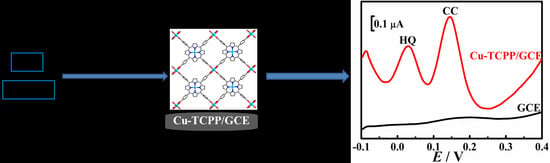
Cu-TCPP Nanosheets-Sensitized Electrode for Simultaneous Determination of Hydroquinone and Catechol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Instruments
2.3. Construction of Cu-TCPP Nanosheets Modified Electrode
3. Results
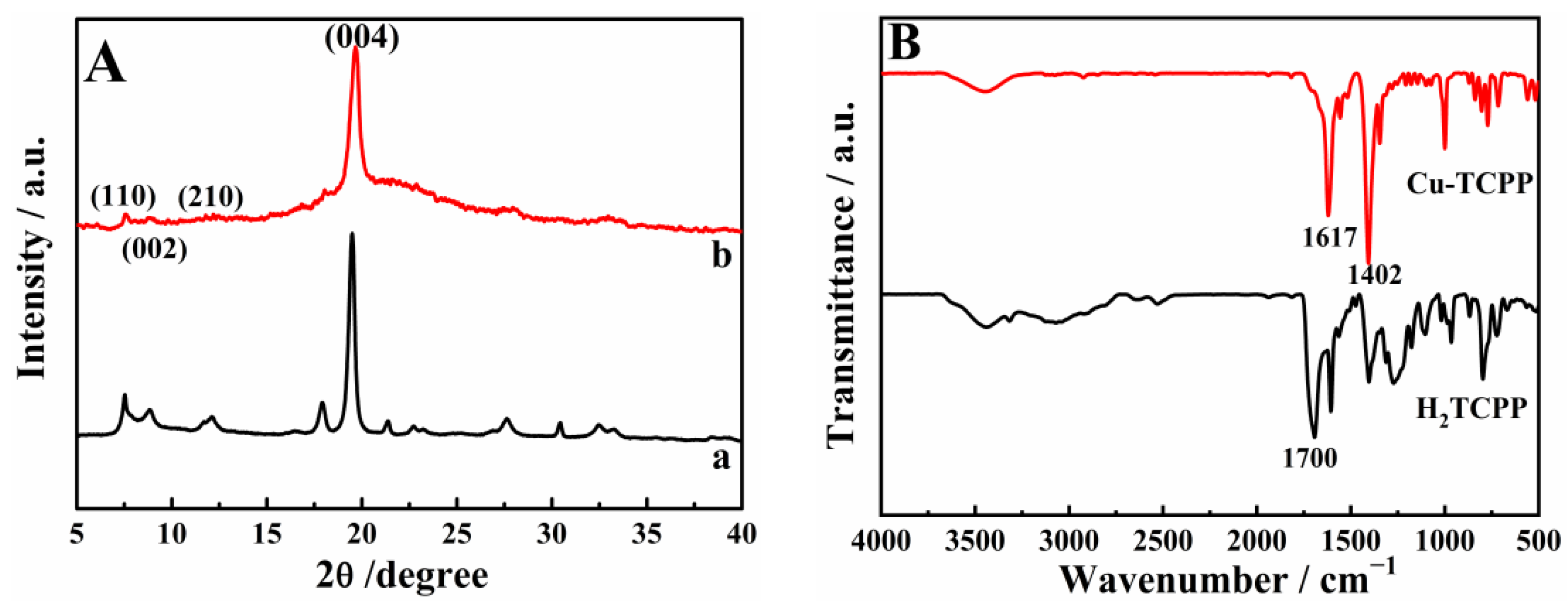
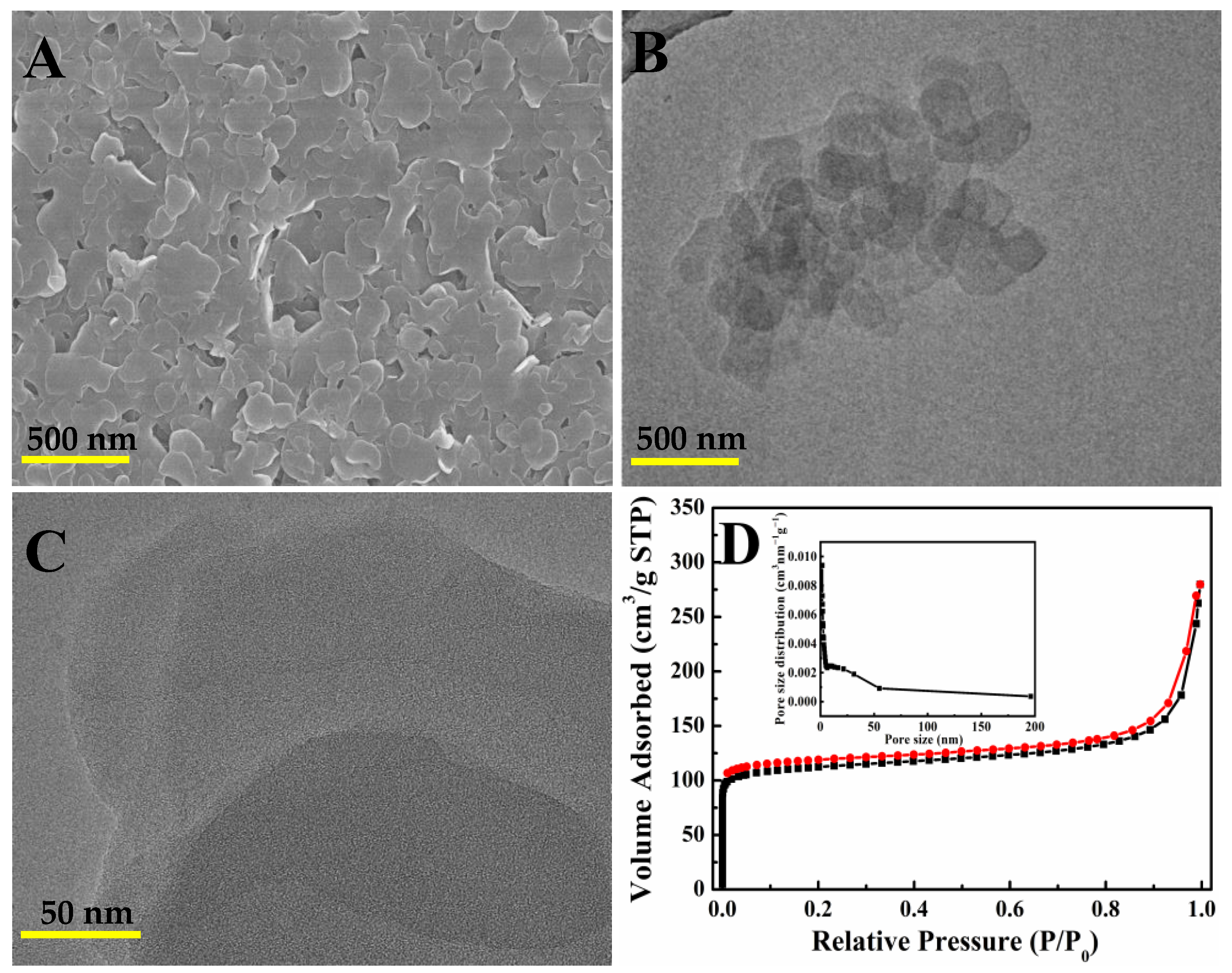
3.1. Characterization of Cu-TCPP Nanosheets
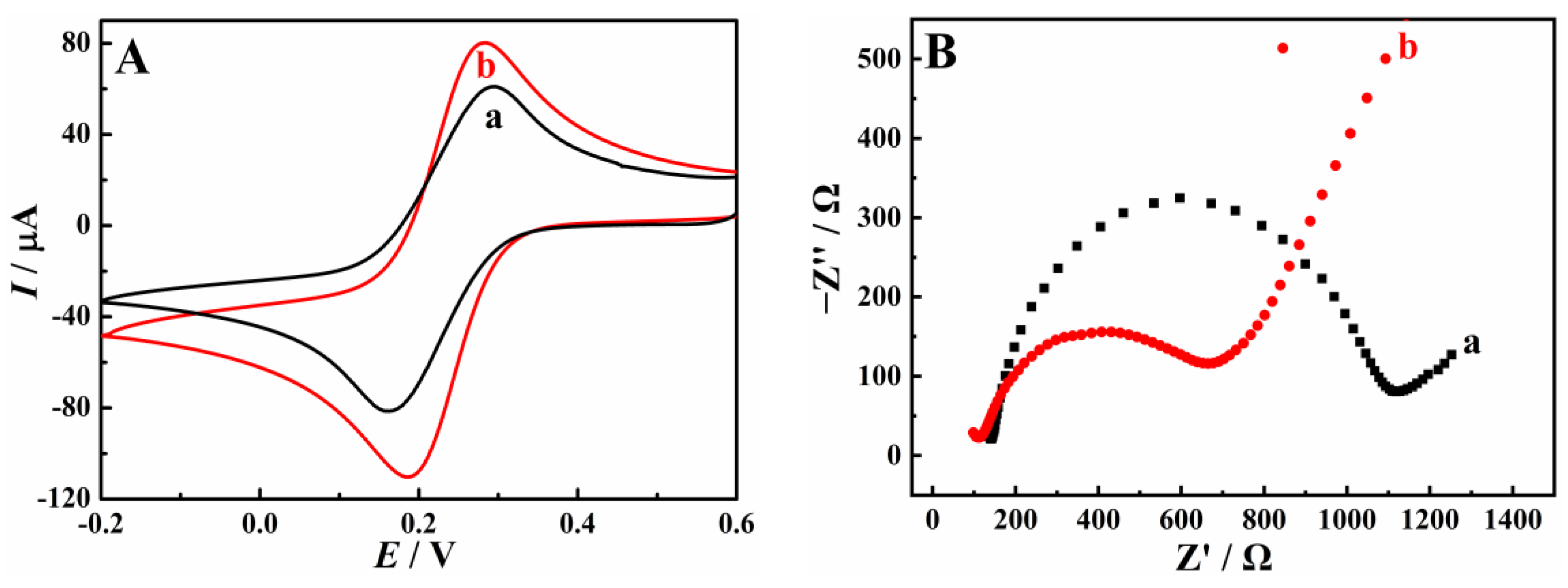
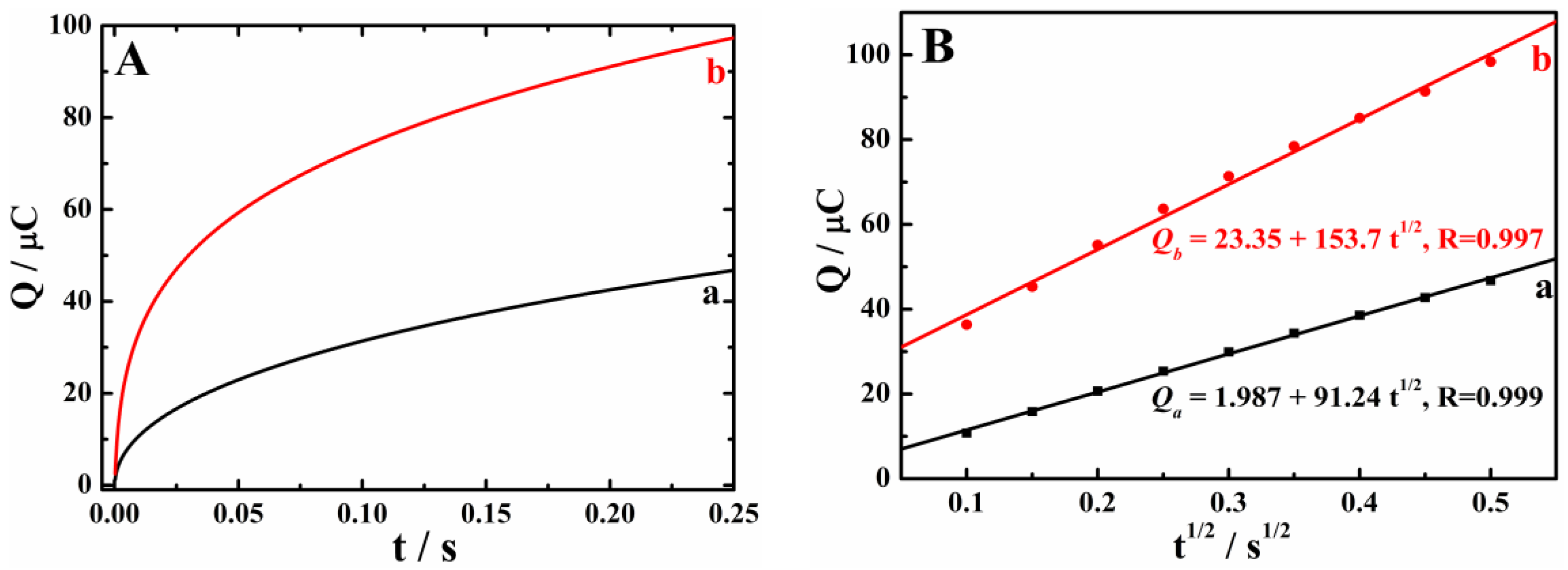
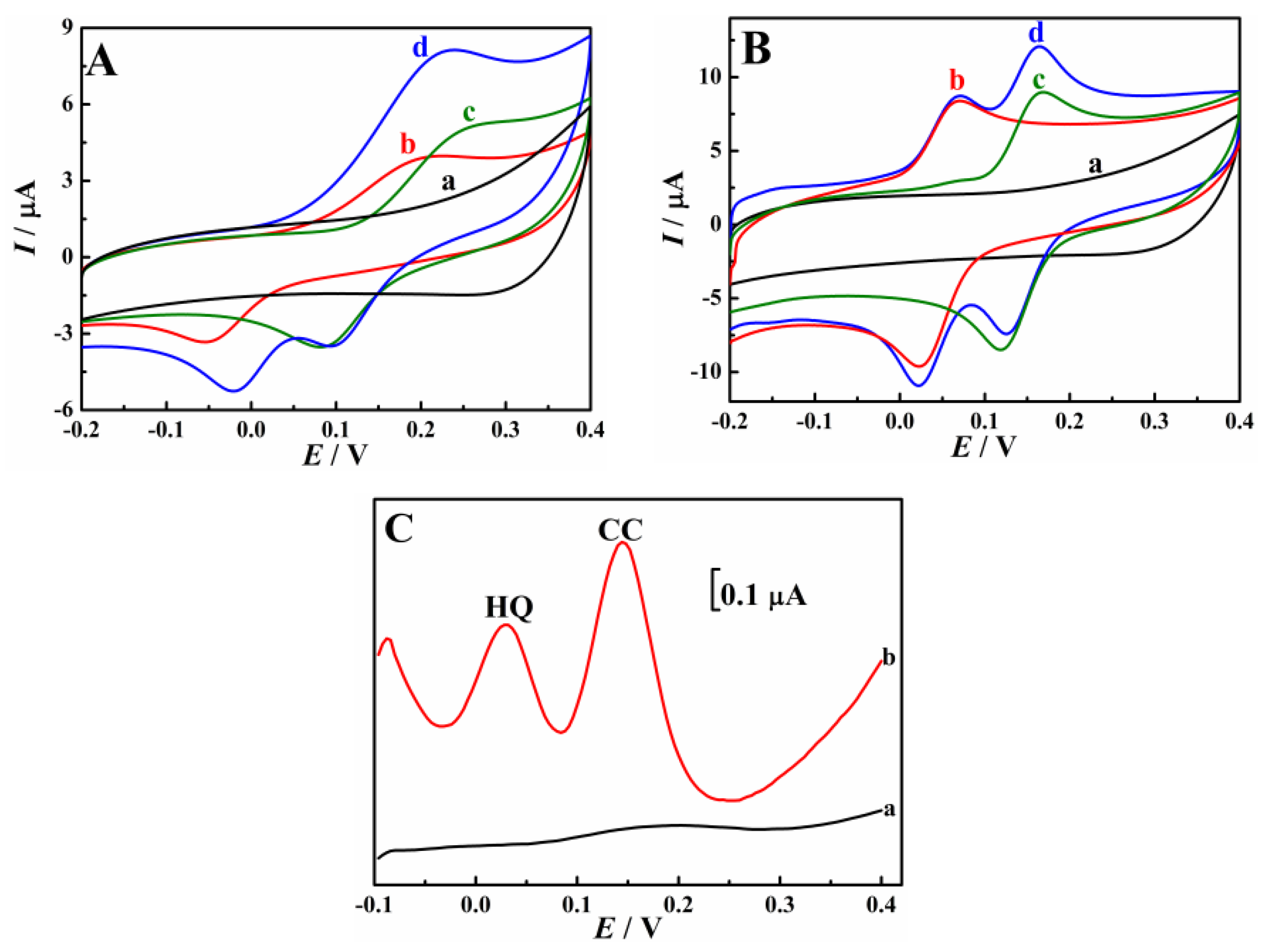
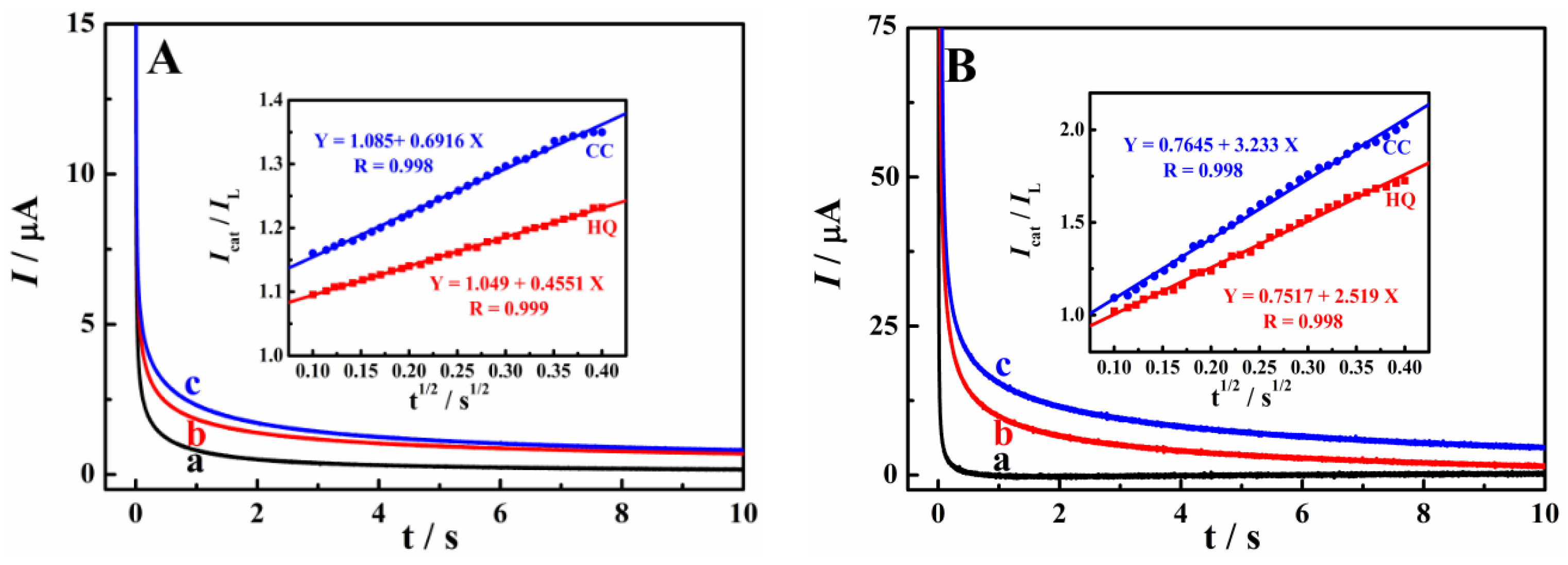
3.2. Enhanced Oxidation of HQ and CC on Cu-TCPP/GCE
3.3. Electrochemical Reaction Mechanism of HQ and CC on Cu-TCPP/GCE
3.4. Simultaneous Determination of HQ and CC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stillman, W.D.; Varella-Garcia, M.; Irons, R.D. The benzene metabolites hydroquinone and catechol act in synergy to induce dose-dependent hypoploidy and-5q31 in a human cell line. Leuk. Lymphoma 1999, 35, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.R.; Hu, X.Y.; Lu, Q.L.; Yang, Y.Y.; Linghu, S.; Zhang, X.Y. Study on the differences in sludge toxicity and microbial community structure caused by catechol, resorcinol and hydroquinone with metagenomic analysis. J. Environ. Manag. 2022, 302, 114027. [Google Scholar] [CrossRef] [PubMed]
- Marrubini, G.; Calleri, E.; Coccini, T.; Castoldi, A.F.; Manzo, L. Direct analysis of phenol, catechol and hydroquinone in human urine by coupled-column HPLC with fluorimetric detection. Chromatographia 2005, 62, 25–31. [Google Scholar] [CrossRef]
- Moldoveanu, S.C.; Kiser, M. Gas chromatography/mass spectrometry versus liquid chromatography/fluorescence detection in the analysis of phenols in mainstream cigarette smoke. J. Chromatogr. A 2007, 1141, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, P.; Vasantha, R.A.; Sunitha, K.R. A sensitive and selective spectrophotometric estimation of catechol derivatives in pharmaceutical preparations. Talanta 2001, 55, 1039–1046. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, Q.L.; Myint, A.; Ge, X.W.; Liu, L.J. Chemiluminescence of cerium(IV)-rhodamine 6G-phenolic compound system. J. Photochem. Photobiol. A Chem. 2006, 181, 238–245. [Google Scholar] [CrossRef]
- Pistonesi, M.F.; Di Nezio, M.S.; Centurion, M.E.; Lista, A.G.; Fragoso, W.D.; Pontes, M.J.C.; Araujo, M.C.U.; Band, B.S.F. Determination of phenol, resorcinol and hydroquinone in air samples by synchronous fluorescence using partial least-squares. Talanta 2006, 69, 1265–1268. [Google Scholar] [CrossRef]
- Han, T.; Zhu, S.; Wang, S.; Wang, B.; Zhang, X.; Wang, G. Fluorometric methods for determination of H2O2, glucose and cholesterol by using MnO2 nanosheets modified with 5-carboxyfluorescein. Microchim. Acta 2019, 186, 269. [Google Scholar] [CrossRef]
- Cao, M.F.; Zou, Y.J.; Zhang, Y.Y.; Zeng, T.; Wan, Q.J.; Lai, G.S.; Yang, Y.J. Robust and selective electrochemical sensing of hazardous photographic developing agents using a MOF-derived 3D porous flower-like Co3O4@C/graphene nanoplate composite. Electrochim. Acta 2022, 409, 139967. [Google Scholar] [CrossRef]
- Liu, Z.H.; Liao, D.; Yu, J.G.; Jiang, X.Y. An electrochemical sensor based on oxygen-vacancy cobalt-aluminum layered double hydroxides and hydroxylated multiwalled carbon nanotubes for catechol and hydroquinone detection. Microchem. J. 2022, 175, 107216. [Google Scholar] [CrossRef]
- Rao, Q.H.; Hu, F.X.; Gan, L.-Y.; Guo, C.X.; Liu, Y.H.; Zhang, C.M.; Chen, C.J.; Yang, H.B.; Li, C.M. Boron-nitrogen-Co-doping nanocarbons to create rich electroactive defects toward simultaneous sensing hydroquinone and catechol. Electrochim. Acta 2022, 402, 139427. [Google Scholar] [CrossRef]
- Yang, X.; He, C.L.; Lin, Y.; Qiu, Y.J.; Li, P.F.; Chen, Y.D.; Huang, B.; Zheng, X.Y. Dihydroxybenzene isomers electrochemical sensor based on activated carbon sensitive material activated by mechanochemistry and low-dosage phosphoric acid. Anal. Methods 2022, 14, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Yang, J.; Xu, P.P.; Liu, H.; Zhang, L.L.; Zhang, S.H.; Tian, L. Gold nanoparticles decorated biochar modified electrode for the high-performance simultaneous determination of hydroquinone and catechol. Sens. Actuator B Chem. 2020, 306, 127590. [Google Scholar] [CrossRef]
- Tashkhourian, J.; Daneshi, M.; Nami-Ana, F.; Behbahani, M.; Bagheri, A. Simultaneous determination of hydroquinone and catechol at gold nanoparticles mesoporous silica modified carbon paste electrode. J. Hazard. Mater. 2016, 318, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, Y.Y.; Qu, J.Y.; Qu, J.H.; Li, S.F. Selective sensing of hydroquinone and catechol based on multiwalled carbon nanotubes/polydopamine/gold nanoparticles composites. Sens. Actuator B Chem. 2016, 223, 501–508. [Google Scholar] [CrossRef]
- Liu, L.T.; Zhou, Y.L.; Liu, S.; Xu, M.T. The applications of metal-organic frameworks in electrochemical sensors. ChemElectroChem 2018, 5, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Chuang, C.-H.; Kung, C.-W. Metal-organic frameworks toward electrochemical sensors: Challenges and opportunities. Electroanalysis 2020, 32, 1885–1895. [Google Scholar] [CrossRef]
- Yuan, B.Q.; Zhang, J.C.; Zhang, R.C.; Shi, H.Z.; Wang, N.; Li, J.W.; Ma, F.J.; Zhang, D.J. Cu-based metal-organic framework as a novel sensing platform for the enhanced electro-oxidation of nitrite. Sens. Actuator B-Chem. 2016, 222, 632–637. [Google Scholar] [CrossRef]
- Chen, S.S.; Wang, C.H.; Zhang, M.; Zhang, W.X.; Qi, J.W.; Sun, X.Y.; Wang, L.J.; Li, J.S. N-doped Cu-MOFs for efficient electrochemical determination of dopamine and sulfanilamide. J. Hazard. Mater. 2020, 390, 122157. [Google Scholar] [CrossRef]
- Li, C.L.; Wu, K.B. Cu-BTC frameworks based electrochemical sensor for hazardous malachite green in aquaculture. Anal. Chim. Acta 2021, 1162, 338473. [Google Scholar] [CrossRef]
- Sun, D.; Deng, Q.H.; Long, J.H. Highly sensitive electrochemical sensor for estradiol based on the signal amplification strategy of Cu-BDC frameworks. J. Solid State Electrochem. 2018, 22, 487–493. [Google Scholar] [CrossRef]
- Cai, Y.J.; Li, X.Y.; Wu, K.B.; Yang, X.F. Electrochemical sensing performance of Eu-BTC and Er-BTC frameworks toward sunset yellow. Anal. Chim. Acta 2019, 1062, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Hai, G.T.; Jia, X.L.; Zhang, K.Y.; Liu, X.; Wu, Z.Y.; Wang, G. High-performance oxygen evolution catalyst using two-dimensional ultrathin metal-organic frameworks nanosheets. Nano Energy 2018, 44, 345–352. [Google Scholar] [CrossRef]
- Chakraborty, G.; Park, I.H.; Medishetty, R.; Vittal, J.J. Two-dimensional metal-organic framework materials: Synthesis, structures, properties and applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Bai, J.; Bo, X.J.; Guo, L.P. A novel electrochemical sensor based on 2D CuTCPP nanosheets and platelet ordered mesoporous carbon composites for hydroxylamine and chlorogenic acid. Anal. Chim. Acta 2019, 1075, 71–80. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, S.M.; Luo, L.Z.; Zhou, Y.; Lu, X.C.; Zhang, G.; Ye, H.; Gu, J.J.; Cao, F.F. Ultrathin two-dimension metal-organic framework nanosheets/multi-walled carbon nanotube composite films for the electrochemical detection of H2O2. J. Electroanal. Chem. 2019, 835, 178–185. [Google Scholar] [CrossRef]
- La, D.D.; Thi, H.P.N.; Kim, Y.S.; Rananaware, A.; Bhosale, S.V. Facile fabrication of Cu(II)-porphyrin MOF thin films from tetrakis(4-carboxyphenyl)porphyrin and Cu(OH)2 nanoneedle array. Appl. Surf. Sci. 2017, 424, 145–450. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhao, S.Y.; Peng, Z.M.; Li, Z.; Chen, L.; Gan, P. Cu2O nanoparticles anchored on carbon for the efficient removal of propofol from operating room wastewater via peroxymonosulfate activation: Efficiency, mechanism, and pathway. RSC Adv. 2021, 11, 20983–20991. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, F.; Zhang, G.W.; Luo, H.Y.; Liu, L.; Shen, M.H.; Yang, Y.Y. A novel two-dimensional coordination polymer-polypyrrole hybrid material as a high-performance electrode for flexible supercapacitor. Chem. Eng. J. 2018, 334, 2547–2557. [Google Scholar] [CrossRef]
- Xu, G.; Otsubo, K.; Yamada, T.; Sakaida, S.; Kitagawa, H. Superprotonic conductivity in a highly oriented crystalline metal-organic framework nanofilm. J. Am. Chem. Soc. 2013, 135, 7438–7441. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Ji, L.D.; Wang, J.; Wu, K.B.; Yang, N.J. Tunable electrochemistry of electrosynthesized copper metal-organic frameworks. Adv. Funct. Mater. 2018, 28, 1706961. [Google Scholar] [CrossRef]
- Hu, P.; Zhu, X.M.; Luo, X.H.; Hu, X.F.; Ji, L.D. Cathodic electrodeposited Cu-BTC MOFs assembled from Cu(II) and trimesic acid for electrochemical determination of bisphenol A. Microchim. Acta 2020, 187, 145. [Google Scholar] [CrossRef] [PubMed]
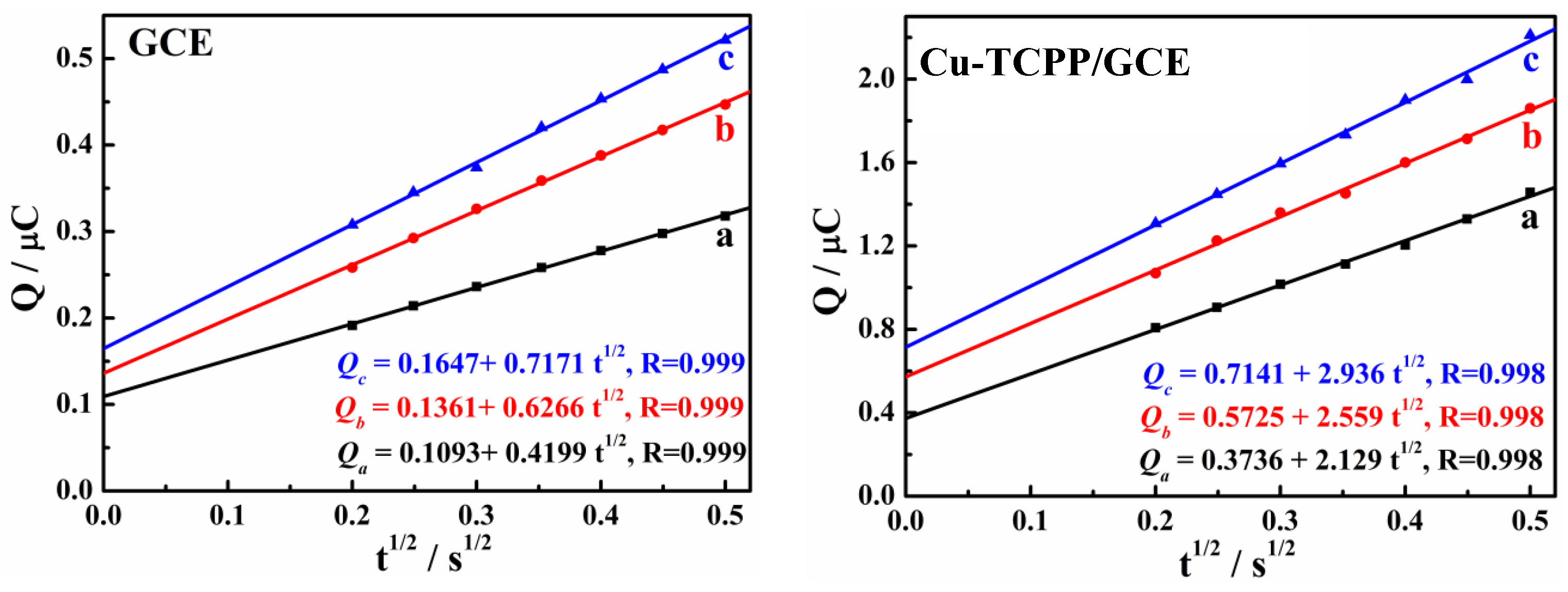
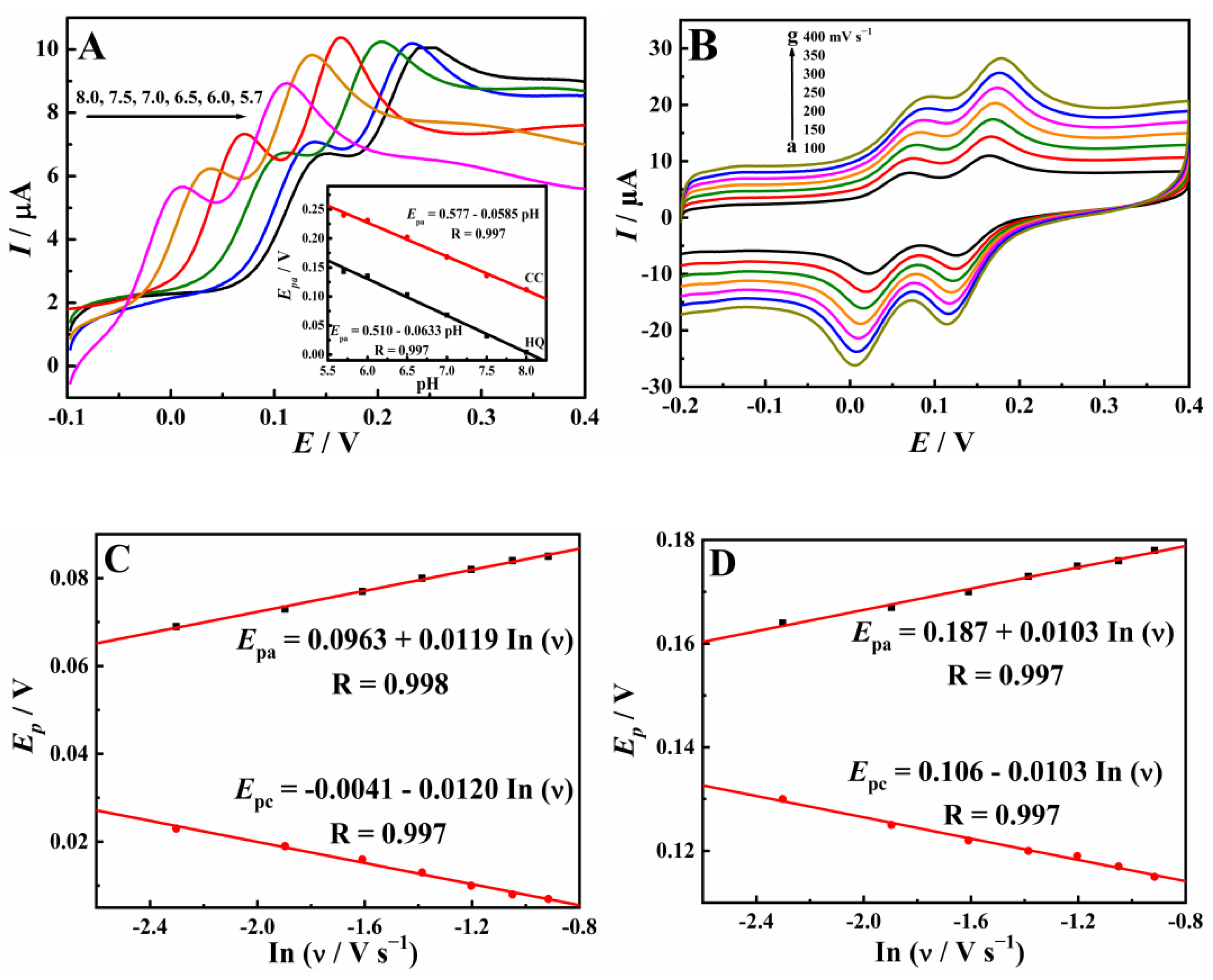
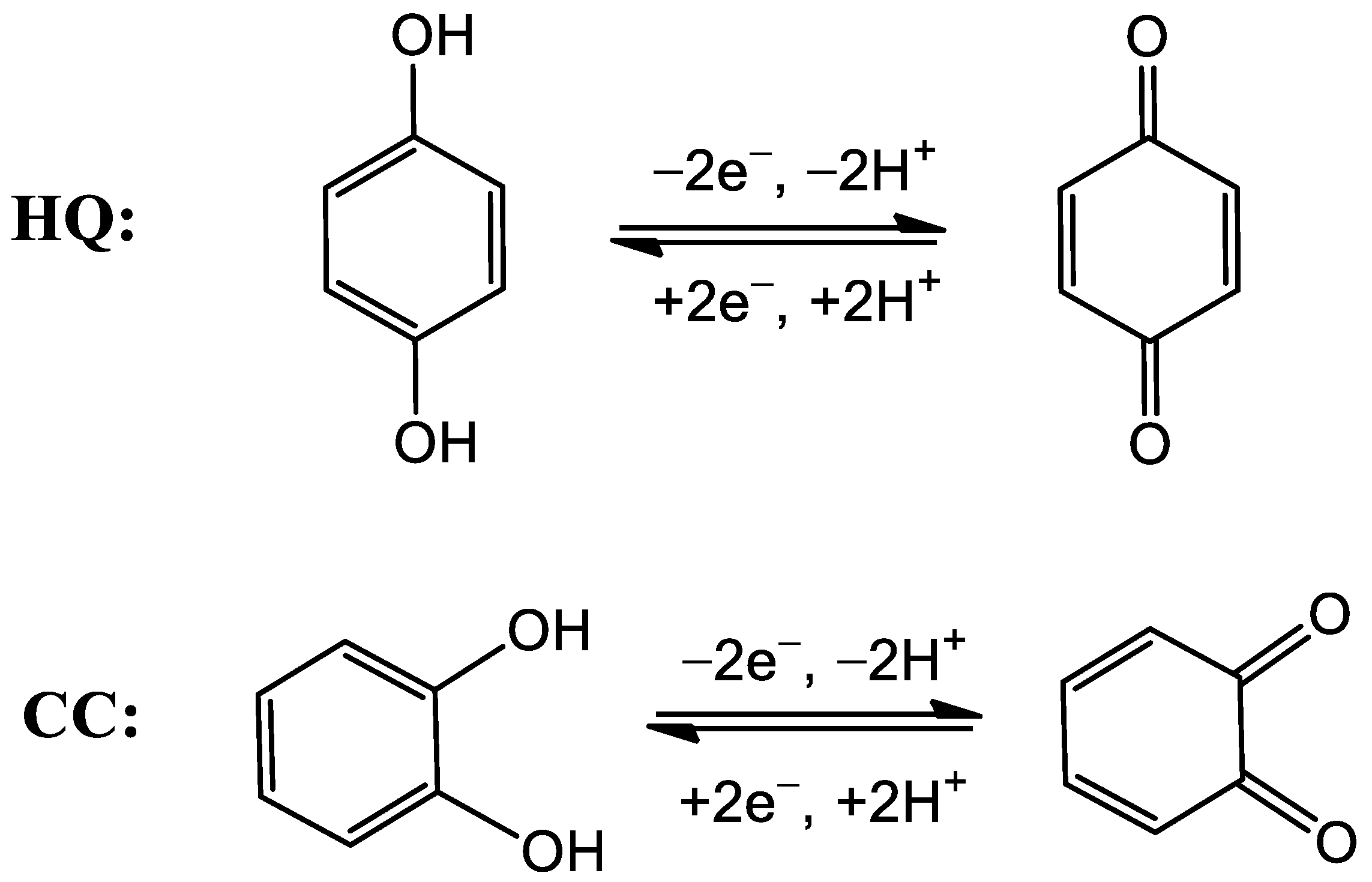
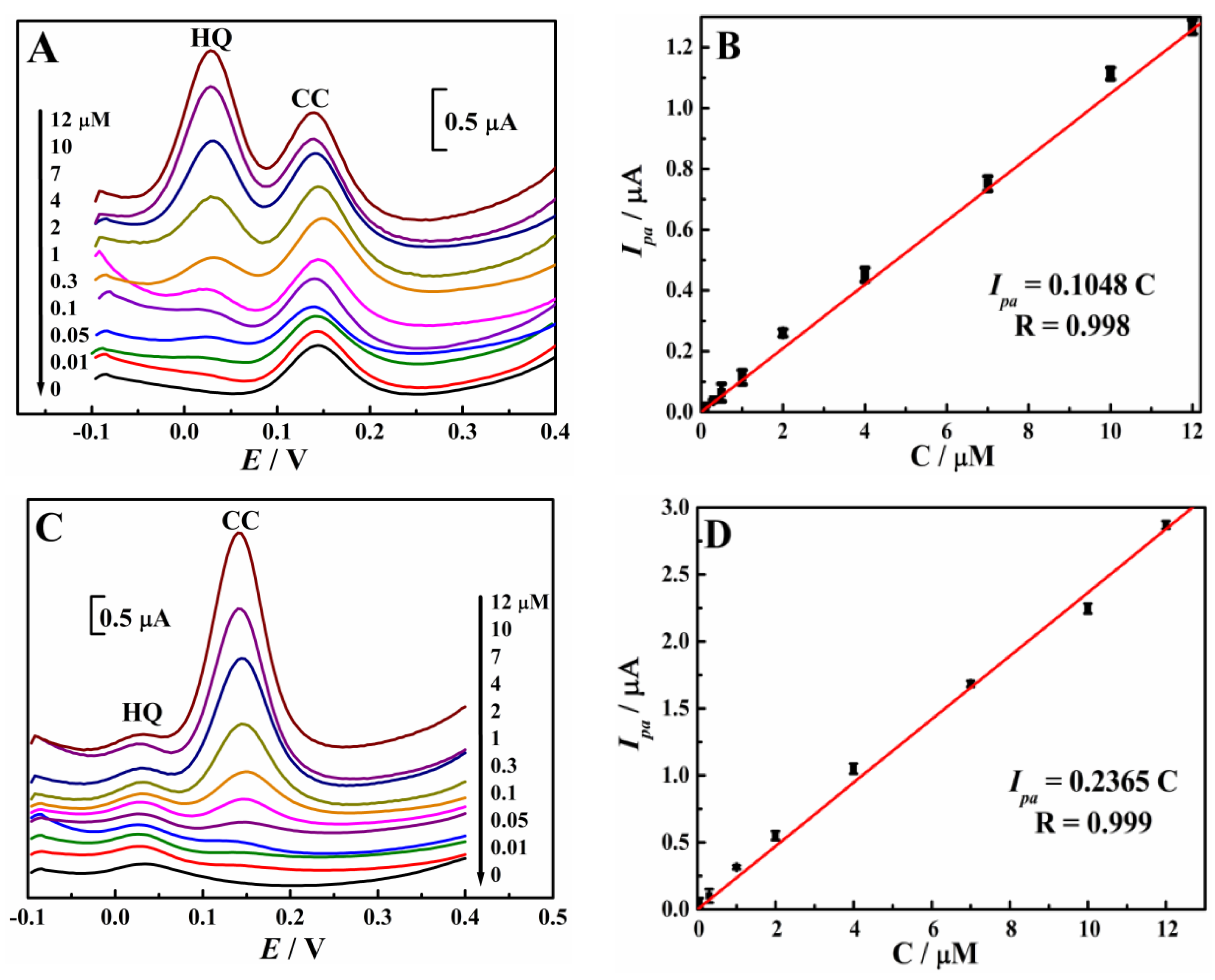
| Electrode | Qads (µC) | |
|---|---|---|
| HQ | CC | |
| GCE | 0.0268 | 0.0554 |
| Cu-TCPP/GCE | 0.1989 | 0.3405 |
| Modified Electrode | Linear Range (µM) | LOD (nM) | Ref. | ||
|---|---|---|---|---|---|
| HQ | CC | HQ | CC | ||
| Co3O4@C/GNPs/GCE | 0.04–30 | 0.5–30 | 14.7 | 169 | [7] |
| OV-LDHs/H-MWCNTs/GCE | 0.5–150 | 0.5–150 | 76 | 74 | [8] |
| BNC/GCE | 0.099–8400, 8400–43,340 | 0.049–1750, 1750–5110 | 33.3 | 16.3 | [9] |
| MCPBAC/GCE | 0.6–100, 100–600 | 0.6–100, 100–600 | 200 | 200 | [10] |
| WBC/Au/GCE | 0.008–1.0, 1.0–7.0 | 0.01–1.0, 1.0–7.0 | 2.0 | 4.0 | [12] |
| AuNPs-MPS/CPE | 10.0–1000.0 | 30.0–1000.0 | 1200 | 1100 | [13] |
| CS/MWCNTs/PDA/AuNPs/GCE | 0.1–10 | 0.1–10 | 35 | 47 | [14] |
| Cu-TCPP/GCE | 0.01–12 | 0.01–12 | 3.4 | 2.3 | This work |
| Samples | Added (nM) | Found (nM) | Recovery (%) | |||
|---|---|---|---|---|---|---|
| HQ | CC | HQ | CC | HQ | CC | |
| A | 50.00 | 50.00 | 47.92 | 48.12 | 95.84 | 96.24 |
| 250.0 | 250.0 | 258.7 | 263.2 | 103.5 | 105.3 | |
| 500.0 | 500.0 | 486.3 | 478.6 | 97.26 | 95.72 | |
| B | 1000 | 1000 | 1045 | 1032 | 104.5 | 103.2 |
| 3000 | 3000 | 2894 | 2963 | 96.47 | 98.77 | |
| 5000 | 5000 | 5227 | 5304 | 104.5 | 106.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, L.; Wang, Q.; Peng, L.; Li, X.; Zhu, X.; Hu, P. Cu-TCPP Nanosheets-Sensitized Electrode for Simultaneous Determination of Hydroquinone and Catechol. Materials 2022, 15, 4625. https://doi.org/10.3390/ma15134625
Ji L, Wang Q, Peng L, Li X, Zhu X, Hu P. Cu-TCPP Nanosheets-Sensitized Electrode for Simultaneous Determination of Hydroquinone and Catechol. Materials. 2022; 15(13):4625. https://doi.org/10.3390/ma15134625
Chicago/Turabian StyleJi, Liudi, Qi Wang, Lianhui Peng, Xiaoyu Li, Xiaoming Zhu, and Peng Hu. 2022. "Cu-TCPP Nanosheets-Sensitized Electrode for Simultaneous Determination of Hydroquinone and Catechol" Materials 15, no. 13: 4625. https://doi.org/10.3390/ma15134625
APA StyleJi, L., Wang, Q., Peng, L., Li, X., Zhu, X., & Hu, P. (2022). Cu-TCPP Nanosheets-Sensitized Electrode for Simultaneous Determination of Hydroquinone and Catechol. Materials, 15(13), 4625. https://doi.org/10.3390/ma15134625