Dealumination and Characterization of Natural Mordenite-Rich Tuffs
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Raw Material
2.2. Dealumination
2.3. Characterization
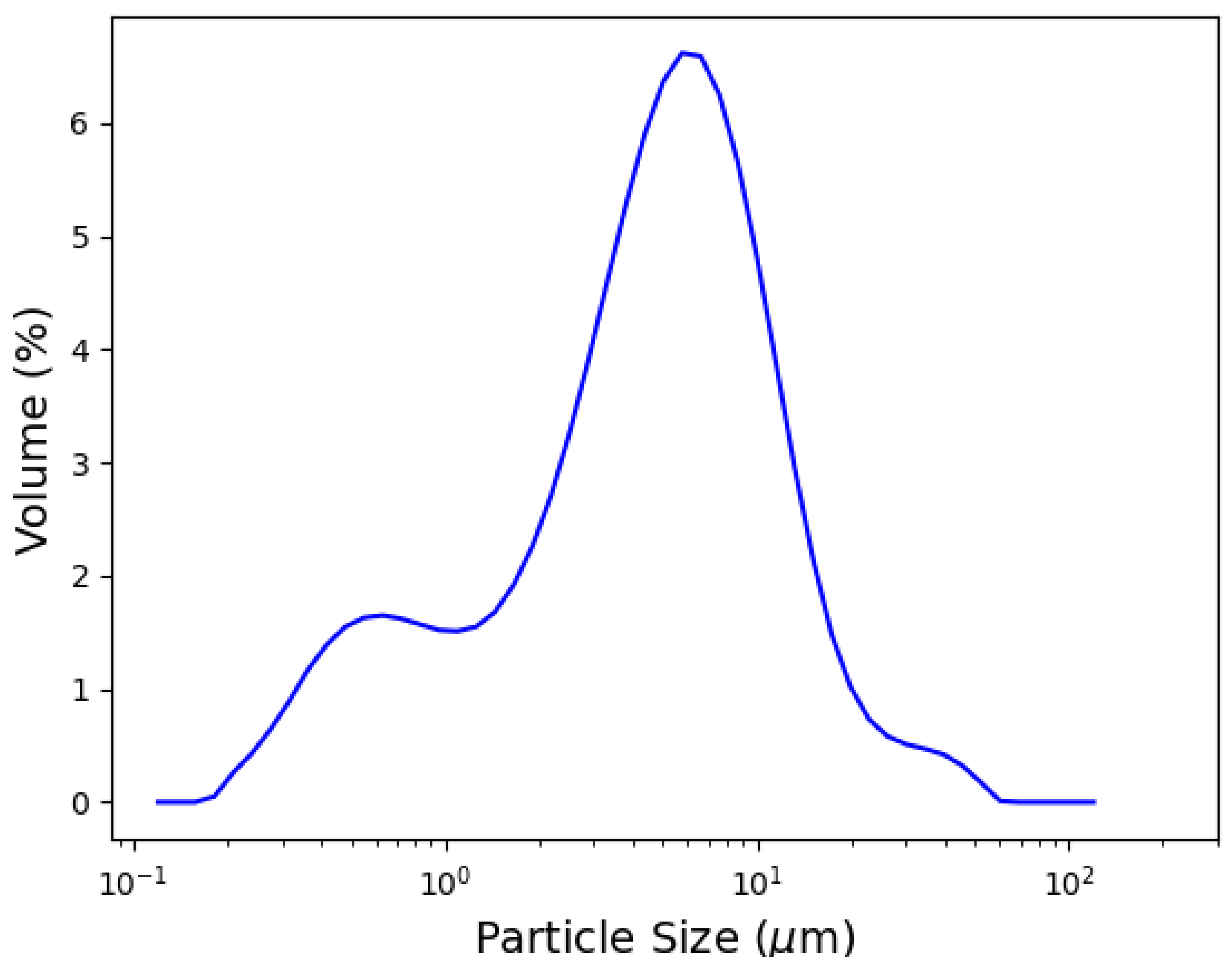
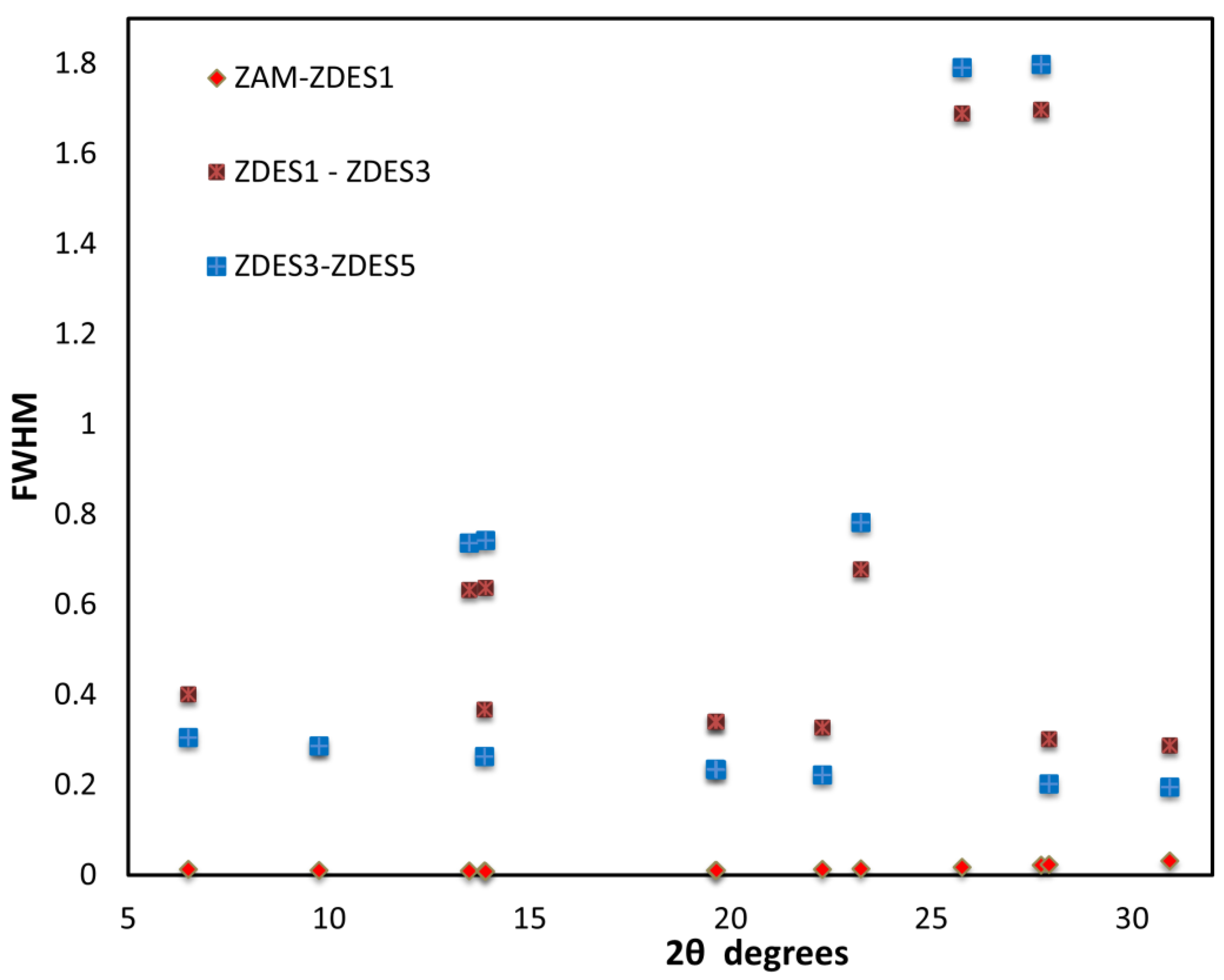
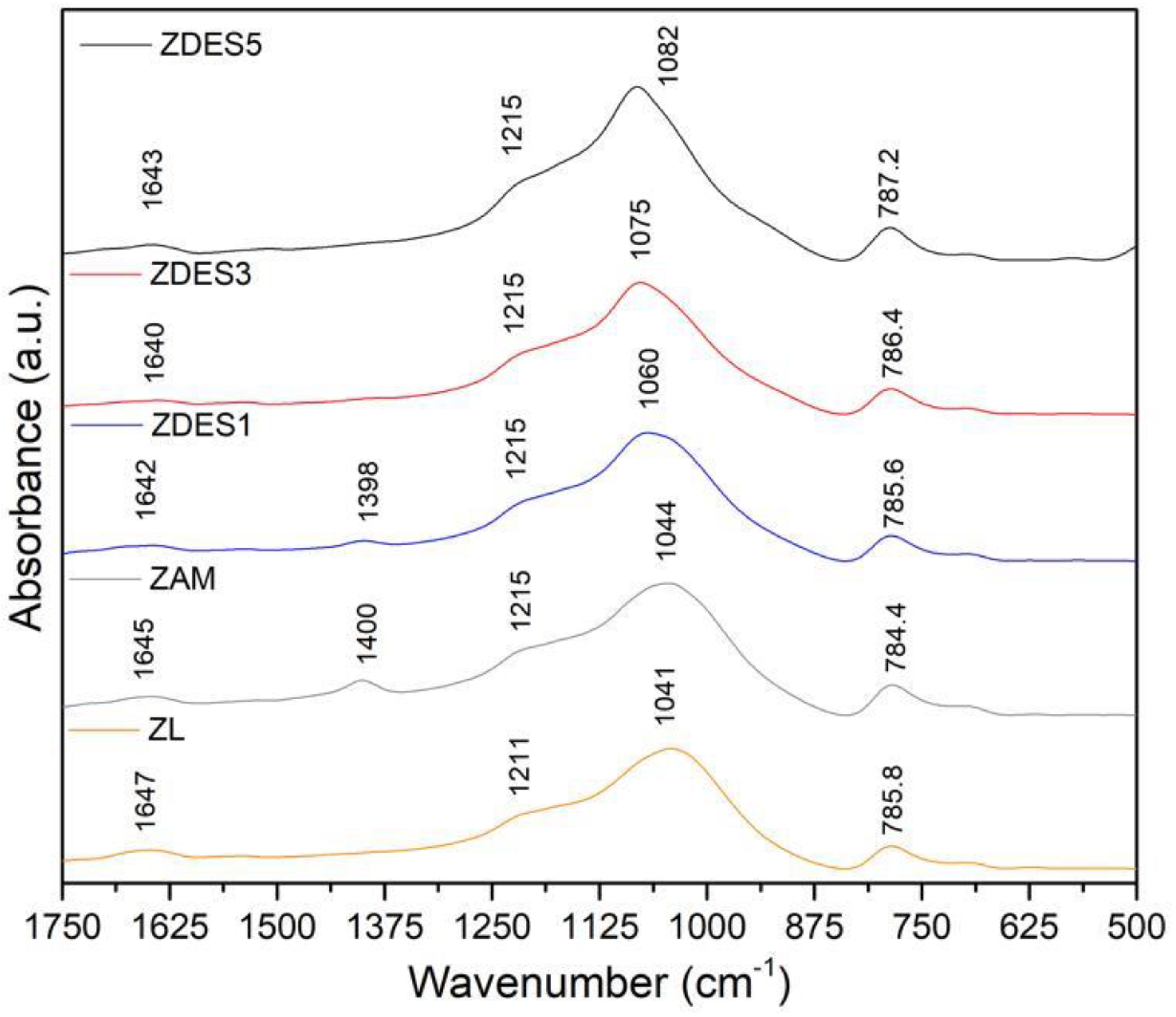
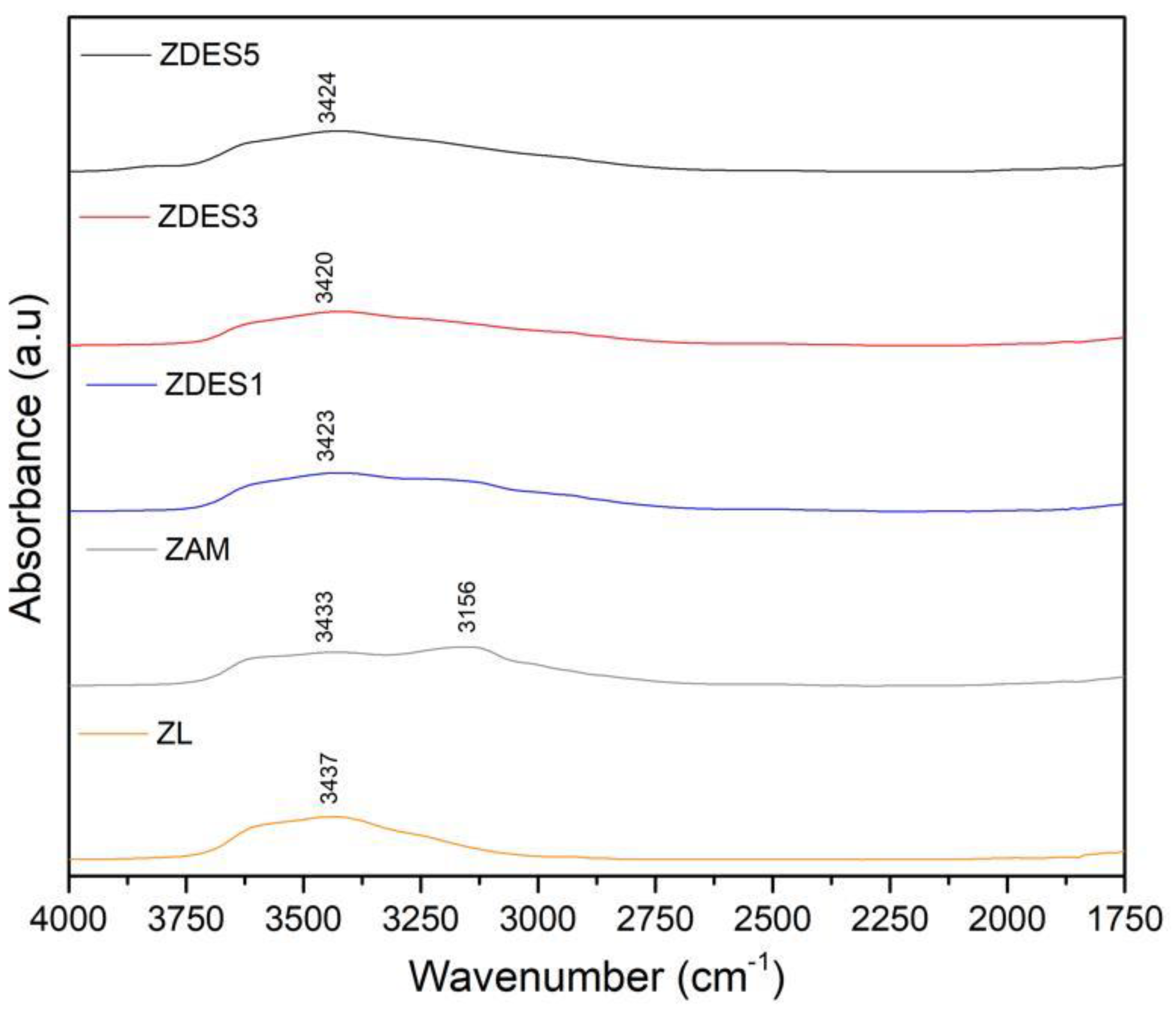
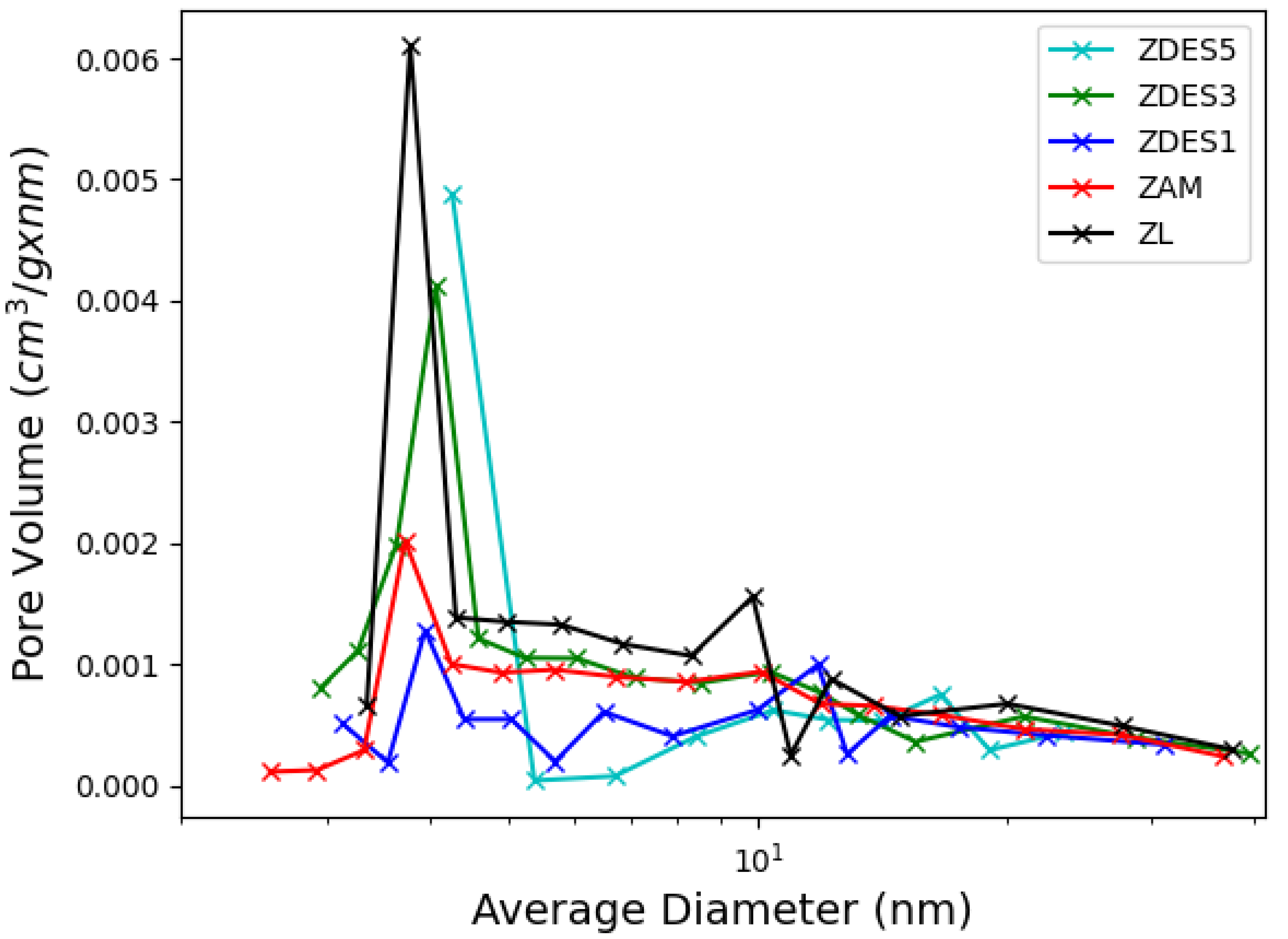
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yilmaz, B.; Müller, U. Catalytic Applications of Zeolites in Chemical Industry. Top. Catal. 2009, 52, 888–895. [Google Scholar] [CrossRef] [Green Version]
- Čejka, J.; Kubička, D. Zeolites and Other Micro- and Mesoporous Molecular Sieves. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley Online Library: Hoboken, NJ, USA, 2000; ISBN 9780471238966. [Google Scholar]
- Ates, A.; Hardacre, C. The Effect of Various Treatment Conditions on Natural Zeolites: Ion Exchange, Acidic, Thermal and Steam Treatments. J. Colloid Interface Sci. 2012, 372, 130–140. [Google Scholar] [CrossRef] [PubMed]
- van Donk, S.; Janssen, A.H.; Bitter, J.H.; de Jong, K.P. Generation, Characterization, and Impact of Mesopores in Zeolite Catalysts. Catal. Rev. 2003, 45, 297–319. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Basabe, Y.; Rodriguez-Iznaga, I.; De Menorval, L.C.; Llewellyn, P.; Maurin, G.; Lewis, D.W.; Binions, R.; Autie, M.; Ruiz-Salvador, A.R. Step-Wise Dealumination of Natural Clinoptilolite: Structural and Physicochemical Characterization. Microporous Mesoporous Mater. 2010, 135, 187–196. [Google Scholar] [CrossRef]
- Beier, G.K.; Belen’kaya, I.M.; Dubinin, M.M.; Khange, F. Structural Changes in Mordenite during Its Dealuminization. Bull. Acad. Sci. USSR Div. Chem. Sci. 1982, 31, 1300–1305. [Google Scholar] [CrossRef]
- Boveri, M.; Márquez-Álvarez, C.; Laborde, M.Á.; Sastre, E. Steam and Acid Dealumination of Mordenite. Characterization and Influence on the Catalytic Performance in Linear Alkylbenzene Synthesis. Catal. Today 2006, 114, 217–225. [Google Scholar] [CrossRef]
- Viswanadham, N.; Kumar, M. Effect of Dealumination Severity on the Pore Size Distribution of Mordenite. Microporous Mesoporous Mater. 2006, 92, 31–37. [Google Scholar] [CrossRef]
- Saxena, S.K.; Viswanadham, N. Enhanced Catalytic Properties of Mesoporous Mordenite for Benzylation of Benzene with Benzyl Alcohol. Appl. Surf. Sci. 2017, 392, 384–390. [Google Scholar] [CrossRef]
- González, M.D.; Cesteros, Y.; Salagre, P. Comparison of Dealumination of Zeolites Beta, Mordenite and ZSM-5 by Treatment with Acid under Microwave Irradiation. Microporous Mesoporous Mater. 2011, 144, 162–170. [Google Scholar] [CrossRef]
- Narayanan, S.; Tamizhdurai, P.; Mangesh, V.L.; Ragupathi, C.; Santhana Krishnan, P.; Ramesh, A. Recent Advances in the Synthesis and Applications of Mordenite Zeolite—Review. RSC Adv. 2021, 11, 250–267. [Google Scholar] [CrossRef]
- Cao, X.; Wang, R.; Wang, K.; Gu, Z.; Wang, F. Enhancing the Catalytic Properties of Mordenites via an Alkali–Acid Treatment and by Loading Nickel–Cerium during o -Ethyltoluene Isomerization. ACS Omega 2021, 6, 22688–22699. [Google Scholar] [CrossRef] [PubMed]
- Supamathanon, N.; Khabuanchalad, S. Development of K-Mordenite Catalyst for Biodiesel Production from Soybean Oil. Mater. Today Proc. 2019, 17, 1412–1422. [Google Scholar] [CrossRef]
- Hu, L.; Wei, X.-Y.; Kang, Y.-H.; Guo, X.-H.; Xu, M.-L.; Zong, Z.-M. Mordenite-Supported Ruthenium Catalyst for Selective Hydrodeoxygenation of Lignin Model Compounds and Lignin-Derived Bio-Oil to Produce Cycloalkanes. J. Energy Inst. 2021, 96, 269–279. [Google Scholar] [CrossRef]
- Fernandes, L.D.; Bartl, P.E.; Monteiro, J.F.; da Silva, J.G.; de Menezes, S.C.; Cardoso, M.J.B. The Effect of Cyclic Dealumination of Mordenite on Its Physicochemical and Catalytic Properties. Zeolites 1994, 14, 533–540. [Google Scholar] [CrossRef]
- Xu, F.; Lv, J.; Chen, C.; Hong, Z.; Zhao, G.; Miao, L.; Yang, W.; Zhu, Z. Effect of Steam Treatment on the Properties of Mordenite and Its Catalytic Performance in a DME Carbonylation Reaction. Ind. Eng. Chem. Res. 2022, 61, 1258–1266. [Google Scholar] [CrossRef]
- Viswanadham, N.; Dixit, L.; Gupta, J.K.; Garg, M.O. Effect of Acidity and Porosity Changes of Dealuminated Mordenites on N-Hexane Isomerization. J. Mol. Catal. A Chem. 2006, 258, 15–21. [Google Scholar] [CrossRef]
- Giudici, R.; Kouwenhoven, H.W.; Prins, R. Comparison of Nitric and Oxalic Acid in the Dealumination of Mordenite. Appl. Catal. A Gen. 2000. [Google Scholar] [CrossRef]
- Sawa, M.; Niwa, M.; Murakami, Y. Change of Pore-Opening Structure of Mordenite upon Dealumination by Hydrochloric Acid. Zeolites 1992, 12, 175–179. [Google Scholar] [CrossRef]
- Ha, B.H.; Guidot, J.; Barthomeuf, D. Changes in Mordenite upon Various Pretreatments. Part 1—Structural Rearrangements. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1979, 75, 1245–1253. [Google Scholar] [CrossRef]
- González, M.D.; Cesteros, Y.; Salagre, P.; Medina, F.; Sueiras, J.E. Effect of Microwaves in the Dealumination of Mordenite on Its Surface and Acidic Properties. Microporous Mesoporous Mater. 2009, 118, 341–347. [Google Scholar] [CrossRef]
- Kurniawan, T.; Setiawan, A.; Putri, N.A.; Irawan, A.; Nandiyanto, A.B.D.; Bindar, Y. Catalytic Pyrolysis of Coconut Oil Soap Using Zeolites for Bio-Hydrocarbon Production. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Fani, K.; Lycourghiotis, S.; Bourikas, K.; Kordouli, E. Biodiesel Upgrading to Renewable Diesel over Nickel Supported on Natural Mordenite Catalysts. Ind. Eng. Chem. Res. 2021, 60, 18695–18706. [Google Scholar] [CrossRef]
- Machiels, L.; Morante, F.; Snellings, R.; Calvo, B.; Canoira, L.; Paredes, C.; Elsen, J. Zeolite Mineralogy of the Cayo Formation in Guayaquil, Ecuador. Appl. Clay Sci. 2008, 42, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Lelyweg, A. X’Pert HighScore Plus, X’Pert HighScore Plus 2002.
- Snellings, R.; Machiels, L.; Mertens, G.; Elsen, J. Rietveld Refinement Strategy for Quantitative Phase Analysis of Partially Amorphous Zeolitized Tuffaceous Rocks. Geol. Belg. 2010, 13, 183–196. [Google Scholar]
- Cumming, G.; Fidler, F.; Vaux, D.L. Error Bars in Experimental Biology. J. Cell Biol. 2007, 177, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Streiner, D.L. Maintaining Standards: Differences between the Standard Deviation and Standard Error, and When to Use Each. Can. J. Psychiatry 1996, 41, 498–502. [Google Scholar] [CrossRef]
- Ortega, K.; Hernandez, M.A.; Portillo, R.; Ayala, E.; Romero, O.; Rojas, F.; Rubio, E.; Pestryakov, A.; Petranovskii, V. Adsorption of Ar and N2 on Dealuminated Mordenite Tuffs. Procedia Chem. 2015, 15, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, S.; Vijaya, J.J.; Sivasanker, S.; Alam, M.; Tamizhdurai, P.; Kennedy, L.J. Characterization and Catalytic Reactivity of Mordenite—Investigation of Selective Oxidation of Benzyl Alcohol. Polyhedron 2015, 89, 289–296. [Google Scholar] [CrossRef]
- Fejes, P.; Hannus, I.; Kiricsi, I.; Pfeifer, H.; Freude, D.; Oehme, W. Thermal Stability of Hydroxy Groups in Dealuminated Mordenites. Zeolites 1985, 5, 45–48. [Google Scholar] [CrossRef]
- Hong, Y.; Fripiat, J.J. Microporous Characteristics of HY, H-ZSM-5 and H-Mordenite Dealuminated by Calcination. Microporous Mater. 1995, 4, 323–334. [Google Scholar] [CrossRef]
- Moreno, S.; Poncelet, G. Dealumination of Small- and Large-Port Mordenites: A Comparative Study. Microporous Mater. 1997, 12, 197–222. [Google Scholar] [CrossRef]
- Rodriguez-Fuentes, G.; de Ménorval, L.C.; Reguera, E.; Chávez Rivas, F. Solid State Multinuclear NMR Study of Iron Species in Natural and Modified Clinoptilolite from Tasajera Deposit (Cuba). Microporous Mesoporous Mater. 2008, 111, 577–590. [Google Scholar] [CrossRef]
- Perraki, T.; Orfanoudaki, A. Mineralogical Study of Zeolites from Pentalofos Area, Thrace, Greece. Appl. Clay Sci. 2004, 25, 9–16. [Google Scholar] [CrossRef]
- Tomazović, B.; Ćeranić, T.; Sijarić, G. The Properties of the NH4-Clinoptilolite. Part 1. Zeolites 1996, 16, 301–308. [Google Scholar] [CrossRef]
- Wakabayashi, F.; Kondo, J.; Wada, A.; Domen, K.; Hirose, C. FT-IR Studies of the Interaction between Zeolitic Hydroxyl Groups and Small Molecules. 1. Adsorption of Nitrogen on H-Mordenite at Low Temperature. J. Phys. Chem. 1993, 97, 10761–10768. [Google Scholar] [CrossRef]
- Sokol, A.A.; Catlow, C.R.A.; Garcés, J.M.; Kuperman, A. Transformation of Hydroxyl Nests in Microporous Aluminosilicates upon Annealing. J. Phys. Condens. Matter 2004, 16, S2781–S2794. [Google Scholar] [CrossRef]
- Pour, A.N.; Rashidi, A.M.; Jozani, K.J.; Mohajeri, A.; Khorami, P. Support Effects on the Chemical Property and Catalytic Activity of Co-Mo HDS Catalyst in Sulfur Recovery. J. Nat. Gas Chem. 2010, 19, 91–95. [Google Scholar] [CrossRef]
- Qiherima; Li, H.; Yuan, H.; Zhang, Y.; Xu, G. Effect of Alumina Support on the Formation of the Active Phase of Selective Hydrodesulfurization Catalysts Co-Mo/Al2O3. Chin. J. Catal. 2011, 32, 240–249. [Google Scholar] [CrossRef]
- Speight, J.G. Chapter 2—Thermal Cracking BT—Heavy and Extra-Heavy Oil Upgrading Technologies; Gulf Professional Publishing: Boston, MA, USA, 2013; pp. 15–38. ISBN 978-0-12-404570-5. [Google Scholar]
- Erdoğan Alver, B.; Esenli, F. Acid Treated Mordenites as Adsorbents of C2H4 and H2 Gases. Microporous Mesoporous Mater. 2017, 244, 67–73. [Google Scholar] [CrossRef]



| Samples | a (Å) | b (Å) | c (Å) | α, β, γ (ο) | Volume* (Å3) |
|---|---|---|---|---|---|
| ZL | 18.042 (1) | 20.397 (2) | 7.481 (2) | 90 | 2775.19 |
| ZAM | 18.143 (2) | 20.384 (3) | 7.500 (1) | 90 | 2774.10 |
| ZDES1 | 18.182 (2) | 20.371 (1) | 7.492 (3) | 90 | 2775.12 |
| ZDES3 | 18.181 (1) | 20.382 (2) | 7.494 (2) | 90 | 2777.18 |
| ZDES5 | 18.161 (3) | 20.322 (2) | 7.473 (4) | 90 | 2758.20 |
| wt.% | ZL | ZAM | ZDES1 | ZDES3 | ZDES5 |
|---|---|---|---|---|---|
| O | 42.70 (0.61) | 42.87 (0.71) | 43.93 (0.61) | 43.88 (1.03) | 43.81 (1.37) |
| Al | 9.15 (0.30) | 9.34 (0.26) | 8.19 (0.35) | 7.42 (0.22) | 5.38 (0.21) |
| Si | 40.21 (0.92) | 45.70 (0.69) | 46.59 (0.77) | 47.49 (1.08) | 49.99 (1.19) |
| Na | 1.18 (0.33) | 0.07 (0.01) | 0.06 (0.01) | 0.05 (0.01) | 0.05 (0.01) |
| K | 1.96 (0.78) | 0.42 (0.06) | 0.38 (0.08) | 0.39 (0.11) | 0.25 (0.05) |
| Ca | 2.88 (0.52) | 0.14 (0.01) | 0.12 (0.01) | 0.08 (0.01) | 0.11 (0.02) |
| Mg | 0.61 (0.15) | 0.25 (0.05) | 0.17 (0.03) | 0.18 (0.04) | 0.12 (0.01) |
| Fe | 1.32 (0.33) | 1.22 (0.60) | 0.56 (0.13) | 0.50 (0.17) | 0.30 (0.05) |
| Sample | BET Area (m2/g) | Microporous Area (m2/g) | Porous Volume (cm3/g) | Microporous Volume (cm3/g) | Average Pore Diameter (Å) | ||
|---|---|---|---|---|---|---|---|
| 4Vp/A | BJH Ads | BJH Des | |||||
| * | ** | *** | |||||
| ZL | 9.7 | 0.2 | 0.055 | 0.0001 | 226.79 | 303.44 | 157.79 |
| ZAM | 6.21 | - | 0.0288 | - | 185.63 | 185.98 | 127.89 |
| ZDES1 | 6.65 | 0.01 | 0.0402 | - | 242.11 | 288.68 | 162.97 |
| ZDES3 | 10.52 | - | 0.032 | - | 121.73 | 152.57 | 124.41 |
| ZDES5 | 36.94 | 20.46 | 0.0442 | 0.091 | 47.85 | 169.69 | 128.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adriano, A.; Cornejo, M.H.; Baykara, H.; Ludeña, E.V.; Brito, J.L. Dealumination and Characterization of Natural Mordenite-Rich Tuffs. Materials 2022, 15, 4654. https://doi.org/10.3390/ma15134654
Adriano A, Cornejo MH, Baykara H, Ludeña EV, Brito JL. Dealumination and Characterization of Natural Mordenite-Rich Tuffs. Materials. 2022; 15(13):4654. https://doi.org/10.3390/ma15134654
Chicago/Turabian StyleAdriano, Armando, Mauricio H. Cornejo, Haci Baykara, Eduardo V. Ludeña, and Joaquín L. Brito. 2022. "Dealumination and Characterization of Natural Mordenite-Rich Tuffs" Materials 15, no. 13: 4654. https://doi.org/10.3390/ma15134654
APA StyleAdriano, A., Cornejo, M. H., Baykara, H., Ludeña, E. V., & Brito, J. L. (2022). Dealumination and Characterization of Natural Mordenite-Rich Tuffs. Materials, 15(13), 4654. https://doi.org/10.3390/ma15134654







