Surface Modification of Synthetic Zeolites with Ca and HDTMA Compounds with Determination of Their Phytoavailability and Comparison of CEC and AEC Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Methodology
2.3. Modification of the Material after the Synthesis (AS)
2.3.1. Modification by Sorption of Ca Ions
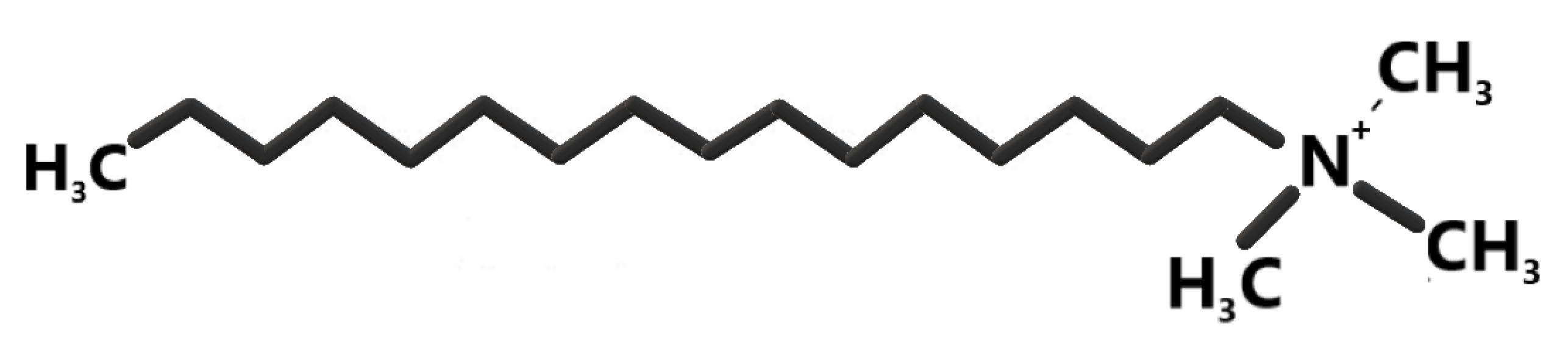
2.3.2. Modification by HDTMA
2.4. Methodology for Determining the Cation Exchange Capacity (CEC) and the Anion Exchange Capacity (AEC)
2.4.1. Determination of the Cation Exchange Capacity of CECs by Sorption of the Quaternary Ammonium Salt Hexadecyltrimethylammonium Bromide (HDTMA)
2.4.2. Determination of the Cation Exchange Capacity (CEC) by Concentration of NH4 Ions
2.4.3. Determination of the Anion-Exchange Capacity (AEC) by the Phosphate Method
2.5. Determination of the Sorption Capacity of Unmodified and Modified Samples towards NO3, PO4, SO4 Ions
2.6. Determination of the Phytoavailability of Adsorbed Ions NO3, PO4, SO4
3. Results and Discussion
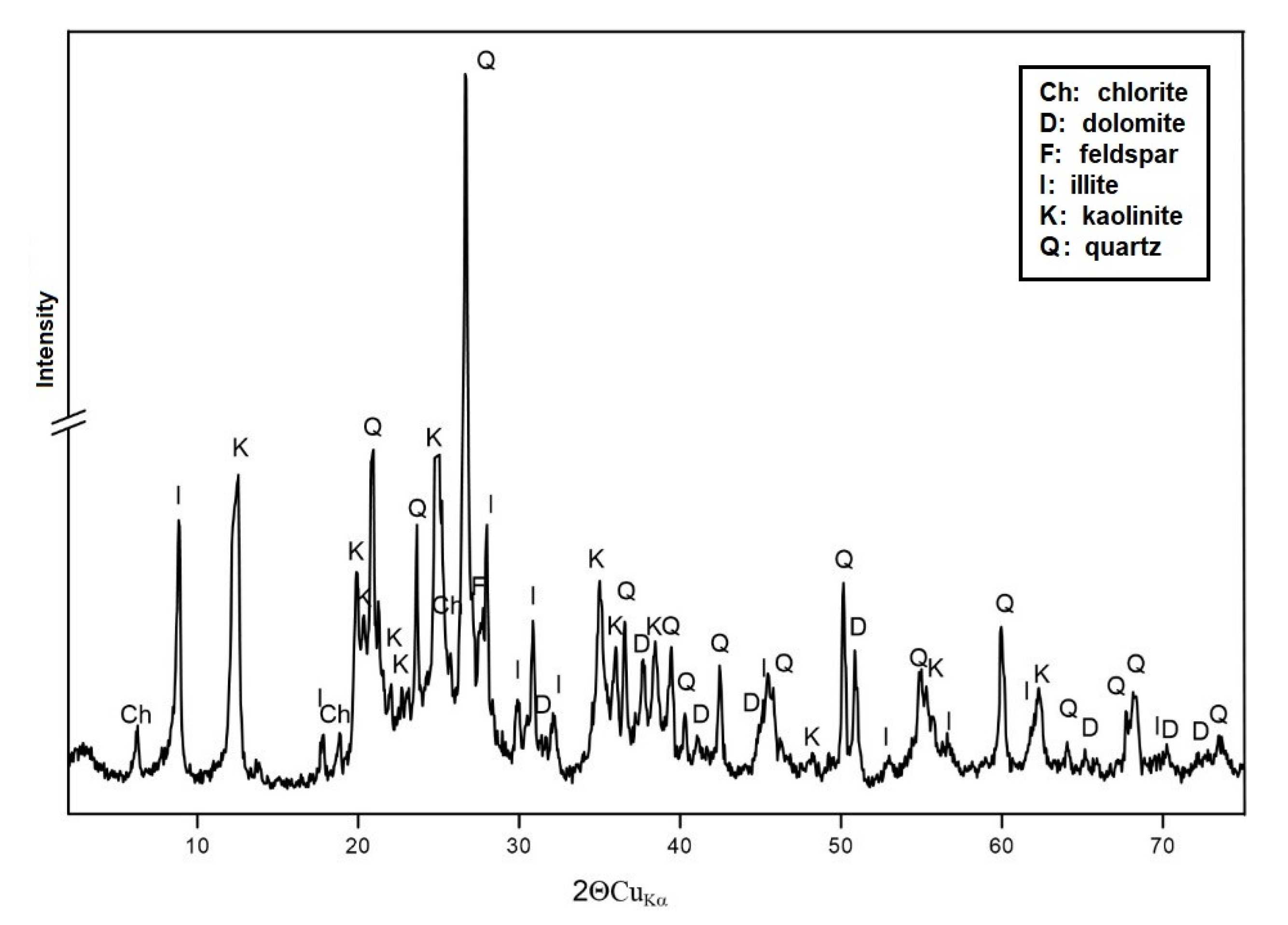
3.1. Characteristics of RM and AS Materials
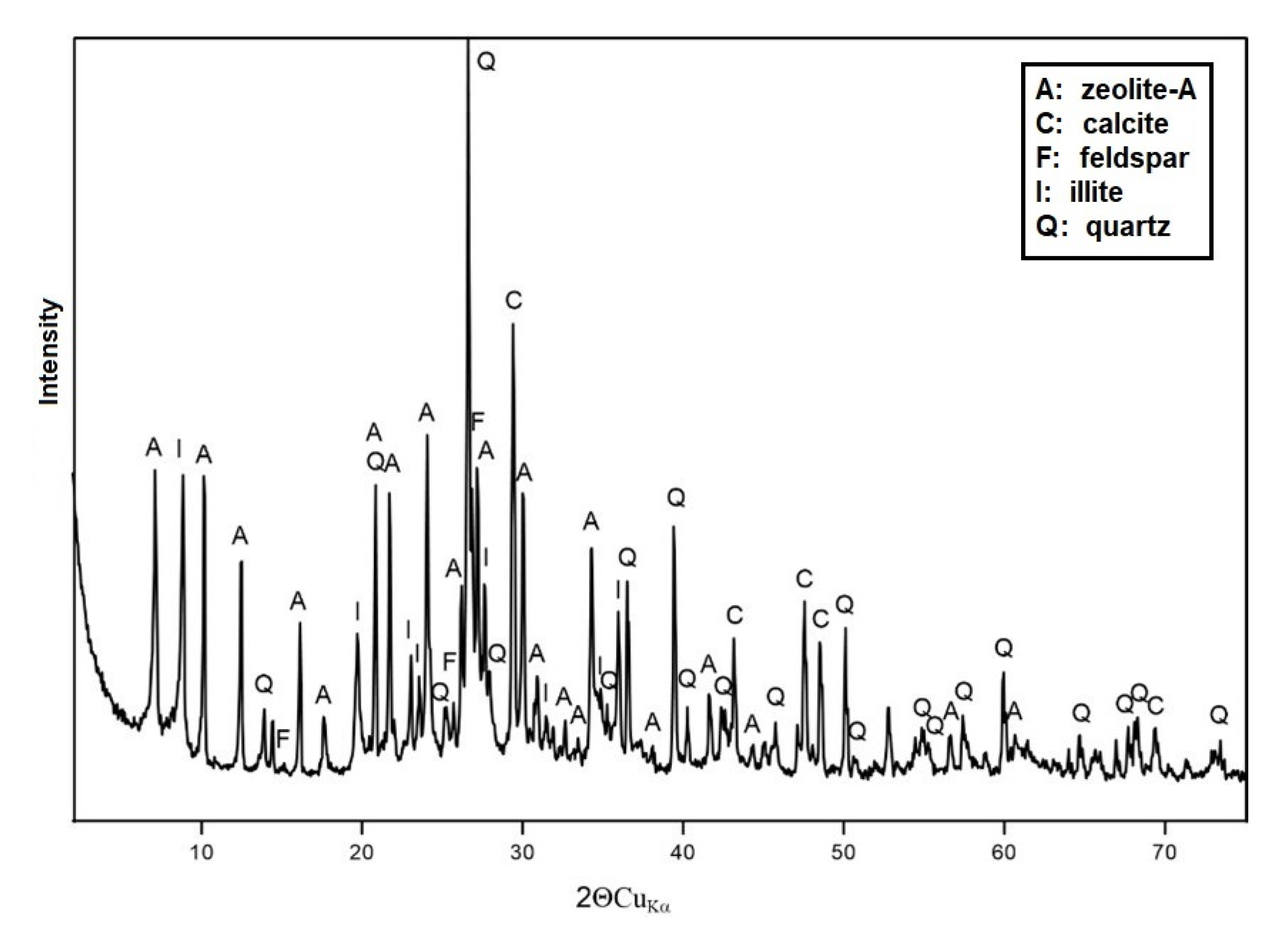
3.1.1. XRD Analysis
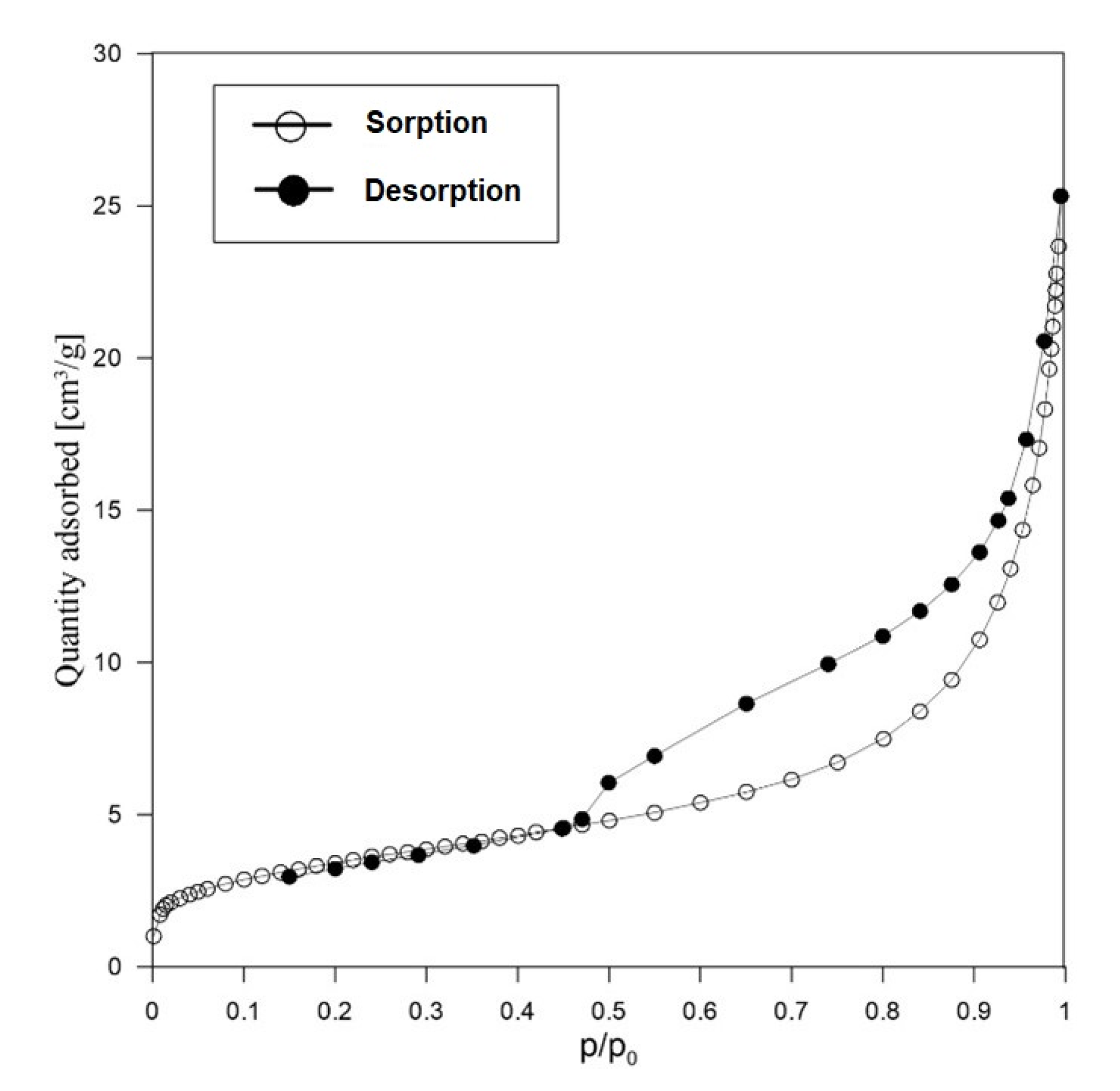
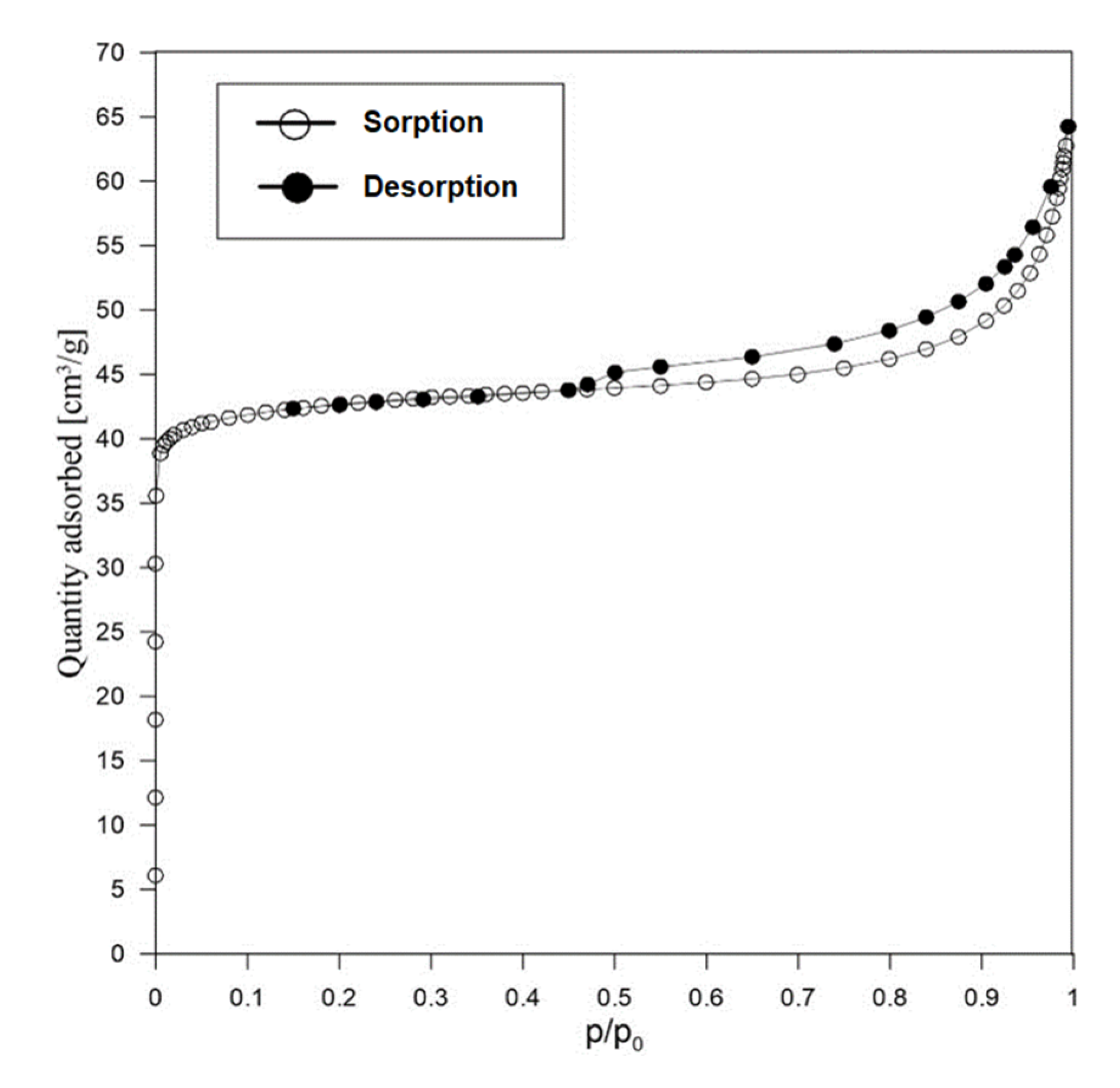
3.1.2. Texture and Morphology (N2 Adsorption/Desorption and SEM)
3.2. Modification Results for M-Ca and M-HDTMA Samples
3.3. CEC and AEC
| Sample | CEC (meq/100 g) | AEC (meq/100 g) | Raw Material | Synthesis Conditions | References | ||
|---|---|---|---|---|---|---|---|
| Solids to Solution/ Activator Ratio | Activator | Temperature/ TIME | |||||
| AS | 138.93 | 19.86 | Calcined Coal Shale | 1.5 g/5 g NaOH | NaOH | 80 °C/24 h | In article |
| FBB Met. N | 79.57 | 84.26 | Fluidized ash | 10 g/0.1 dm3 NaOH | 3 M NaOH | 21 °C/30 days | [49] |
| FBB Met. F | 104.32 | 83.24 | Fluidized ash | S/NaOH—0.83 | NaOH | 100 °C/24 h (activation) 60 °C/24 h (crystallization). | [49] |
| KL SŁ WF | 68.73 | 17.38 | Slovak clinoptilolite | no treatment | no treatment | no treatment | [50] |
| AA01A5M160-2H | 18.95 | 84.90 | Fly ash fluidized bed “Green Block”, forest + sunflower biomass | 2.5 g/10 mL | 2 M NaOH | 140 °C (autoclave)—24 h | [50] |
| AA04A5M160-2H | 110.78 | 54.87 | Fly ash from co-burning, 20% of the biomass including 20% chips, 80% sunflower + straw | 2.5 g/10 mL | 2 M NaOH | 140 °C (autoclave)—24 h | [50] |
3.4. Results of Sorption, Desorption, and Phytoavailability of NO3, PO4, SO4
4. Conclusions
- for nitrogen and phosphorus compounds, M-HDTMA samples had the best phytoavailability (97%),
- for the sample M-Ca, the phytoavailability oscillated in the limits of 83% (for nitrogen compounds) and 89% (for phosphorus compounds), and
- the phytoavailability of sulphates for the modified samples (M-Ca and M-HDTMA) oscillated around 60%.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsuneki, H.; Kirishiki, M.; Oku, T. Development of 2,2′-Iminodiethanol Selective Production Process Using Shape-Selective Pentasil-Type Zeolite Catalyst. Bull. Chem. Soc. Jpn. 2007, 80, 1075–1090. [Google Scholar] [CrossRef]
- Niwa, M.; Katada, N.; Okumura, K. Introduction to Zeolite Science and Catalysis; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2010; Volume 141, pp. 1–8. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th Revised ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Bandura, L.; Franus, M.; Wdowin, M.; Franus, W. Removal of Enviromental Pollutions Using Zeolites from Fly Ash: A Review. Fresenius Environ. Bull. 2015, 24, 854–866. [Google Scholar]
- Wang, L.; Xu, S.; He, S.; Xiao, F.S. Rational Construction of Metal Nanoparticles Fixed in Zeolite Crystals as Highly Efficient Heterogeneous Catalysts. Nano Today 2018, 20, 74–83. [Google Scholar] [CrossRef]
- Rhodes, C.J. Properties and Applications of Zeolites. Sci. Prog. 2010, 93, 223–284. [Google Scholar] [CrossRef]
- Jia, X.; Khan, W.; Wu, Z.; Choi, J.; Yip, A.C.K. Modern Synthesis Strategies for Hierarchical Zeolites: Bottom-up versus Top-down Strategies. Adv. Powder Technol. 2019, 30, 467–484. [Google Scholar] [CrossRef]
- Lin, Q.F.; Gao, Z.R.; Lin, C.; Zhang, S.; Chen, J.; Li, Z.; Liu, X.; Fan, W.; Li, J.; Chen, X.; et al. A Stable Aluminosilicate Zeolite with Intersecting Three-Dimensional Extra-Large Pores. Science 2021, 374, 1605–1608. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolites and Catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Mohandes, F. From Zeolite to Host-Guest Nanocomposite Materials. In Advances in Diverse Industrial Applications of Nanocomposites; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Munthali, M.W.; Elsheikh, M.A.; Johan, E.; Matsue, N. Proton Adsorption Selectivity of Zeolites in Aqueous Media: Effect of Si/Al Ratio of Zeolites. Molecules 2014, 19, 20468–20481. [Google Scholar] [CrossRef]
- Wajima, T. Ion Exchange Properties of Japanese Natural Zeolites in Seawater. Anal. Sci. 2013, 29, 139–141. [Google Scholar] [CrossRef][Green Version]
- Kwakye-Awuah, B.; Labik, L.K.; Nkrumah, I.; Williams, C. Removal of Ammonium Ions by Laboratory-Synthesized Zeolite Linde Type A Adsorption from Water Samples Affected by Mining Activities in Ghana. J. Water Health 2014, 12, 151–160. [Google Scholar] [CrossRef]
- Kılıç, Z.; Atakol, O.; Aras, S.; Cansaran-Duman, D.; Emregul, E. Biosorption Properties of Zinc(II) from Aqueous Solutions by Pseudevernia furfuracea (L.) Zopf. J. Air Waste Manag. Assoc. 2014, 64, 1112–1121. [Google Scholar] [CrossRef]
- Katsou, E.; Malamis, S.; Haralambous, K.J.; Loizidou, M. Use of Ultrafiltration Membranes and Aluminosilicate Minerals for Nickel Removal from Industrial Wastewater. J. Membr. Sci. 2010, 360, 234–249. [Google Scholar] [CrossRef]
- Ursini, O.; Lilla, E.; Montanari, R. The Investigation on Cationic Exchange Capacity of Zeolites: The Use as Selective Ion Trappers in the Electrokinetic Soil Technique. J. Hazard. Mater. 2006, 137, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Terbouche, A.; Ramdane-Terbouche, C.A.; Hauchard, D.; Djebbar, S. Evaluation of Adsorption Capacities of Humic Acids Extracted from Algerian Soil on Polyaniline for Application to Remove Pollutants Such as Cd(II), Zn(II) and Ni(II) and Characterization with Cavity Microelectrode. J. Environ. Sci. 2011, 23, 1095–1103. [Google Scholar] [CrossRef]
- Xu, H.; Wu, P. Two-Dimensional Zeolites in Catalysis: Current State-of-the-Art and Perspectives. Catal. Rev.—Sci. Eng. 2021, 63, 234–301. [Google Scholar] [CrossRef]
- Burton, A. Recent Trends in the Synthesis of High-Silica Zeolites. Sci. Eng. 2017, 60, 132–175. [Google Scholar] [CrossRef]
- Jiao, G.F.; Pu, M.; Chen, B.H. Theoretical Study of Formation Mechanism of Aluminosilicate in the Synthesis of Zeolites. Struct. Chem. 2008, 19, 481–487. [Google Scholar] [CrossRef]
- Feng, S.H.; Li, G.H. Hydrothermal and Solvothermal Syntheses. In Modern Inorganic Synthetic Chemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 73–104. [Google Scholar] [CrossRef]
- Park, M.; Choi, C.L.; Lim, W.T.; Kim, M.C.; Choi, J.; Heo, N.H. Molten-Salt Method for the Synthesis of Zeolitic Materials: I. Zeolite Formation in Alkaline Molten-Salt System. Microporous Mesoporous Mater. 2000, 37, 81–89. [Google Scholar] [CrossRef]
- Alkan, M.; Hopa, Ç.; Yilmaz, Z.; Güler, H. The Effect of Alkali Concentration and Solid/Liquid Ratio on the Hydrothermal Synthesis of Zeolite NaA from Natural Kaolinite. Microporous Mesoporous Mater. 2005, 86, 176–184. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Ayora, C.; Pereira, C.F.; Janssen-Jurkovicová, M. Utilization of Zeolites Synthesized from Coal Fly Ash for the Purification of Acid Mine Waters. Environ. Sci. Technol. 2001, 35, 3526–3534. [Google Scholar] [CrossRef]
- Moreno, N.; Querol, X.; Ayora, C.; Alastuey, A.; Fernández-Pereira, C.; Janssen-Jurkovicová, M. Potential Environmental Applications of Pure Zeolitic Material Synthesized from Fly Ash. J. Environ. Eng. 2001, 127, 994–1002. [Google Scholar] [CrossRef][Green Version]
- Querol, X.; Moreno, N.; Umaña, J.C.; Alastuey, A.; Hernández, E.; López-Soler, A.; Plana, F. Synthesis of Zeolites from Coal Fly Ash: An Overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Murayama, N.; Yamamoto, H.; Shibata, J. Mechanism of Zeolite Synthesis from Coal Fly Ash by Alkali Hydrothermal Reaction. Int. J. Miner. Process. 2002, 64, 1–17. [Google Scholar] [CrossRef]
- Wdowin, M.; Baran, P.; Panek, R.; Zarębska, K.; Franus, W. Analiza Możliwości Oczyszczania Gazów Wylotowych z Hg 0 i CO 2 Na Zeolitach Syntetycznych Otrzymanych z Popiołów Lotnych. Environ. Protect. 2015, 17, 1306–1319. [Google Scholar]
- Wdowin, M.; Macherzyński, M.; Panek, R.; Górecki, J.; Franus, W. Investigation of the Sorption of Mercury Vapour from Exhaust Gas by an Ag-X Zeolite. Clay Miner. 2015, 50, 31–40. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Sun, S.; Gao, J.; Zhang, H.; Zhang, Z.; Wang, Z. Disinfection and Removal Performance for Escherichia Coli, Toxic Heavy Metals and Arsenic by Wood Vinegar-Modified Zeolite. Ecotoxicol. Environ. Saf. 2019, 174, 129–136. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wendell, K.; Zhu, J.; Li, J.L.; Yu, X.; Zhang, Z. Synthesis of Nano-Zeolite from Coal Fly Ash and Its Potential for Nutrient Sequestration from Anaerobically Digested Swine Wastewater. Bioresour. Technol. 2012, 110, 79–85. [Google Scholar] [CrossRef]
- Chałupnik, S.; Franus, W.; Wysocka, M.; Gzyl, G. Application of Zeolites for Radium Removal from Mine Water. Environ. Sci. Pollut. Res. 2013, 20, 7900–7906. [Google Scholar] [CrossRef]
- Sobuś, N.; Diichuk, V.; Kobasa, I.M. Characteristics of the Structure of Natural Zeolites and Their Potential Application in Catalysis and Adsorption Processes. Tech. Trans. 2020, 2020, e2020043. [Google Scholar] [CrossRef]
- Li, L.; Roy, S.J.; Zou, Y.; Bowman, R.S. Long-Term Chemical and Biological Stability of Surfactant-Modified Zeolite. Environ. Sci. Technol. 1998, 32, 2628–2632. [Google Scholar] [CrossRef]
- Haggerty, G.M.; Bowman, R.S. Sorption of Chromate and Other Inorganic Anions by Organo-Zeolite. Environ. Sci. Technol. 1994, 28, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Sparks, D.L.; Scrivner, N.C. Sorption and Desorption of Quaternary Amine Cations on Clays. Environ. Sci. Technol. 2002, 27, 1625–1631. [Google Scholar] [CrossRef]
- Xu, S.; Boyd, S.A. Cationic Surfactant Adsorption by Swelling and Nonswelling Layer Silicates. Langmuir 1995, 11, 2508–2514. [Google Scholar] [CrossRef]
- Li, H.; Laine, A.; O’Keeffe, M.; Yaghi, O.M. Supertetrahedral Sulfide Crystals with Giant Cavities and Channels. Science 1999, 283, 1145–1147. [Google Scholar] [CrossRef]
- Li, Z.; Bowman, R.S. Sorption of Perchloroethylene by Surfactant-Modified Zeolite as Controlled by Surfactant Loading. Environ. Sci. Technol. 1998, 32, 2278–2282. [Google Scholar] [CrossRef]
- Muir, B.; Wołowiec, M.; Bajda, T.; Nowak, P. The Removal of Organic Compounds by Natural and Synthetic Surface-Functionalized Zeolites: A Mini-Review. Mineralogia 2017, 48, 145–156. [Google Scholar] [CrossRef]
- Groisman, L.; Rav-Acha, C.; Gerstl, Z.; Mingelgrin, U. Sorption of Organic Compounds of Varying Hydrophobicities from Water and Industrial Wastewater by Long- and Short-Chain Organoclays. Appl. Clay Sci. 2004, 24, 159–166. [Google Scholar] [CrossRef]
- Łach, M. Geopolymer foams—Will they ever become a viable alternative to popular insulation materials?—A critical opinion. Materials 2021, 14, 3568. [Google Scholar] [CrossRef]
- Łach, M.; Grela, A.; Komar, N.; Mikuła, J.; Hebda, M. Calcined Post-Production Waste as Materials Suitable for the Hydrothermal Synthesis of Zeolites. Materials 2019, 12, 2742. [Google Scholar] [CrossRef] [PubMed]
- Gougazeh, M.; Buhl, J.C. Synthesis and Characterization of Zeolite A by Hydrothermal Transformation of Natural Jordanian Kaolin. J. Assoc. Arab Univ. Basic Appl. Sci. 2014, 15, 35–42. [Google Scholar] [CrossRef]
- Loiola, A.R.; Andrade, J.C.R.A.; Sasaki, J.M.; da Silva, L.R.D. Structural Analysis of Zeolite NaA Synthesized by a Cost-Effective Hydrothermal Method Using Kaolin and Its Use as Water Softener. J. Colloid Interface Sci. 2012, 367, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Grela, A.; Łach, M.; Korniejenko, K.; Mierzwiński, D.; Bajda, T.; Hebda, M.; Figiela, B. Management of Mining Wastes through Their Transformation into Useful Sorbent. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Wroclaw, Poland, 23–25 June 2021; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 942. [Google Scholar] [CrossRef]
- Grela, A.; Łach, M.; Mikuła, J.; Hebda, M. Thermal Analysis of the Products of Alkali Activation of Fly Ash from CFB Boilers. J. Ther. Anal. Calorim. 2016, 124, 1609–1621. [Google Scholar] [CrossRef]
- Łach, M.; Grela, A.; Bajda, T.; Mierzwiński, D.; Komar, N.; Mikuła, J. Production of Zeolite Sorbents from Burning and Co-Burning Biomass with Coal. E3SWC 2018, 44, 00097. [Google Scholar] [CrossRef]
- Khoshgoftar, A.H.; Shariatmadari, H.; Karimian, N.; Kalbasi, M.; van der Zee, S.E.A.T.M.; Parker, D.R. Salinity and Zinc Application Effects on Phytoavailability of Cadmium and Zinc. Soil Sci. Soc. Am. J. 2004, 68, 1885–1889. [Google Scholar] [CrossRef]


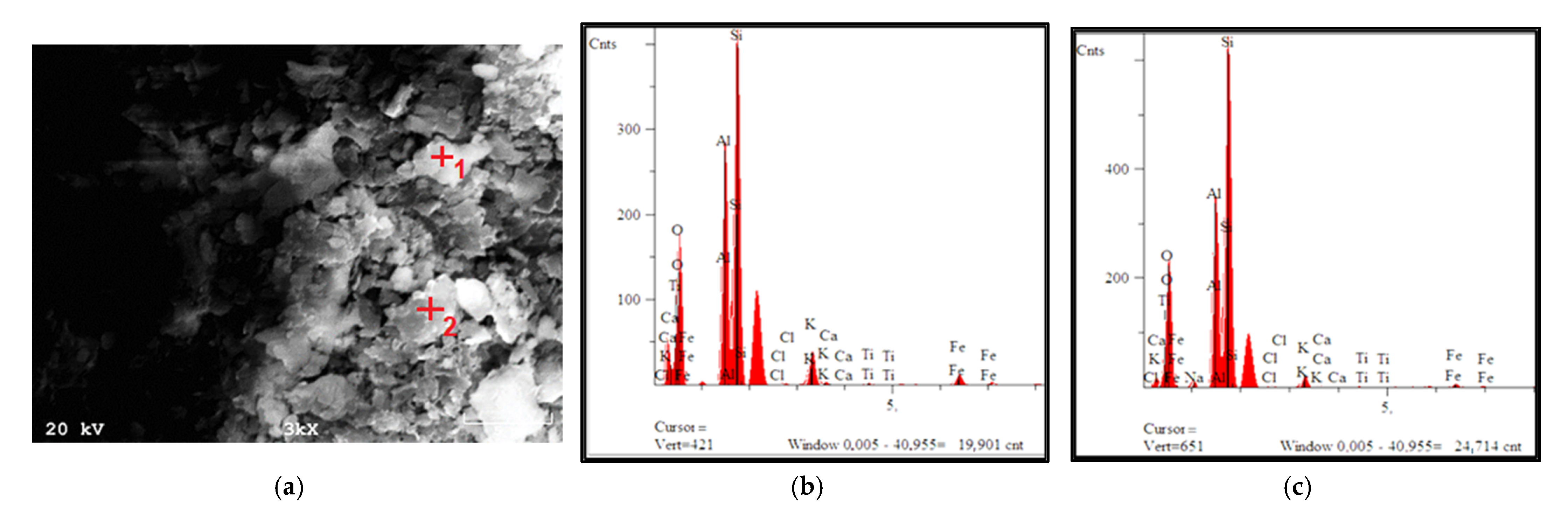
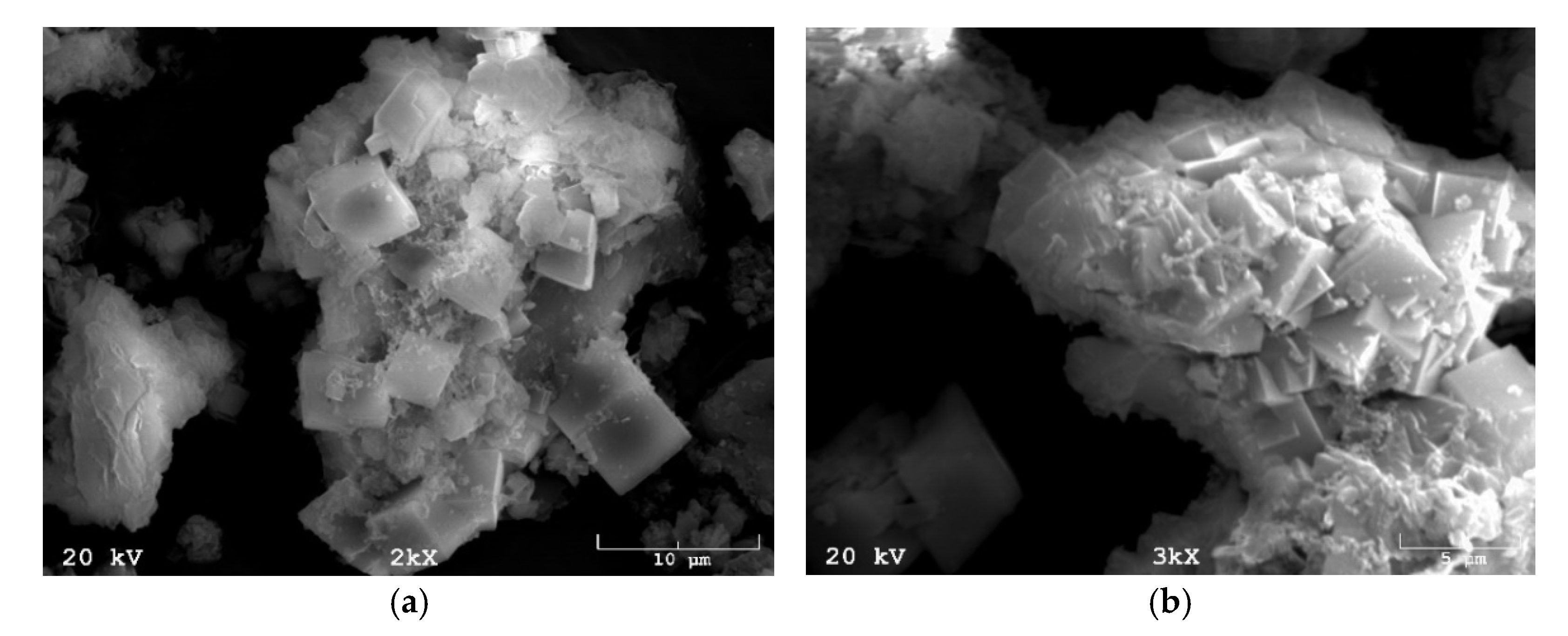
| Name of Mine | Petrographic Composition | Loss of Calcination | ||||
|---|---|---|---|---|---|---|
| Clay | Siltstones | Sandstones | Clay Siderites | Coal | ||
| (%) | (%) | (%) | (%) | (%) | (%) | |
| KWK Rydułtowy—Anna Ruch Rydułtowy | 46 | 26 | 28 | 0 | 0 | 3.3 |
| Name of Sample | |||
|---|---|---|---|
| RM | AS | M-Ca | M-HDTMA |
| Raw material | After synthesis | Modified Ca | Modified HDTMA |
| Sample | Porous Texture Parameters | ||||
|---|---|---|---|---|---|
| SBET (m2/g) | |||||
| RM (reference) | 12.0 | 0.035 | 0.005 | 0.020 | 0.010 |
| AS | 172.0 | 0.096 | 0.065 | 0.019 | 0.008 |
| Sample | AS | M-Ca |
|---|---|---|
| Concentration of Ca (meq/100 g) | 154.2 | 273.4 |
| Sorption (mmol/kg) | |||
|---|---|---|---|
| Sample | NO3 | PO4 | SO4 |
| AS | 213.6 | 871.6 | 854.1 |
| M-Ca | 218.7 | 105.9 | 1513.8 |
| M-HDTMA | 31.6 | 560.1 | 1165.6 |
| Desorption (mmol/kg) | |||
|---|---|---|---|
| Sample | NO3 | PO4 | SO4 |
| AS | 199.5 | 788.4 | 752.5 |
| M-Ca | 181.2 | 93.9 | 910.5 |
| M-HDTMA | 30.8 | 526.3 | 741.6 |
| Desorption (%) | |||
|---|---|---|---|
| Sample | NO3 | PO4 | SO4 |
| AS | 93 | 90 | 88 |
| M-Ca | 83 | 89 | 60 |
| M-HDTMA | 97 | 94 | 63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łach, M.; Grela, A.; Pławecka, K.; Guigou, M.D.; Mikuła, J.; Komar, N.; Bajda, T.; Korniejenko, K. Surface Modification of Synthetic Zeolites with Ca and HDTMA Compounds with Determination of Their Phytoavailability and Comparison of CEC and AEC Parameters. Materials 2022, 15, 4083. https://doi.org/10.3390/ma15124083
Łach M, Grela A, Pławecka K, Guigou MD, Mikuła J, Komar N, Bajda T, Korniejenko K. Surface Modification of Synthetic Zeolites with Ca and HDTMA Compounds with Determination of Their Phytoavailability and Comparison of CEC and AEC Parameters. Materials. 2022; 15(12):4083. https://doi.org/10.3390/ma15124083
Chicago/Turabian StyleŁach, Michał, Agnieszka Grela, Kinga Pławecka, Martin Duarte Guigou, Janusz Mikuła, Norbert Komar, Tomasz Bajda, and Kinga Korniejenko. 2022. "Surface Modification of Synthetic Zeolites with Ca and HDTMA Compounds with Determination of Their Phytoavailability and Comparison of CEC and AEC Parameters" Materials 15, no. 12: 4083. https://doi.org/10.3390/ma15124083
APA StyleŁach, M., Grela, A., Pławecka, K., Guigou, M. D., Mikuła, J., Komar, N., Bajda, T., & Korniejenko, K. (2022). Surface Modification of Synthetic Zeolites with Ca and HDTMA Compounds with Determination of Their Phytoavailability and Comparison of CEC and AEC Parameters. Materials, 15(12), 4083. https://doi.org/10.3390/ma15124083










