A Comparative Mini-Review on Transition Metal Oxides Applied for the Selective Catalytic Ammonia Oxidation (NH3-SCO)
Abstract
1. Introduction
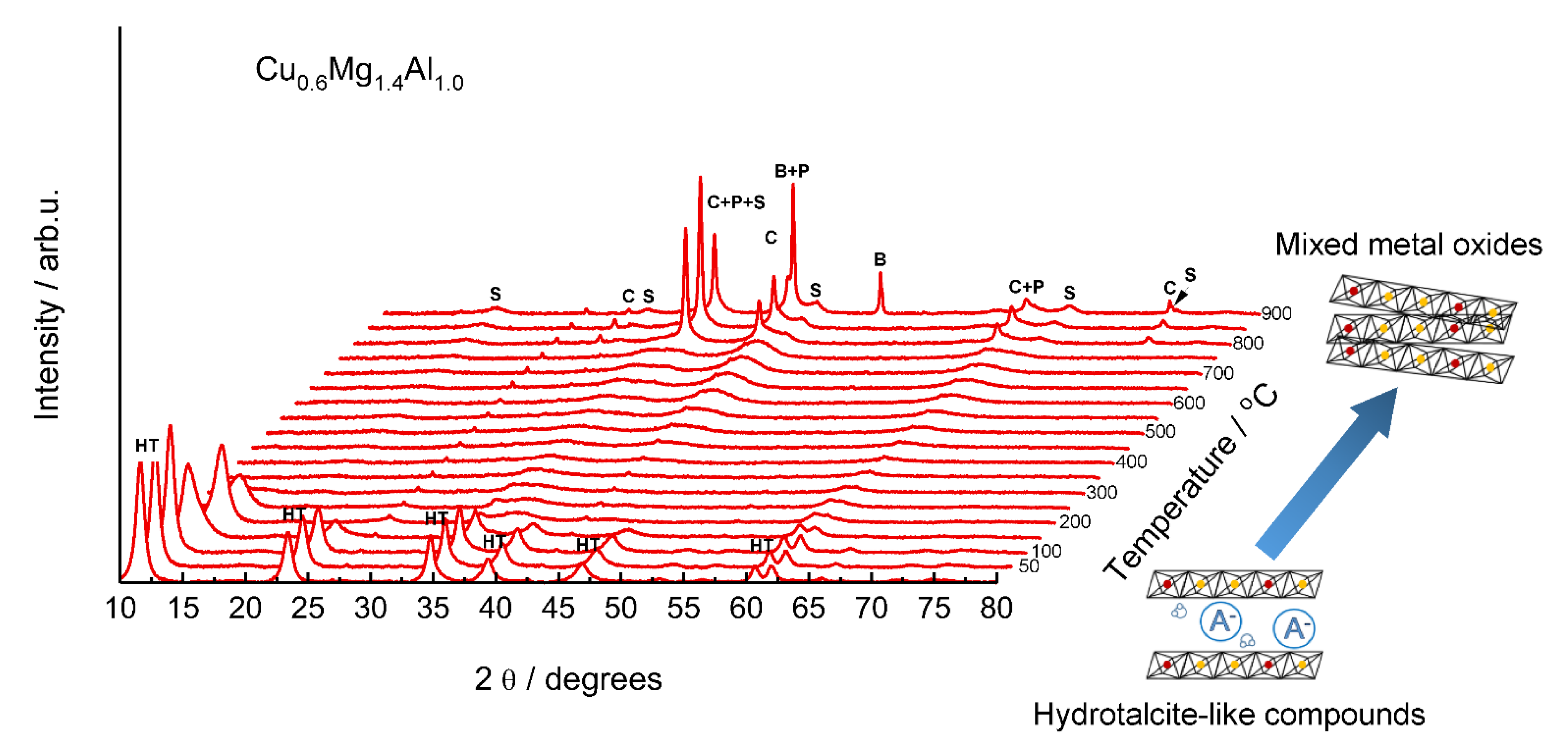
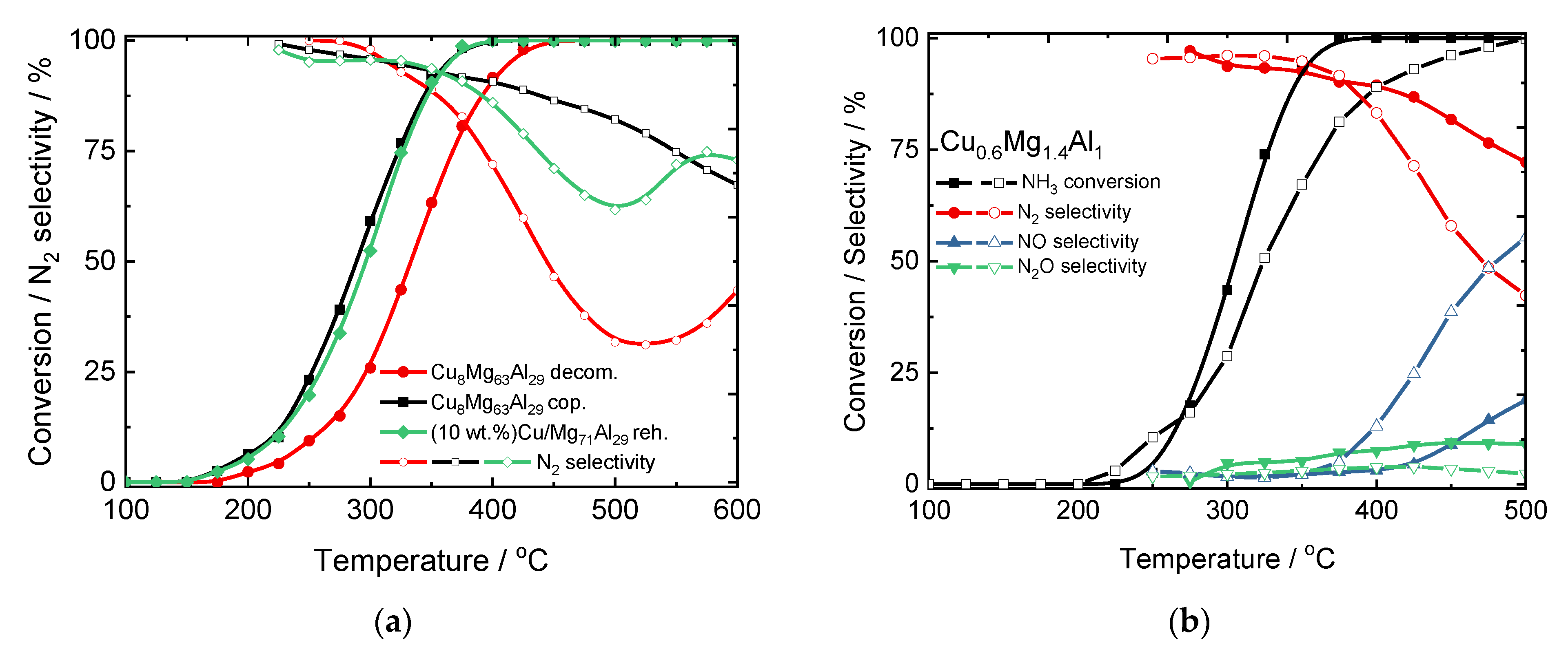
2. Hydrotalcite-Derived Mixed Metal Oxides
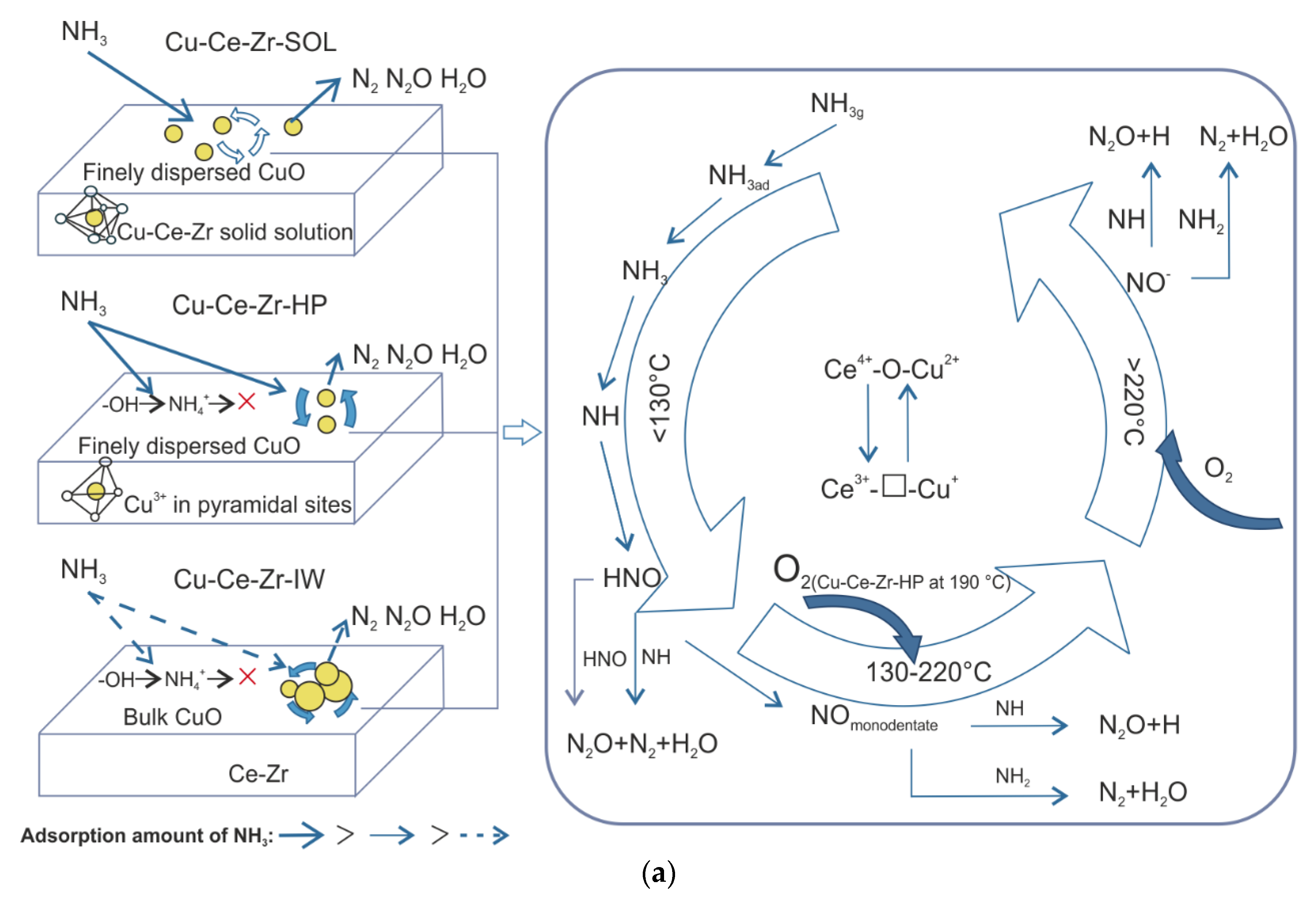
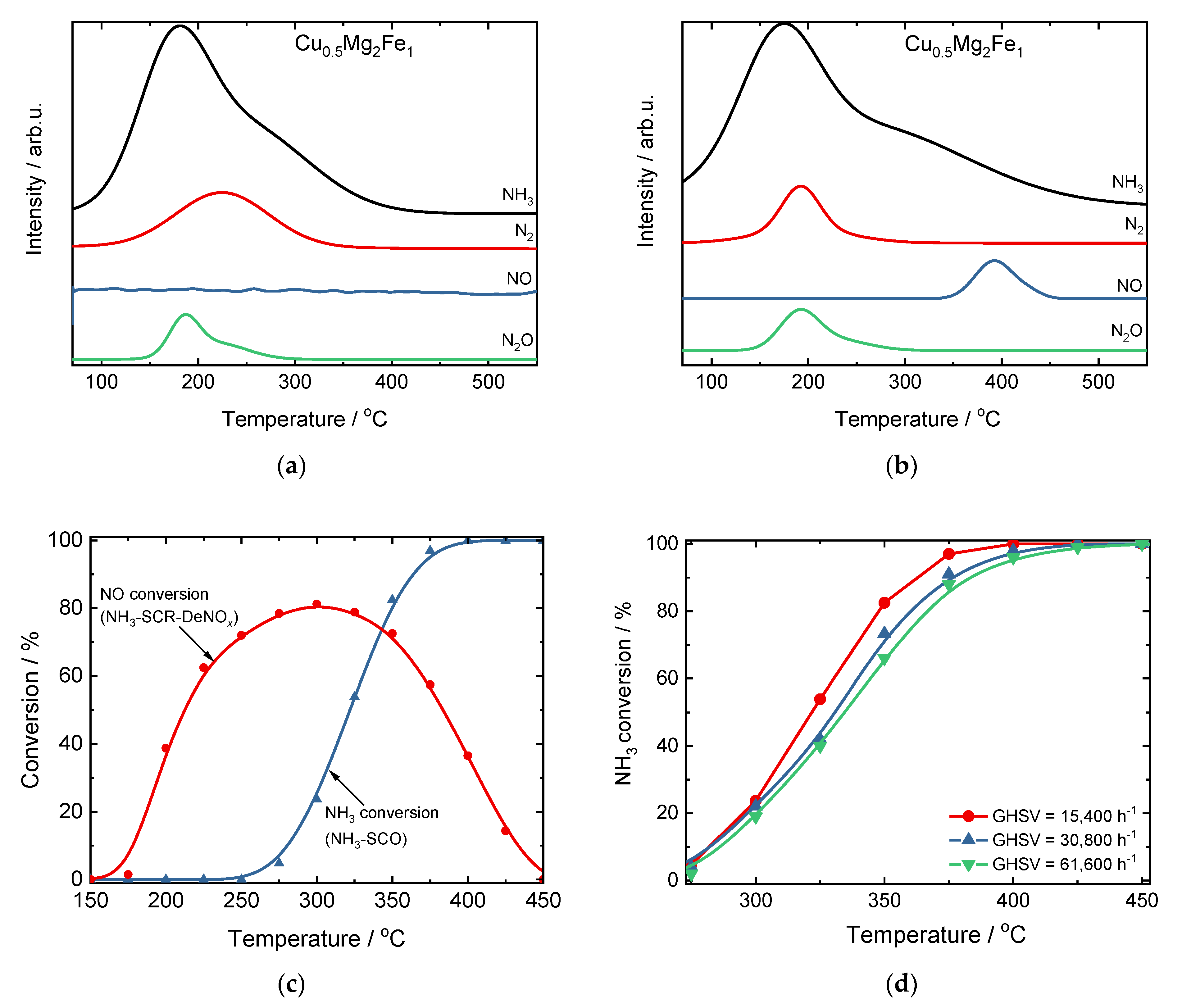
3. Other Metal Oxides

| Pos. | Sample | Preparation | Reaction Conditions | Operation Temperature for Achieving 100% NH3 Conversion/°C | N2 Selectivity/% | Refs. |
|---|---|---|---|---|---|---|
| Hydrotalcite-derived mixed metal oxides | ||||||
| 1 | CuMgAl n(Cu)/n(Mg)/n(Al) = 4.6/66.4/29, mol.% | Coprecipitation, calcination, 650 °C, air, 14 h | 0.5 vol.% NH3, 1.75 vol.% O2, He balance, GHSV 10,000–12,000 h−1 | 500 | >80 | [29] |
| 2 | CuMgAl n(Cu)/n(Mg)/n(Al) = 5/66/29, mol.% | Coprecipitation, calcination, 600 °C, air, 16 h | 0.5 vol.% NH3, 2.5 vol.% O2, He balance, GHSV 30,000 h−1 | 400–650 | >80 | [30] |
| 3 | CuMgFe n(Cu)/n(Mg)/n(Fe) = 0.5/2/1, mol.% | Coprecipitation, calcination, 600 °C, air, 12 h | 0.5 vol.% NH3, 2.5 vol.% O2, He balance, GHSV 15,400 h−1 | 400–450 | >70 | [31] |
| 4 | CuMgAl n(Cu)/n(Mg)/n(Al) = 8/63/29, mol.% | Coprecipitation, calcination, 600 °C, air, 6 h | 0.5 vol.% NH3, 2.5 vol.% O2, Ar balance, WHSV 24,000 mL h−1 g−1 * 0.5 vol.% NH3, 2.5 vol.% O2, N2 balance, WHSV 137,000–140,000 mL h−1 g−1 ** 0.5 vol.% NH3, 2.5 vol.% O2, 10 vol.% CO2, 5 vol.% H2O, N2 balance, WHSV 137,000–140,000 mL h−1 g−1 | 400–600 * 450–600 ** 600 | >60 * >60 ** >55 | [34] *,** [111] |
| 5 | CuMgAl n(Cu)/n(Mg)/n(Al) = 0.6/1.4/1.0, mol.% | Coprecipitation, calcination, 600 °C, *900 °C, air, 12 h | 0.5 vol.% NH3, 2.5 vol.% O2, He balance, WHSV 24,000 mL h−1 g−1 | 375–500 * 500 | >70 * >40 | [28] |
| 6 | CuMgAl n(Cu)/n(Mg)/n(Al) = 5/62/33, mol.% | Coprecipitation, calcination, 600 °C, 800 °C *, air, 12 h | 0.5 vol.% NH3, 2.5 vol.% O2, He balance, WHSV 24,000 mL h−1 g−1 | 475–500 * 475–500 | >60 * >85 | [35] |
| 7 | GaCuMgAl * CeCuMgAl n(Ga/Ce)/n(Cu)/n(Mg)/n(Al) = 0.25/5/65.75/29 | Coprecipitation, calcination, 600 °C, air, 6 h | 0.5 vol.% NH3, 2.5 vol.% O2, Ar balance, WHSV 24,000 mL h−1 g−1 | 375–500 * 375–500 | >80 * >50 | [36] |
| 8 | CuMgAl n(Cu)/n(Mg)/n(Al) = 10–15/52–57/33, mol.% * (4.1 wt.%)CeCuMgAl n(Cu)/n(Mg)/n(Al) = 5/62/33, mol.% | Coprecipitation, calcination, 800 °C, air, 9 h * Impregnation, calcination, 800 C, air, 9 h | 0.035 vol.% NH3, 20 vol.% O2, N2 balance, WHSV 30,000 mL h−1 g−1 | 350 * 350 | <20 * <70 | [37] |
| 9 | CuMgAl n(Cu)/n(Mg)/n(Al) = 5/62/33, mol.% * (3 wt.%)CeCuMgAl ** (0.5 wt.% Ce)CuMgAl | Coprecipitation, calcination, 600 °C, air, 12 h, *,** Impregnation, calcination, air, 600 °C, 12 h | 0.5 vol.% NH3, 2.5 vol.% O2, He balance, WHSV 24,000 mL h−1 g−1 | 500–600 * 500–600 ** 450–600 | >60 * >75 ** >55 | [38] |
| 10 | CuZnAl n(Cu)/n(Zn)/n(Al) = 10–15/52/33, mol.% * (8.14 wt.%)CeCuMgAl | 0.035 vol.% NH3, 20 vol.% O2, N2 balance, WHSV 30,000 mL h−1 g−1 | 350 * 350 | <30 * <40 | [39] | |
| 11 | CoMnAl n(Co)/n(Mn)/n(Al) = 4/1/1 | Coprecipitation, calcination, 500 °C, air, 4 h; * Mechanochemical method, calcination, 500 °C, air, 4 h | 0.5 vol.% NH3, 2.5 vol.% O2, He balance, WHSV 24,000 mL h−1 g−1 | 250–500 * 250–500 | >40 * >45 | [40] |
| Other metal oxides | ||||||
| 12 | CuO/monolith | Precursors calcination on the monolith, 600 °C, air, 6 h | 0.05 vol.% NH3, 3 vol.% O2, N2 balance, GHSV 40,000 h−1 | 450–550 | 67–85 | [45] |
| 13 | (10 wt.%)V/TiO2 | Impregnation, calcination, 550 °C, air, 6 h | 0.05 vol.% NH3, 2.5 vol.% O2, N2 balance, GHSV 35,385 h−1 | 225–300 | - | [83] |
| 14 | (10 wt.%)Cu/TiO2 | 200–300 | - | |||
| 15 | (10 wt.%)Cu/TiO2 | Impregnation, rotary evaporator, calcination, 450 °C, air, 3 h | 0.04 vol.% NH3, 10 vol.% O2, He balance, GHSV 50,000 h−1 * 0.04 vol.% NH3, 10 vol.% O2, 3 vol.% H2O, He balance, GHSV 50,000 h−1 | 250–300 * 350–375 | >95 * >95 | [65] |
| 16 | (10 wt.%)Cu/Al2O3 | 400 | >95 | |||
| 17 | (10–15 wt.%)Cu/Al2O3 | Impregnation, calcination, 600 °C, air, 24 h | 1.14 vol.% NH3, 8.21 vol.% O2, He balance, WHSV 2 240 mL h−1 g−1 | 350 | >90 | [61] |
| 18 | (10 wt.%)Cu/Al2O3 | Impregnation, calcination, 600 °C, air, 24 h | 1.14 vol.% NH3, 8.21 vol.% O2, He balance, WHSV 2240 mL h−1 g−1 * 1.14 vol.% NH3, 8.21 vol.% O2, He balance, WHSV 2240 mL h−1 g−1 | 350 * 350 | 94 * 95 | [62] |
| 19 | (10 wt.%)Cu/Al2O3 | Impregnation, calcination, 600 °C, air, 3 h, * Cu(CH3COO)2 as precursor ** Cu(NO3)2 as precursor | 0.1 vol.% NH3, 10 vol.% O2, He balance, GHSV 50,000 h−1 | * 350–400 ** 375–400 | * >85 ** >95 | [70] |
| 20 | (10 wt.%)Cu/Al2O3 | Impregnation, calcination, 600 °C, air, 6 h | 0.5 vol.% NH3, 2.5 vol.% O2, N2 balance, WHSV 137,000–140,000 mL h−1 g−1 * 0.5 vol.% NH3, 2.5 vol.% O2, 10 vol.% CO2, 5 vol.% H2O, N2 balance, WHSV 137,000–140,000 mL h−1 g−1 | 450–600 * 600 | >60 * >50 | [111] |
| 21 | (10 wt.%)Cu/Al2O3 | Impregnation, calcination, 600 °C, air, 12 h | 0.5 vol.% NH3, 2.5 vol.% O2 Ar balance, WHSV 24,000 mL h−1 g−1 | 425–500 | >75 | [112] |
| 22 | (10 wt.%)Cu/Al2O3 * (10 wt.%)Cu/Al2O3 | Imprgnation, rotary evaporation, calcination, 500 °C, air, 2 h * Impregnation, rotary evaporation, 500 °C, H2/N2, 2 h; 0.05 vol.% NH3, 5 vol.% O2, N2 balance | 0.05 vol.% NH3, 5 vol.% O2 N2 balance, GHSV 60,000 h−1 | 330 * 300–330 | not shown * not shown | [9] |
| 23 | (1.3 wt.%)Cu/Al2O3 * (1 wt.%)Cu/CeOx/Li2O/Al2O3 | Impregnation, calcination, 350 °C, air, time not given; homogenous deposition precipitation, H2 reduction, 400 °C, 2 h | 2 vol.% NH3, 2 vol.% O2, Ar balance, GHSV 2500 h−1 | 400 * 325–400 | 100 * 100 | [76] |
| 24 | (3.4 wt.%)Cu/Al2O3 | Impregnation, calcination, 450 °C, air, 5 h | 0.54 vol.% NH3, 8 vol.% O2, He balance, WHSV 240 mL h−1 g−1 | 400–450 | not shown | [69] |
| 25 | (20 wt.%)Cu/Al2O3/monolith | Impregnation, calcination, 800 °C, air, 4 h | 0.04 vol.% NH3, 8.2 vol.% O2, 1.3 vol.% CH4, 3.9 vol.% CO2, 4.1 vol.% CO, 2.9 vol.% H2, GHSV 100,000 h−1 | 400–500 | 0 | [71] |
| 26 | (1 wt.%)PbO-(4.3 wt.%)Cu/Al2O3 | Impregnation, calcination, 450 °C, air, time not shown | 0.54 vol.% NH3, 8 vol.% O2, He balance, WHSV 800 mL h−1 g−1 | 325 | 95 | [68] |
| 27 | (1–2 wt.%)Cu/η-Al2O3 | Impregnation, Rotary evaporator, calcination, 500 °C, air, 10 h; pre-treatment conditions: 20 vol.% O2/He, 550 °C, 1 h | 0.1 vol.% NH3, 8 vol.% O2, 3.5 vol.% H2O, He balance, WHSV 250,000 mL h−1 g−1 | 550 | not shown | [75] |
| 28 | CuO/CNTs (carbon nanotubes, 9.85 wt.% Cu) | Impregnation, ultrasonic treatmnet, evaporation, 350 °C, He, 3 h | 0.1 vol.% NH3, 2 vol.% O2, He balance, WHSV 60,000 mL h−1 g−1 | 189–250 | >98 | [63] |
| 29 | Cu/graphene (2.57–3.42 wt.%) | Impregnation, ultrasonic treatment, 400 °C, N2, 3 h, * Cu(CH3COO)2 H2O as precursor ** Cu(NO3)2·H2O as precursor | 0.05 vol.% NH3, 1 vol.% O2, N2 balance, GHSV 35,000 h−1 | * 300 ** 250–300 | * >80 ** >80 | [72] |
| 30 | (5 wt.%)Ni/Al2O3 | Impregnation, calcination, 800 °C, air, 8 h | 0.1 vol.% NH3, 18 vol.% O2, N2 balance, GHSV 61,000 h−1 | 550–800 | >55 | [78] |
| 31 | (5 wt.%)Mn/Al2O3 | 300–800 | >55 | |||
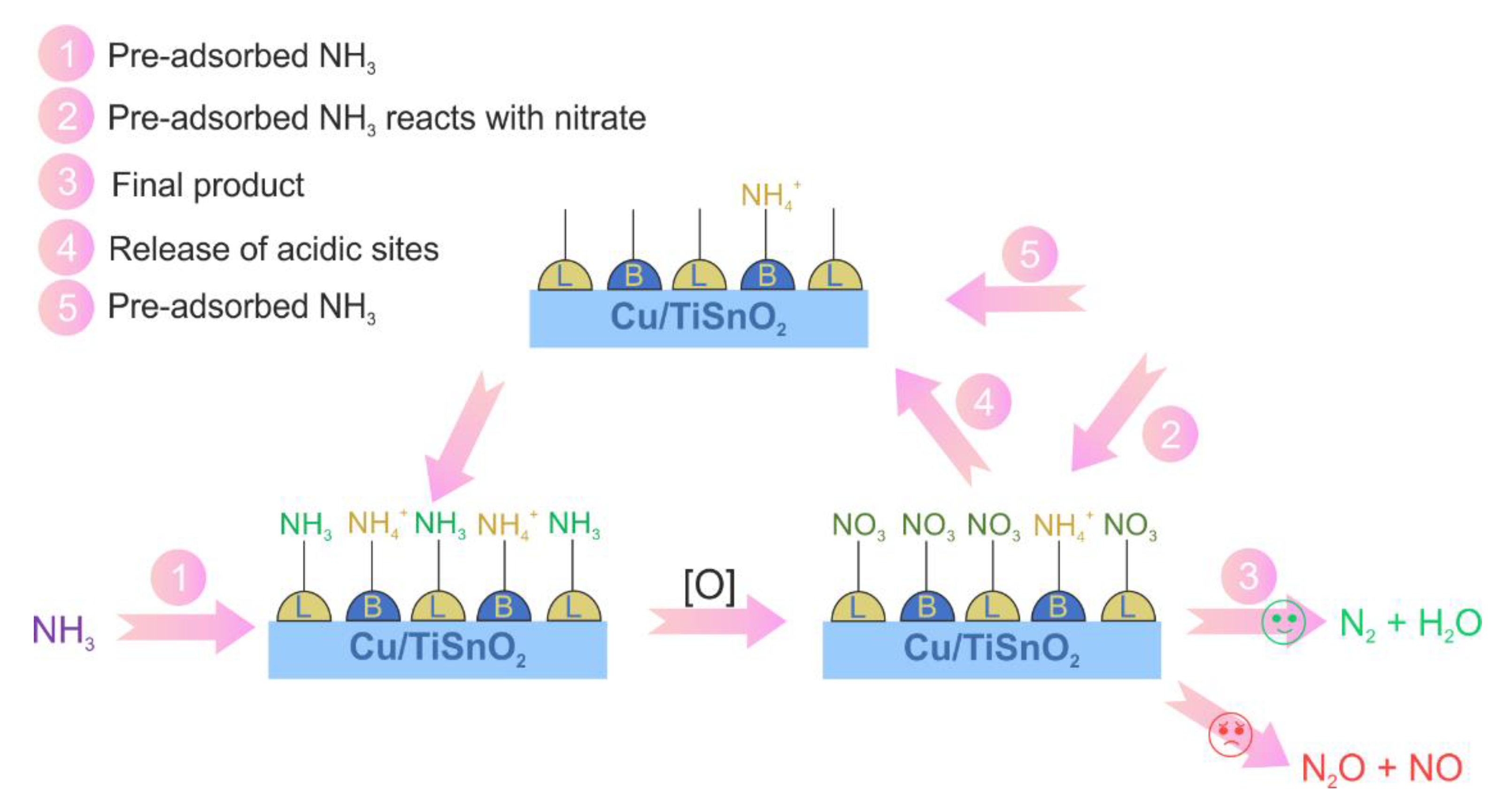
| 32 | (10.5 wt.%)CuO/TiSnO2 | Impregnation, calcination, 450 °C, air, 4 h | 0.05 vol.% NH3, 3 vol.% O2, N2 balance, WHSV 60,000 mL h−1 g−1 | 300–400 | >70 | [85] |
| 33 | (5 wt.%)CuOx/La2Ce2O7 | Impregnation, calcination, 600 °C, air, 1 h | 0.05 vol.% NH3, 5 vol.% O2, N2 balance, GHSV 20,000 h−1 | 275–425 | >80 | [67] |
| 34 | (10 wt.%)Ce/(2 wt.%)V/TiO2 | Impregnation, calcination, 400 °C, air, 4 h; pre-treatment conditions: 8 vol.% O2/N2, 400 °C, 0.5 h | 0.02 vol.% NH3, 8 vol.% O2, 6 vol.% H2O, N2 balance, GHSV 120,000 h−1 | 300–350 | >90 | [95] |
| 35 | Ce0.4Zr0.6O2 | Surfactant-templated method, calcination, 550 °C, air, 3 h | 0.1 vol.% NH3, 10 vol.% O2, He balance, GHSV 40,000 h−1 | 360–380 | >90 | [93] |
| 36 | (6 wt.%)Cu-Ce-Zr n(Si)/n(Al) = 4 | Sol-gel method, calcination, 450 °C, air, 3 h | 0.1 vol.% NH3, 10 vol.% O2, He balance, GHSV 40,000 h−1 | 230 | >90 | [97] |
| 37 | CuO-Fe2O3 n(Cu)/n(Fe) = 1:1 | Sol-gel method, calcination, 500 °C, air, 4 h | 0.08 vol.% NH3, 5 vol.% O2, Ar balance, GHSV 60,000 h−1 | 225–300 | >80 | [87] |
| 38 | CuO-Fe2O3 n(Cu)/n(Fe) = 5:5 | Sol-gel method, calcination, 400 °C, air, 4 h | 0.08 vol.% NH3, 5 vol.% O2, Ar balance, GHSV 60,000 h−1 | 250–300 | >80 | [86] |
| 39 | CuFe2O4 (8.59 wt.% Cu, 7.45 wt.% Fe) | Hard-template method, 600 °C, air, 6 h | 0.1 vol.% NH3, 0.2 vol.% O2, He balance, GHSV 35,000 h−1 | 350–600 | >75 | [88] |
| 40 | CuO-CeO2 n(Cu)/n(Ce) = 6/4 | Coprecipitation, calcination, 500 °C, air, 4 h | 0.1 vol.% NH3, 4 vol.% O2, 12 vol.% H2O, He balance, WHSV 92,000 mL h−1 g−1 | 400 | 82 | [99] |
| 41 | CuO-CeO2 (10 wt.% Cu) | Surfactant templated method, 500 °C, air, 3 h | 0.1 vol.% NH3, 10 vol.% O2, He balance, GHSV 40,000 h−1 | 250–300 | >90 | [100] |
| 42 | (1 wt.%)Cu-PILC-Verm (Alumina pillared vermiculites) | Ion-exchange, calcination, 450 °C, air, 3 h | 0.5 vol.% NH3, 2.5 vol.% O2, He balance, WHSV 24,000 mL h−1 g−1 | 500–550 | >95 | [89] |
| 43 | (5.7 wt.%)Fe-PILC-Phlog (Alumina pillared phlogopite) | 500–550 | >70 | |||
| 44 | (0.59 wt.%)Cu-PCH (Porous clay heterostructures) | Ion-exchange, 450 °C, air, 3 h | 0.5 vol.% NH3, 2.5 vol.% O2, He balance, WHSV 24,000 mL h−1 g−1 | 500–550 | >90 | [53] |
| 45 | (1.43 wt.%)Cu-PCH (Porous clay heterostructures) | 400–550 | >90 | [90] | ||
| 46 | Cu/attapulgite (5–10 wt.% Cu) | Impregnation, 400 °C, air, 4 h | 0.005 vol.% NH3, 4 vol.% O2, N2 balance, GHSV 150,000 h−1 | 450–500 | >75 | [92] |
| 47 | natural manganese ore | Fluidization, 12 h | 0.05 vol.% NH3, 3 vol.% O2, He balance, GHSV 15,000–80,000 h−1 | 240 | >70 | [103] |
| 48 | MnO2 | Calcination, 400 °C, air, 2 h | 210 | >60 | ||
| 49 | Cu-Mn/TiO2 n(Cu)/n(Mn) = 20/80 | Impregnation, rotary evaporator, calcination, 550 °C, air, 2 h | 0.06 vol.% NH3, 6 vol.% O2, N2 balance, WHSV 200,000 mL h−1 g−1 | 307 | not shown | [104] |
| 50 | MnOx-TiO2 (27.8 wt.% Mn) | Sol-gel method, calcination, 500 °C, air, 4 h | 0.05 vol.% NH3, 5 vol.% O2, He balance, WHSV 240,000 mL h−1 g−1 | 200–350 | >60 | [106] |
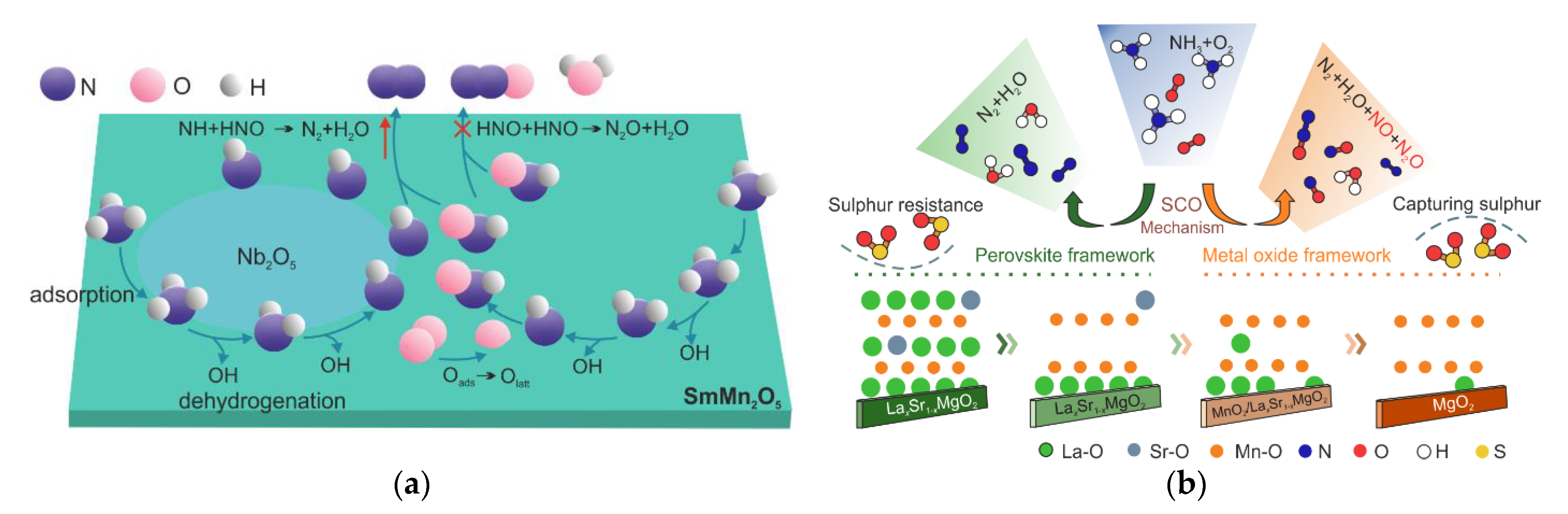
| 51 | SmMn2O5 | Organic solution combustion methods, 700 °C, air, 8 h | 0.05 vol.% NH3, 10 vol.% O2, N2 balance, WHSV 120,000 mL h−1 g−1 | 175–250 | >45 | [108] |
| 52 | (5.0 wt.%)Nb2O5/SmMn2O5 | Impregnation, 450 °C, air, 2 h | 200–250 | >60 | ||
| 53 | (30 wt.%)SmMn2O5/Cu-SAPO | Grinding the mixture; * after hydrothermal aging treatment conditions: 21 vol.% O2, 10 vol.% H2O, N2 balance, 800 °C, 5 h | 0.05 vol.% NH3, 21 vol.% O2, N2 balance, GHSV 100,000 h−1 | 225–400 * 300–400 | >20 * not shown | [109] |
| 54 | LaxSr1−xMnO3 | Hydrothermal method, 400 °C, air, 2 h, post-treatment in 3 M HNO3 | 0.05 vol.% NH3, 3 vol.% O2, N2 balance, WHSV 120,000 mL h−1 g−1 | 300–450 | not shown | [110] |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Environment Agency. Available online: https://www.eea.europa.eu/publications/national-emission-reduction-commitments-directive (accessed on 30 May 2022).
- Arasu, P.T.; Khalaf, A.L.; Aziz, S.H.A.; Yaacob, M.H.; Noor, A.S.M. Optical fiber based ammonia gas sensor with carbon nanotubes sensing enhancement. In Proceedings of the 2017 IEEE Region 10 Symposium (TENSYMP), Cochin, India, 14–16 July 2017; pp. 1–4. [Google Scholar]
- Chmielarz, L.; Jabłońska, M. Advances in selective catalytic oxidation of ammonia to dinitrogen: A review. RSC Adv. 2015, 5, 43408–43431. [Google Scholar] [CrossRef]
- Jabłońska, M.; Palkovits, R. Copper based catalysts for the selective ammonia oxidation into nitrogen and water vapour-Recent trends and open challenges. Appl. Catal. B Environ. 2016, 181, 332–351. [Google Scholar] [CrossRef]
- Jabłońska, M. Progress on noble metal-based catalysts dedicated to the selective catalytic ammonia oxidation into nitrogen and water vapor (NH3-SCO). Molecules 2021, 26, 6461. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, M. Progress on selective catalytic ammonia oxidation (NH3-SCO) over Cu-containing zeolite-based catalysts. ChemCatChem 2002, 12, 4490–4500. [Google Scholar] [CrossRef]
- Torp, T.K.; Hansen, B.B.; Vennestrøm, P.N.R.; Janssens, T.V.W.; Jensen, A.D. Modeling and optimization of multi-functional ammonia slip catalysts for diesel exhaust aftertreatment. Emiss. Control Sci. Technol. 2021, 7, 7–25. [Google Scholar] [CrossRef]
- Wang, F.; Ma, J.; He, G.; Chen, M.; Zhang, C.; He, H. Nanosize effect of Al2O3 in Ag/Al2O3 catalyst for the selective catalytic oxidation of ammonia. ACS Catal. 2018, 8, 2670–2682. [Google Scholar] [CrossRef]
- Lan, T.; Deng, J.; Zhang, X.; Wang, F.; Liu, X.; Cheng, D.; Zhang, D. Unraveling the promotion effects of dynamically constructed CuOx-OH interfacial sites in the selective catalytic oxidation of ammonia. ACS Catal. 2022, 12, 3955–3964. [Google Scholar] [CrossRef]
- Gao, F.; Liu, Y.; Sani, Z.; Tang, X.; Yi, H.; Zhao, S.; Yu, Q.; Zhou, Y. Advances in selective catalytic oxidation of ammonia (NH3-SCO) to dinitrogen in excess oxygen: A review on typical catalysts, catalytic performances and reaction mechanisms. J. Environ. Chem. Eng. 2020, 9, 104575–104595. [Google Scholar] [CrossRef]
- Rives, V.; Ulibarri, M.A. Layered double hydroxides (LDH) intercalated with metal coordination compounds and oxometalates. Coord. Chem. Rev. 1999, 181, 61–120. [Google Scholar] [CrossRef]
- Evans, D.G.; Slade, R.C.T. Structural aspects of layered double hydroxides. In Layered Double Hydroxides; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–87. [Google Scholar]
- Basile, F.; Fornasari, G.; Gazzano, M.; Vaccari, A. Synthesis and thermal evolution of hydrotalcite-type compounds containing noble metals. Appl. Clay Sci. 2000, 16, 185–200. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Catalysis by layered materials: A review. Microporous Mesoporous Mater. 2008, 107, 3–15. [Google Scholar] [CrossRef]
- Kannan, S. Catalytic applications of hydrotalcite-like materials and their derived forms. Catal. Surv. Asia 2006, 10, 117–137. [Google Scholar] [CrossRef]
- Jabłońska, M.; Palkovits, R. Nitrogen oxide removal over hydrotalcite-derived mixed metal oxides. Catal. Sci. Technol. 2016, 6, 49–72. [Google Scholar] [CrossRef]
- Jabłońska, M.; Palkovits, R. It is no laughing matter: Nitrous oxide formation in diesel engines and advances in its abatement over rhodium-based catalysts. Catal. Sci. Technol. 2016, 6, 7671–7687. [Google Scholar] [CrossRef]
- Schmidt-Szałowski, K.; Krawczyk, K.; Petryk, J. The properties of cobalt oxide catalyst for ammonia oxidation. Appl. Catal. A Gen. 1998, 175, 147–157. [Google Scholar] [CrossRef]
- Petryk, J.; Kołakowska, E. Cobalt oxide catalysts for ammonia oxidation activated with cerium and lanthanum. Appl. Catal. B Environ. 2000, 24, 121–128. [Google Scholar] [CrossRef]
- Escandón, L.S.; Ordóńez, S.; Díez, F.V.; Sastre, H. Ammonia oxidation over conventional combustion catalysts. React. Kinet. Catal. Lett. 2002, 76, 61–68. [Google Scholar] [CrossRef]
- Zakharchenko, N.I. Catalytic Properties of the Fe2O3-MnO System for Ammonia Oxidation. Kinet. Catal. 2001, 42, 679–685. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Isupova, L.A.; Zolotarskii, I.A.; Bobrova, L.N.; Noskov, A.S.; Parmon, V.N.; Brushtein, E.A.; Telyatnikova, T.V.; Chernyshev, V.I.; Lunin, V.V. Oxide catalysts for ammonia oxidation in nitric acid production: Properties and perspectives. Appl. Catal. A Gen. 2000, 204, 59–87. [Google Scholar] [CrossRef]
- Noskov, A.S.; Zolotarskii, I.A.; Pokrovskaya, S.A.; Korotkikh, V.N.; Slavinskaya, E.M.; Mokrinskii, V.V.; Kashkin, V.N. Ammonia oxidation into nitrous oxide over Mn/Bi/Al catalyst: I. Single cooling tube experiments. Chem. Eng. J. 2003, 91, 235–242. [Google Scholar] [CrossRef]
- Slavinskaya, E.M.; Veniaminov, S.A.; Notté, P.; Ivanova, A.S.; Boronin, A.I.; Chesalov, Y.A.; Polukhina, I.A.; Noskov, A.S. Studies of the mechanism of ammonia oxidation into nitrous oxide over MnBiO/α-Al2O3 catalyst. J. Catal. 2004, 222, 129–142. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Jin, W. Recent advances in the synthesis of layered, double-hydroxide-Based materials and their applications in hydrogen and oxygen evolution. Energy Technol. 2016, 4, 354–368. [Google Scholar] [CrossRef]
- Newman, S.P.; Jones, W.; O’Connor, P.; Stamires, D.N. Synthesis of the 3R 2 polytype of a hydrotalcite-like mineral. J. Mater. Chem. 2002, 12, 153–155. [Google Scholar] [CrossRef]
- Jabłońska, M.; Chmielarz, L.; Węgrzyn, A.; Guzik, K.; Piwowarska, Z.; Witkowski, S.; Walton, R.I.; Dunne, P.W.; Kovanda, F. Thermal transformations of Cu-Mg (Zn)-Al(Fe) hydrotalcite-like materials into metal oxide systems and their catalytic activity in selective oxidation of ammonia to dinitrogen. J. Therm. Anal. Calorim. 2013, 114, 731–747. [Google Scholar] [CrossRef]
- Trombetta, M.; Ramis, G.; Busca, G.; Montanari, B.; Vaccari, A. Ammonia adsorption and oxidation on Cu/Mg/Al mixed oxide catalysts prepared via hydrotalcite-type precursors. Langmuir 1997, 13, 4628–4637. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Rafalska-Łasocha, A.; Dziembaj, R. Selective oxidation of ammonia to nitrogen on transition metal containing mixed metal oxides. Appl. Catal. B Environ. 2005, 58, 235–244. [Google Scholar] [CrossRef]
- Chmielarz, L.; Węgrzyn, A.; Wojciechowska, M.; Witkowski, S.; Michalik, M. Selective catalytic oxidation (SCO) of ammonia to nitrogen over hydrotalcite originated Mg-Cu-Fe mixed metal oxides. Catal. Lett. 2011, 141, 1345–1354. [Google Scholar] [CrossRef]
- Górecka, S.; Pacultová, K.; Górecki, K.; Smýkalová, A.; Pamin, K.; Obalová, L. Cu-Mg-Fe-O-(Ce) complex oxides as catalysts of selective catalytic oxidation of ammonia to dinitrogen (NH3-SCO). Catalysts 2020, 10, 153. [Google Scholar] [CrossRef]
- Chmielarz, L.; Jabłońska, M.; Strumiński, A.; Piwowarska, Z.; Węgrzyn, A.; Witkowski, S.; Michalik, M. Selective catalytic oxidation of ammonia to nitrogen over Mg-Al, Cu-Mg-Al and Fe-Mg-Al mixed metal oxides doped with noble metals. Appl. Catal. B Environ. 2013, 130, 152–162. [Google Scholar] [CrossRef]
- Jabłońska, M.; Nocuń, M.; Gołąbek, K.; Palkovits, R. Effect of preparation procedures on catalytic activity and selectivity of copper-based mixed oxides in selective catalytic oxidation of ammonia into nitrogen and water vapour. Appl. Surf. Sci. 2017, 423, 498–508. [Google Scholar] [CrossRef]
- Basąg, S.; Piwowarska, Z.; Kowalczyk, A.; Węgrzyn, A.; Baran, R.; Gil, B.; Michalik, M.; Chmielarz, L. Cu-Mg-Al hydrotalcite-like materials as precursors of effective catalysts for selective oxidation of ammonia to dinitrogen—The influence of Mg/Al ratio and calcination temperature. Appl. Clay Sci. 2016, 129, 122–130. [Google Scholar] [CrossRef]
- Jabłońska, M.; Nothdurft, K.; Nocuń, M.; Girman, V.; Palkovits, R. Redox-performance correlations in Ag-Cu-Mg-Al, Ce-Cu-Mg-Al, and Ga-Cu-Mg-Al hydrotalcite derived mixed metal oxides. Appl. Catal. B Environ. 2017, 207, 385–396. [Google Scholar] [CrossRef]
- Górecka, S.; Pacultová, K.; Smýkalová, A.; Fridrichová, D.; Górecki, K.; Rokicińska, A.; Kuśtrowski, P.; Žebrák, R.; Obalová, L. Role of the Cu content and Ce activating effect on catalytic performance of Cu-Mg-Al and Ce/Cu-Mg-Al oxides in ammonia selective catalytic oxidation. Appl. Surf. Sci. 2022, 573, 151540–151555. [Google Scholar] [CrossRef]
- Basąg, S.; Kocoł, K.; Piwowarska, Z.; Rutkowska, M.; Baran, R.; Chmielarz, L. Activating effect of cerium in hydrotalcite derived Cu-Mg-Al catalysts for selective ammonia oxidation and the selective reduction of NO with ammonia. React. Kinet. Mech. Catal. 2017, 121, 225–240. [Google Scholar] [CrossRef]
- Górecka, S.; Pacultová, K.; Fridrichová, D.; Górecki, K.; Bílková, T.; Žebrák, R.; Obalová, L. Catalytic oxidation of ammonia over cerium-modified copper aluminium zinc mixed oxides. Materials 2021, 14, 6581. [Google Scholar] [CrossRef]
- Ludvíková, J.; Jabłońska, M.; Jirátová, K.; Chmielarz, L.; Balabánová, J.; Kovanda, F.; Obalová, L. Co-Mn-Al mixed oxides as catalysts for ammonia oxidation to N2O. Res. Chem. Intermed. 2016, 42, 2669–2690. [Google Scholar] [CrossRef]
- Il’Chenko, N.I.; Golodets, G.I. Catalytic oxidation of ammonia: I. Reaction kinetics and mechanism. J. Catal. 1975, 39, 57–72. [Google Scholar] [CrossRef]
- Il’chenko, N.I.; Golodets, G.I. Catalytic oxidation of ammonia: II. Relationship between catalytic properties of substances and surface oxygen bond energy. General regularities in catalytic oxidation of ammonia and organic substances. J. Catal. 1975, 39, 73–86. [Google Scholar] [CrossRef]
- Il’chenko, N.I. Catalytic oxidation of ammonia. Russ. Chem. Rev. 1976, 45, 1119. [Google Scholar] [CrossRef]
- Hinokuma, S.; Shimanoe, H.; Matsuki, S.; Kawano, M.; Kawabata, Y.; Machida, M. Catalytic activity and selectivities of metal oxides and Pt/Al2O3 for NH3 combustion. Chem. Lett. 2016, 45, 179–181. [Google Scholar] [CrossRef]
- Gandhi, H.S.; Shelef, M. Selectivity for nitrogen formation in NH3 oxidation in wet and dry systems over mixed molybdenum oxides. J. Catal. 1975, 40, 312–317. [Google Scholar] [CrossRef]
- Chen, J.P.; Yang, R.T. Role of WO3 in mixed V2O5-WO3/TiO2 catalysts for selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. A Gen. 1992, 80, 135–148. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F. Oxidation of NH3 on V/Ti oxide based catalysts prepared by precipitation. Catal. Today 1989, 4, 253–265. [Google Scholar] [CrossRef]
- Biermann, J.J.; Janssen, F.J. Low temperature isotopic exchange of molecular oxygen via the reaction of NO, NH3 and O2 over supported vanadia and molybdena catalysts. Catal. Lett. 1989, 2, 385–393. [Google Scholar] [CrossRef]
- Nova, I.; Dall’Acqua, L.; Lietti, L.; Giamello, E.; Forzatti, P. Study of thermal deactivation of a de-NOx commercial catalyst. Appl. Catal. B Environ. 2001, 35, 31–42. [Google Scholar] [CrossRef]
- Due-Hansen, J.; Kustov, A.L.; Christensen, C.H.; Fehrmann, R. Impact of support and potassium-poisoning on the V2O5-WO3/ZrO2 catalyst performance in ammonia oxidation. Catal. Commun. 2009, 10, 803–806. [Google Scholar] [CrossRef]
- Ueshima, M.; Sano, K.; Ikeda, M.; Yoshino, K.; Okamura, J. New technology for selective catalytic oxidation of ammonia to nitrogen. Res. Chem. Intermed. 1998, 24, 133–141. [Google Scholar] [CrossRef]
- Sazonova, N.N.; Simakov, A.V.; Nikoro, T.A.; Barannik, G.B.; Lyakhova, V.F.; Zheivot, V.I.; Ismagilov, Z.R.; Veringa, H. Selective catalytic oxidation of ammonia to nitrogen. React. Kinet. Catal. Lett. 1996, 57, 71–79. [Google Scholar] [CrossRef]
- Liu, W.; Long, Y.; Liu, S.; Zhou, Y.; Tong, X.; Yin, Y.; Li, X.; Hu, K.; Hu, J. Promotional effect of Ce in NH3-SCO and NH3-SCR reactions over Cu-Ce/SCR catalysts. J. Ind. Eng. Chem. 2021, 107, 197–206. [Google Scholar] [CrossRef]
- Liu, W.; Long, Y.; Tong, X.; Yin, Y.; Li, X.; Hu, J. Transition metals modified commercial SCR catalysts as efficient catalysts in NH3-SCO and NH3-SCR reactions. Mol. Catal. 2021, 515, 111888–111897. [Google Scholar] [CrossRef]
- Yuan, R.-M.; Fu, G.; Xu, X.; Wan, H.-L. Mechanisms for selective catalytic oxidation of ammonia over vanadium oxides. J. Phys. Chem. C 2011, 115, 21218–21229. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Q.; Ma, D.; Liu, X.; You, Z.; Qiu, G.; Lv, X. Periodic DFT study on the adsorption and deoxygenation process of NH3 on V2O5 (001) surface. JOM 2022, 74, 1870–1877. [Google Scholar] [CrossRef]
- Carley, A.F.; Davies, P.R.; Roberts, M.W. An STM-XPS study of ammonia oxidation: The molecular architecture of chemisorbed imide ‘strings’ at Cu (110) surfaces. Chem. Commun. 1998, 17, 1793–1794. [Google Scholar] [CrossRef]
- Mayer, R.W.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R. Investigation of ammonia oxidation over copper with in situ NEXAFS in the soft X-ray range: Influence of pressure on the catalyst performance. Catal. Lett. 2001, 74, 115–119. [Google Scholar] [CrossRef]
- Mayer, R.W.; Melzer, M.; Hävecker, M.; Knop-Gericke, A.; Urban, J.; Freund, H.-J.; Schlögl, R. Comparison of oxidized polycrystalline copper foil with small deposited copper clusters in their behavior in ammonia oxidation: An investigation by means of in situ NEXAFS spectroscopy in the soft X-ray range. Catal. Lett. 2003, 86, 245–260. [Google Scholar] [CrossRef]
- Hirabayashi, S.; Ichihashi, M. Gas-phase reactions of copper oxide cluster cations with ammonia: Selective catalytic oxidation to nitrogen and water molecules. J. Phys. Chem. A 2018, 122, 4801–4807. [Google Scholar] [CrossRef]
- Gang, L.; Van Grondelle, J.; Anderson, B.G.; Van Santen, R.A. Selective low temperature NH3 oxidation to N2 on copper-based catalysts. J. Catal. 1999, 186, 100–109. [Google Scholar] [CrossRef]
- Gang, L.; Anderson, B.G.; Van Grondelle, J.; Van Santen, R.A. NH3 oxidation to nitrogen and water at low temperatures using supported transition metal catalysts. Catal. Today 2000, 61, 179–185. [Google Scholar] [CrossRef]
- Song, S.; Jiang, S. Selective catalytic oxidation of ammonia to nitrogen over CuO/CNTs: The promoting effect of the defects of CNTs on the catalytic activity and selectivity. Appl. Catal. B Environ. 2012, 117, 346–350. [Google Scholar] [CrossRef]
- Hung, C.-M. Synthesis, characterization and performance of CuO/La2O3 composite catalyst for ammonia catalytic oxidation. Powder Technol. 2009, 196, 56–61. [Google Scholar] [CrossRef]
- He, S.; Zhang, C.; Yang, M.; Zhang, Y.; Xu, W.; Cao, N.; He, H. Selective catalytic oxidation of ammonia from MAP decomposition. Sep. Purif. Technol. 2007, 58, 173–178. [Google Scholar] [CrossRef]
- Kaddouri, A.; Dupont, N.; Gélin, P.; Auroux, A. Selective oxidation of gas phase ammonia over copper chromites catalysts prepared by the sol-gel process. Catal. Commun. 2011, 15, 32–36. [Google Scholar] [CrossRef]
- Kong, X.; Li, Z.; Shao, Y.; Ren, X.; Li, K.; Wu, H.; Lv, C.; Lv, C.; Zhu, S. Modulate the superficial structure of La2Ce2O7 catalyst with anchoring CuOx species for the selective catalytic oxidation of NH3. J. Mater. Sci. Technol. 2022, 111, 1–8. [Google Scholar] [CrossRef]
- Curtin, T.; O’Regan, F.; Deconinck, C.; Knüttle, N.; Hodnett, B.K. The catalytic oxidation of ammonia: Influence of water and sulfur on selectivity to nitrogen over promoted copper oxide/alumina catalysts. Catal. Today 2000, 55, 189–195. [Google Scholar] [CrossRef]
- Lenihan, S.; Curtin, T. The selective oxidation of ammonia using copper-based catalysts: The effects of water. Catal. Today 2009, 145, 85–89. [Google Scholar] [CrossRef]
- Liang, C.; Li, X.; Qu, Z.; Tade, M.; Liu, S. The role of copper species on Cu/γ-Al2O3 catalysts for NH3-SCO reaction. Appl. Surf. Sci. 2012, 258, 3738–3743. [Google Scholar] [CrossRef]
- Kušar, H.M.J.; Ersson, A.G.; Vosecký, M.; Järås, S.G. Selective catalytic oxidation of NH3 to N2 for catalytic combustion of low heating value gas under lean/rich conditions. Appl. Catal. B Environ. 2005, 58, 25–32. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Yi, H.; Yu, Q.; Gao, F.; Zhang, R.; Li, C.; Chu, C. Effects of copper-precursors on the catalytic activity of Cu/graphene catalysts for the selective catalytic oxidation of ammonia. Appl. Surf. Sci. 2017, 412, 37–44. [Google Scholar] [CrossRef]
- Darvell, L.I.; Heiskanen, K.; Jones, J.M.; Ross, A.B.; Simell, P.; Williams, A. An investigation of alumina-supported catalysts for the selective catalytic oxidation of ammonia in biomass gasification. Catal. Today 2003, 81, 681–692. [Google Scholar] [CrossRef]
- Jones, J.M.; Pourkashanian, M.; Williams, A.; Backreedy, R.I.; Darvell, L.I.; Simell, P.; Heiskanen, K.; Kilpinen, P. The selective oxidation of ammonia over alumina supported catalysts-experiments and modelling. Appl. Catal. B Environ. 2005, 60, 139–146. [Google Scholar] [CrossRef]
- Jraba, N.; Makhlouf, T.; Delahay, G.; Tounsi, H. Catalytic activity of Cu/η-Al2O3 catalysts prepared from aluminum scraps in the NH3-SCO and in the NH3-SCR of NO. Environ. Sci. Pollut. Res. 2022, 29, 9053–9064. [Google Scholar] [CrossRef]
- Lippits, M.J.; Gluhoi, A.C.; Nieuwenhuys, B.E. A comparative study of the selective oxidation of NH3 to N2 over gold, silver and copper catalysts and the effect of addition of Li2O and CeOx. Catal. Today 2008, 137, 446–452. [Google Scholar] [CrossRef]
- Machida, M.; Tokudome, Y.; Maeda, A.; Sato, T.; Yoshida, H.; Ohyama, J.; Fujii, K.; Ishikawa, N. A comparative study of various transition metal overlayer catalysts for low-temperature NH3 oxidation under dry and wet conditions. Catal. Today 2022, 384, 70–75. [Google Scholar] [CrossRef]
- Amblard, M.; Burch, R.; Southward, B.W.L. The selective conversion of ammonia to nitrogen on metal oxide catalysts under strongly oxidising conditions. Appl. Catal. B Environ. 1999, 22, 159–166. [Google Scholar]
- Nassos, S.; Svensson, E.E.; Nilsson, M.; Boutonnet, M.; Järås, S. Microemulsion-prepared Ni catalysts supported on cerium-lanthanum oxide for the selective catalytic oxidation of ammonia in gasified biomass. Appl. Catal. B Environ. 2006, 64, 96–102. [Google Scholar] [CrossRef]
- Nassos, S.; Svensson, E.E.; Boutonnet, M.; Järås, S.G. The influence of Ni load and support material on catalysts for the selective catalytic oxidation of ammonia in gasified biomass. Appl. Catal. B Environ. 2007, 74, 92–102. [Google Scholar] [CrossRef]
- Wang, W.Q.; Tang, X.L.; Yi, H.H.; Hu, J.L. A Comparative study of the selective oxidation of NH3 to N2 over transition metal catalysts. Adv. Mater. Res. 2013, 798, 239–244. [Google Scholar]
- Jabłońska, M. Selective catalytic oxidation of ammonia into nitrogen and water vapour over transition metals modified Al2O3, TiO2 and ZrO2. Chem. Pap. 2015, 69, 1141–1155. [Google Scholar] [CrossRef]
- Duan, K.; Tang, X.; Yi, H.; Zhang, Y.; Ning, P. Comparative study on low temperature selective catalytic oxidation of ammonia over transition metals supported on TiO2. In Proceedings of the 2010 International Conference on Management and Service Science, Wuhan, China, 24–26 August 2010; pp. 1–4. [Google Scholar]
- Jabłońska, M.; Ciptonugroho, W.; Góra-Marek, K.; Al-Shaal, M.G.; Palkovits, R. Preparation, characterization and catalytic performance of Ag-modified mesoporous TiO2 in low-temperature selective ammonia oxidation into nitrogen and water vapour. Microporous Mesoporous Mater. 2017, 245, 31–44. [Google Scholar] [CrossRef]
- Ge, S.; Liu, X.; Liu, J.; Liu, H.; Liu, H.; Chen, X.; Wang, G.; Chen, J.; Zhang, G.; Zhang, Y.; et al. Synthesis of TixSn1-xO2 mixed metal oxide for copper catalysts as high-efficiency NH3 selective catalytic oxidation. Fuel 2022, 314, 123061–123071. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Ning, P.; Song, Z.; Liu, X.; Duan, Y. In situ DRIFTS studies on CuO-Fe2O3 catalysts for low temperature selective catalytic oxidation of ammonia to nitrogen. Appl. Surf. Sci. 2017, 419, 733–743. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Zhang, T.; Wang, J.; Wei, G.; Liu, M.; Ning, P. Structural tuning and NH3-SCO performance optimization of CuO-Fe2O3 catalysts by impact of thermal treatment. Appl. Surf. Sci. 2019, 485, 81–91. [Google Scholar] [CrossRef]
- Yue, W.; Zhang, R.; Liu, N.; Chen, B. Selective catalytic oxidation of ammonia to nitrogen over orderly mesoporous CuFe2O4 with high specific surface area. Chin. Sci. Bull. 2014, 59, 3980–3986. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Piwowarska, Z.; Michalik, M.; Dudek, B.; Dziembaj, R. Natural micas intercalated with Al2O3 and modified with transition metals as catalysts of the selective oxidation of ammonia to nitrogen. Top. Catal. 2009, 52, 1017–1022. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Drozdek, M.; Dziembaj, R.; Cool, P.; Vansant, E.F. Selective catalytic oxidation of ammonia into nitrogen over PCH modified with copper and iron species. Catal. Today 2006, 114, 319–325. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Dziembaj, R.; Cool, P.; Vansant, E.F. Selective catalytic reduction of NO with ammonia over porous clay heterostructures modified with copper and iron species. Catal. Today 2007, 119, 181–186. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Liu, S.; Chen, J.; Jia, W. The catalytic properties of Cu modified attapulgite in NH3-SCO and NH3-SCR reactions. Appl. Surf. Sci. 2019, 480, 537–547. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, Z.; Quan, X.; Wang, H. Selective catalytic oxidation of ammonia to nitrogen over ceria-zirconia mixed oxides. Appl. Catal. A Gen. 2012, 411, 131–138. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.H.; Hong, S.C. Influence of calcination temperature on Ce/TiO2 catalysis of selective catalytic oxidation of NH3 to N2. Appl. Catal. A Gen. 2014, 470, 189–198. [Google Scholar] [CrossRef]
- Lee, S.M.; Hong, S.C. Promotional effect of vanadium on the selective catalytic oxidation of NH3 to N2 over Ce/V/TiO2 catalyst. Appl. Catal. B Environ. 2015, 163, 30–39. [Google Scholar] [CrossRef]
- Chen, W.; Ma, Y.; Qu, Z.; Liu, Q.; Huang, W.; Hu, X.; Yan, N. Mechanism of the selective catalytic oxidation of slip ammonia over Ru-modified Ce-Zr complexes determined by in situ diffuse reflectance infrared Fourier transform spectroscopy. Environ. Sci. Technol. 2014, 48, 12199–12205. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, Z.; Zhang, X.; Wang, H. Role of different coordinated Cu and reactive oxygen species on the highly active Cu-Ce-Zr mixed oxides in NH3-SCO: A combined in situ EPR and O2-TPD approach. Catal. Sci. Technol. 2016, 6, 4491–4502. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Wang, Z.; Qu, Z. Adsorption and surface reaction pathway of NH3 selective catalytic oxidation over different Cu-Ce-Zr catalysts. Appl. Surf. Sci. 2018, 447, 40–48. [Google Scholar] [CrossRef]
- Lou, J.-C.; Hung, C.-M.; Yang, S.-F. Selective catalytic oxidation of ammonia over copper-cerium composite catalyst. J. Air Waste Manag. Assoc. 2004, 54, 68–76. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Qu, Z.; Quan, X.; Li, Z.; Wang, H.; Fan, R. Selective catalytic oxidation of ammonia to nitrogen over CuO-CeO2 mixed oxides prepared by surfactant-templated method. Appl. Catal. B Environ. 2013, 134, 153–166. [Google Scholar] [CrossRef]
- Hung, C.-M. Catalytic decomposition of ammonia over bimetallic CuO/CeO2 nanoparticle catalyst. Aerosol Air Qual. Res. 2008, 8, 447–458. [Google Scholar] [CrossRef]
- Hung, C.-M. Selective catalytic oxidation of ammonia to nitrogen on CuO-CeO2 bimetallic oxide catalysts. Aerosol Air Qual. Res. 2006, 6, 150–169. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, S.B.; Hong, S.C. Characterization and reactivity of natural manganese ore catalysts in the selective catalytic oxidation of ammonia to nitrogen. Chemosphere 2003, 50, 1115–1122. [Google Scholar] [CrossRef]
- Wöllner, A.; Lange, F.; Schmelz, H.; Knözinger, H. Characterization of mixed copper-manganese oxides supported on titania catalysts for selective oxidation of ammonia. Appl. Catal. A Gen. 1993, 94, 181–203. [Google Scholar] [CrossRef]
- Kaijiao, D.; Xiaolong, T.; Honnghong, Y.; Ping, N.; Lida, W. Rare earth oxide modified Cu-Mn compounds supported on TiO2 catalysts for low temperature selective catalytic oxidation of ammonia and in lean oxygen. J. Rare Earths 2010, 28, 338–342. [Google Scholar]
- Song, D.; Shao, X.; Yuan, M.; Wang, L.; Zhan, W.; Guo, Y.; Guo, Y.; Lu, G. Selective catalytic oxidation of ammonia over MnOx-TiO2 mixed oxides. RSC Adv. 2016, 6, 88117–88125. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Selective catalytic oxidation of ammonia to nitrogen over Fe2O3-TiO2 prepared with a sol-gel method. J. Catal. 2002, 207, 158–165. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Ma, X.; Tang, Y.; Zhao, Y.; Zhang, A.; Wang, C.; Du, C.; Shan, B. Selective catalytic oxidation of ammonia over AMn2O5 (A = Sm, Y, Gd) and reaction selectivity promotion through Nb decoration. J. Catal. 2021, 402, 10–21. [Google Scholar] [CrossRef]
- Dong, A.; Yang, Z.; Wang, W. Mixed catalyst SmMn2O5/Cu-SAPO-34 for NH3-selective catalytic oxidation. ACS Omega 2022, 7, 8633–8639. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Y.; Yang, Q.; Xiong, S.; Li, J.; Crittenden, J. Performance of modified LaxSr1-x MnO3 perovskite catalysts for NH3 oxidation: TPD, DFT, and kinetic studies. Environ. Sci. Technol. 2018, 52, 7443–7449. [Google Scholar] [CrossRef]
- Jabłońska, M.; Wolkenar, B.; Beale, A.M.; Pischinger, S.; Palkovits, R. Comparison of Cu-Mg-Al-Ox and Cu/Al2O3 in selective catalytic oxidation of ammonia (NH3-SCO). Catal. Commun. 2018, 110, 5–9. [Google Scholar] [CrossRef]
- Jabłońska, M.; Beale, A.M.; Nocuń, M.; Palkovits, R. Ag-Cu based catalysts for the selective ammonia oxidation into nitrogen and water vapour. Appl. Catal. B Environ. 2018, 232, 275–287. [Google Scholar] [CrossRef]

| Cations | Cations’ Ratio | Compounds Identified |
|---|---|---|
| CuAl | 1.0/1.0 | Amorphous species |
| CuZnAl | 2.0/1.0/1.0 | HT + R |
| CuZnAl | 3.3/1.6/1.0 | HT + R |
| CuZnAl | 1.6/0.8/1.0 | HT + R |
| CuZnAl | 1.5/1.5/1.0 | HT (HT + R) |
| CuZnAl | 1.2/1.2/1.0 | HT |
| CuZnAl | 0.8/0.8/1.0 | HT |
| CuCr | 1.0/1.0 | Amorphous species |
| CuZnCr | 1.5/1.5/1.0 | HT |
| CuCoCr | 2.0/2.0/1.0 | HT + M |
| CuCoCr | 1.5/1.5/1.0 | HT |
| CuZnCr | 1.5/1.5/1.0 | HT |
| CuMgCr | 1.5/1.5/1.0 | HT |
| CuMnCr | 1.5/1.5/1.0 | MnCO3 + HT |
| CuCoZnCr | 1.4/0.1/1.5/1.0 | HT |
| CuZnAlCr | 3.0/3.0/1.0/1.0 | HT |
| CuZnFe | 1.5/1.5/1.0 | Au |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłońska, M.; Mollá Robles, A. A Comparative Mini-Review on Transition Metal Oxides Applied for the Selective Catalytic Ammonia Oxidation (NH3-SCO). Materials 2022, 15, 4770. https://doi.org/10.3390/ma15144770
Jabłońska M, Mollá Robles A. A Comparative Mini-Review on Transition Metal Oxides Applied for the Selective Catalytic Ammonia Oxidation (NH3-SCO). Materials. 2022; 15(14):4770. https://doi.org/10.3390/ma15144770
Chicago/Turabian StyleJabłońska, Magdalena, and Alejandro Mollá Robles. 2022. "A Comparative Mini-Review on Transition Metal Oxides Applied for the Selective Catalytic Ammonia Oxidation (NH3-SCO)" Materials 15, no. 14: 4770. https://doi.org/10.3390/ma15144770
APA StyleJabłońska, M., & Mollá Robles, A. (2022). A Comparative Mini-Review on Transition Metal Oxides Applied for the Selective Catalytic Ammonia Oxidation (NH3-SCO). Materials, 15(14), 4770. https://doi.org/10.3390/ma15144770






