Usage of Converter Gas as a Substitute Fuel for a Tunnel Furnace in Steelworks
Abstract
:1. Introduction
- justification of converter gas to be used as a substitute fuel in the steel manufacturing process,
- tools for modeling and chemistry reduction methods, which can be implemented for converter gas modeling with the aid of a skeletal combustion mechanism.
- testing and verification of a skeletal reduced kinetic combustion mechanism obtained with the aid of the DRGEP method,
- modeling combustion of BOFG in a tunnel furnace with aid of this tested and verified mechanism with a focus on ecological aspects,
- economic benefits from applying the BOFG to the tunnel furnace.
- the advantage of detailed mechanisms is the high accuracy and detail of the results obtained, and the disadvantage is the extended calculation time and associated procedure costs;
- the advantage of simplified mechanisms is the short calculation time, and the disadvantage is the lower accuracy of the results obtained;
- advantages of skeletal mechanisms include: the same accuracy of results as for detailed models and much shorter computation time.
2. Materials and Methods
2.1. Experimental Setup
2.2. Calculation Procedure
- pressure of 1 atm;
- initial temperature of 298 K;
- equivalence ratio of 0.7–1.1;
- residence time of 0.001–100 s.
2.3. Testing and Verification of the RKM_Konnov 0.6 Mechanism
3. Results and Discussion
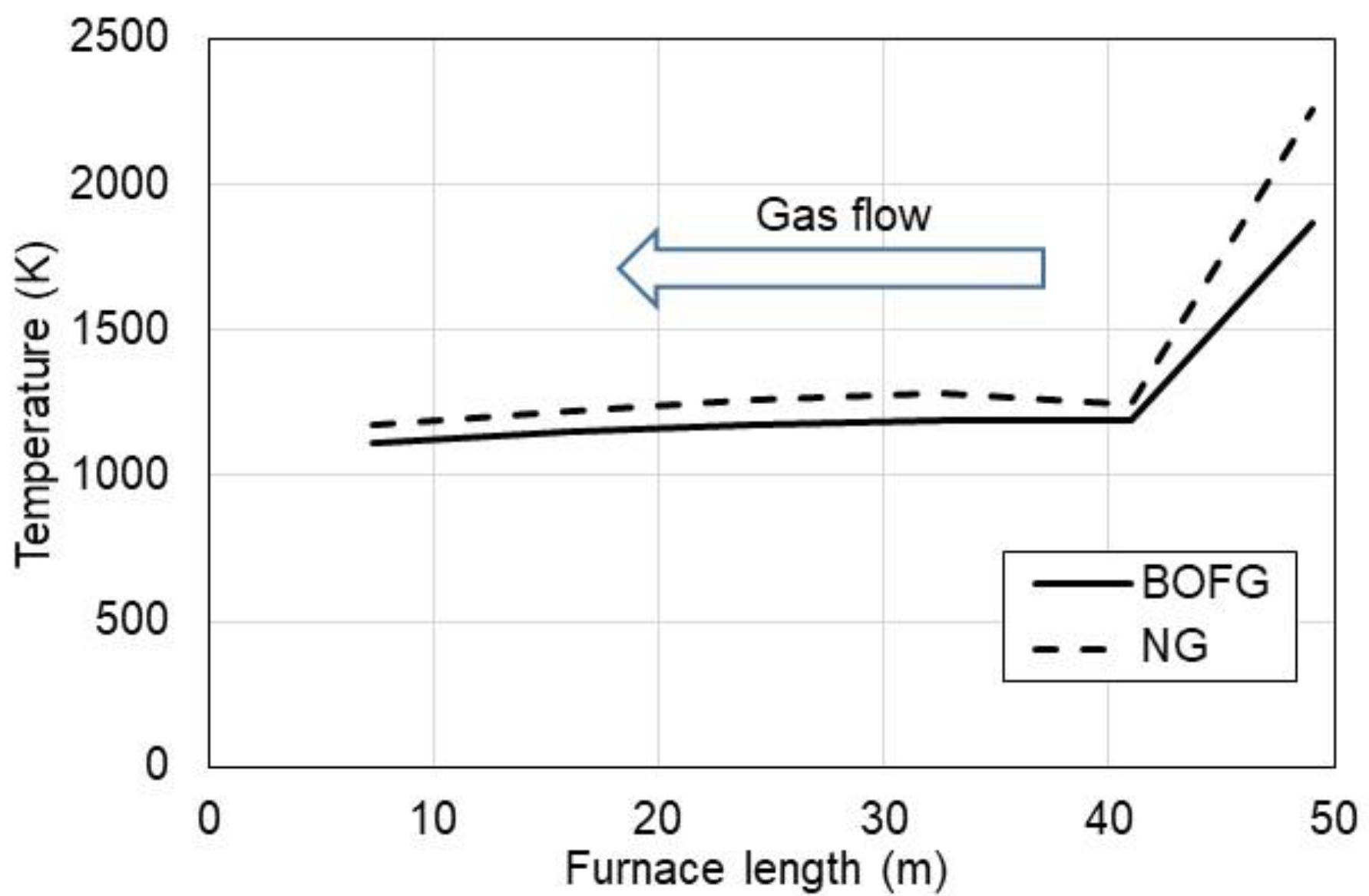
3.1. Ecological Aspect of Use of BOFG
- constant furnace power P = 7 MWth;
- BOFG composition: 3% H2, 64% CO, 15% CO2, and 18% N2;
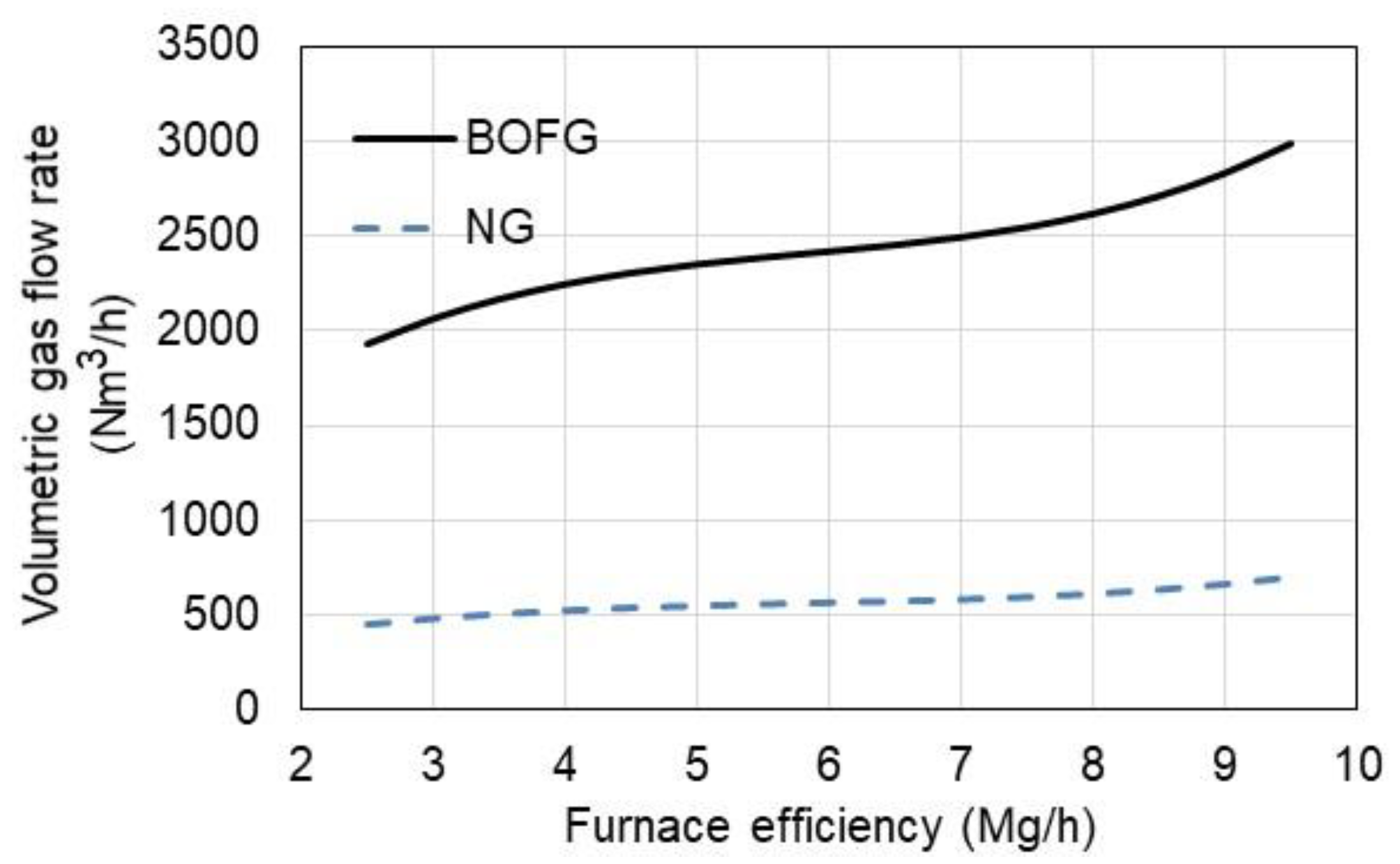
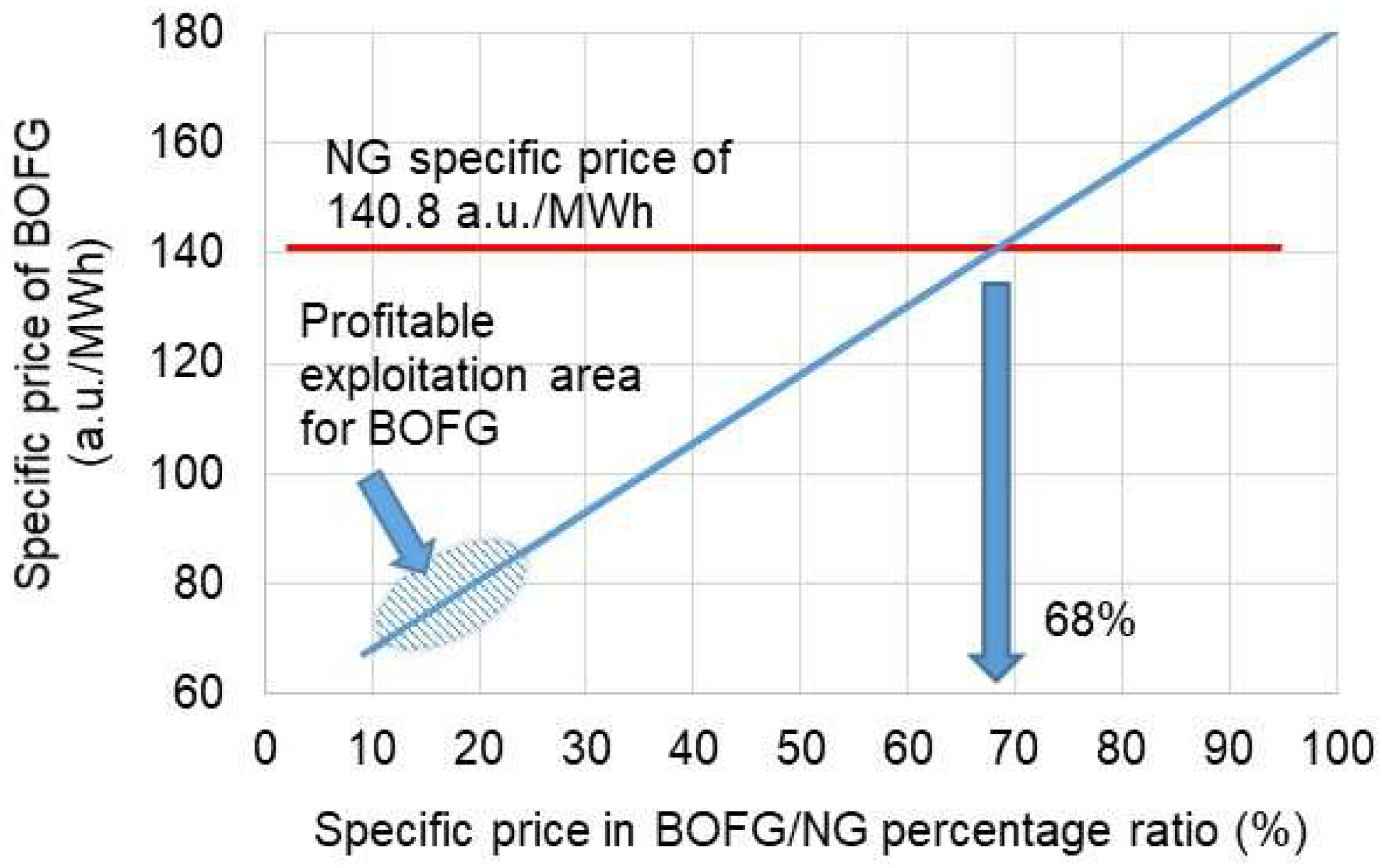
3.2. Economic Aspect of the Use of BOFG
4. Conclusions
- Combustion kinetic mechanism RKM_Konnov 0.6 was found as a reliable tool for modeling converter gas (BOFG) in a tunnel furnace. The validation procedure was conducted on a real test bed working on natural gas. Good conformity between numerical and experimental data was obtained.
- Simulations of converter gas combustion carried out for the current operating parameters of the tunnel furnace show promising results for nitric oxide emission. NO at the exit of the furnace was found to be more than three times lower in comparison with the NO emission from the combustion of natural gas in this furnace.
- Carbon dioxide emission was nearly three times higher from the combustion of converter gas if compared to natural gas. However, one should note that converter gas is a by-product from steel manufacturing and due to its high CO content (over 60%) it cannot be released into the ambient air. Therefore, converter gas is combusted and CO2 is formed anyway. The proposed idea was to utilize converter gas as substitute for of natural gas. Thus, the CO2 reduction comes from eliminating natural gas from fueling a tunnel furnace.
- By applying BOFG instead of natural gas for heating a tunnel furnace, it should bring significant financial savings regarding gas purchasing costs.
- Taking the above into account, BOFG, due to its high energy potential, may become an attractive fuel, even though it contains a high carbon fraction in CO and CO2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Yang, B.; Shen, X.; Xie, Z.; Sun, F. Thermodynamic Optimization Opportunities for the Recovery and Utilization of Residual Energy and Heat in China’s Iron and Steel Industry: A Case Study. Appl. Therm. Eng. 2015, 86, 151–160. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Qin, X.; Sun, F. Exergy Loss Minimization for a Blast Furnace with Comparative Analyses for Energy Flows and Exergy Flows. Energy 2015, 93, 10–19. [Google Scholar] [CrossRef]
- Polish Central Statistical Office. Statistical Yearbook of Industry; Statistical Publishing Establishment: Warsaw, Poland, 2018. Available online: https://stat.gov.pl/download/gfx/portalinformacyjny/en/defaultaktualnosci/3328/5/12/1/statistical_yearbook_of_industry_2018.pdf (accessed on 10 January 2021).
- Zhai, Y.; Li, S.; Yan, W.; Wei, X.; Zhang, L.; Wang, Y. Effects of Water Vapor and Temperature on NOx and CO Emissions during Converter Gas Combustion. Fuel 2019, 256, 115914. [Google Scholar] [CrossRef]
- Xie, J.; Zhou, J.; Jiang, X.; Zhang, H. Evaluation of Mixed Heat Effect of Fuel Gas and Coke in Steelmaking Flue. J. Therm. Anal. Calorim. 2019, 137, 245–252. [Google Scholar] [CrossRef]
- Niesler, M.; Oleksiak, B. Oddziaływanie Przemysłu Na Środowisko Naturalne. Część I. Hutnictwo Żelaza i Stali; Instytut Metalurgii Żelaza: Gliwice, Poland, 2012; ISBN 9788391689325. [Google Scholar]
- Ren, B.; Wang, G.; Zuo, H.; Xue, Q.; She, X.; Wang, J. In-Situ Catalytic Reforming of Converter Gas in Converter Flue Based on Thermochemical Energy Storage: Kinetics and Numerical Simulation. J. Energy Storage 2022, 48, 103693. [Google Scholar] [CrossRef]
- Halkos, G.E.; Tzeremes, N.G. Measuring the Effect of Kyoto Protocol Agreement on Countries’ Environmental Efficiency in CO2 Emissions: An Application of Conditional Full Frontiers. J. Product. Anal. 2014, 41, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, A. CO2 Abatement in the Iron and Steel Industry; IEA Clean Coal Centre, 2012; ISBN 978-92-9029-513-6. Available online: https://usea.org/sites/default/files/012012_CO2 abatement in the iron and steel industry_ccc193.pdf (accessed on 30 May 2022).
- Biermann, M.; Ali, H.; Sundqvist, M.; Larsson, M.; Normann, F.; Johnsson, F. Excess Heat-Driven Carbon Capture at an Integrated Steel Mill—Considerations for Capture Cost Optimization. Int. J. Greenh. Gas Control 2019, 91, 102833. [Google Scholar] [CrossRef]
- Mittal, D.; Kundoo, J.K.; Bisen, A.K.; Tiwari, H.P. Perspectives on the Reducing CO2 Emissions of Gas-Based DRI in Steelmaking. Metall. Res. Technol. 2022, 119, 404. [Google Scholar] [CrossRef]
- Mahato, N.; Agarwal, H.; Jain, J. Reduction of Specific Heat Consumption by Modification of Reversal Cycle Period of Coke Oven Battery. Mater. Today Proc. 2022, 61, 1149–1153. [Google Scholar] [CrossRef]
- Yi, Z.; Hu, X. Numerical Simulation of By-Product Fuel Combustion in a Tangential Gas-Fired Boiler: Effect of Proportion and Angle on Coke Oven Gas. J. Energy Eng. 2022, 148, 04022006. [Google Scholar] [CrossRef]
- Onarheim, K.; Arasto, A. Staged Implementation of Alternative Processes in an Existing Integrated Steel Mill for Improved Performance and Reduced CO2 Emissions—Part I: Technical Concept Analysis. Int. J. Greenh. Gas Control 2016, 45, 163–171. [Google Scholar] [CrossRef]
- de Castro, J.A.; de Oliveira, E.M.; de Campos, M.F.; Takano, C.; Yagi, J. ichiro Analyzing Cleaner Alternatives of Solid and Gaseous Fuels for Iron Ore Sintering in Compacts Machines. J. Clean. Prod. 2018, 198, 654–661. [Google Scholar] [CrossRef]
- Mousa, E.A.; Babich, A.; Senk, D. Utilization of Coke Oven Gas and Converter Gas in the Direct Reduction of Lump Iron Ore. Metall. Mater. Trans. B 2014, 45, 617–628. [Google Scholar] [CrossRef]
- Tsupari, E.; Kärki, J.; Arasto, A.; Lilja, J.; Kinnunen, K.; Sihvonen, M. Oxygen Blast Furnace with CO2 Capture and Storage at an Integrated Steel Mill—Part II: Economic Feasibility in Comparison with Conventional Blast Furnace Highlighting Sensitivities. Int. J. Greenh. Gas Control 2015, 32, 189–196. [Google Scholar] [CrossRef]
- Rieger, J.; Weiss, C.; Rummer, B. Modelling and Control of Pollutant Formation in Blast Stoves. J. Clean. Prod. 2015, 88, 254–261. [Google Scholar] [CrossRef]
- Musiał, D. Coke and Blast Furnace Gases: Ecological and Economic Benefits of Use in Heating Furnaces. Combust. Sci. Technol. 2020, 192, 1015–1027. [Google Scholar] [CrossRef]
- Mariños Rosado, D.J.; Rojas Chávez, S.B.; Amaro Gutierrez, J.; Mayworm de Araújo, F.H.; de Carvalho, J.A.; Mendiburu, A.Z. Energetic Analysis of Reheating Furnaces in the Combustion of Coke Oven Gas, Linz-Donawitz Gas and Blast Furnace Gas in the Steel Industry. Appl. Therm. Eng. 2020, 169, 114905. [Google Scholar] [CrossRef]
- Matino, I.; Dettori, S.; Colla, V.; Weber, V.; Salame, S. Forecasting Blast Furnace Gas Production and Demand through Echo State Neural Network-Based Models: Pave the Way to off-Gas Optimized Management. Appl. Energy 2019, 253, 113578. [Google Scholar] [CrossRef]
- Rimar, M.; Kulikov, A.; Fedak, M.; Yeromin, O.; Sukhyy, K.; Gupalo, O.; Belyanovskaya, E.; Berta, R.; Smajda, M.; Ratnayake, M.R. Mathematical Model of a Heating Furnace Implemented with Volumetric Fuel Combustion. Processes 2020, 8, 469. [Google Scholar] [CrossRef]
- Yao, L.; Zhu, R.; Tang, Y.; Wei, G.; Dong, K. Effect of Furnace Gas Composition on Characteristics of Supersonic Oxygen Jets in the Converter Steelmaking Process. Materials 2020, 13, 3353. [Google Scholar] [CrossRef]
- Musiał, D. Optimization of the Coke Oven Gas Combustion Process in an Experimental Heating Chamber. Przem. Chem. 2021, 100, 347–349. [Google Scholar] [CrossRef]
- Zajemska, M.; Urbańczyk, P.; Poskart, A.; Urbaniak, D.; Radomiak, H.; Musiał, D.; Golański, G.; Wyleciał, T. The Impact of Co-Firing Sunflower Husk Pellets with Coal in a Boiler on the Chemical Composition of Flue Gas. Energy Fuels 2016 2017, 14, 02021. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Norinaga, K.; Watanabe, H.; Kitagawa, T. Prediction of Hot Coke Oven Gas Reforming by LES Coupled with the Extended Flamelet/Progress Variable Approach. Fuel 2018, 231, 234–243. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Westbrook, C.K.; Mehl, M.; Pitz, W.J.; Togbe, C.; Dagaut, P.; Wang, H.; Oehlschlaeger, M.A.; Niemann, U.; Seshadri, K.; et al. Comprehensive Chemical Kinetic Modeling of the Oxidation of 2-Methylalkanes from C7 to C20. Combust. Flame 2011, 158, 2338–2357. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Q.; Li, Y.; Liu, J. A Minimal Skeletal Mechanism for Combustion of CH4/O2/CO2 Mixtures. Energy Fuels 2016, 30, 1407–1414. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Li, L.; Wan, K.; Cen, K. Reaction Mechanism Reduction for Ozone-Enhanced CH4/Air Combustion by a Combination of Directed Relation Graph with Error Propagation, Sensitivity Anassumption. Energies 2018, 11, 1470. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Yang, W.; Fan, L.; Zhou, D.; Ma, X. Development of a Skeletal Mechanism for Heavy-Duty Engines Fuelled by Diesel and Natural Gas. Appl. Therm. Eng. 2017, 123, 1060–1071. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J. Application of Jacobian Defined Direct Interaction Coefficient in DRGEP-Based Chemical Mechanism Reduction Methods Using Different Graph Search Algorithms. Combust. Flame 2016, 174, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Niemeyer, K.E.; Sung, C.-J.; Raju, M.P. Skeletal Mechanism Generation for Surrogate Fuels Using Directed Relation Graph with Error Propagation and Sensitivity Analysis. Combust. Flame 2010, 157, 1760–1770. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Yu, L.; Xu, L.; Mao, Y.; Lu, X. Workbench for the Reduction of Detailed Chemical Kinetic Mechanisms Based on Directed Relation Graph and Its Deduced Methods: Methodology and n-Cetane as an Example. Energy Fuels 2018, 32, 7169–7178. [Google Scholar] [CrossRef]
- Li, R.; He, G.; Zhang, D.; Qin, F. Skeletal Kinetic Mechanism Generation and Uncertainty Analysis for Combustion of Iso-Octane at High Temperatures. Energy Fuels 2018, 32, 3842–3850. [Google Scholar] [CrossRef]
- Konnov, A.A. Implementation of the NCN Pathway of Prompt-NO Formation in the Detailed Reaction Mechanism. Combust. Flame 2009, 156, 2093–2105. [Google Scholar] [CrossRef]
- Ziauddin, M.; Balakrishna, A.; Vlachos, D.G.; Schmidt, L.D. Ignition of Methane Flames in Oxygen near Inert Surfaces: Effects of Composition, Pressure, Preheat and Residence Time. Combust. Flame 1997, 110, 377–391. [Google Scholar] [CrossRef]
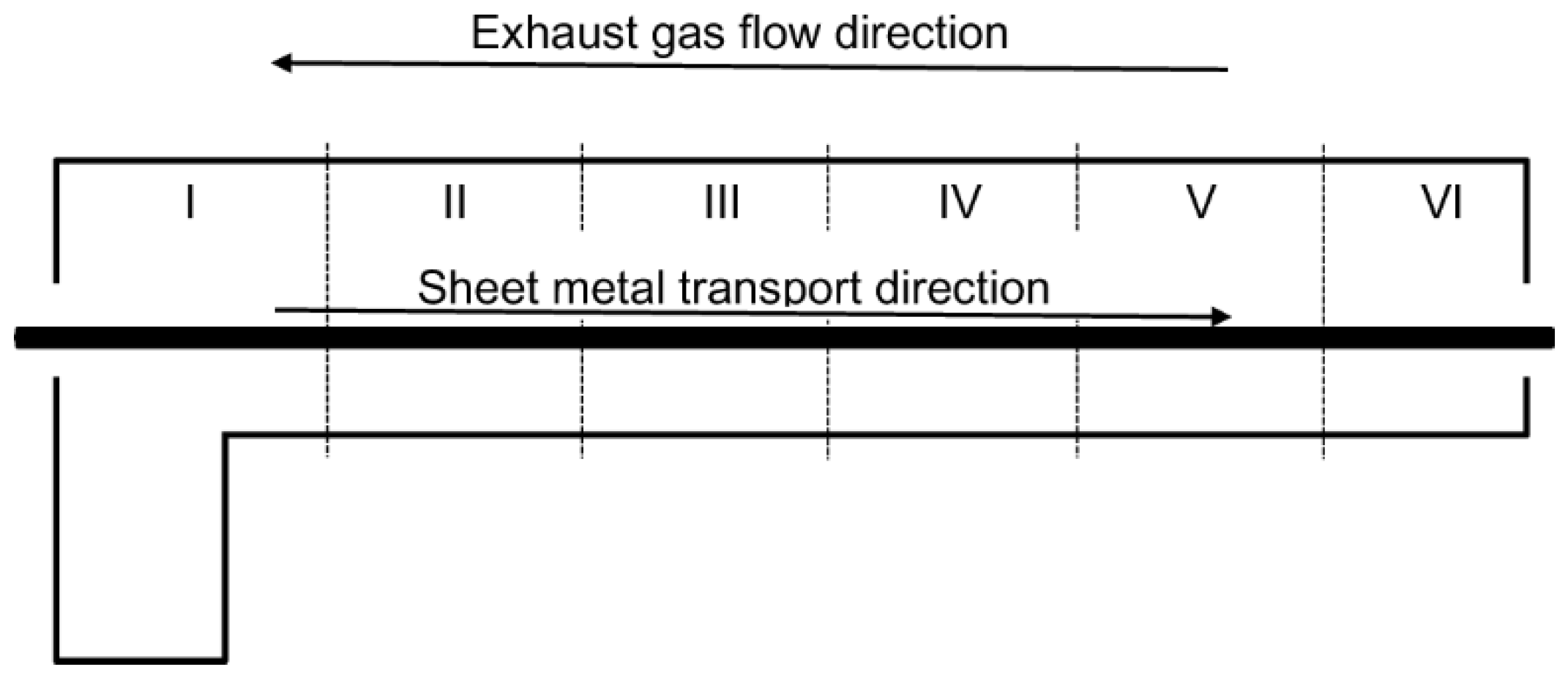





| Parameters | Zone No. and Furnace Length (m) | |||||
|---|---|---|---|---|---|---|
| I 7.3 | II 16 | III 24 | IV 32.5 | V 41 | VI 49 | |
| Gas flowrate, , | 0.068 | 0.035 | 0.026 | 0.019 | 0.031 | 0.016 |
| Air flowrate, , | 0.681 | 0.353 | 0.256 | 0.186 | 0.261 | 0.136 |
| Temperature of steel sheets, | 1113 | 1153 | 1173 | 1193 | 1193 | 1193 |
| Excess air ratio, | 1.05 | 1.05 | 1.05 | 1.05 | 0.90 | 0.90 |
| Parameters | Zone No. and Furnace Length (m) | |||||
|---|---|---|---|---|---|---|
| I 7.3 | II 16 | III 24 | IV 32.5 | V 41 | VI 49 | |
| Gas flowrate, , | 0.292 | 0.151 | 0.110 | 0.079 | 0.130 | 0.068 |
| Air flowrate, , | 0.489 | 0.253 | 0.184 | 0.134 | 0.187 | 0.098 |
| Excess air ratio, | 1.05 | 1.05 | 1.05 | 1.05 | 0.90 | 0.90 |
| Residence time, | 11.35 | 16.60 | 22.86 | 31.78 | 45.80 | 116.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musial, D.; Szwaja, M.; Kurtyka, M.; Szwaja, S. Usage of Converter Gas as a Substitute Fuel for a Tunnel Furnace in Steelworks. Materials 2022, 15, 5054. https://doi.org/10.3390/ma15145054
Musial D, Szwaja M, Kurtyka M, Szwaja S. Usage of Converter Gas as a Substitute Fuel for a Tunnel Furnace in Steelworks. Materials. 2022; 15(14):5054. https://doi.org/10.3390/ma15145054
Chicago/Turabian StyleMusial, Dorota, Magdalena Szwaja, Marek Kurtyka, and Stanislaw Szwaja. 2022. "Usage of Converter Gas as a Substitute Fuel for a Tunnel Furnace in Steelworks" Materials 15, no. 14: 5054. https://doi.org/10.3390/ma15145054
APA StyleMusial, D., Szwaja, M., Kurtyka, M., & Szwaja, S. (2022). Usage of Converter Gas as a Substitute Fuel for a Tunnel Furnace in Steelworks. Materials, 15(14), 5054. https://doi.org/10.3390/ma15145054







