How Porphyromonas gingivalis Navigate the Map: The Effect of Surface Topography on the Adhesion of Porphyromonas gingivalis on Biomaterials
Abstract
:1. Introduction
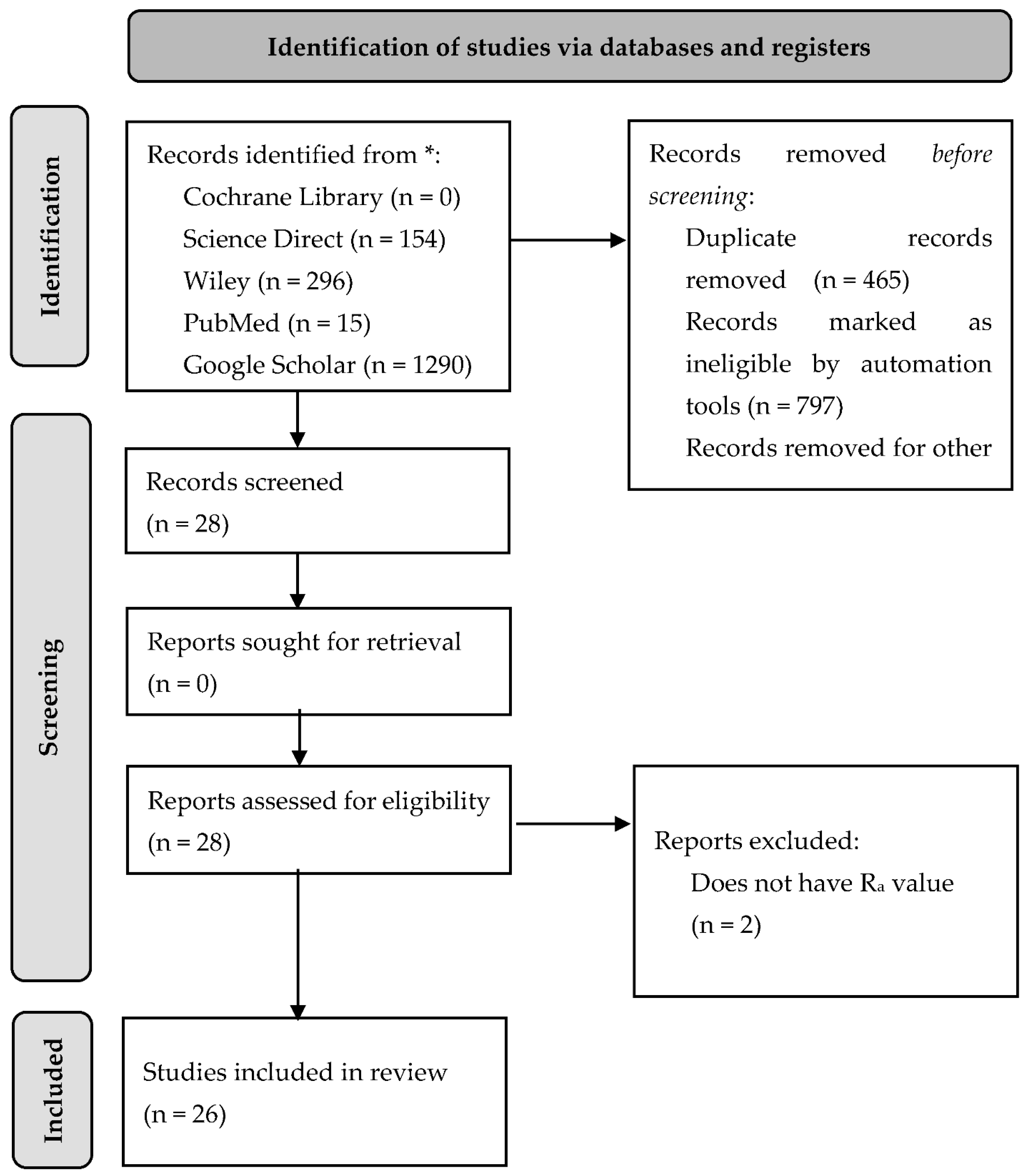
2. Materials and Methods
3. Discussion
3.1. Porphyromonas gingivalis Structure and Characteristic
3.1.1. Fimbriae
3.1.2. Capsule
3.1.3. Cell-Wall
3.2. Biofilm Formation
3.3. Surface Topography
3.4. Subperiosteal Implant Materials and Surface Modification
3.5. How Porphyromonas gingivalis Responds to Topography
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nguyen, T.; Caruhel, J.B.; Khonsari, R. A subperiosteal maxillary implant causing severe osteolysis. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Minichetti, J.C. Analysis of HA-coated Subperiosteal Implants. J. Oral Implantol. 1999, 07631, 111–116. [Google Scholar] [CrossRef]
- Schou, S.; Pallesen, L.; Hjørting-Hansen, E.; Pedersen, C.S.; Fibæk, B. A 41-year history of a mandibular subperiosteal implant. Clin. Oral Implant. Res. 2000, 11, 171–178. [Google Scholar] [CrossRef]
- Cerea, M.; Dolcini, G.A. Custom-Made Direct Metal Laser Sintering Titanium Subperiosteal Implants: A Retrospective Clinical Study on 70 Patients. Biomed. Res. Int. 2018, 2018, 5420391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misch, C.E. Disadvantages of the maxillary subperiosteal implant. Dent. Today 1990, 9, 34–35. [Google Scholar]
- Mapkar, M.A.; Syed, R. Revisiting the maxillary subperiosteal implant prosthesis: A case study. J. Dent. Implant. 2015, 5, 113. [Google Scholar] [CrossRef]
- Rams, T.E.; Balkin, B.E.; Roberts, T.W.; Molzan, A.K. Microbiological aspects of human mandibular subperiosteal dental implants. J. Oral Implantol. 2013, 39, 714–722. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Clin. Periodontol. 2018, 45, S246–S266. [Google Scholar] [CrossRef] [Green Version]
- Misch, C.M. Personalised medicine: Applications for dental. Int. J. Oral Implant. 2021, 14, 119–120. [Google Scholar]
- Mombelli, A. Etiology, diagnosis, and treatment considerations in peri-implantitis. Curr. Opin. Periodontol. 1997, 4, 127–136. [Google Scholar]
- Donelli, G. (Ed.) Biofilm-Based Healthcare-Associated Infections; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, E.; Torosian, J.; Slots, J. Microbial differences in 2 clinically distinct types of failures of osseointegrated implants Clin Oral Impl Res. Clin. Oral Implant. Res. 1991, 1991, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Noiri, Y.; Yamaguchi, M.; Asahi, Y.; Maezono, H.; Ebisu, S.; Hayashi, M. Inhibition of polysaccharide synthesis by the sinR orthologue PGN_0088 is indirectly associated with the penetration of Porphyromonas gingivalis biofilms by macrolide antibiotics. Microbiology 2015, 161, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuson, H.H.; Weibel, D.B. Bacteria-surface interactions. Soft Matter 2013, 9, 4368–4380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, Z.; Nasazky, E.; Boyan, B.D. Surface microtopography regulates osteointegration: The role of implant surface microtopography in osteointegration. Alpha Omegan 2005, 98, 9–19. [Google Scholar]
- Bermejo, P.; Sánchez, M.C.; Llama-Palacios, A.; Figuero, E.; Herrera, D.; Alonso, M.S. Biofilm formation on dental implants with different surface micro-topography: An in vitro study. Clin. Oral Implant. Res. 2019, 30, 725–734. [Google Scholar] [CrossRef]
- Beacchey, E.H. Bacterial Adherence: Adhesin-Receptor Interactions Mediating the Attachment of Bacteria to Mucosal Surfaces. J. Infect. Dis. 1981, 143, 325–345. [Google Scholar] [CrossRef]
- Fang, D.; Yuran, S.; Reches, M.; Catunda, R.; Levin, L.; Febbraio, M. A peptide coating preventing the attachment of Porphyromonas gingivalis on the surfaces of dental implants. J. Periodontal Res. 2020, 55, 503–510. [Google Scholar] [CrossRef]
- Garcia, D.R.; Deckey, D.G.; Zega, A.; Mayfield, C.; Spake, C.S.L.; Emanuel, T.; Daniels, A.; Jarrell, J.; Glasser, J.; Born, C.T.; et al. Analysis of growth and biofilm formation of bacterial pathogens on frequently used spinal implant materials. Spine Deform. 2020, 8, 351–359. [Google Scholar] [CrossRef]
- Schwarz, F.; Sculean, A.; Wieland, M.; Horn, N.; Nuesry, E.; Bube, C.; Becker, J. Effects of hydrophilicity and microtopography of titanium implant surfaces on initial supragingival plaque biofilm Formation. A pilot study. Mund-Kiefer-Und Gesichtschirurgie 2007, 11, 333–338. [Google Scholar] [CrossRef]
- Lee, S.W.; Phillips, K.S.; Gu, H.; Kazemzadeh-Narbat, M.; Ren, D. How microbes read the map: Effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 2021, 268, 120595. [Google Scholar] [CrossRef] [PubMed]
- Mitik-Dineva, N.; Wang, J.; Mocanasu, R.C.; Stoddart, P.R.; Crawford, R.J.; Ivanova, E.P. Impact of nano-topography on bacterial attachment. Biotechnol. J. 2008, 3, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Rismanchian, M.; Babashahi, A.; Goroohi, H.; Shahabouee, M.; Yaghini, J.; Badrian, H. Microflora around teeth and dental implants. Dent. Res. J. 2012, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.R.; Renvert, S. Cluster of bacteria associated with peri-implantitis. Clin. Implant. Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincón, M.V.; Gómez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Mei, F.; Xie, M.; Huang, X.; Long, Y.; Lu, X.; Wang, X.; Chen, L. Porphyromonas gingivalis and its systemic impact: Current status. Pathogens 2020, 9, 944. [Google Scholar] [CrossRef]
- Bogino, P.C.; De, M.; Oliva, M.; Sorroche, F.G. The Role of Bacterial Biofilms and Surface Components in Plant-Bacterial Associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef] [Green Version]
- Enersen, M.; Nakano, K.; Amano, A. Porphyromonas gingivalis fimbriae. J. Oral Microbiol. 2013, 5, 20265. [Google Scholar] [CrossRef]
- Hamada, S.; Amano, A.; Kimura, S.; Nakagawa, I.; Kawabata, S.; Morisaki, I. The importance of fimbriae in the virulence and ecology of some oral bacteria. Oral Microbiol. Immunol. 1998, 13, 129–138. [Google Scholar] [CrossRef]
- Amano, A.; Nakagawa, I.; Okahashi, N.; Hamada, N. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J. Periodontal Res. 2004, 39, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Thanassi, D.G.; Nuccio, S.-P.; So, S.S.K.; Bäumler, A.J. Fimbriae: Classification and Biochemistry. EcoSal Plus 2007, 2. [Google Scholar] [CrossRef] [PubMed]
- Sojar, H.T.; Sharma, A.; Genco, R.J. Porphyromonas gingivalis fimbriae bind to cytokeratin of epithelial cells. Infect. Immun. 2002, 70, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.S.; Silveira, V.R.; Rego, R.O. Analysis of Porphyromonas gingivalis fimA genotypes in severe periodontitis patients. Braz. Oral Res. 2020, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nagano, K.; Hasegawa, Y.; Abiko, Y.; Yoshida, Y.; Murakami, Y.; Yoshimura, F. Porphyromonas gingivalis FimA Fimbriae: Fimbrial Assembly by fimA Alone in the fim Gene Cluster and Differential Antigenicity among fimA Genotypes. PLoS ONE 2012, 7, e43722. [Google Scholar] [CrossRef]
- Dickinson, D.P.; Kubiniec, M.A.; Yoshimura, F.; Genco, R.J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J. Bacteriol. 1988, 170, 1658–1665. [Google Scholar] [CrossRef] [Green Version]
- Ikai, R.; Hasegawa, Y.; Izumigawa, M.; Nagano, K.; Yoshida, Y.; Kitai, N.; Lamont, R.J.; Yoshimura, F.; Murakami, Y. Mfa4, an Accessory Protein of Mfa1 Fimbriae, Modulates Fimbrial Biogenesis, Cell Auto- Aggregation, and Biofilm Formation in Porphyromonas gingivalis. PLoS ONE 2015, 10, e139454. [Google Scholar] [CrossRef]
- Singh, A.; Wyant, T.; Anaya-Bergman, C.; Aduse-Opoku, J.; Brunner, J.; Laine, M.L.; A Curtis, M.; Lewis, J.P. The capsule of porphyromonas gingivalis leads to a reduction in the host inflammatory response, evasion of phagocytosis, and increase in Virulence. Infect. Immun. 2011, 79, 4533–4542. [Google Scholar] [CrossRef] [Green Version]
- Brunner, J.; Scheres, N.; El Idrissi, N.B.; Deng, D.M.; Laine, M.L.; van Winkelhoff, A.J.; Crielaard, W. The capsule of Porphyromonas gingivalis reduces the immune response of human gingival fibroblasts. BMC Microbiol. 2010, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Septiwidyati, T.R.; Bachtiar, E.W. The Role of Porphyromonas GingivalisVirulence Factors in Periodontitis Immunopathogenesis. Dentika Dent. J. 2020, 23, 6–12. [Google Scholar] [CrossRef]
- Hayashi, J.; Hamada, N.; Kuramitsu, H.K. The autolysin of Porphyromonas gingivalis is involved in outer membrane vesicle release. FEMS Microbiol. Lett. 2002, 216, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Hirota, K.; Yoshida, K.; Weng, Y.; He, Y. Outer membrane vesicles of Porphyromonas gingivalis: Novel communication tool and strategy. Jpn. Dent. Sci. Rev. 2021, 57, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Cecil, J.D.; Brien-simpson, N.M.O.; Lenzo, J.C.; Holden, J.A.; Reynolds, E.C. Outer Membrane Vesicles Prime and Activate Macrophage Inflammasomes and Cytokine Secretion In Vitro and In Vivo. Front. Immunol. 2017, 8, 1017. [Google Scholar] [CrossRef] [Green Version]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Majumdar, S.; Pal, S. A Physical Insight of Biofilms. In Model Organisms for Microbial Pathogenesis, Biofilm Formation and Antimicrobial Drug Discovery; Siddhardha, B., Dyavaiah, M., Syed, A., Eds.; Springer: Singapore, 2020; pp. 37–46. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 1–22. [Google Scholar] [CrossRef]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef]
- Mabboux, F.; Ponsonnet, L.; Morrier, J.J.; Jaffrezic, N.; Barsotti, O. Surface free energy and bacterial retention to saliva-coated dental implant materials—An in vitro study. Colloids Surf. B Biointerfaces 2004, 39, 199–205. [Google Scholar] [CrossRef]
- Sri, A.K.; Deepika, G.; Nishanthini, J.; Hikku, G.S.; Jeyasubramanian, K.; Murugesan, R. Super-hydrophobicity: Mechanism, fabrication and its application in medical implants to prevent biomaterial associated infections. J. Ind. Eng. Chem. 2020, 92, 1–17. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, B.; Liu, Y.; Suo, X.; Li, H. Influence of surface topography on bacterial adhesion: A review (Review). Biointerphases 2018, 13, 060801. [Google Scholar] [CrossRef] [Green Version]
- Xin, Q. Friction and lubrication in diesel engine system design. Diesel Engine Syst. Des. 2013, 651–758. [Google Scholar] [CrossRef]
- Rudawska, A. Assessment of surface preparation for the bonding/adhesive technology. Surf. Treat. Bond. Technol. 2019, 227–275. [Google Scholar] [CrossRef]
- Cassie, B.D. Of porous surfaces. Trans. Faraday Soc. 1944, 5, 546–551. [Google Scholar] [CrossRef]
- Pidhatika, B.; Möller, J.; Benetti, E.M.; Konradi, R.; Rakhmatullina, E.; Mühlebach, A.; Zimmermann, R.; Werner, C.; Vogel, V.; Textor, M. The role of the interplay between polymer architecture and bacterial surface properties on the microbial adhesion to polyoxazoline-based ultrathin films. Biomaterials 2010, 31, 9462–9472. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q. Role of surface roughness in the wettability, surface energy and flotation kinetics of calcite. Powder Technol. 2020, 371, 55–63. [Google Scholar] [CrossRef]
- Zortuk, M.; Kesim, S.; Kaya, E.; Özbilge, H. Bacterial Adhesion of Porphyromonas Gingivalis on Provisional Fixed Prosthetic Materials. Dent. Res. J. 2010, 7, 35–40. [Google Scholar]
- Alagatu, A.; Dhapade, D.; Gajbhiye, M.; Panjrekar, R.; Raut, A. Review of different material and surface modification techniques for dental implants. Mater. Today Proc. 2022, 60, 2245–2249. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, N.; Weber, K.K. Dental Implants; StatPearls Publishing: Treasure Island, FL, USA, 2021; Available online: http://europepmc.org/books/NBK470448 (accessed on 5 April 2022).
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.F.; Cardaropoli, G.; Thompson, V.P.; Lemons, J.E. Basic research methods and current trends of dental implant surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 579–596. [Google Scholar] [CrossRef]
- Özcan, M.; Hämmerle, C. Titanium as a reconstruction and implant material in dentistry: Advantages and pitfalls. Materials 2012, 5, 1528–1545. [Google Scholar] [CrossRef] [Green Version]
- Webber, L.P.; Chan, H.L.; Wang, H.L. Will zirconia implants replace titanium implants? Appl. Sci. 2021, 11, 6776. [Google Scholar] [CrossRef]
- Han, A.; Tsoi, J.K.H.; Matinlinna, J.P.; Chen, Z. Influence of grit-blasting and hydrofluoric acid etching treatment on surface characteristics and biofilm formation on zirconia. Coatings 2017, 7, 130. [Google Scholar] [CrossRef]
- Yeo, I.-S. Reality of Dental Implant Surface Modification: A Short Literature Review. Open Biomed. Eng. J. 2014, 8, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.-W.; Zhong, Y.; Wang, L. Multi-scale surface treatments of titanium implants for rapid osseointegration: A review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Grandin, H.M.; Berner, S.; Dard, M. A review of Titanium Zirconium (TiZr) alloys for use in endosseous dental implants. Materials 2012, 5, 1348–1360. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kang, I.-G.; Cheon, K.-H.; Lee, S.; Park, S.; Kim, H.-E.; Han, C.-M. Stable sol–gel hydroxyapatite coating on zirconia dental implant for improved osseointegration. J. Mater. Sci. Mater. Med. 2021, 32, 81. [Google Scholar] [CrossRef]
- Pardun, K.; Treccani, L.; Volkmann, E.; Streckbein, P.; Heiss, C.; Destri, G.L.; Marletta, G.; Rezwan, K. Mixed zirconia calcium phosphate coatings for dental implants: Tailoring coating stability and bioactivity potential. Mater. Sci. Eng. C 2015, 48, 337–346. [Google Scholar] [CrossRef]
- Munro, T.; Miller, C.M.; Antunes, E.; Sharma, D. Interactions of osteoprogenitor cells with a novel zirconia implant surface. J. Funct. Biomater. 2020, 11, 50. [Google Scholar] [CrossRef]
- Oliva, J.; Oliva, X.; Oliva, J.D. Five-year success rate of 831 consecutively placed Zirconia dental implants in humans: A comparison of three different rough surfaces. Int. J. Oral Maxillofac. Implant. 2010, 25, 336–344. [Google Scholar]
- Delgado-Ruíz, R.A.; Calvo-Guirado, J.L.; Moreno, P.; Guardia, J.; Gomez-Moreno, G.; Mate-Sánchez, J.E.; Ramirez-Fernández, P.; Chiva, F. Femtosecond laser microstructuring of zirconia dental implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96, 91–100. [Google Scholar] [CrossRef]
- Tuna, T.; Wein, M.; Swain, M.; Fischer, J.; Att, W. Influence of ultraviolet photofunctionalization on the surface characteristics of zirconia-based dental implant materials. Dent. Mater. 2015, 31, e14–e24. [Google Scholar] [CrossRef]
- Robinson, F.G.; Knoernschild, K.L.; Sterrett, J.D.; Tompkins, R. Porphyromonas gingivalis endotoxin affinity for dental ceramics. J. Prosthet. Dent. 1996, 75, 217–227. [Google Scholar] [CrossRef]
- Verran, J.; Boyd, R.D. The relationship between substratum surface roughness and microbiological and organic soiling: A review. Biofouling 2001, 17, 59–71. [Google Scholar] [CrossRef]
- Xu, Z.; He, Y.; Zeng, X.; Zeng, X.; Huang, J.; Lin, X.; Chen, J. Enhanced Human Gingival Fibroblast Response and Reduced Porphyromonas gingivalis Adhesion with Titania Nanotubes. Biomed Res. Int. 2020, 2020, 5651780. [Google Scholar] [CrossRef] [PubMed]
- Pier-Francesco, A.; Adams, R.J.; Waters, M.G.J.; Williams, D.W. Titanium surface modification and its effect on the adherence of Porphyromonas gingivalis: An in vitro study. Clin. Oral Implant. Res. 2006, 17, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Mukaddam, K.; Astasov-Frauenhoffer, M.; Fasler-Kan, E.; Marot, L.; Kisiel, M.; Meyer, E.; Köser, J.; Waser, M.; Bornstein, M.M.; Kühl, S. Effect of a nanostructured titanium surface on gingival cell adhesion, viability and properties against P. gingivalis. Materials 2021, 14, 7686. [Google Scholar] [CrossRef]
- Daw, A.E.; Colombo, J.S.; Rowe, W.G.; Waddington, R.J.; Thomas, D.W.; Kazi, H.A.; Williams, D.W.; Moseley, R. Differential cellular and microbial responses to nano-/micron-scale titanium surface roughness induced by hydrogen peroxide treatment. J. Biomater. Appl. 2013, 28, 144–160. [Google Scholar] [CrossRef]
- Kim, M.L.; Jeong, C.M.; Jeon, Y.C.; Byon, E.; Jeong, Y.; Cho, L.R. The effects of Mg-ion implantation and sandblasting on Porphyromonas gingivalis attachment. Clin. Oral Implant. Res. 2012, 23, 245–252. [Google Scholar] [CrossRef]
- Batsukh, N.; Feng, S.W.; Lee, W.F.; Leu, S.-J.; Tsai, P.-Y.; Ho, K.-N.; Lin, C.T.; Su, C.-H.; Chang, W.-J. Effects of Porphyromonas gingivalis on Titanium Surface by Different Clinical Treatment. J. Med. Biol. Eng. 2017, 37, 35–44. [Google Scholar] [CrossRef]
- Mukaddam, K.; Astasov-Frauenhoffer, M.; Fasler-Kan, E.; Marot, L.; Kisiel, M.; Steiner, R.; Sanchez, F.; Meyer, E.; Köser, J.; Bornstein, M.M.; et al. Novel Titanium Nanospike Structure Using Low-Energy Helium Ion Bombardment for the Transgingival Part of a Dental Implant. Nanomaterials 2022, 12, 1065. [Google Scholar] [CrossRef]
- de Avila, E.D.; de Molon, R.S.; Lima, B.P.; Lux, R.; Shi, W.; Junior, M.J.; Spolidorio, D.M.P.; Vergani, C.E.; Junior, F.D.A.M. Impact of physical chemical characteristics of abutment implant surfaces on bacteria adhesion. J. Oral Implantol. 2016, 42, 153–158. [Google Scholar] [CrossRef]
- Teughels, W.; van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Surface Free Energy. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.-W., Eds.; Springer: Boston, MA, USA, 2013; pp. 3443–3448. [Google Scholar] [CrossRef]
- di Giulio, M.; Traini, T.; Sinjari, B.; Nostro, A.; Caputi, S.; Cellini, L. Porphyromonas gingivalis biofilm formation in different titanium surfaces, an in vitro study. Clin. Oral Implant. Res. 2016, 27, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lai, Y.; Huang, W.; Huang, S.; Xu, Z.; Chen, J.; Wu, D. Biofunctionalization of microgroove titanium surfaces with an antimicrobial peptide to enhance their bactericidal activity and cytocompatibility. Colloids Surf. B Biointerfaces 2015, 128, 552–560. [Google Scholar] [CrossRef]
- Barão, V.A.R.; Yoon, C.J.; Mathew, M.T.; Yuan, J.C.-C.; Wu, C.D.; Sukotjo, C. Attachment of Porphyromonas gingivalis to Corroded Commercially Pure Titanium and Titanium-Aluminum-Vanadium Alloy. J. Periodontol. 2014, 85, 1275–1282. [Google Scholar] [CrossRef]
- Jeyachandran, Y.; Venkatachalam, S.; Karunagaran, B.; Narayandass, S.; Mangalaraj, D.; Bao, C.; Zhang, C. Bacterial adhesion studies on titanium, titanium nitride and modified hydroxyapatite thin films. Mater. Sci. Eng. C 2007, 27, 35–41. [Google Scholar] [CrossRef]
- Jeyachandran, Y.L.; Narayandass, S.K.; Mangalaraj, D.; Bao, C.Y.; Martin, P.J. The effect of surface composition of titanium films on bacterial adhesion. Biomed. Mater. 2006, 1, L1–L5. [Google Scholar] [CrossRef]
- Bollen, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Llama-Palacios, A.; Sánchez, M.C.; Díaz, L.; Cabal, B.; Suárez, M.; Moya, J.; Torrecillas, R.; Figuero, E.; Sanz, M.; Herrera, D. In vitro biofilm formation on different ceramic biomaterial surfaces: Coating with two bactericidal glasses. Dent. Mater. 2019, 35, 883–892. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardhani, R.; Diana, R.; Pidhatika, B. How Porphyromonas gingivalis Navigate the Map: The Effect of Surface Topography on the Adhesion of Porphyromonas gingivalis on Biomaterials. Materials 2022, 15, 4988. https://doi.org/10.3390/ma15144988
Ardhani R, Diana R, Pidhatika B. How Porphyromonas gingivalis Navigate the Map: The Effect of Surface Topography on the Adhesion of Porphyromonas gingivalis on Biomaterials. Materials. 2022; 15(14):4988. https://doi.org/10.3390/ma15144988
Chicago/Turabian StyleArdhani, Retno, Rasda Diana, and Bidhari Pidhatika. 2022. "How Porphyromonas gingivalis Navigate the Map: The Effect of Surface Topography on the Adhesion of Porphyromonas gingivalis on Biomaterials" Materials 15, no. 14: 4988. https://doi.org/10.3390/ma15144988
APA StyleArdhani, R., Diana, R., & Pidhatika, B. (2022). How Porphyromonas gingivalis Navigate the Map: The Effect of Surface Topography on the Adhesion of Porphyromonas gingivalis on Biomaterials. Materials, 15(14), 4988. https://doi.org/10.3390/ma15144988





