Fabrication of Mn-Doped SrTiO3/Carbon Fiber with Oxygen Vacancy for Enhanced Photocatalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Experiment Section
2.1. Preparation of Photocatalytic Material
2.2. Characterization Method
2.3. Photocatalytic Evolution
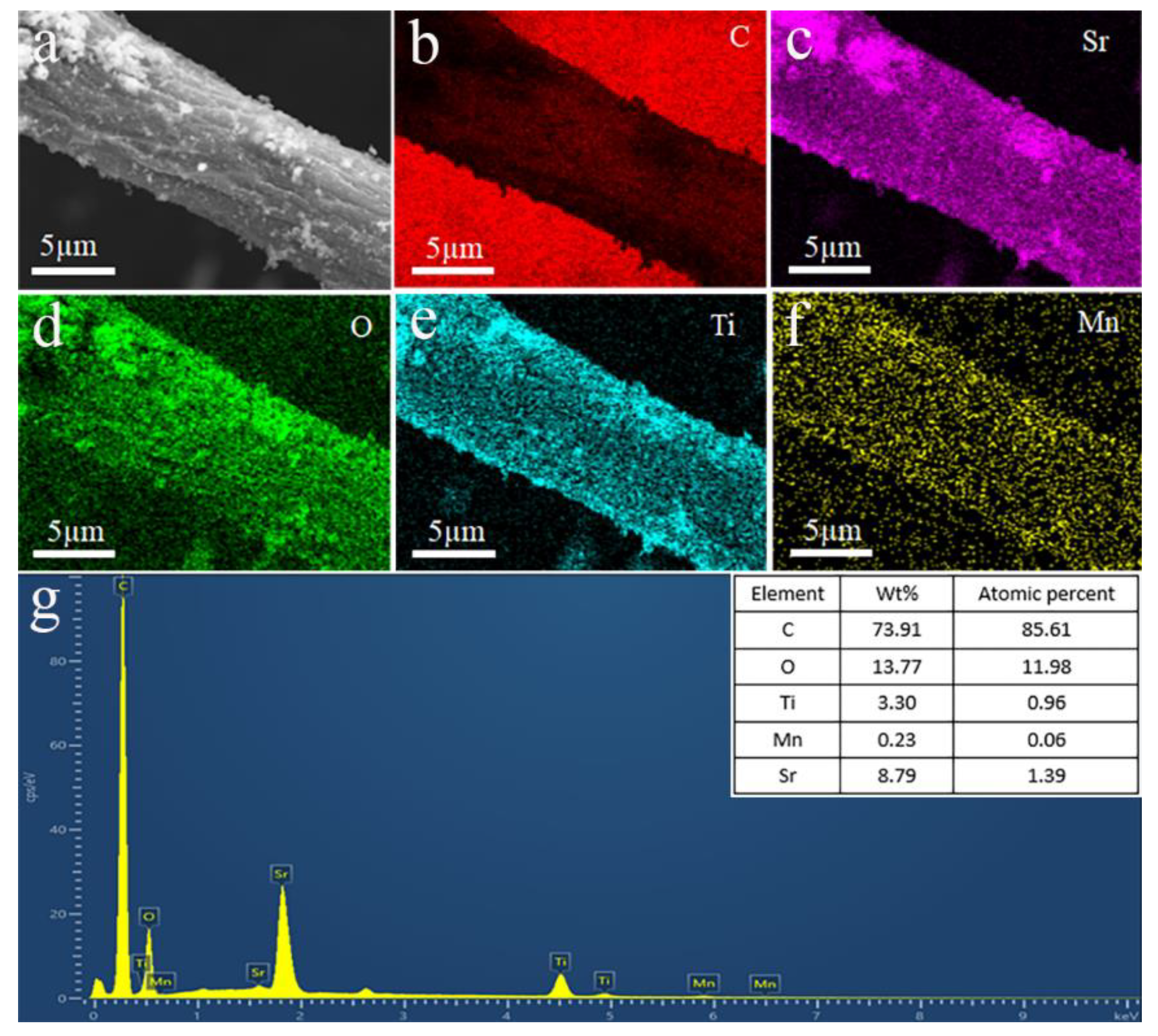
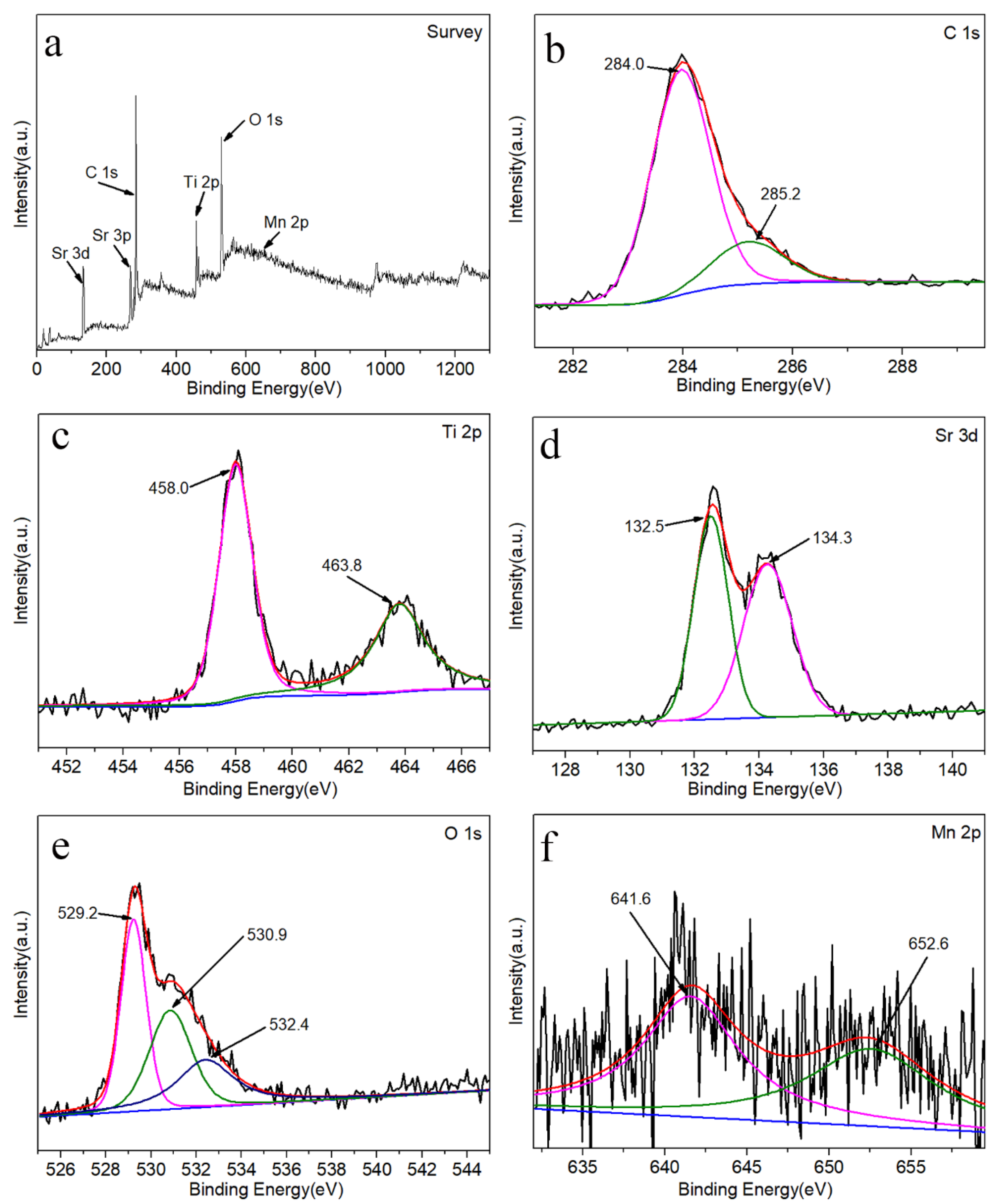
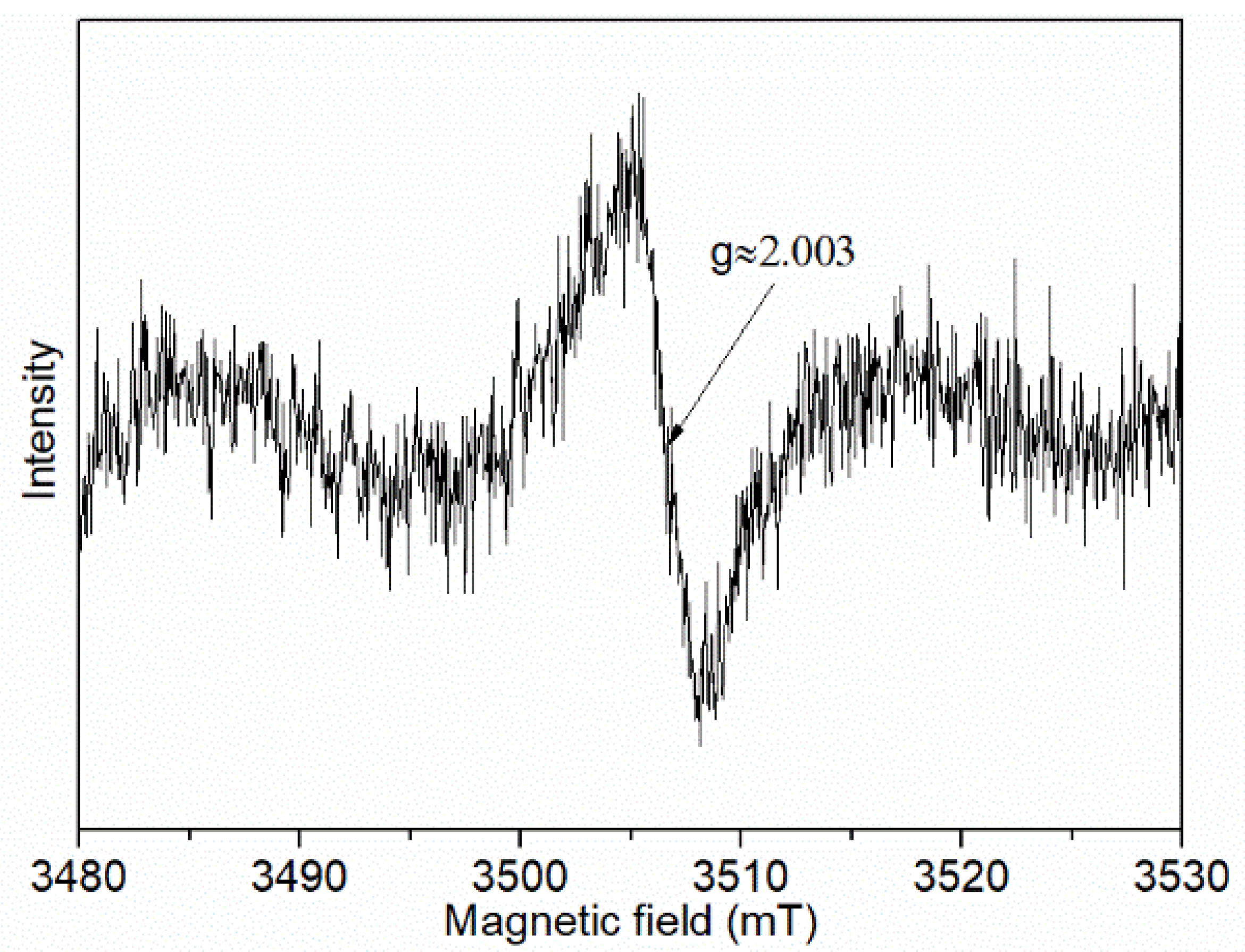
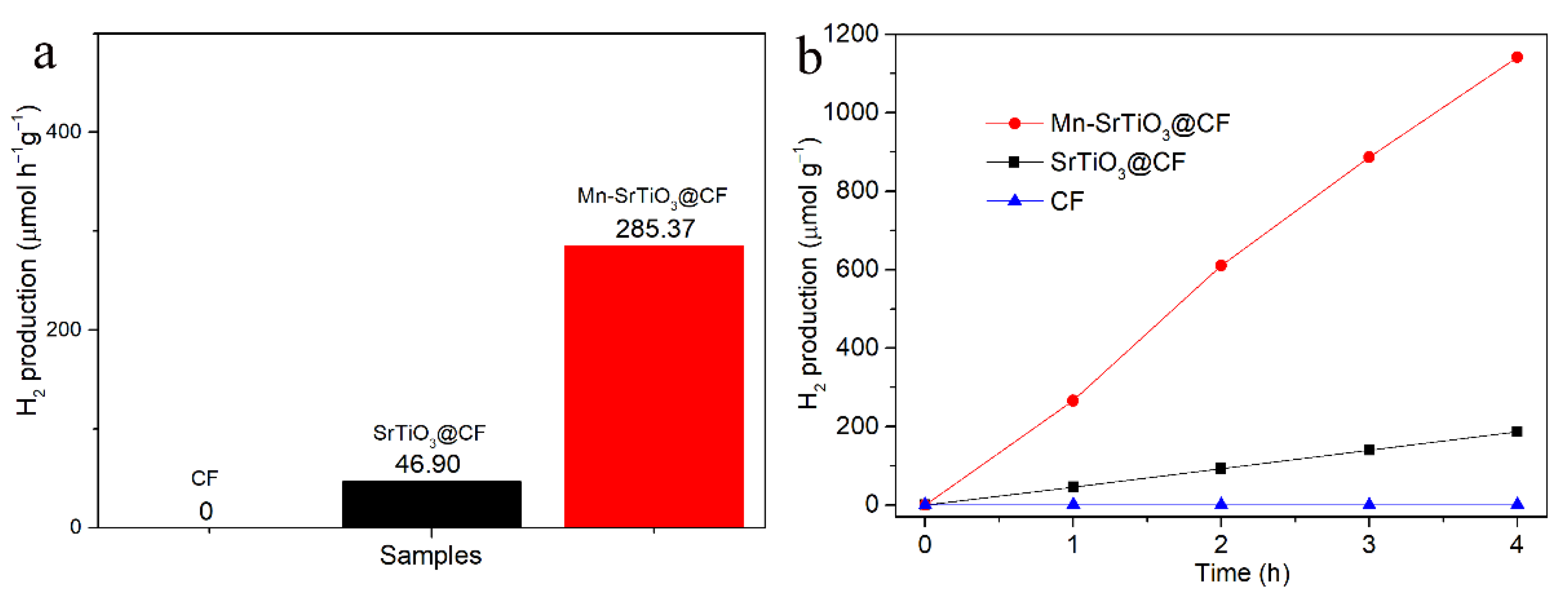
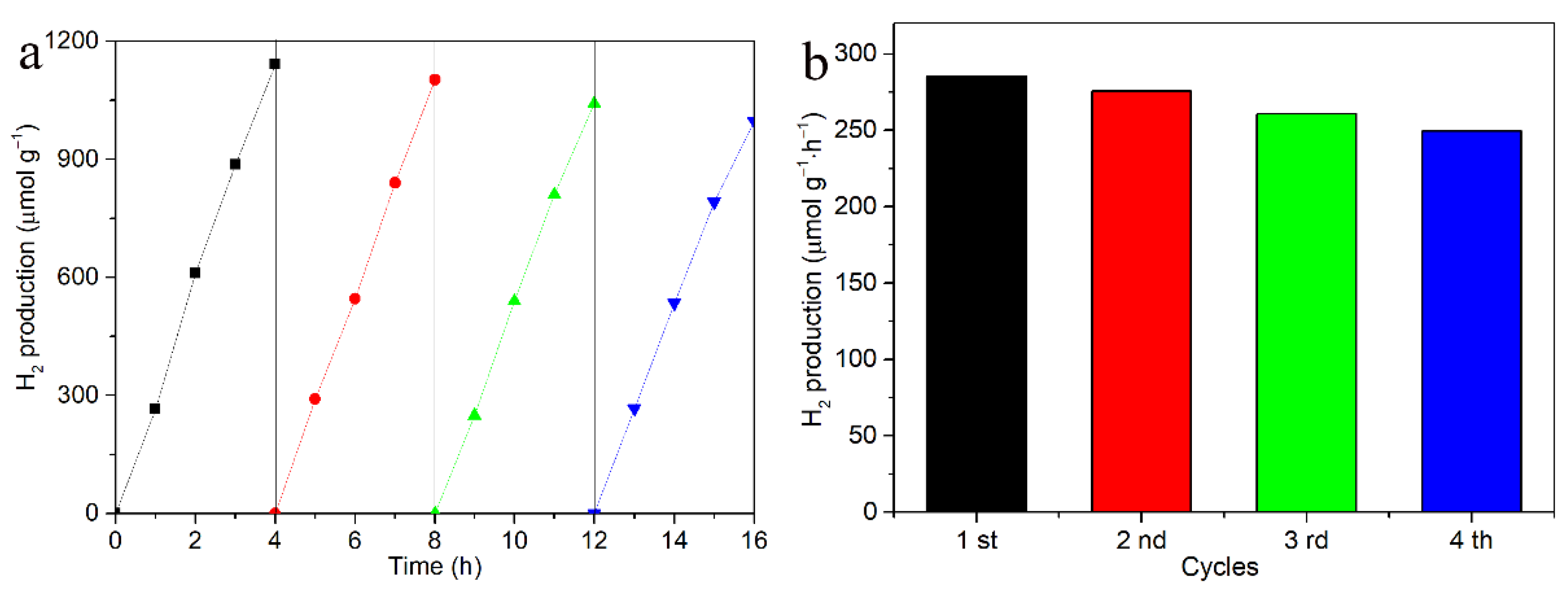
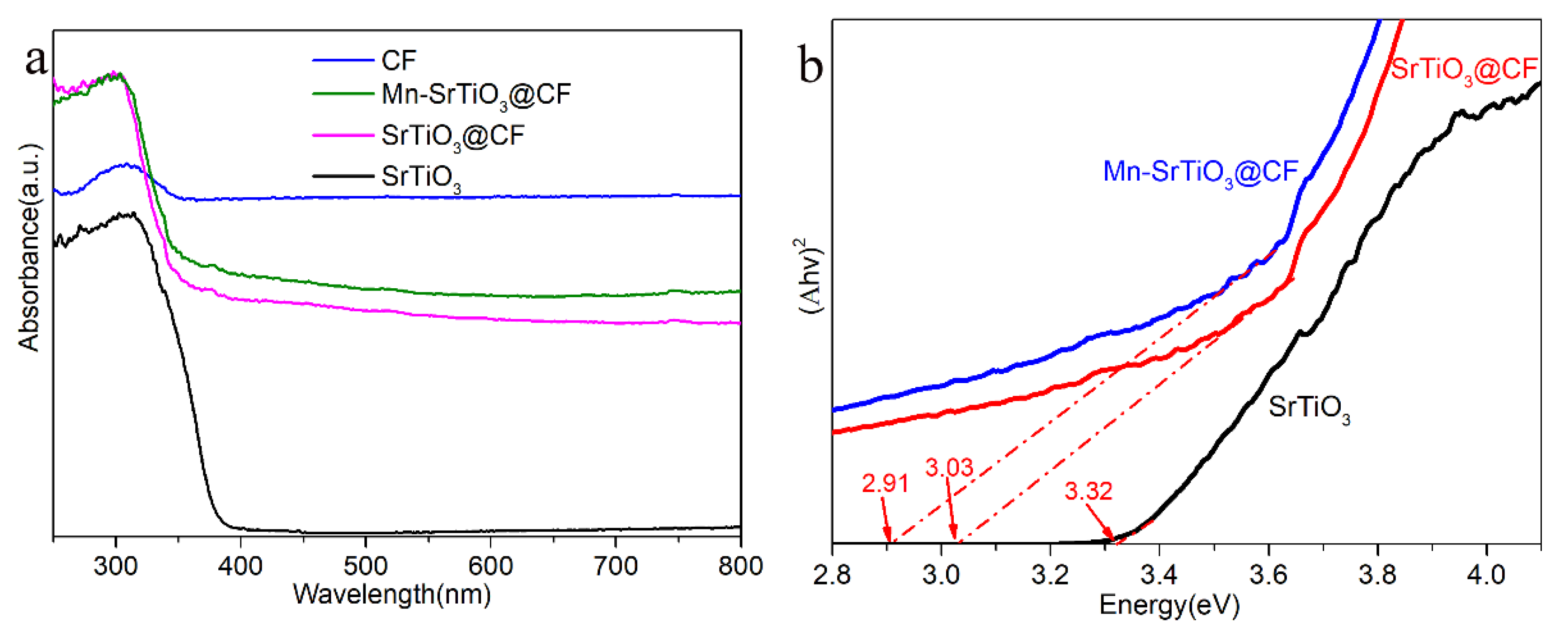
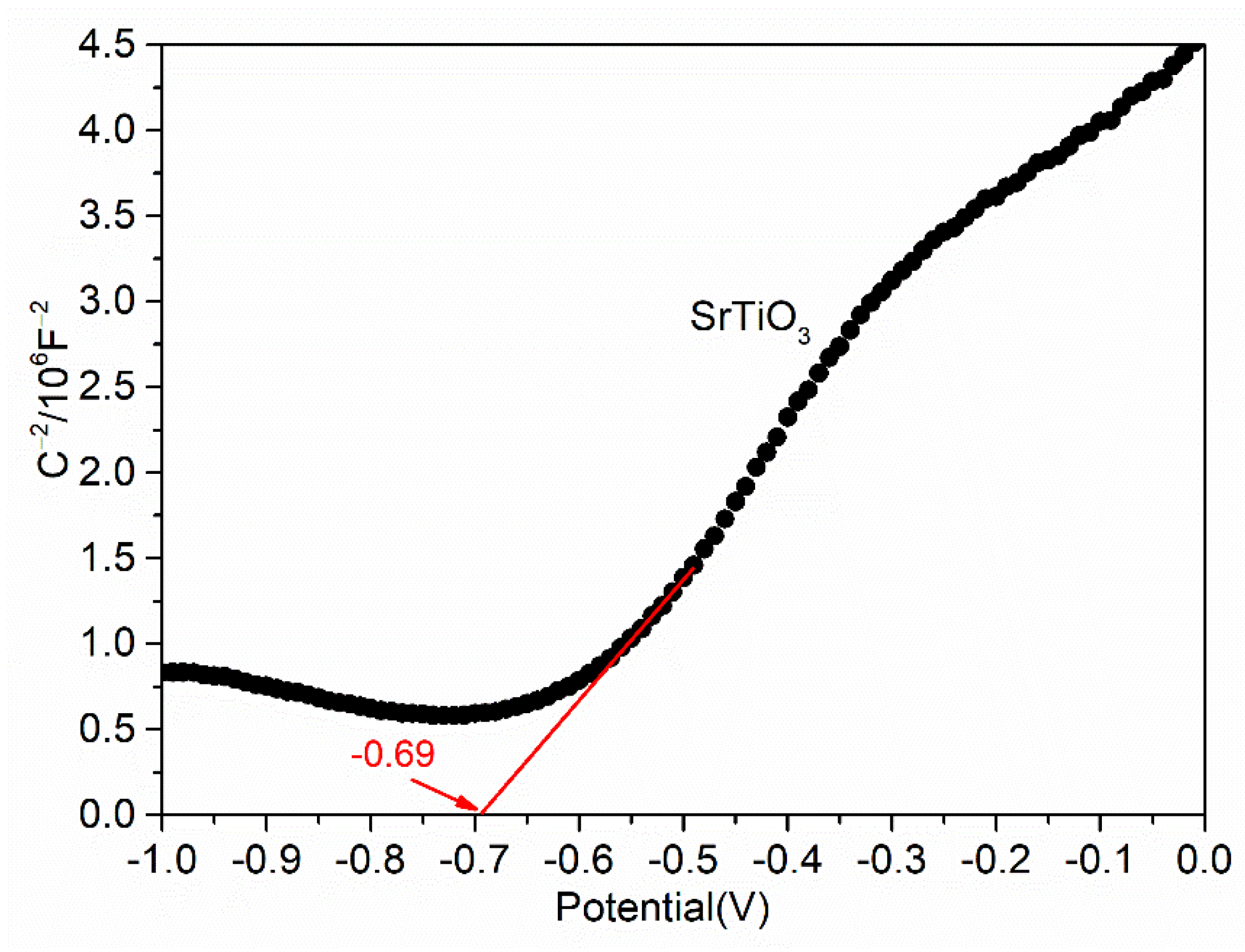
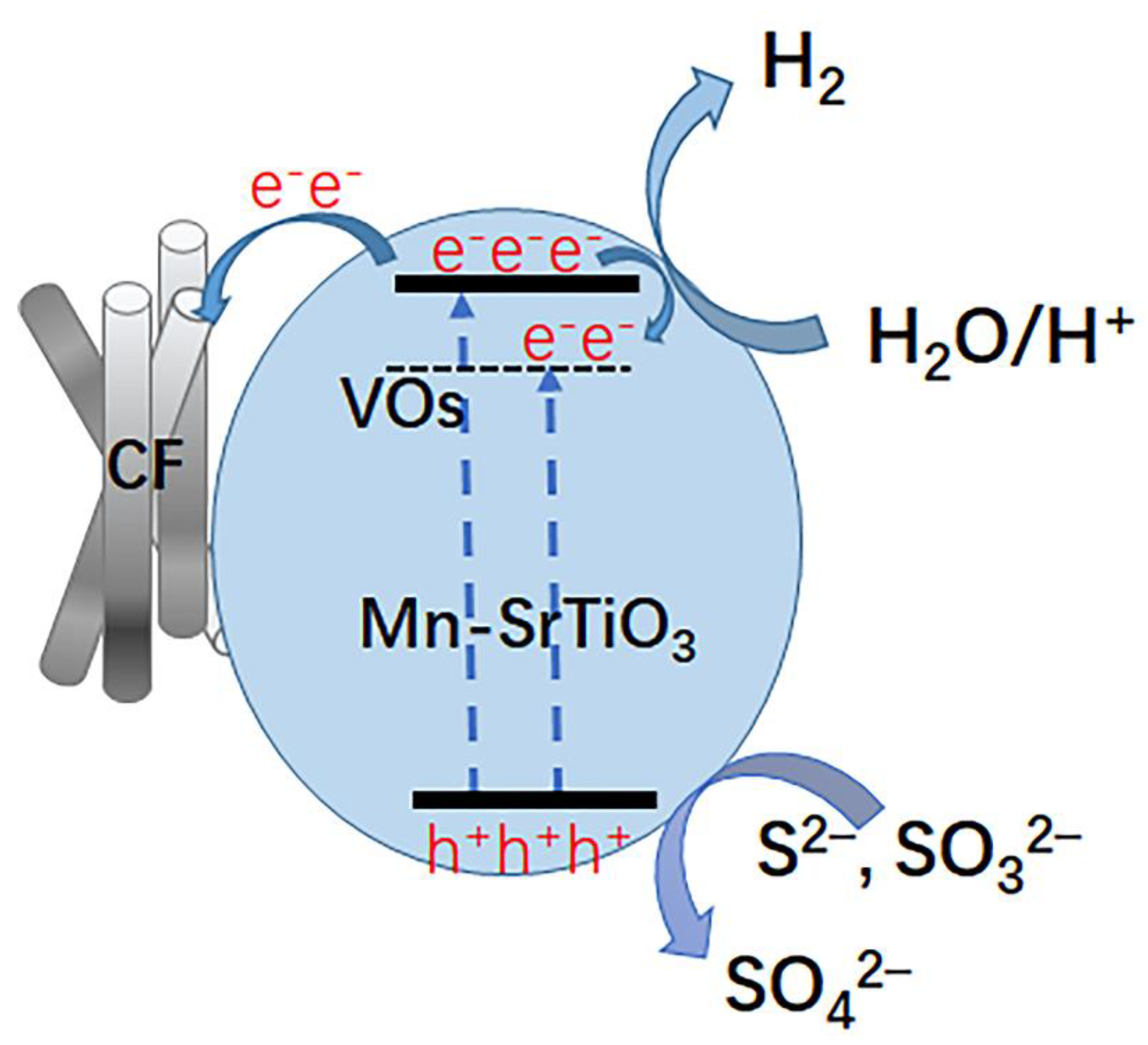
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phoon, B.L.; Lai, C.W.; Juan, J.C.; Show, P.L.; Chen, W.H. A review of synthesis and morphology of SrTiO3 for energy and other applications. Int. J. Energy Res. 2019, 43, 5151–5174. [Google Scholar] [CrossRef]
- Liang, Y. Recent advanced development of metal-loaded mesoporous organosilicas as catalytic nanoreactors. Nanoscale Adv. 2021, 3, 6827–6868. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Czyżewski, A.; Wanag, A.; Dubicki, M.; Sadłowski, M.; Wróbel, R.J.; Morawski, A.W. Photocatalytic oxidation of nitric oxide over AgNPs/TiO2-loaded carbon fiber cloths. J. Environ. Manag. 2020, 262, 110343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Ding, C.; Sun, Y.; Lin, Y.; Sun, W.; Luo, C. Synthesis of surface plasma photocatalyst Ag loaded TiO2 nanowire arrays/graphene oxide coated carbon fiber composites and enhancement of the photocatalytic activity for tetracycline hydrochloride degradation. J. Photochem. Photobiol. A Chem. 2017, 342, 94–101. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, W.; Chen, Y. Structure and photocatalytic properties of Mn-doped TiO2 loaded on wood-based activated carbon fiber composites. Materials 2017, 10, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opoku, F.; Govender, K.K.; van Sittert, C.G.C.E.; Govender, P.P. Enhancing Charge Separation and Photocatalytic Activity of Cubic SrTiO3 with Perovskite-Type Materials MTaO3 (M = Na, K) for Environmental Remediation: A First-Principles Study. ChemistrySelect 2017, 2, 6304–6316. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, Z.; Li, B.; Li, S.; Yue, J.; Zhao, G.; Muhler, M.; Wang, X. Solvent effects on photocatalytic anaerobic oxidation of benzyl alcohol over Pt-loaded defective SrTiO3 nanoparticles. ACS Appl. Nano Mater. 2021, 4, 9254–9264. [Google Scholar] [CrossRef]
- Wu, Y.; He, T. Ag loading induced visible light photocatalytic activity for pervoskite SrTiO3 nanofibers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 199, 283–289. [Google Scholar] [CrossRef]
- Grabowska, E.; Marchelek, M.; Klimczuk, T.; Lisowski, W.; Zaleska-Medynska, A. TiO2/SrTiO3 and SrTiO3 microspheres decorated with Rh, Ru or Pt nanoparticles: Highly UV–vis responsible photoactivity and mechanism. J. Catal. 2017, 350, 159–173. [Google Scholar] [CrossRef]
- Li, X.; Ge, Z.; Xue, F.; Liu, H.; Lyu, B.; Liu, M. Lattice-oriented contact in Pd/SrTiO3 heterojunction for rapid electron transfer during photocatalytic H2 production. Mater. Res. Bull. 2020, 123, 110722. [Google Scholar] [CrossRef]
- Tolod, K.; Bajamundi, C.; de Leon, R.; Sreearunothai, P.; Khunphonoi, R.; Grisdanurak, N. Visible light-driven photocatalytic hydrogen production using Cu-doped SrTiO3. Energy Sour. Part A Recovery Util. Environ. Eff. 2016, 38, 286–294. [Google Scholar] [CrossRef]
- Chiang, T.H.; Lyu, H.; Hisatomi, T.; Goto, Y.; Takata, T.; Katayama, M.; Minegishi, T.; Domen, K. Efficient photocatalytic water splitting using Al-doped SrTiO3 coloaded with molybdenum oxide and rhodium–chromium oxide. ACS Catal. 2018, 8, 2782–2788. [Google Scholar] [CrossRef]
- Wu, G.; Li, P.; Xu, D.; Luo, B.; Hong, Y.; Shi, W.; Liu, C. Hydrothermal synthesis and visible-light-driven photocatalytic degradation for tetracycline of Mn-doped SrTiO3 nanocubes. Appl. Surf. Sci. 2015, 333, 39–47. [Google Scholar] [CrossRef]
- Thanh, T.D.; Phan, T.; Van Minh, N.; Lee, J.S.; Yu, S. Influence of Mn Doping on the Crystal Structure, and Optical and Magnetic Properties of SrTiO3 Compounds. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar]
- Bentour, H.; El Yadari, M.; El Kenz, A.; Benyoussef, A. DFT study of electronic and optical properties of (S–Mn) co-doped SrTiO3 for enhanced photocatalytic hydrogen production. Solid State Commun. 2020, 312, 113893. [Google Scholar] [CrossRef]
- Yang, C.; Liu, T.; Cheng, Z.; Gan, H.; Chen, J. Study on Mn-doped SrTiO3 with first principle calculation. Phys. B Condens. Matter 2012, 407, 844–848. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Zhu, W.-b.; Coker, E.N.; Li, B.; Zheng, M.; Yu, W.; Fan, H.; Sun, Z. Oxygen vacancy enhanced photocatalytic activity of pervoskite SrTiO3. ACS Appl. Mater. Interfaces 2014, 6, 19184–19190. [Google Scholar] [CrossRef]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygen vacancies in materials. TrAC Trends Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Sarkar, A.; Khan, G.G. The formation and detection techniques of oxygen vacancies in titanium oxide-based nanostructures. Nanoscale 2019, 11, 3414–3444. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ai, Z.; Jia, F.; Zhang, L. Oxygen vacancy-mediated photocatalysis of BiOCl: Reactivity, selectivity, and perspectives. Angew. Chem. Int. Ed. 2018, 57, 122–138. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Qin, C.; Tang, X.; Zhong, J.; Li, J.; Chen, J. Spiral carbon fibers modified Bi2WO6 with enhanced photocatalytic activity. J. Phys. Chem. Solids 2020, 141, 109430. [Google Scholar] [CrossRef]
- Zhang, C.; Han, P.; Lu, X.; Mao, Q.; Qu, J.; Li, Y. Preparation and photocatalytic activity characterization of activated carbon fiber–BiVO4 composites. RSC Adv. 2018, 8, 24665–24672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, G.; Abas, A.; Wang, S. Photocatalytic Degradation and Hydrogen Production of TiO2/Carbon Fiber Composite Using Bast as a Carbon Fiber Source. Int. J. Photoenergy 2018, 2018, 4954039. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Ouyang, S.; Xu, H.; Li, D.; Zhang, X.; Li, Y.; Ye, J. Designing Au surface-modified nanoporous-single-crystalline SrTiO3 to optimize diffusion of surface plasmon resonance-induce photoelectron toward enhanced visible-light photoactivity. ACS Appl. Mater. Interfaces 2016, 8, 9506–9513. [Google Scholar] [CrossRef]
- Ramos, M.; Bonelli, P.; Cukierman, A.; Carrott, M.R.; Carrott, P. Influence of thermal treatment conditions on porosity development and mechanical properties of activated carbon cloths from a novel nanofibre-made fabric. Mater. Chem. Phys. 2009, 116, 310–314. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Zeng, B.; Li, C.; Han, H. EPR study of charge separation associated states and reversibility of surface bound superoxide radicals in SrTiO3 photocatalyst. J. Energy Chem. 2022, 70, 388–393. [Google Scholar] [CrossRef]
- Xia, Y.; He, Z.; Su, J.; Liu, Y.; Tang, B. Fabrication and photocatalytic property of novel SrTiO3/Bi5O7I nanocomposites. Nanoscale Res. Lett. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, X.; Pan, F.; Deng, B.; Liu, Z.; Zhang, X.; Huang, C.; Xiang, Z.; Lu, W. Mace-like carbon fiber/ZnO nanorod composite derived from typha orientalis for lightweight and high-efficient electromagnetic wave absorber. Adv. Compos. Hybrid Mater. 2021, 4, 1002–1014. [Google Scholar] [CrossRef]
- Jiang, D.; Sun, X.; Wu, X.; Shi, L.; Du, F. Hydrothermal synthesis of single-crystal Cr-doped SrTiO3 for efficient visible-light responsive photocatalytic hydrogen evolution. Mater. Res. Express 2020, 7, 015047. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Chen, J.; Li, G.; An, T.; Yamashita, H. Fabrication of Au/TiO2 nanowires@ carbon fiber paper ternary composite for visible-light photocatalytic degradation of gaseous styrene. Catal. Today 2017, 281, 621–629. [Google Scholar] [CrossRef]
- Wong, C.P.P.; Lai, C.W.; Lee, K.M.; Pan, G.T.; Huang, C.M.; Juan, J.C.; Yang, T.C.K. Enhancement of discharge capacity and energy density by oxygen vacancies in nickel doped SrTiO3 as cathode for rechargeable alkaline zinc battery. Electrochim. Acta 2022, 404, 139705. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Y.; Yu, Y.; Zhang, B. Oxygen vacancy engineering in photocatalysis. Sol. RRL 2020, 4, 2000037. [Google Scholar] [CrossRef]
- Hao, L.; Huang, H.; Zhang, Y.; Ma, T. Oxygen vacant semiconductor photocatalysts. Adv. Funct. Mater. 2021, 31, 2100919. [Google Scholar] [CrossRef]
- Li, C.-Q.; Yi, S.-S.; Liu, Y.; Niu, Z.-L.; Yue, X.-Z.; Liu, Z.-Y. In-situ constructing S-scheme/Schottky junction and oxygen vacancy on SrTiO3 to steer charge transfer for boosted photocatalytic H2 evolution. Chem. Eng. J. 2021, 417, 129231. [Google Scholar] [CrossRef]
- Yang, H.; Sun, Y.; Pei, W.; Wang, X.; Luo, Y.; Jin, Y.; Zhang, Y.; Yi, X.; Li, Q.; Fan, F. Insight into energy level modulation via Mn doping solid solutions for enhanced photocatalytic hydrogen production. Inorg. Chem. Commun. 2022, 135, 109041. [Google Scholar] [CrossRef]
- Suárez-Vázquez, S.I.; Cruz-López, A.; Molina-Guerrero, C.E.; Sánchez-Vázquez, A.I.; Macías-Sotelo, C. Effect of dopant loading on the structural and catalytic properties of Mn-doped SrTiO3 catalysts for catalytic soot combustion. Catalysts 2018, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; He, C.; Zuo, S.; Yan, X.; Dai, D.; Zhang, Y.; Yao, C. Photocatalytic nitrogen fixation over fluoride/attapulgite nanocomposite: Effect of upconversion and fluorine vacancy. Sol. Energy 2019, 191, 251–262. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Dong, X.; Zheng, N.; Ma, H.; Zhang, X. Fabrication of In2O3/In2S3 microsphere heterostructures for efficient and stable photocatalytic nitrogen fixation. Appl. Catal. B Environ. 2019, 257, 117932. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Y.; Chen, X.; Wang, Q.; Guo, L.; Zhang, S.; Zhong, Q. One-step hydrothermal synthesis of a novel 3D BiFeWOx/Bi2WO6 composite with superior visible-light photocatalytic activity. Green Chem. 2018, 20, 3014–3023. [Google Scholar] [CrossRef]
- Wang, H.; Yong, D.; Chen, S.; Jiang, S.; Zhang, X.; Shao, W.; Zhang, Q.; Yan, W.; Pan, B.; Xie, Y. Oxygen-vacancy-mediated exciton dissociation in BiOBr for boosting charge-carrier-involved molecular oxygen activation. J. Am. Chem. Soc. 2018, 140, 1760–1766. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Niu, J.; Zhang, K.-Q.; Yao, M. Fabrication of Mn-Doped SrTiO3/Carbon Fiber with Oxygen Vacancy for Enhanced Photocatalytic Hydrogen Evolution. Materials 2022, 15, 4723. https://doi.org/10.3390/ma15134723
Hu Q, Niu J, Zhang K-Q, Yao M. Fabrication of Mn-Doped SrTiO3/Carbon Fiber with Oxygen Vacancy for Enhanced Photocatalytic Hydrogen Evolution. Materials. 2022; 15(13):4723. https://doi.org/10.3390/ma15134723
Chicago/Turabian StyleHu, Qi, Jiantao Niu, Ke-Qin Zhang, and Mu Yao. 2022. "Fabrication of Mn-Doped SrTiO3/Carbon Fiber with Oxygen Vacancy for Enhanced Photocatalytic Hydrogen Evolution" Materials 15, no. 13: 4723. https://doi.org/10.3390/ma15134723
APA StyleHu, Q., Niu, J., Zhang, K.-Q., & Yao, M. (2022). Fabrication of Mn-Doped SrTiO3/Carbon Fiber with Oxygen Vacancy for Enhanced Photocatalytic Hydrogen Evolution. Materials, 15(13), 4723. https://doi.org/10.3390/ma15134723





