Use of Carbonated Water as Kneading in Mortars Made with Recycled Aggregates
Abstract
:1. Introduction
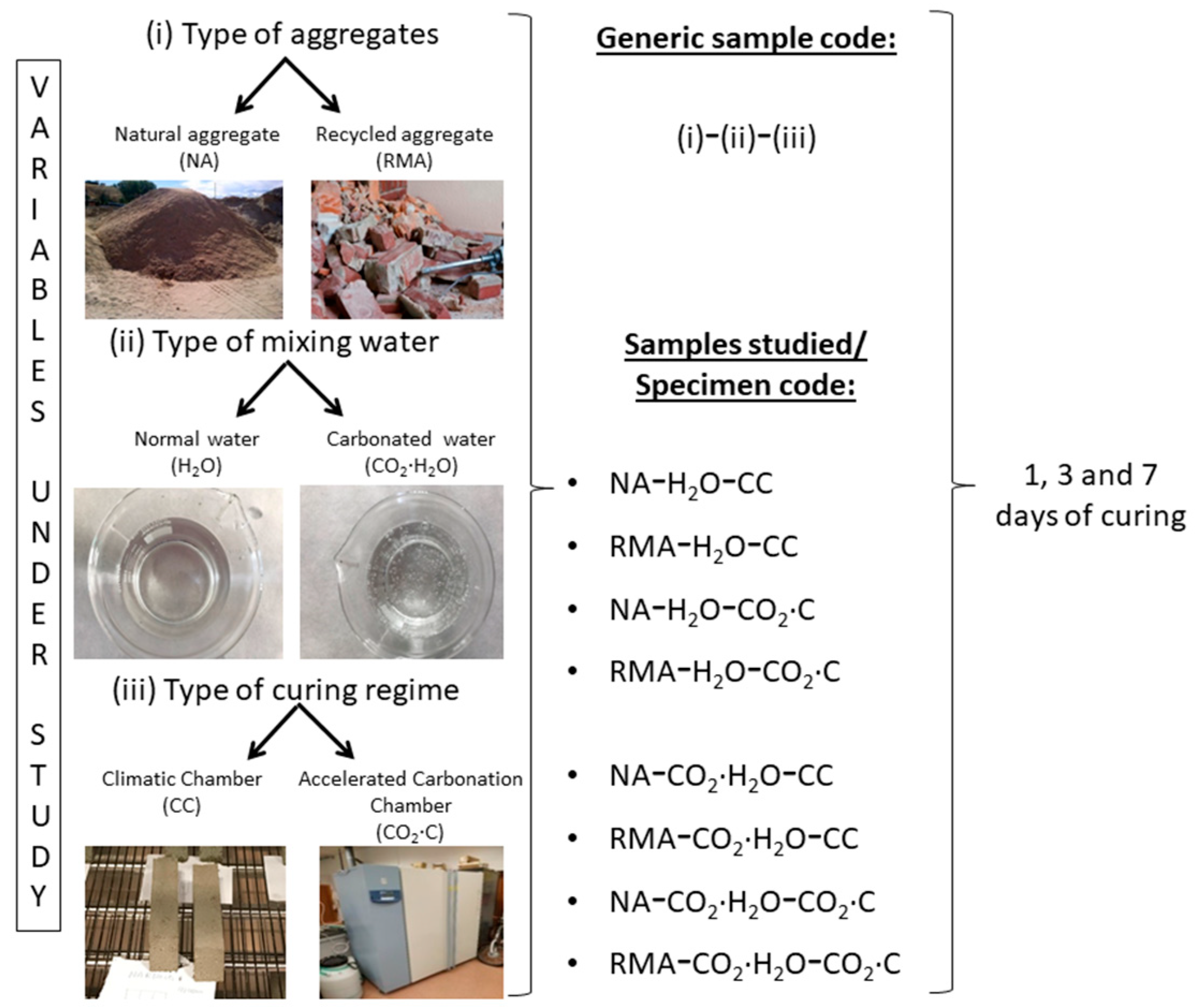
2. Materials and Methods
2.1. Materials
2.2. Experimental Design and Curing Conditions
2.3. Kneading Process
2.4. Test Methods
3. Results and Discussion
3.1. Characterization of Raw Materials
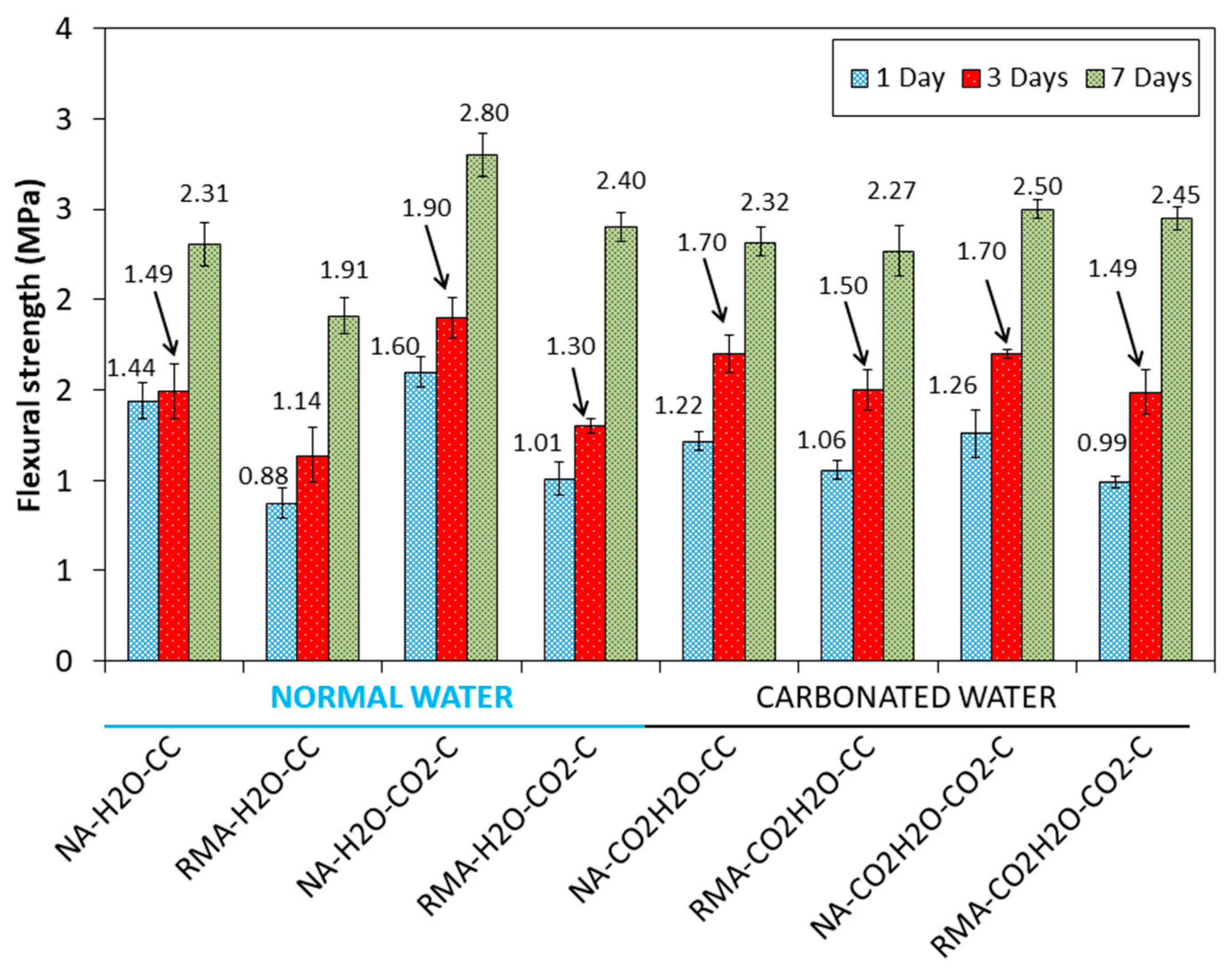
3.2. Compressive and Flexural Strength
3.3. DBD and APW
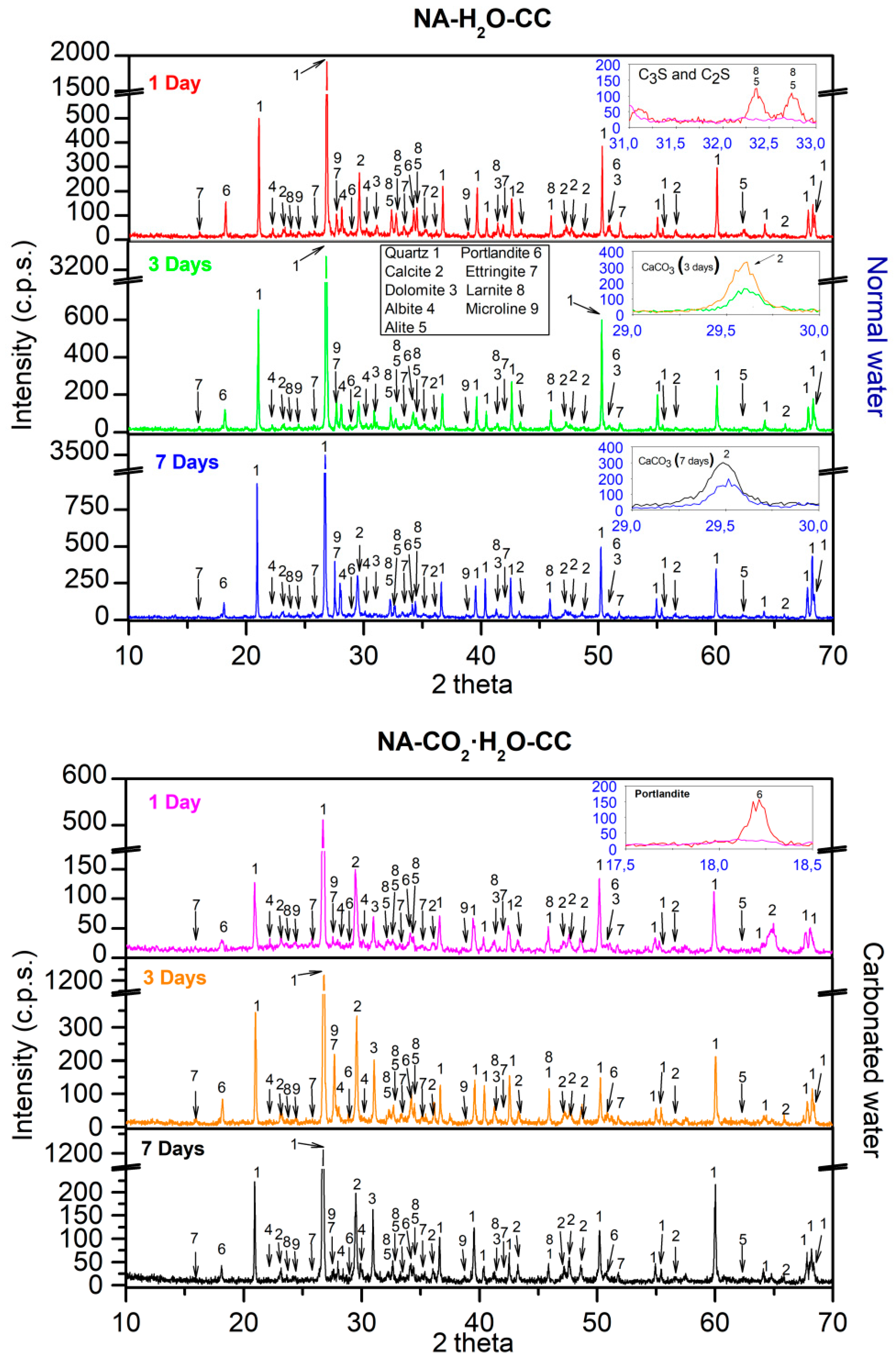
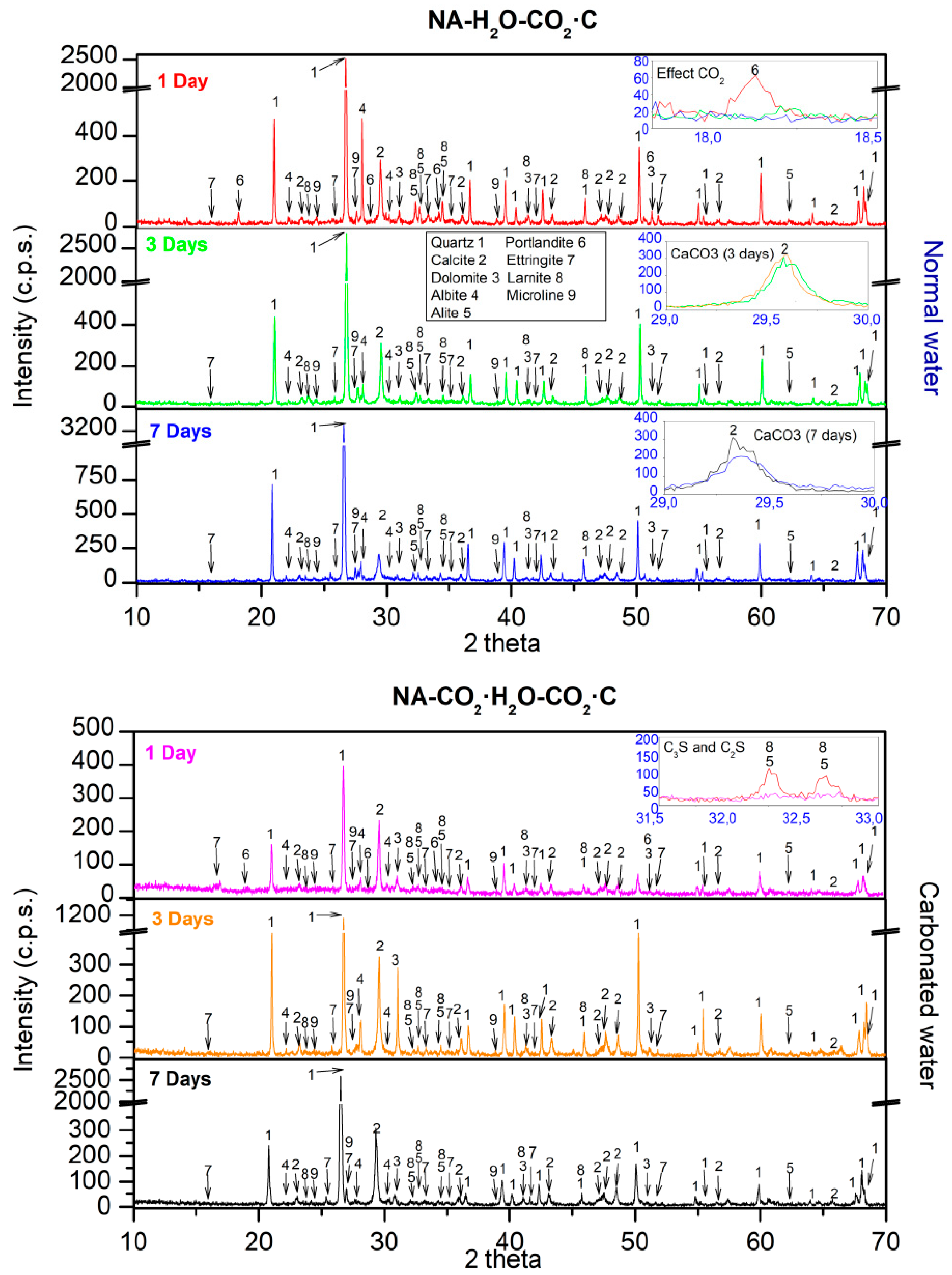
3.4. XRD
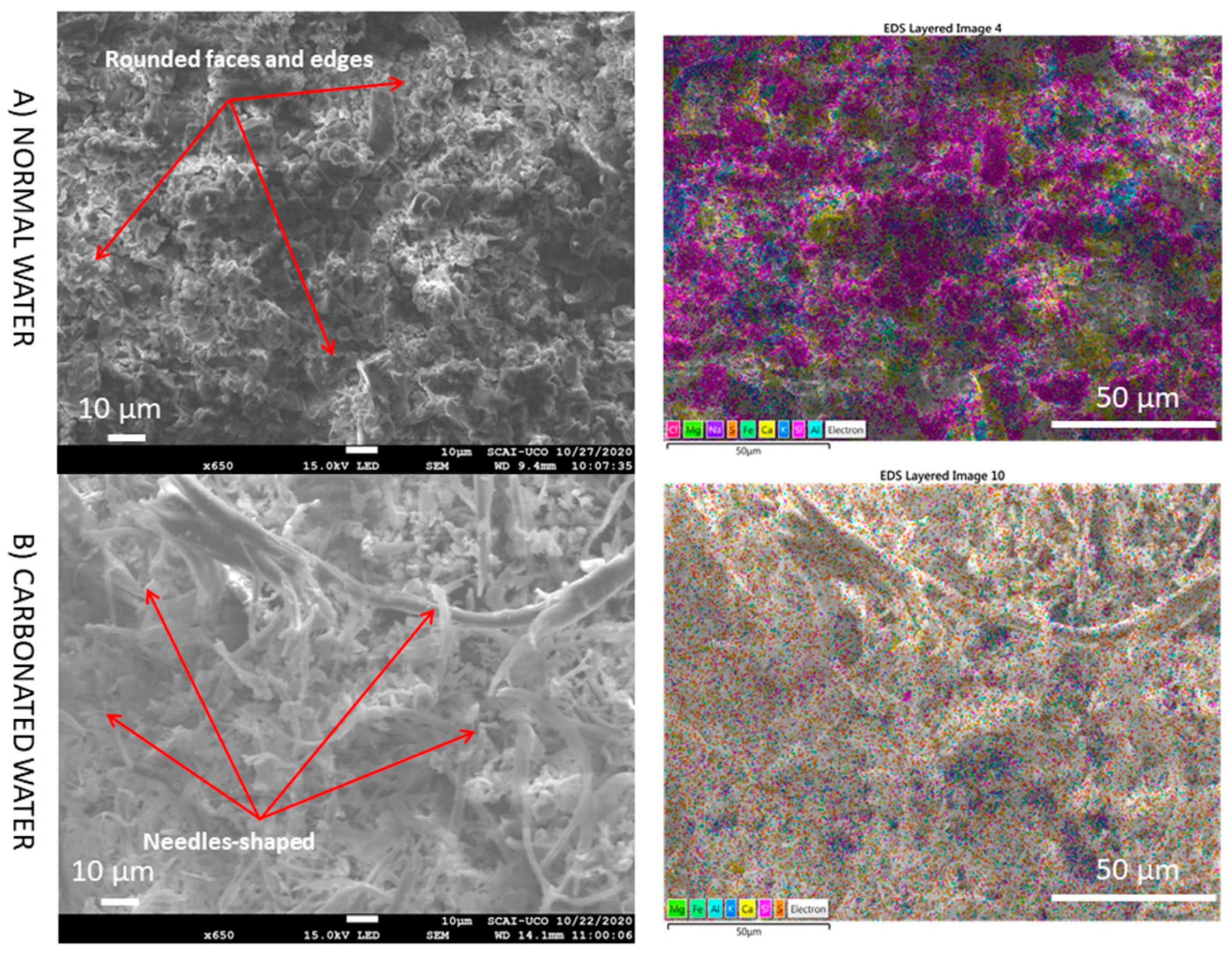
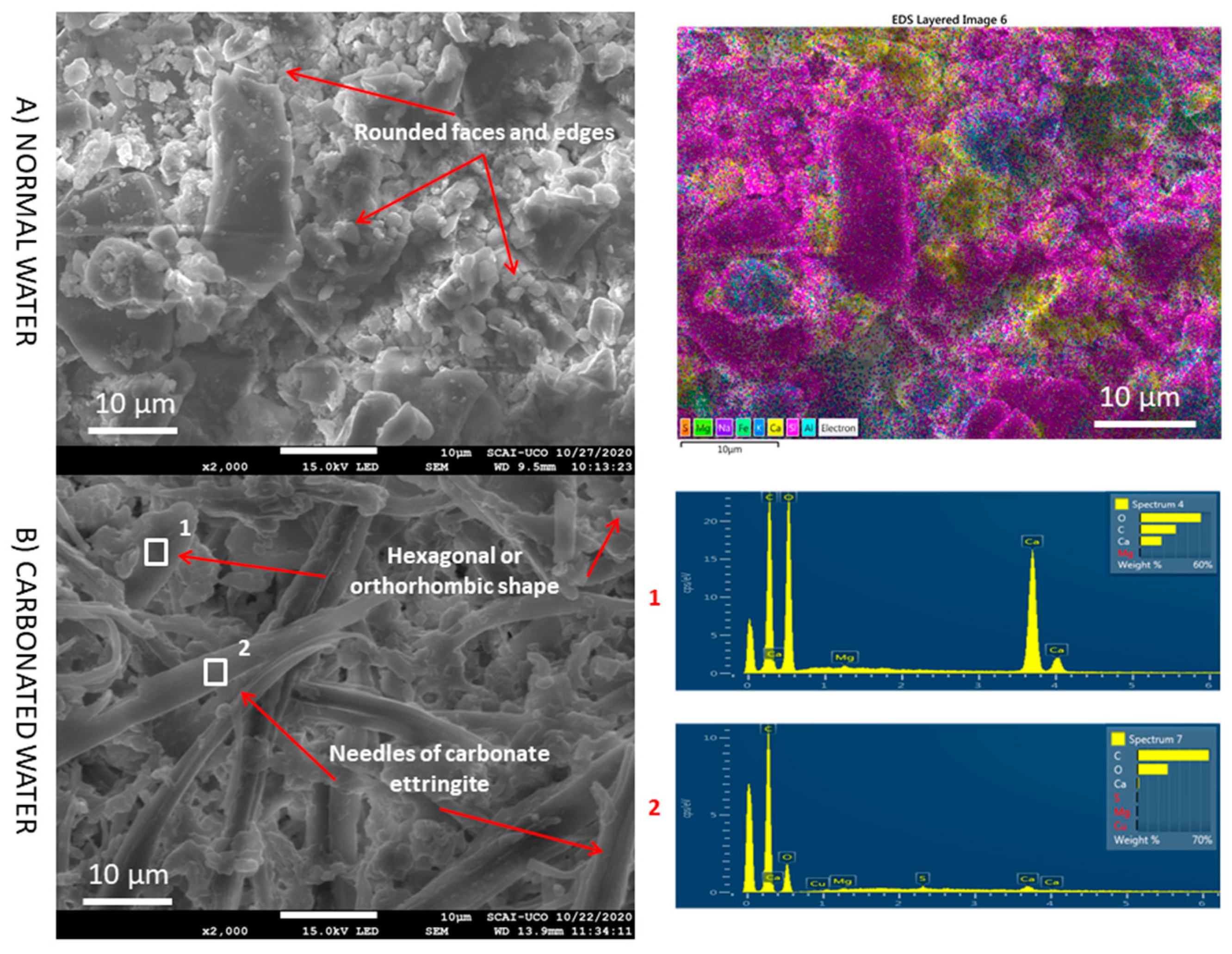
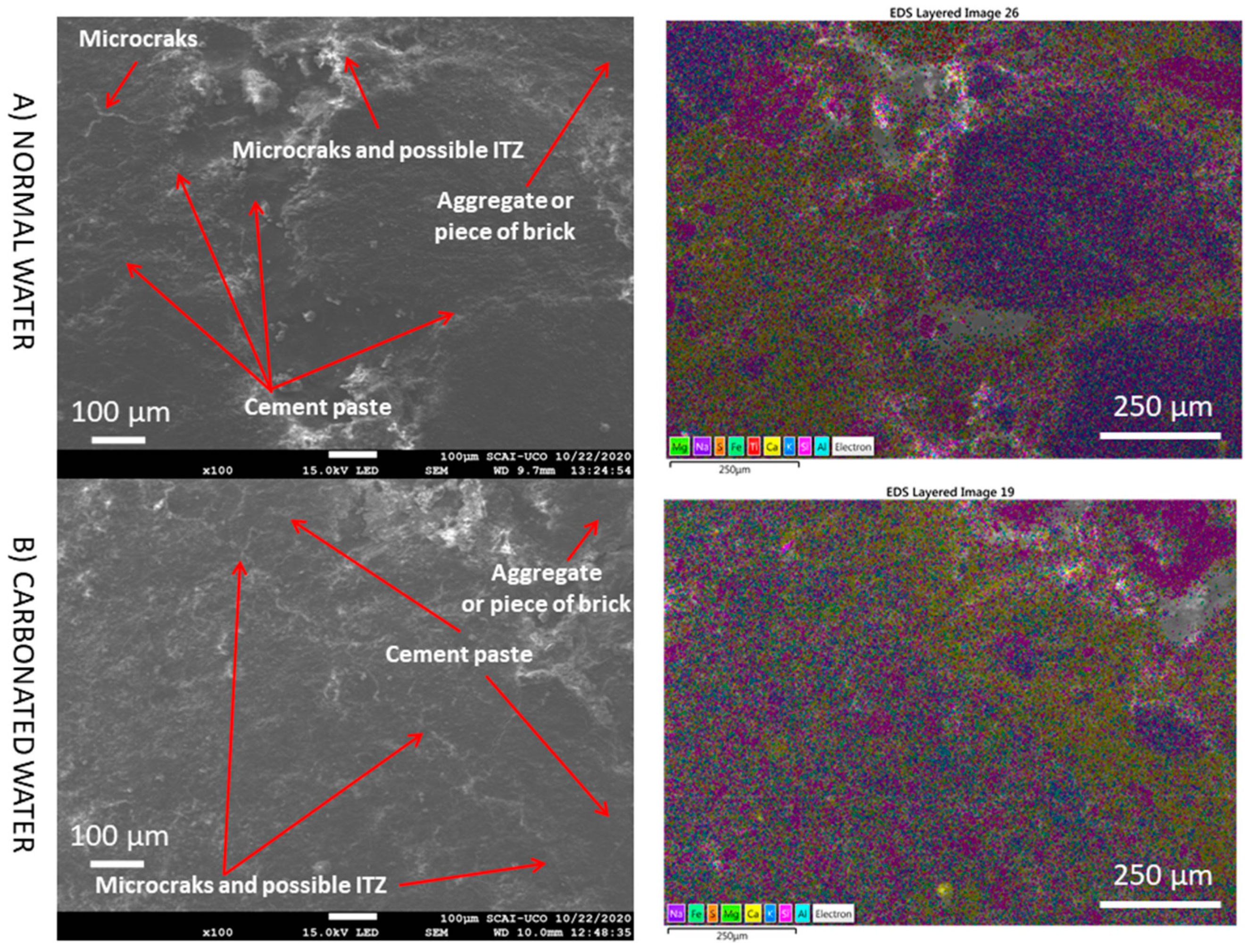
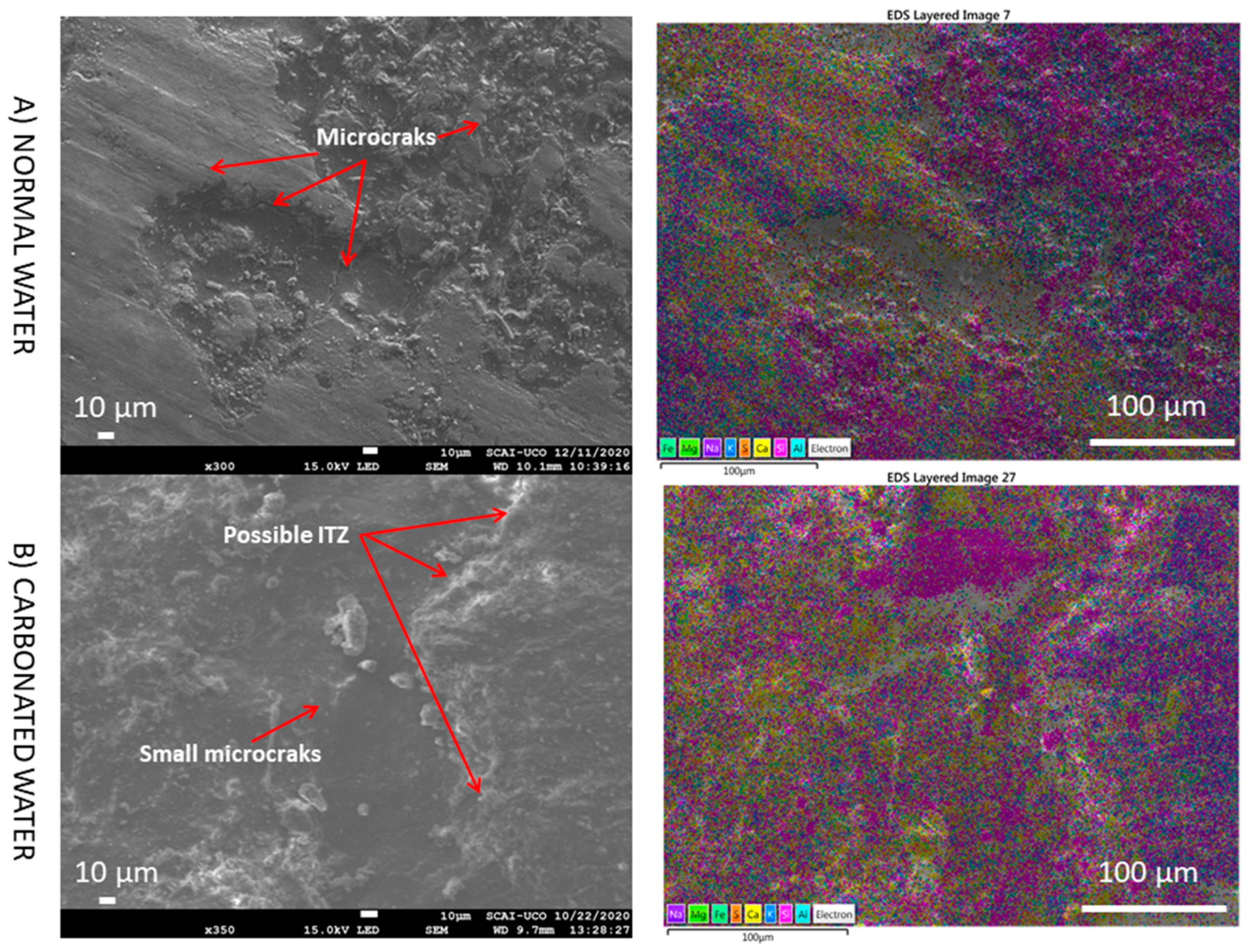
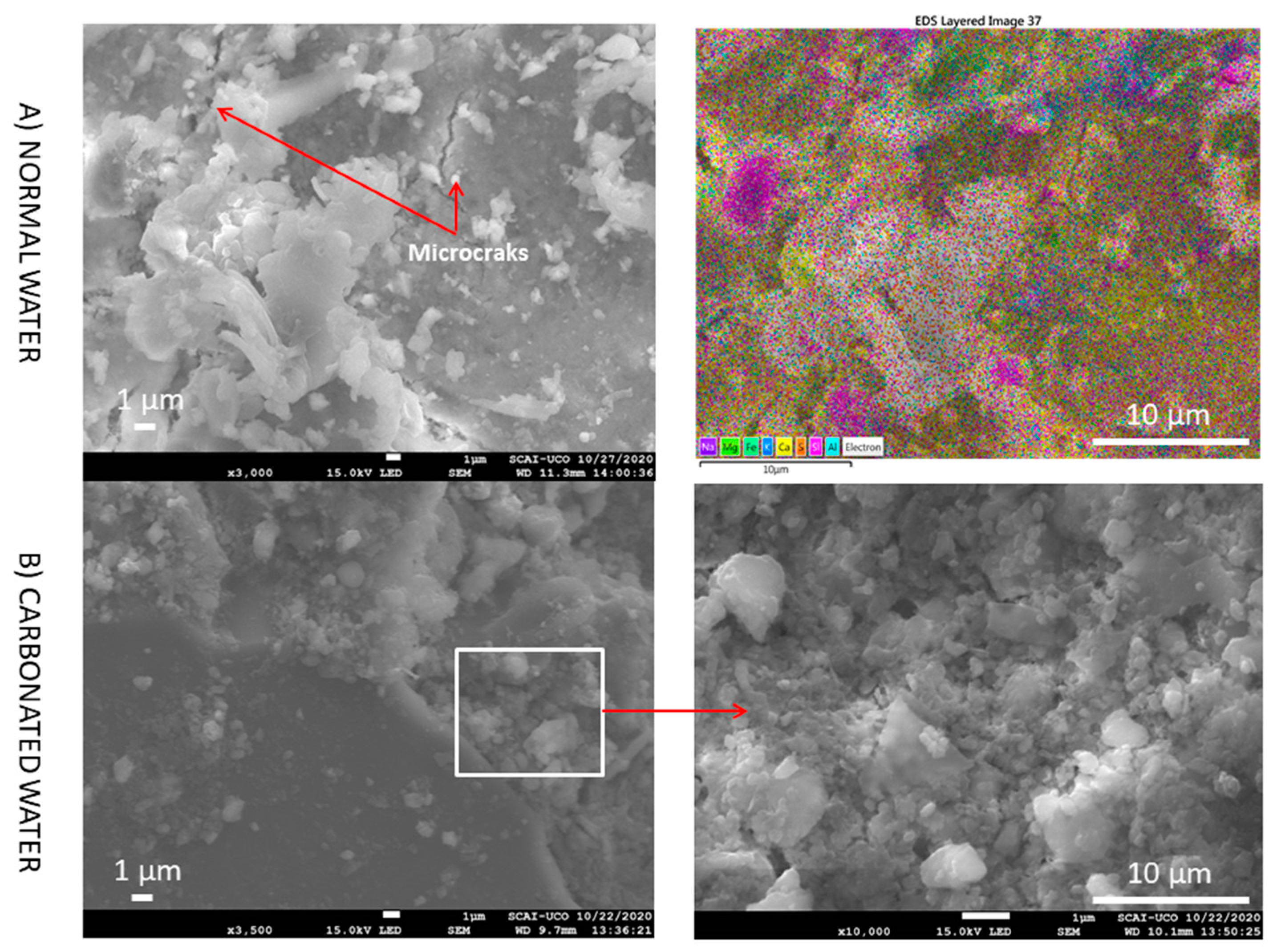
3.5. SEM
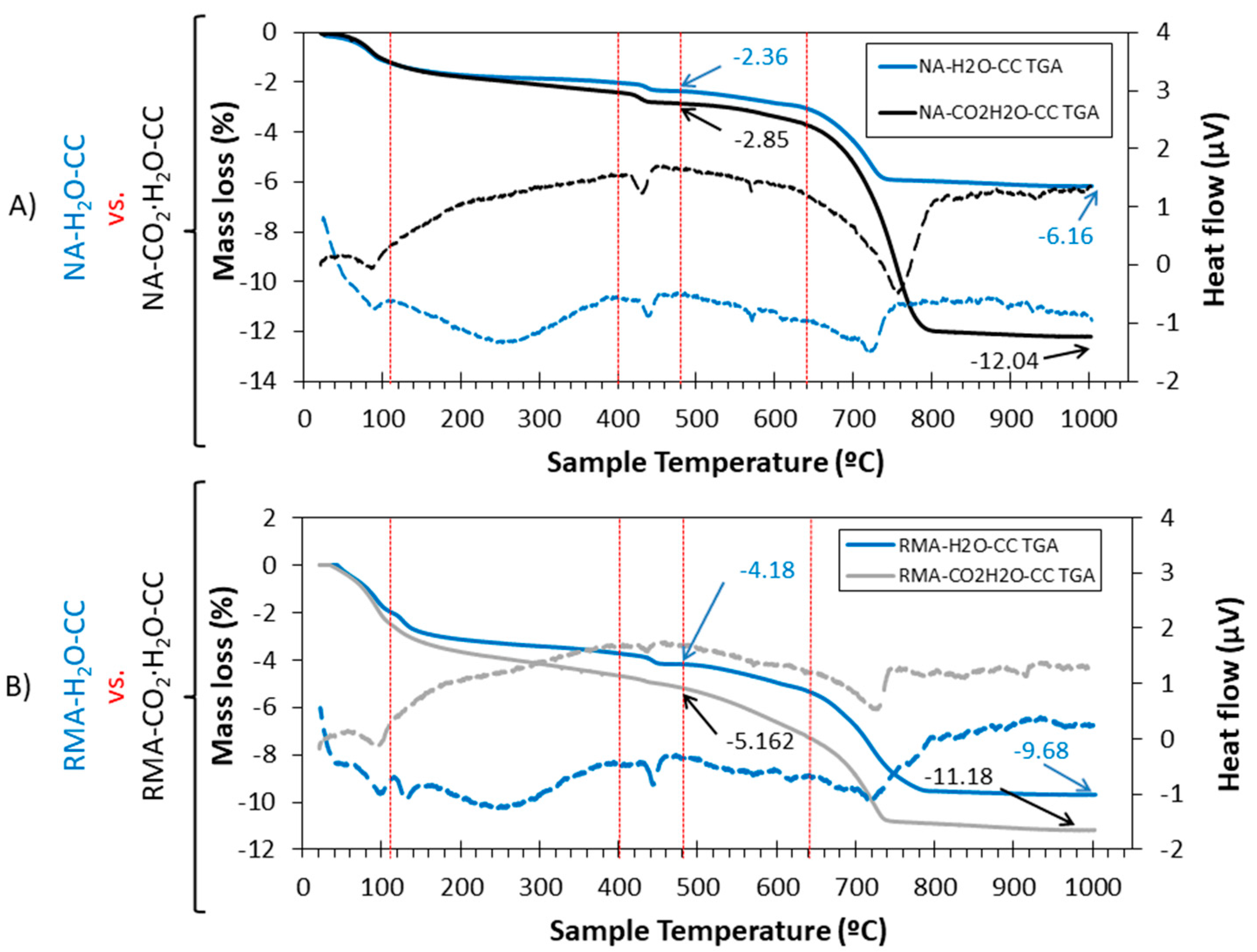
3.6. TGA-DTA
4. Conclusions
- Carbonated water worsened mechanical properties at 1 d of curing with NA under the CC regime, compared to normal water. The phases of CaCO3 and Ca(OH)2 in the RMA, acted as a buffer for carbonated water.
- The low pH value of carbonated water and accelerated carbonation (CO2·C) further lowers the pH, and negatively affects the strength at 1 d of normal curing for all the mixes. The simultaneous utilization of carbonated water as kneading water and subsequent curing in CO2 is not recommended.
- In all the mixtures studied, the effect of carbonated water increased the DBD (due to carbonation) and APW, indicating that carbonated water generated additional porosity. The carbonation reaction that occurs with carbonated water under CC explains the increase in mechanical strength at 7 d of curing for NA and RMA. A greater intensity in the CaCO3 peaks (XRD) and increased weight loss of calcite decomposition (TGA/DTA) was also observed.
- The presence of interlaced needles of ettringite carbonate observed by SEM and the increased presence of calcite (due to the carbonation produced by CO2 in the carbonated water) resulted in better mechanical properties than normal water. Carbonated water on the microstructure of the RMA results in the filling of microcracks (shown in the SEM images). Ettringite carbonate was not observed in this case because of portlandite in RMA, which consumed CO2 from carbonated water. Carbonated water as kneading water using RMA could allow for the production of precast CBM products with good mechanical properties without the need for CO2 curing chamber.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lippiatt, N.; Ling, T.-C.; Pan, S.-Y. Towards carbon-neutral construction materials: Carbonation of cement-based materials and the future perspective. J. Build. Eng. 2020, 28, 101062. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.; Poon, C.S.; Shi, C.J. Mechanism for rapid hardening of cement pastes under coupled CO2-water curing regime. Cem. Concr. Compos. 2019, 97, 78–88. [Google Scholar] [CrossRef]
- Wang, R.; Yu, N.; Li, Y. Methods for improving the microstructure of recycled concrete aggregate: A review. Constr. Build. Mater. 2020, 242, 118164. [Google Scholar] [CrossRef]
- Burek, J.; Nutter, D. Life cycle assessment of grocery, perishable, and general merchandise multi-facility distribution center networks. Energy Build. 2018, 174, 388–401. [Google Scholar] [CrossRef]
- Lippiatt, N.; Ling, T.-C.; Eggermont, S. Combining hydration and carbonation of cement using super-saturated aqueous CO2 solution. Constr. Build. Mater. 2019, 229, 116825. [Google Scholar] [CrossRef]
- Higuchi, T.; Morioka, M.; Yoshioka, I.; Yokozeki, K. Development of a new ecological concrete with CO2 emissions below zero. Constr. Build. Mater. 2014, 67, 338–343. [Google Scholar] [CrossRef]
- Sanjuán, M.; Andrade, C.; Mora, P.; Zaragoza, A. Carbon Dioxide Uptake by Mortars and Concretes Made with Portuguese Cements. Appl. Sci. 2020, 10, 646. [Google Scholar] [CrossRef] [Green Version]
- Kaliyavaradhan, S.K.; Ling, T.-C.; Mo, K.H. CO2 sequestration of fresh concrete slurry waste: Optimization of CO2 uptake and feasible use as a potential cement binder. J. CO2 Util. 2020, 42, 101330. [Google Scholar] [CrossRef]
- Yuan, J. Vertical Profiles of Carbon Dioxide in the Lower Troposphere at Manua Loa Observatory, Hawaii, Determined with a Multi-Copter Drone. In Proceedings of the Ocean Sciences Meeting, San Diego, CA, USA, 16–21 February 2020. [Google Scholar]
- Qiu, R.; Zhang, H.; Zhou, X.; Guo, Z.; Wang, G.; Yin, L.; Liang, Y. A multi-objective and multi-scenario optimization model for operation control of CO2-flooding pipeline network system. J. Clean. Prod. 2020, 247, 119157. [Google Scholar] [CrossRef]
- Yu, S.; Horing, J.; Liu, Q.; Dahowski, R.; Davidson, C.; Edmonds, J.; Liu, B.; McJeon, H.; McLeod, J.; Patel, P.; et al. CCUS in China’s mitigation strategy: Insights from integrated assessment modeling. Int. J. Greenh. Gas Control 2019, 84, 204–218. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Cantador-Fernández, D.; Jiménez, J.; Fernández, J. Mitigation of CO2 emissions by hydrotalcites of Mg3Al-CO3 at 0 °C and high pressure. Appl. Clay Sci. 2020, 202, 105950. [Google Scholar] [CrossRef]
- Liang, C.; Pan, B.; Ma, Z.; He, Z.; Duan, Z. Utilization of CO2 curing to enhance the properties of recycled aggregate and prepared concrete: A review. Cem. Concr. Compos. 2019, 105, 103446. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.-C. Potential of CO2 sequestration through construction and demolition (C&D) waste—An overview. J. CO2 Util. 2017, 20, 234–242. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Zheng, J.; Ling, T.-C. Carbon dioxide sequestration on recycled aggregates. In Carbon Dioxide Sequestration in Cementitious Construction Materials; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar] [CrossRef]
- Quesada Carballo, L.; del Rosario Perez Perez, M.; Cantador Fernandez, D.; Caballero Amores, A.; Fernandez Rodriguez, J.M. Optimum Particle Size of Treated Calcites for CO2 Capture in a Power Plant. Materials 2019, 12, 1284. [Google Scholar] [CrossRef] [Green Version]
- Hang, J.; Shi, C.; Li, Y.; Pan, X.; Poon, C.-S.; Xie, Z. Performance Enhancement of Recycled Concrete Aggregates through Carbonation. J. Mater. Civ. Eng. 2015, 27. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Carbonation Curing versus Steam Curing for Precast Concrete Production. J. Mater. Civ. Eng. 2012, 24, 1221–1229. [Google Scholar] [CrossRef]
- Boumaaza, M.; Huet, B.; Turcry, P.; Aït-Mokhtar, A. The CO2-binding capacity of synthetic anhydrous and hydrates: Validation of a test method based on the instantaneous reaction rate. Cem. Concr. Res. 2020, 135, 106113. [Google Scholar] [CrossRef]
- Dos Santos, V.; Tonoli, G.H.D.; Mármol, G.; Savastano, H. Fiber-cement composites hydrated with carbonated water: Effect on physical-mechanical properties. Cem. Concr. Res. 2019, 124, 105812. [Google Scholar] [CrossRef]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Zhan, B.; Poon, C.S.; Liu, Q.; Kou, S.; Shi, C. Experimental study on CO2 curing for enhancement of recycled aggregate properties. Constr. Build. Mater. 2014, 67, 3–7. [Google Scholar] [CrossRef]
- Kou, S.-C.; Zhan, B.-J.; Poon, C.-S. Use of a CO2 curing step to improve the properties of concrete prepared with recycled aggregates. Cem. Concr. Compos. 2014, 45, 22–28. [Google Scholar] [CrossRef]
- Shi, C.; He, F.; Wu, Y. Effect of pre-conditioning on CO2 curing of lightweight concrete blocks mixtures. Constr. Build. Mater. 2012, 26, 257–267. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Fernández-Rodríguez, J.M.; Jiménez, J.R. Use of carbonated water to improve the mechanical properties and reduce the carbon footprint of cement-based materials with recycled aggregates. J. CO2 Util. 2022, 57, 101886. [Google Scholar] [CrossRef]
- Kashef-Haghighi, S.; Shao, Y.; Ghoshal, S. Mathematical modeling of CO2 uptake by concrete during accelerated carbonation curing. Cem. Concr. Res. 2015, 67, 1–10. [Google Scholar] [CrossRef]
- He, P.; Shi, C.; Tu, Z.; Poon, C.S.; Zhang, J. Effect of further water curing on compressive strength and microstructure of CO2-cured concrete. Cem. Concr. Compos. 2016, 72, 80–88. [Google Scholar] [CrossRef]
- Zhan, B.J.; Poon, C.S.; Shi, C.J. Materials characteristics affecting CO2 curing of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2016, 67, 50–59. [Google Scholar] [CrossRef]
- Zhan, B.; Poon, C.; Shi, C. CO2 curing for improving the properties of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2013, 42, 1–8. [Google Scholar] [CrossRef]
- Liu, S.; Guan, X.; Zhang, S.; Xu, C.; Li, H.; Zhang, J. Sintering red mud based imitative ceramic bricks with CO2 emissions below zero. Mater. Lett. 2017, 191, 222–224. [Google Scholar] [CrossRef]
- Monkman, S.; Shao, Y. Carbonation Curing of Slag-Cement Concrete for Binding CO2 and Improving Performance. J. Mater. Civ. Eng. 2010, 22, 296–304. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y. Studies on some factors affecting CO2 curing of lightweight concrete products. Resour. Conserv. Recycl. 2008, 52, 1087–1092. [Google Scholar] [CrossRef]
- Ben Ghacham, A.; Pasquier, L.-C.; Cecchi, E.; Blais, J.-F.; Mercier, G. Valorization of waste concrete through CO2 mineral carbonation: Optimizing parameters and improving reactivity using concrete separation. J. Clean. Prod. 2017, 166, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Shi, C.; Farzadnia, N.; Hu, X.; Zheng, J. Properties and microstructure of CO2 surface treated cement mortars with subsequent lime-saturated water curing. Cem. Concr. Compos. 2019, 99, 89–99. [Google Scholar] [CrossRef]
- Pan, X.; Shi, C.; Hu, X.; Ou, Z. Effects of CO2 surface treatment on strength and permeability of one-day-aged cement mortar. Constr. Build. Mater. 2017, 154, 1087–1095. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Kalinowska-Wichrowska, K.; Fernández, J.M.; Jiménez, J.R. Accelerated carbonation of fresh cement-based products containing recycled masonry aggregates for CO2 sequestration. J. CO2 Util. 2021, 46, 101461. [Google Scholar] [CrossRef]
- Kwasny, J.; Basheer, P.A.M.; Russell, M.; Doherty, W.; Owens, K.; Ward, N. CO2 sequestration in cement-based materials during mixing process using carbonated water and gaseous CO2. In Proceedings of the 4th International Conference on the Durability of Concrete Structures, West Lafayette, IN, USA, 24–26 July 2014; pp. 72–79. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fu, T.; Wang, R. An assessment of microcracks in the interfacial transition zone of recycled concrete aggregates cured by CO2. Constr. Build. Mater. 2020, 236, 117543. [Google Scholar] [CrossRef]
- Amin, M.; Tayeh, B.A.; Agwa, I.S. Effect of using mineral admixtures and ceramic wastes as coarse aggregates on properties of ultrahigh-performance concrete. J. Clean. Prod. 2020, 273, 123073. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Soomro, M.; Evangelista, A.C.J. A review of recycled aggregate in concrete applications (2000–2017). Constr. Build. Mater. 2018, 172, 272–292. [Google Scholar] [CrossRef]
- Ferreira, R.L.S.; Anjos, M.A.S.; Maia, C.; Pinto, L.; de Azevedo, A.R.G.; de Brito, J. Long-term analysis of the physical properties of the mixed recycled aggregate and their effect on the properties of mortars. Constr. Build. Mater. 2020, 274, 121796. [Google Scholar] [CrossRef]
- Pavlu, T.; Fortova, K.; Divis, J.; Hajek, P. The Utilization of Recycled Masonry Aggregate and Recycled EPS for Concrete Blocks for Mortarless Masonry. Materials 2019, 12, 1923. [Google Scholar] [CrossRef] [Green Version]
- Mora-Ortiz, R.S.; Munguía-Balvanera, E.; Díaz, S.A.; Magaña-Hernández, F.; Del Angel-Meraz, E.; Bolaina-Juárez, Á. Mechanical Behavior of Masonry Mortars Made with Recycled Mortar Aggregate. Materials 2020, 13, 2373. [Google Scholar] [CrossRef]
- Cuenca-Moyano, G.M.; Martín-Pascual, J.; Martín-Morales, M.; Valverde-Palacios, I.; Zamorano, M. Effects of water to cement ratio, recycled fine aggregate and air entraining/plasticizer admixture on masonry mortar properties. Constr. Build. Mater. 2020, 230, 116929. [Google Scholar] [CrossRef]
- Tan, J.; Cai, J.; Li, X.; Pan, J.; Li, J. Development of eco-friendly geopolymers with ground mixed recycled aggregates and slag. J. Clean. Prod. 2020, 256, 120369. [Google Scholar] [CrossRef]
- Cantero, B.; del Bosque, I.S.; Matías, A.; Medina, C. Statistically significant effects of mixed recycled aggregate on the physical-mechanical properties of structural concretes. Constr. Build. Mater. 2018, 185, 93–101. [Google Scholar] [CrossRef]
- Zhang, L.; Sojobi, A.; Kodur, V.; Liew, K.M. Effective utilization and recycling of mixed recycled aggregates for a greener environment. J. Clean. Prod. 2019, 236, 117600. [Google Scholar] [CrossRef]
- Cantero, B.; Bravo, M.; de Brito, J.; del Bosque, I.S.; Medina, C. Mechanical behaviour of structural concrete with ground recycled concrete cement and mixed recycled aggregate. J. Clean. Prod. 2020, 275, 122913. [Google Scholar] [CrossRef]
- Jiménez, J.R.; Ayuso, J.; López, M.; Fernández, J.M.; De Brito, J.M.C.L. Use of fine recycled aggregates from ceramic waste in masonry mortar manufacturing. Constr. Build. Mater. 2013, 40, 679–690. [Google Scholar] [CrossRef]
- Ledesma, E.F.; Jiménez, J.R.; Ayuso, J.; Fernández, J.M.; de Brito, J. Maximum feasible use of recycled sand from construction and demolition waste for eco-mortar production–Part-I: Ceramic masonry waste. J. Clean. Prod. 2015, 87, 692–706. [Google Scholar] [CrossRef]
- Silva, R.; de Brito, J.; Dhir, R. Performance of cementitious renderings and masonry mortars containing recycled aggregates from construction and demolition wastes. Constr. Build. Mater. 2016, 105, 400–415. [Google Scholar] [CrossRef]
- Silva, Y.F.; Robayo, R.A.; Mattey, P.E.; Delvasto, S. Properties of self-compacting concrete on fresh and hardened with residue of masonry and recycled concrete. Constr. Build. Mater. 2016, 124, 639–644. [Google Scholar] [CrossRef]
- Pacheco, J.; de Brito, J.; Chastre, C.; Evangelista, L. Experimental investigation on the variability of the main mechanical properties of concrete produced with coarse recycled concrete aggregates. Constr. Build. Mater. 2019, 201, 110–120. [Google Scholar] [CrossRef]
- Silva, R.V.; Jiménez, J.; Agrela, F.; de Brito, J. Real-scale applications of recycled aggregate concrete. In New Trends in Eco-efficient and Recycled Concrete; Woodhead Publishing: Sawston, UK, 2018; pp. 573–589. [Google Scholar] [CrossRef]
- Lopez-Uceda, A.; Ayuso, J.; Jiménez, J.R.; Galvín, A.P.; Del Rey, I. Feasibility study of roller compacted concrete with recycled aggregates as base layer for light-traffic roads. Road Mater. Pavement Des. 2018, 21, 276–288. [Google Scholar] [CrossRef]
- Sáez del Bosque, I.F.; Van den Heede, P.; De Belie, N.; de Rojas, M.S.; Medina, C. Carbonation of concrete with construction and demolition waste based recycled aggregates and cement with recycled content. Constr. Build. Mater. 2020, 234, 117336. [Google Scholar] [CrossRef]
- UNE-EN-1097-6:2013; Tests for Mechanical and Physical Properties of Aggregates. Part 6: Determination of Particle Density and Water Absorption. CEN: Brussels, Belgium, 2013.
- UNE-EN-197-1:2011; Part 1: Composition, Specifications and Conformity Criteria for Common Cements. BSI: London, UK, 2011.
- ASTM-C-144; Standard Specification for Aggregate for Masonry Mortar. American Society for Testing and Materials: West Conshohocken, PA, USA, 2004.
- EN-1015-6:1999; Methods of Test Mortar for Mansory. Part 6: Determination of Bulk Density of Fresh Mortar. SIST: Ljubljana, Slovenia, 1999.
- EN-1015-11:2000; Methods of Test for Mortar for Mansory. Part 11: Determination of Flexural and Compressive Strenght of Hardened Mortar. SIS: Stockholm, Sweden, 2000.
- JCPDS. Joint Committee on Power Diffraction Standard-International Centre for Diffraction. 2003. [Google Scholar]
- EN-1015-10:2000; Methods of Test Mortar for Mansory. Part 6: Determination of Bulk Density of Hardened Mortar. AENOR: Madrid, Spain, 2000.
- UNE-83980:2014; Concrete Durability. Test Methods. Determination of the Water Absorption, Density and Accesible Porosity for Water in Concrete. Spanish Association for Standardization: Madrid, Spain, 2014.
- Roncero, J.; Valls, S.; Gettu, R. Study of the influence of superplasticizers on the hydration of cement paste using nuclear magnetic resonance and X-ray diffraction techniques. Cem. Concr. Res. 2002, 32, 103–108. [Google Scholar] [CrossRef]
- Tang, S.; Cai, X.; He, Z.; Shao, H.; Li, Z.; Chen, E. Hydration process of fly ash blended cement pastes by impedance measurement. Constr. Build. Mater. 2016, 113, 939–950. [Google Scholar] [CrossRef]
- Abed, M.; Nemes, R.; Lublóy, E. Performance of Self-Compacting High-Performance Concrete Produced with Waste Materials after Exposure to Elevated Temperature. J. Mater. Civ. Eng. 2020, 32, 05019004. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Ríos, J.D.; De La Concha, A.M.; Cifuentes, H.; Jiménez, J.R.; Fernández, J.M. Effect of moderate temperatures on compressive strength of ultra-high-performance concrete: A microstructural analysis. Cem. Concr. Res. 2021, 140, 106303. [Google Scholar] [CrossRef]
- Esquinas, R.; Motos-Pérez, D.; Jiménez, M.; Ramos, C.; Jiménez, J.R.; Fernández, J. Mechanical and durability behaviour of self-compacting concretes for application in the manufacture of hazardous waste containers. Constr. Build. Mater. 2018, 168, 442–458. [Google Scholar] [CrossRef]
- Sumra, Y.; Payam, S.; Zainah, I. The pH of Cement-based Materials: A Review. J. Wuhan Univ. Technol. Sci. Ed. 2020, 35, 908–924. [Google Scholar] [CrossRef]
- Hou, W.; Bao, J. Evaluation of cement retarding performance of cellulosic sugar acids. Constr. Build. Mater. 2019, 202, 522–527. [Google Scholar] [CrossRef]
- Bao, H.; Xu, G.; Wang, Q.; Peng, Y.; Liu, J. Study on the deterioration mechanism of cement-based materials in acid water containing aggressive carbon dioxide. Constr. Build. Mater. 2020, 243, 118233. [Google Scholar] [CrossRef]
- Li, X.; Shui, Z.; Sun, T.; Liu, K.; Wang, X. The hydration mechanism of cement-based materials served in marine environment during early-age magnesium precipitation. Constr. Build. Mater. 2020, 230, 117010. [Google Scholar] [CrossRef]
- Lothenbach, B.; Winnefeld, F. Thermodynamic modelling of the hydration of Portland cement. Cem. Concr. Res. 2006, 36, 209–226. [Google Scholar] [CrossRef]
- Jiménez, J.R.; Fernández-Ledesma, E.; Ayuso, J.; Corinaldesi, V.; Iglesias, F.J. A proposal for the maximum use of recycled concrete sand in masonry mortar design. Mater. Construcción 2016, 66, e075. [Google Scholar] [CrossRef] [Green Version]
- Duan, Z.; Hou, S.; Xiao, J.; Li, B. Study on the essential properties of recycled powders from construction and demolition waste. J. Clean. Prod. 2020, 253, 119865. [Google Scholar] [CrossRef]
- Martínez, P.S.; Cortina, M.G.; Martínez, F.F.; Sánchez, A.R. Comparative study of three types of fine recycled aggregates from construction and demolition waste (CDW), and their use in masonry mortar fabrication. J. Clean. Prod. 2016, 118, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Corominas, A.; Etxeberria, M. Properties of high performance concrete made with recycled fine ceramic and coarse mixed aggregates. Constr. Build. Mater. 2014, 68, 618–626. [Google Scholar] [CrossRef]
- Silva, J.; de Brito, J.; Veiga, R. Recycled Red-Clay Ceramic Construction and Demolition Waste for Mortars Production. J. Mater. Civ. Eng. 2010, 22, 236–244. [Google Scholar] [CrossRef]
- Li, X.; Ling, T.-C. Instant CO2 curing for dry-mix pressed cement pastes: Consideration of CO2 concentrations coupled with further water curing. J. CO2 Util. 2020, 38, 348–354. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Cantador-Fernández, D.; Jiménez, J.R.; Fernández, J.M. Potential CO2 capture in one-coat limestone mortar modified with Mg3Al–CO3 calcined hydrotalcites using ultrafast testing technique. Chem. Eng. J. 2021, 415, 129077. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Fernández, D.C.; Fernández, J.M.; Jiménez, J.R. The combined effect of CO2 and calcined hydrotalcite on one-coat limestone mortar properties. Constr. Build. Mater. 2020, 280, 122532. [Google Scholar] [CrossRef]
- Zhao, K.; Liang, Y.; Ji, T.; Lu, Y.; Lin, X. Effect of activator types and concentration of CO2 on the steel corrosion in the carbonated alkali-activated slag concrete. Constr. Build. Mater. 2020, 262, 120044. [Google Scholar] [CrossRef]
- Vogler, N.; Lindemann, M.; Drabetzki, P.; Kühne, H.-C. Alternative pH-indicators for determination of carbonation depth on cement-based concretes. Cem. Concr. Compos. 2020, 109, 103565. [Google Scholar] [CrossRef]
- Seigneur, N.; Kangni-Foli, E.; Lagneau, V.; Dauzères, A.; Poyet, S.; Le Bescop, P.; L’Hôpital, E.; de Lacaillerie, J.-B.D. Predicting the atmospheric carbonation of cementitious materials using fully coupled two-phase reactive transport modelling. Cem. Concr. Res. 2020, 130, 105966. [Google Scholar] [CrossRef] [Green Version]
- De Hita, P.R.; Pérez-Gálvez, F.; Morales-Conde, M.J.; Pedreño-Rojas, M.A. Characterisation of recycled ceramic mortars for use in prefabricated beam-filling pieces in structural floors. Mater. Construcción 2019, 69, e189. [Google Scholar] [CrossRef]
- Gonçalves, T.; Silva, R.V.; De Brito, J.; Fernández, J.M.; Esquinas, A.R. Mechanical and durability performance of mortars with fine recycled concrete aggregates and reactive magnesium oxide as partial cement replacement. Cem. Concr. Compos. 2020, 105, 103420. [Google Scholar] [CrossRef]
- Lozano-Lunar, A.; Dubchenko, I.; Bashynskyi, S.; Rodero, A.; Fernández, J.; Jiménez, J. Performance of self-compacting mortars with granite sludge as aggregate. Constr. Build. Mater. 2020, 251, 118998. [Google Scholar] [CrossRef]
- Qian, X.; Wang, J.; Fang, Y.; Wang, L. Carbon dioxide as an admixture for better performance of OPC-based concrete. J. CO2 Util. 2018, 25, 31–38. [Google Scholar] [CrossRef]
- Wu, C.-R.; Hong, Z.-Q.; Zhang, J.-L.; Kou, S.-C. Pore size distribution and ITZ performance of mortars prepared with different bio-deposition approaches for the treatment of recycled concrete aggregate. Cem. Concr. Compos. 2020, 111, 103631. [Google Scholar] [CrossRef]
- Dang, J.; Zhao, J.; Pang, S.D.; Zhao, S. Durability and microstructural properties of concrete with recycled brick as fine aggregates. Constr. Build. Mater. 2020, 262, 120032. [Google Scholar] [CrossRef]
- Ye, G.; Liu, X.; De Schutter, G.; Poppe, A.-M.; Taerwe, L. Influence of limestone powder used as filler in SCC on hydration and microstructure of cement pastes. Cem. Concr. Compos. 2007, 29, 94–102. [Google Scholar] [CrossRef]







| Size (mm) | ASTM C 144-04 (Limit) | Fraction Size | Original Percentage Retained | Application of 2 Gaps | Particle Size Distribution Obtained (Passing) |
|---|---|---|---|---|---|
| 4 | 100 | >4 | 0 | 0 | 100 |
| 2 | 88 | 2/4 | 12 | 16 | 84 |
| 1 | 62 | 1/2 | 26 | 35 | 49 |
| 0.5 | 32 | 0.5/1 | 30 | 40 | 9 |
| 0.25 | 8 | 0.25/0.5 | 24 | 0 | 9 |
| 0.125 | 1 | 0.125/0.25 | 7 | 9 | 0 |
| 0.075 | 0 | <0.125 | 1 | 0 | 0 |
| 0.063 | TOTAL | 100 | 100 | - |
| Mixes | NA | RMA 100% | |||
|---|---|---|---|---|---|
| Fraction Size (mm) | Weight Used (g) | BD * (g/cm3) | Volume (cm3) | BD * (g/cm3) | Weight Used (g) |
| >4 | 0 | - | - | - | - |
| 2/4 | 561 | 1.44 | 388.38 | 0.99 | 386 |
| 1/2 | 1216 | 1.49 | 813.53 | 1.05 | 855 |
| 0.5/1 | 1403 | 1.55 | 900.01 | 1.17 | 1053 |
| 0.25/0.5 | 0 | - | - | - | - |
| 0.125/0.25 | 327 | 1.38 | 235.80 | 1.19 | 279 |
| <0.125 | 0 | - | - | - | - |
| TOTAL | 3507 (Equation (7)) | 2574 | |||
| Mortar Type | NA RMA | Cement | Saturation Water | Effective Water | Total Water | w/c | Consistency Index (mm) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| H2O | CO2·H2O | H2O | CO2·H2O | |||||||
| NA-H2O-(*) | 3507 | - | 771 | 28 | - | 308 | - | 336 | 0.4 | 80 ±10 |
| RMA-H2O-(*) | - | 2574 | 771 | 232 | - | 308 | - | 540 | 0.4 | 80 ±10 |
| NA-CO2·H2O-(*) | 3507 | - | 771 | - | 28 | - | 308 | 336 | 0.4 | 80 ±10 |
| RMA-CO2·H2O-(*) | - | 2574 | 771 | - | 232 | - | 308 | 540 | 0.4 | 80 ±10 |
| Oxides | NA | RMA | Cement |
|---|---|---|---|
| F2O | - | 0.74 | - |
| Na2O | 0.82 | 0.71 | 0.29 |
| MgO | 1.06 | 1.65 | 1.00 |
| Al2O3 | 5.82 | 10.79 | 6.59 |
| SiO2 | 49.63 | 34.44 | 18.29 |
| P2O5 | 0.07 | 0.12 | 0.13 |
| SO3 | 0.03 | 2.52 | 4.02 |
| Cl2O3 | - | 0.05 | 0.07 |
| K2O | 1.52 | 2.18 | 1.09 |
| CaO | 5.60 | 12.18 | 45.61 |
| TiO2 | 0.27 | 0.54 | 0.41 |
| Cr2O3 | 0.04 | 0.02 | - |
| MnO2 | 0.04 | 0.06 | 0.05 |
| Fe2O3 | 1.74 | 3.55 | 2.85 |
| CuO | - | - | 0.04 |
| ZnO | - | 0.02 | 0.02 |
| SrO | - | 0.03 | 0.05 |
| Rb2O | - | - | 0.01 |
| BaO | 0.03 | 0.03 | 0.06 |
| BALANCE CO2 | 32.32 | 30.61 | 19.43 |
| TOTAL | 67.68 | 69.39 | 80.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suescum-Morales, D.; Jiménez, J.R.; Fernández-Rodríguez, J.M. Use of Carbonated Water as Kneading in Mortars Made with Recycled Aggregates. Materials 2022, 15, 4876. https://doi.org/10.3390/ma15144876
Suescum-Morales D, Jiménez JR, Fernández-Rodríguez JM. Use of Carbonated Water as Kneading in Mortars Made with Recycled Aggregates. Materials. 2022; 15(14):4876. https://doi.org/10.3390/ma15144876
Chicago/Turabian StyleSuescum-Morales, David, José Ramón Jiménez, and José María Fernández-Rodríguez. 2022. "Use of Carbonated Water as Kneading in Mortars Made with Recycled Aggregates" Materials 15, no. 14: 4876. https://doi.org/10.3390/ma15144876
APA StyleSuescum-Morales, D., Jiménez, J. R., & Fernández-Rodríguez, J. M. (2022). Use of Carbonated Water as Kneading in Mortars Made with Recycled Aggregates. Materials, 15(14), 4876. https://doi.org/10.3390/ma15144876







