 2021, 121, 106985. [Google Scholar] [CrossRef]
- Baron, R.I.; Bercea, M.; Avadanei, M.; Lisa, G.; Biliuta, G.; Coseri, S. Green route for the fabrication of self-healable hydrogels based on tricarboxy cellulose and poly(vinyl alcohol). Int. J. Biol. Macromol. 2019, 123, 744–751. [Google Scholar] [CrossRef]
- Baron, R.I.; Biliuta, G.; Socoliuc, V.; Coseri, S. Affordable Magnetic Hydrogels Prepared from Biocompatible and Biodegradable Sources. Polymers 2021, 13, 1693. [Google Scholar] [CrossRef]
- Chavan, V.B.; Sarwade, B.D.; Varma, A.J. Morphology of cellulose and oxidised cellulose in powder form. Carbohydr. Polym. 2002, 50, 41–45. [Google Scholar] [CrossRef]
- Yang, H.; Alam, M.N.; van de Ven, T.G.M. Highly charged nanocrystalline cellulose and dicarboxylated cellulose from periodate and chlorite oxidized cellulose fibers. Cellulose 2013, 20, 1865–1875. [Google Scholar] [CrossRef]
- Salama, A.; Aljohani, H.A.; Shoueir, K.R. Oxidized cellulose reinforced silica gel: New hybrid for dye adsorption. Mater. Lett. 2018, 230, 293–296. [Google Scholar] [CrossRef]
- Kramar, A.; Ivanovska, A.; Kostić, M. Regenerated Cellulose Fiber Functionalization by Two-step Oxidation Using Sodium Periodate and Sodium Chlorite—Impact on the Structure and Sorption Properties. Fibers Polym. 2021, 22, 2177–2186. [Google Scholar] [CrossRef]
- Liimatainen, H.; Visanko, M.; Sirviö, J.A.; Hormi, O.E.O.; Niinimaki, J. Enhancement of the nanofibrillation of wood cellulose through sequential periodate-chlorite oxidation. Biomacromolecules 2012, 13, 1592–1597. [Google Scholar] [CrossRef]
- Conley, K.; Whitehead, M.A.; van de Ven, T.G.M. Chemically peeling layers of cellulose nanocrystals by periodate and chlorite oxidation. Cellulose 2016, 23, 1553–1563. [Google Scholar] [CrossRef]
- Kekäläinen, K.; Liimatainen, H.; Niinimäki, J. Disintegration of periodate-chlorite oxidized hardwood pulp fibres to cellulose microfibrils: Kinetics and charge threshold. Cellulose 2014, 21, 3691–3700. [Google Scholar] [CrossRef]
- Plappert, S.F.; Liebner, F.W.; Konnerth, J.; Nedelec, J.M. Anisotropic nanocellulose gel–membranes for drug delivery: Tailoring structure and interface by sequential periodate–chlorite oxidation. Carbohydr. Polym. 2019, 226, 115306. [Google Scholar] [CrossRef] [PubMed]
- Patterson, G.; Hsieh, Y. Lo Tunable dialdehyde/dicarboxylate nanocelluloses by stoichiometrically optimized sequential periodate–chlorite oxidation for tough and wet shape recoverable aerogels. Nanoscale Adv. 2020, 2, 5623–5634. [Google Scholar] [CrossRef]
- Ayouch, I.; Barrak, I.; Kassem, I.; Kassab, Z.; Draoui, K.; El Achaby, M. Ultrasonic-mediated production of carboxylated cellulose nanospheres. J. Environ. Chem. Eng. 2021, 9, 106302. [Google Scholar] [CrossRef]
- Abou-Zeid, R.E.; Dacrory, S.; Ali, K.A.; Kamel, S. Novel method of preparation of tricarboxylic cellulose nanofiber for efficient removal of heavy metal ions from aqueous solution. Int. J. Biol. Macromol. 2018, 119, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, J.; Zhang, S.; Yu, J.; Liu, L.; Fan, Y. Surface modification of cellulose originated from different plant sources through TEMPO/Laccase/O2 oxidation. Ind. Crops Prod. 2022, 176, 114295. [Google Scholar] [CrossRef]
- Haunreiter, K.J.; Dichiara, A.B.; Gustafson, R. Nanocellulose by Ammonium Persulfate Oxidation: An Alternative to TEMPO-Mediated Oxidation. ACS Sustain. Chem. Eng. 2022, 10, 3882–3891. [Google Scholar] [CrossRef]
- Pettignano, A.; Leguy, J.; Heux, L.; Jean, B.; Charlot, A.; Fleury, E. Multifunctionalization of cellulose microfibrils through a cascade pathway entailing the sustainable Passerini multi-component reaction. Green Chem. 2020, 22, 7059–7069. [Google Scholar] [CrossRef]
- Tanasa, A.; Suteu, D. Biovegetal wastes used as biosorbent for removal of chemical pollutants from wastewater. Res. J. Agric. Sci. 2021, 53, 227–232. [Google Scholar]
- Mashhadi, S.; Javadian, H.; Ghasemi, M.; Saleh, T.A.; Gupta, V.K. Microwave-induced H2SO4 activation of activated carbon derived from rice agricultural wastes for sorption of methylene blue from aqueous solution. Desalination Water Treat. 2016, 57, 21091–21104, Project: Nanotechnology for water purification. [Google Scholar] [CrossRef]
- Melone, L.; Rossi, B.; Pastori, N.; Panzeri, W.; Mele, A.; Punta, C. TEMPO-Oxidized Cellulose Cross-Linked with Branched Polyethyleneimine: Nanostructured Adsorbent Sponges for Water Remediation. Chempluschem 2015, 80, 1408–1415. [Google Scholar] [CrossRef]
- Fiorati, A.; Grassi, G.; Graziano, A.; Liberatori, G.; Pastori, N.; Melone, L.; Bonciani, L.; Pontorno, L.; Punta, C.; Corsi, I. Eco-design of nanostructured cellulose sponges for sea-water decontamination from heavy metal ions. J. Clean. Prod. 2020, 246, 119009. [Google Scholar] [CrossRef]
- Isogai, A.; Zhou, Y. Diverse nanocelluloses prepared from TEMPO-oxidized wood cellulose fibers: Nanonetworks, nanofibers, and nanocrystals. Curr. Opin. Solid State Mater. Sci. 2019, 23, 101–106. [Google Scholar] [CrossRef]
- Levanič, J.; Šenk, V.P.; Nadrah, P.; Poljanšek, I.; Oven, P.; Haapala, A. Analyzing TEMPO-Oxidized Cellulose Fiber Morphology: New Insights into Optimization of the Oxidation Process and Nanocellulose Dispersion Quality. ACS Sustain. Chem. Eng. 2020, 8, 17752–17762. [Google Scholar] [CrossRef]
- Errokh, A.; Magnin, A.; Putaux, J.L.; Boufi, S. Morphology of the nanocellulose produced by periodate oxidation and reductive treatment of cellulose fibers. Cellulose 2018, 25, 3899–3911. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Liu, H.; Du, C.; Yu, H.; Ru, J.; Zhou, Z. Effect of surface charge content in the TEMPO-oxidized cellulose nanofibers on morphologies and properties of poly(N-isopropylacrylamide)-based composite hydrogels. Ind. Crops Prod. 2016, 92, 227–235. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Nolan, M.; Li, W.; Kundal, L.; Hsiao, B.S. Sequential Oxidation on Wood and Its Application in Pb2+ Removal from Contaminated Water. Polysaccharides 2021, 2, 245–256. [Google Scholar] [CrossRef]
- Sim, G.; Alam, M.N.; Godbout, L.; van de Ven, T. Structure of swollen carboxylated cellulose fibers. Cellulose 2014, 21, 4595–4606. [Google Scholar] [CrossRef]
- Sjöstedt, A.; Wohlert, J.; Larsson, P.T.; Wågberg, L. Structural changes during swelling of highly charged cellulose fibres. Cellulose 2015, 22, 2943–2953. [Google Scholar] [CrossRef]
- Torstensen, J.; Liu, M.; Jin, S.A.; Deng, L.; Hawari, A.I.; Syverud, K.; Spontak, R.J.; Gregersen, Y.W. Swelling and Free-Volume Characteristics of TEMPO-Oxidized Cellulose Nanofibril Films. Biomacromolecules 2018, 19, 1016–1025. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Okita, Y.; Isogai, A. Thermal stabilization of TEMPO-oxidized cellulose. Polym. Degrad. Stab. 2010, 95, 1502–1508. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Varma, A.J. Thermal stability of cellulose and their nanoparticles: Effect of incremental increases in carboxyl and aldehyde groups. Carbohydr. Polym. 2014, 114, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Tarrés, Q.; Boufi, S.; Mutjé, P.; Delgado-Aguilar, M. Enzymatically hydrolyzed and TEMPO-oxidized cellulose nanofibers for the production of nanopapers: Morphological, optical, thermal and mechanical properties. Cellulose 2017, 24, 3943–3954. [Google Scholar] [CrossRef]
- López Durán, V.; Larsson, P.A.; Wågberg, L. Chemical modification of cellulose-rich fibres to clarify the influence of the chemical structure on the physical and mechanical properties of cellulose fibres and thereof made sheets. Carbohydr. Polym. 2018, 182, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Delattre, C.; Michaud, P.; Elboutachfaiti, R.; Courtois, B.; Courtois, J. Production of oligocellouronates by biodegradation of oxidized cellulose. Cellulose 2006, 13, 63–71. [Google Scholar] [CrossRef]
- Konno, N.; Habu, N.; Maeda, I.; Azuma, N.; Isogai, A. Cellouronate (β-1,4-linked polyglucuronate) lyase from Brevundimonas sp. SH203: Purification and characterization. Carbohydr. Polym. 2006, 64, 589–596. [Google Scholar] [CrossRef]
- Konno, N.; Habu, N.; Iihashi, N.; Isogai, A. Purification and characterization of exo-type cellouronate lyase. Cellulose 2008, 15, 453–463. [Google Scholar] [CrossRef]
- Li, J.; Bendi, R.; Malla, R.; Shah, K.J.; Parida, K.; You, Z. Cellulose nanofibers-based green nanocomposites for water environmental sustainability: A review. Emergent Mater. 2021, 4, 1259–1273. [Google Scholar] [CrossRef]
- Bethke, K.; Palantöken, S.; Andrei, V.; Roß, M.; Raghuwanshi, V.S.; Kettemann, F.; Greis, K.; Ingber, T.T.K.; Stückrath, J.B.; Valiyaveettil, S.; et al. Functionalized Cellulose for Water Purification, Antimicrobial Applications, and Sensors. Adv. Funct. Mater. 2018, 28, 1800409. [Google Scholar] [CrossRef]
- Yu, H.; Zheng, L.; Zhang, T.; Ren, J.; Meng, P. Highly TEMPO-oxidized cellulose for removal of ionic and complexed cadmium from a complicated water system. Environ. Sci. Pollut. Res. 2022, 29, 36575–36588. [Google Scholar] [CrossRef]
- Xing, X.; Li, W.; Zhang, J.; Wu, H.; Guan, Y.; Gao, H. TEMPO-oxidized cellulose hydrogel for efficient adsorption of Cu2+ and Pb2+ modified by polyethyleneimine. Cellulose 2021, 28, 7953–7968. [Google Scholar] [CrossRef]
- Fiol, N.; Vásquez, M.G.; Pereira, M.; Tarrés, Q.; Mutjé, P.; Delgado-Aguilar, M. TEMPO-oxidized cellulose nanofibers as potential Cu(II) adsorbent for wastewater treatment. Cellulose 2019, 26, 903–916. [Google Scholar] [CrossRef]
- Köse, K.; Mavlan, M.; Nuruddin, M.; Youngblood, J.P. TEMPO-oxidized cellulose nanofiber based polymeric adsorbent for use in iron removal. Cellulose 2020, 27, 4623–4635. [Google Scholar] [CrossRef]
- Si, R.; Wu, C.; Yu, D.; Ding, Q.; Li, R. Novel TEMPO-oxidized cellulose nanofiber/polyvinyl alcohol/polyethyleneimine nanoparticles for Cu2+ removal in water. Cellulose 2021, 28, 10999–11011. [Google Scholar] [CrossRef]
- Paladini, G.; Venuti, V.; Crupi, V.; Majolino, D.; Fiorati, A.; Punta, C. FTIR-ATR analysis of the H-bond network of water in branched polyethyleneimine/TEMPO-oxidized cellulose nano-fiber xerogels. Cellulose 2020, 27, 8605–8618. [Google Scholar] [CrossRef]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Ur Rashid, T.U.; Kabir, S.M.F. Cellulose-Based Hydrogels for Wastewater Treatment: A Concise Review. Gels 2021, 7, 30. [Google Scholar] [CrossRef]
- Isobe, N.; Chen, X.; Kim, U.J.; Kimura, S.; Wada, M.; Saito, T.; Isogai, A. TEMPO-oxidized cellulose hydrogel as a high-capacity and reusable heavy metal ion adsorbent. J. Hazard. Mater. 2013, 260, 195–201. [Google Scholar] [CrossRef]
- Hosseini, M.; Zaki Dizaji, H.; Taghavi, M.; Babaei, A.A. Preparation of ultra-lightweight and surface-tailored cellulose nanofibril composite cryogels derived from Date palm waste as powerful and low-cost heavy metals adsorbent to treat aqueous medium. Ind. Crops Prod. 2020, 154, 112696. [Google Scholar] [CrossRef]
- Zheng, Q.; Cai, Z.; Gong, S. Green synthesis of polyvinyl alcohol (PVA)–cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J. Mater. Chem. A 2014, 2, 3110–3118. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, P.; Mathew, A.P. Self-Assembled TEMPO Cellulose Nanofibers: Graphene Oxide-Based Biohybrids for Water Purification. ACS Appl. Mater. Interfaces 2017, 9, 21048–21058. [Google Scholar] [CrossRef]
- Teixeira, L.T.; Braz, W.F.; Correia de Siqueira, R.N.; Pandoli, O.G.; Geraldes, M.C. Sulfated and carboxylated nanocellulose for Co+2 adsorption. J. Mater. Res. Technol. 2021, 15, 434–447. [Google Scholar] [CrossRef]
- Corsi, I.; Fiorati, A.; Grassi, G.; Bartolozzi, I.; Daddi, T.; Melone, L.; Punta, C. Environmentally Sustainable and Ecosafe Polysaccharide-Based Materials for Water Nano-Treatment: An Eco-Design Study. Materials 2018, 11, 1228. [Google Scholar] [CrossRef]
- Georgouvelas, D.; Abdelhamid, H.N.; Li, J.; Edlund, U.; Mathew, A.P. All-cellulose functional membranes for water treatment: Adsorption of metal ions and catalytic decolorization of dyes. Carbohydr. Polym. 2021, 264, 118044. [Google Scholar] [CrossRef]
- Bartolozzi, I.; Daddi, T.; Punta, C.; Fiorati, A.; Iraldo, F. Life cycle assessment of emerging environmental technologies in the early stage of development: A case study on nanostructured materials. J. Ind. Ecol. 2020, 24, 101–115. [Google Scholar] [CrossRef]
- Batmaz, R.; Mohammed, N.; Zaman, M.; Minhas, G.; Berry, R.M.; Tam, K.C. Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 2014, 21, 1655–1665. [Google Scholar] [CrossRef]
- Hussain, A.; Li, J.; Wang, J.; Xue, F.; Chen, Y.; Bin Aftab, T.; Li, D. Hybrid Monolith of Graphene/TEMPO-Oxidized Cellulose Nanofiber as Mechanically Robust, Highly Functional, and Recyclable Adsorbent of Methylene Blue Dye. J. Nanomater. 2018, 2018, 5963982. [Google Scholar] [CrossRef]
- Al-Ahmed, Z.A.; Hassan, A.A.; El-Khouly, S.M.; El-Shafey, S.E. TEMPO-oxidized cellulose nanofibers/TiO2 nanocomposite as new adsorbent for Brilliant Blue dye removal. Polym. Bull. 2020, 77, 6213–6226. [Google Scholar] [CrossRef]
- Pottathara, Y.B.; Narwade, V.N.; Bogle, K.A.; Kokol, V. TEMPO-oxidized cellulose nanofibrils–graphene oxide composite films with improved dye adsorption properties. Polym. Bull. 2020, 77, 6175–6189. [Google Scholar] [CrossRef]
- Chen, W.; Li, Q.; Wang, Y.; Yi, X.; Zeng, J.; Yu, H.; Liu, Y.; Li, J. Comparative Study of Aerogels Obtained from Differently Prepared Nanocellulose Fibers. ChemSusChem 2014, 7, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Melone, L.; Altomare, L.; Alfieri, I.; Lorenzi, A.; De Nardo, L.; Punta, C. Ceramic aerogels from TEMPO-oxidized cellulose nanofibre templates: Synthesis, characterization, and photocatalytic properties. J. Photochem. Photobiol. A Chem. 2013, 261, 53–60. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, L.; Liao, Q.; Chen, X.; Qian, Z.; Shen, J.; Liang, J.; Yao, J. Functionalization of cellulose with hyperbranched polyethylenimine for selective dye adsorption and separation. Cellulose 2016, 23, 3785–3797. [Google Scholar] [CrossRef]
- Wang, W.; Bai, Q.; Liang, T.; Bai, H.; Liu, X. Two-Sided Surface Oxidized Cellulose Membranes Modified with PEI: Preparation, Characterization and Application for Dyes Removal. Polymer 2017, 9, 455. [Google Scholar] [CrossRef]
- Riva, L.; Pastori, N.; Panozzo, A.; Antonelli, M.; Punta, C. Nanostructured cellulose-based sorbent materials for water decontamination from organic dyes. Nanomaterials 2020, 10, 1570. [Google Scholar] [CrossRef]
- Wang, M.; Shao, C.; Zhou, S.; Yang, J.; Xu, F. Preparation of carbon aerogels from TEMPO-oxidized cellulose nanofibers for organic solvents absorption. RSC Adv. 2017, 7, 38220–38230. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Sakamoto, H.; Kitamura, T.; Hashimoto, M.; Suye, S. ichiro Structure retention of proteins interacting electrostatically with TEMPO-oxidized cellulose nanofiber surface. Colloids Surf. B Biointerfaces 2019, 183, 110392. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Nakayama, H.; Morita, Y.; Sakamoto, H.; Kitamura, T.; Hashimoto, M.; Suye, S.I. Enhanced and Prolonged Activity of Enzymes Adsorbed on TEMPO-Oxidized Cellulose Nanofibers. ACS Omega 2020, 5, 18826–18830. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, J.; Xin, Y.; Lubineau, G.; Ma, Q.; Jiang, L. Alcohol Recognition by Flexible, Transparent and Highly Sensitive Graphene-Based Thin-Film Sensors. Sci. Rep. 2017, 7, 4317. [Google Scholar] [CrossRef]
- Jradi, K.; Bideau, B.; Chabot, B.; Daneault, C. Characterization of conductive composite films based on TEMPO-oxidized cellulose nanofibers and polypyrrole. J. Mater. Sci. 2012, 47, 3752–3762. [Google Scholar] [CrossRef]
- Masruchin, N.; Park, B.D.; Lee, J.M. Surface modification of TEMPO-oxidized cellulose nanofibrils for composites to give color change in response to pH level. Cellulose 2018, 25, 7079–7090. [Google Scholar] [CrossRef]
- Mackin, R.T.; Fontenot, K.R.; Edwards, J.V.; Prevost, N.T.; Grimm, C.; Condon, B.D.; Liebner, F.; Jordan, J.H.; Easson, M.W.; French, A.D. Synthesis and characterization of TEMPO-oxidized peptide-cellulose conjugate biosensors for detecting human neutrophil elastase. Cellulose 2022, 29, 1293–1305. [Google Scholar] [CrossRef]


| Metal Ion | Sorbent | Qmax (mg/g) | Observations |
|---|---|---|---|
| Cu(II) | TOCNFs | 75 | Cu(II) was reduced to self-assembled Cu(0) nanoparticles |
| TOCNFs membranes | 374 | membranes with high water permeability, mechanical stability, and functionality | |
| TOCNFs hydrogel | 268.2 | pH = 5.0–6.0 | |
| TOCNFs aerogel | 303 | ||
| TOCNFs-PEI | 52.32 | pH = 5.0, T = 30 °C, t = 20 h | |
| TOCNFs-PVA hybrid aerogel | 151.3 | ||
| TOCNFs-alginate | 105.2–204.1 | ||
| TOCNFs-GO | 63.5–68.1 | ||
| Cd(II) | TOCNFs | 140.3 | pH = 5.5 |
| TOCNFs hydrogel | 115 | ||
| Ni(II) | TOCNFs | 49 | pH = 6 |
| Zn(II) | TOCNFs | 66 | pH = 6 |
| Pb(II) | TOCNFs | 137.7 | pH = 5.0 |
| TOCNFs-PVA hybrid aerogel | 110.6 | From solution of mixed heavy metal sals | |
| Hg(II) | TOCNFs-PVA hybrid aerogel | 157.5 | From solution of mixed heavy metal sals |
| Fe(II) | In situ modified TOCNFs membranes | 456 | redox system Fe(II)/Fe(III) |
| Cr(III) | TOCNFs | 58 | pH = 5.0 |
| Cr(VI) | TOCNFs-PAN membrane | 87.5 | pH = 4.0, bichromate solution |
| Cs(I) | TOCNFs hydrogel | 133.8 | |
| Au(III) | TOCNFs hydrogel | 15.44 | pH = 2.0, 48 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duceac, I.A.; Tanasa, F.; Coseri, S. Selective Oxidation of Cellulose—A Multitask Platform with Significant Environmental Impact. Materials 2022, 15, 5076. https://doi.org/10.3390/ma15145076
Duceac IA, Tanasa F, Coseri S. Selective Oxidation of Cellulose—A Multitask Platform with Significant Environmental Impact. Materials. 2022; 15(14):5076. https://doi.org/10.3390/ma15145076
Chicago/Turabian StyleDuceac, Ioana A., Fulga Tanasa, and Sergiu Coseri. 2022. "Selective Oxidation of Cellulose—A Multitask Platform with Significant Environmental Impact" Materials 15, no. 14: 5076. https://doi.org/10.3390/ma15145076
APA StyleDuceac, I. A., Tanasa, F., & Coseri, S. (2022). Selective Oxidation of Cellulose—A Multitask Platform with Significant Environmental Impact. Materials, 15(14), 5076. https://doi.org/10.3390/ma15145076








