Mass/Heat Transfer Analogy Method in the Research on Convective Fluid Flow through a System of Long Square Mini-Channels
Abstract
:1. Introduction
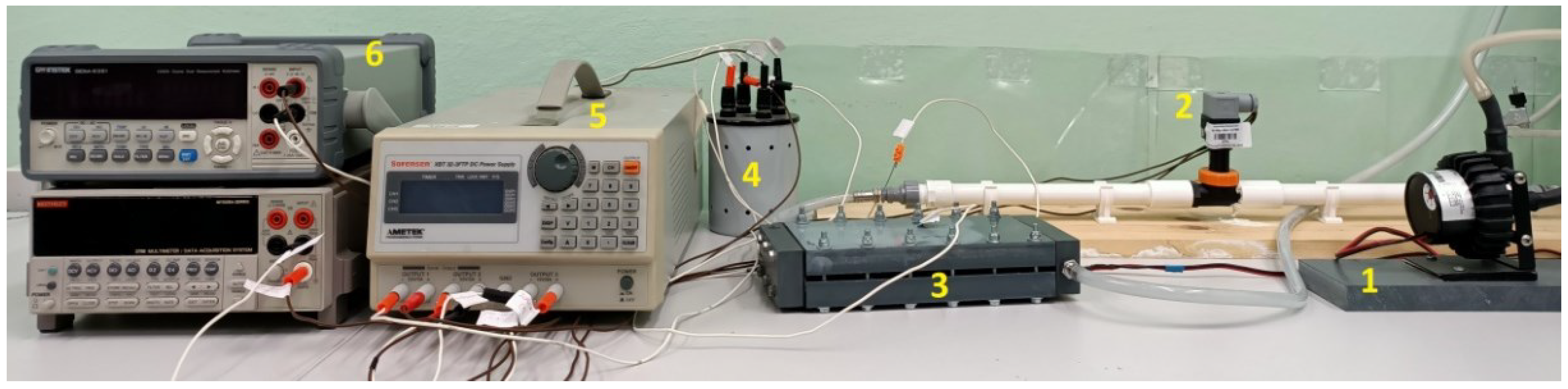
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- -
- The material of the cathode modeling the heat transfer surface has an impact on the flat part of the voltammogram and limiting current value determination may be difficult, especially at higher electrolyte velocities.
- -
- As the considered mini-channels are long, the change of the ion concentration along the channel length should be considered in the mass transfer coefficient calculations.
- -
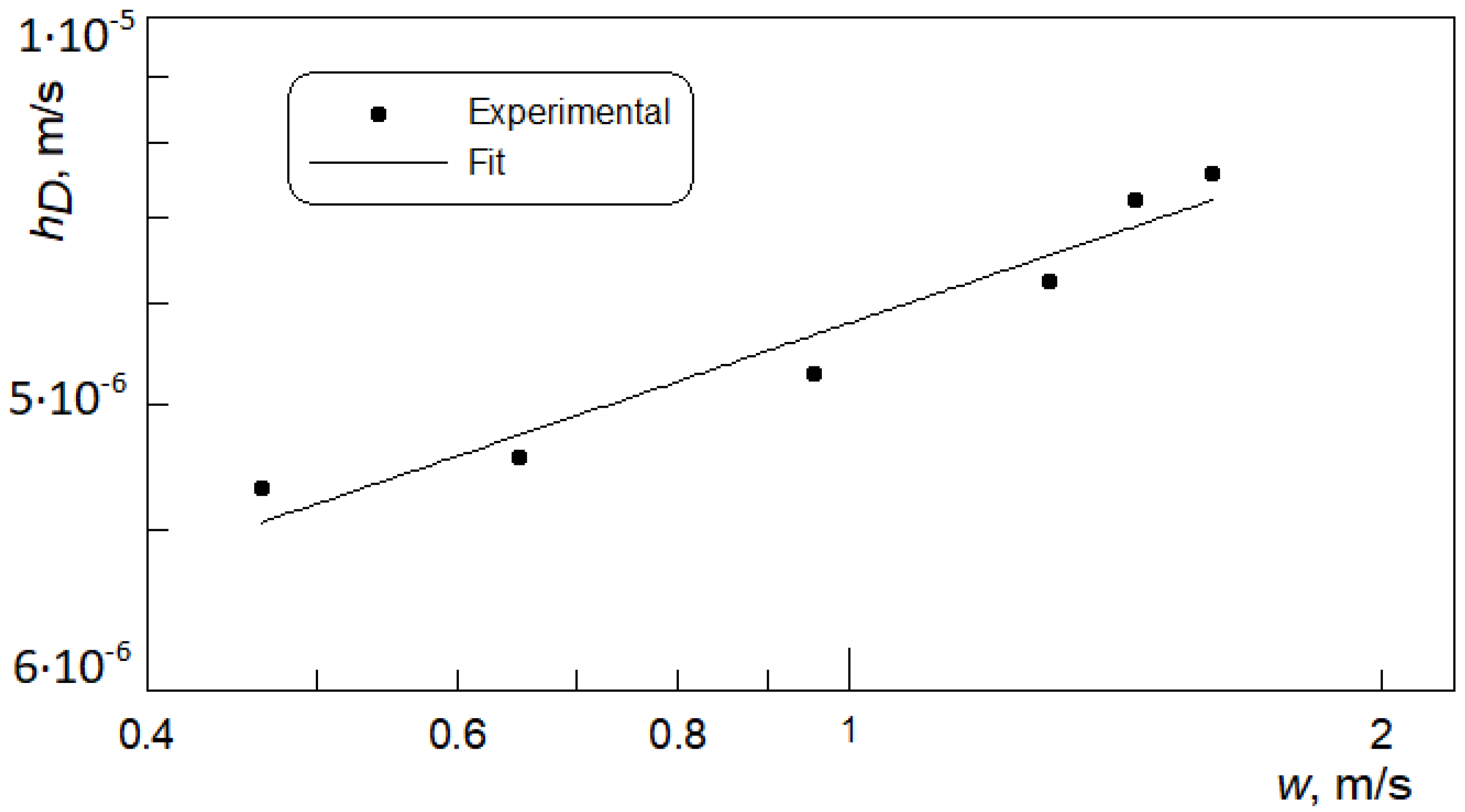
- The received heat transfer coefficients are lower than conventional channels. The specific characteristics of the channel cross section, square and small dimensions, may be the cause of this condition.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | cathode surface area, m2 |
| cp | specific heat, J/(kgK) |
| C | ion concentration, kmol/m3 |
| dH | hydraulic diameter, m |
| D | diffusion coefficient, m2 |
| F | Faraday constant, As/kmol |
| h | heat transfer coefficient, W/(m2K) |
| hM | mass transfeer coefficient, m/s |
| I | current intensity, A |
| Ip | limiting current, A |
| jH, jM | Chiltion–Colburn fcoefficient for heat and mass transfer, respectively |
| k | thermal conductivity, W/(mK) |
| L | channel length, m |
| n | valence charge of reacting ions |
| N | molar flux density, kmol/(m2s) |
| Nu | Nusselt number |
| Pr | Prandtl number |
| Re | Reynolds number |
| Sc | Schmidt number |
| Sh | Sherwood number |
| StH | Stanton number for heat transfer |
| StM | Stanton number for mass transfer |
| U | voltage, V |
| volumetric flow rate, m3/s | |
| w | mean flow velocity, m/s |
| ν | kinematic viscosity, m2/s |
| ρ | deensity, kg/m3 |
References
- Reif-Acherman, S. Early and current experimental methods for determining thermal conductivities of metals. Int. J. Heat Mass Transf. 2014, 77, 542–566. [Google Scholar] [CrossRef]
- Wilk, J.; Smusz, R.; Grosicki, S. Thermphysical properties of water based Cu nanofluids used in special type of coil heat exchanger. Appl. Therm. Eng. 2017, 127, 933–943. [Google Scholar] [CrossRef]
- Xiang, D.; Shen, L.; Wang, H. Investigation on the thermal conductivity of mineral oil-based alumina/aluminum nitride nanofluids. Materials 2019, 12, 4217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Li, X.; Wang, H.; Zhao, F.; Zhang, D.; Chen, Y. Improvement in thermal conductivity of through-plane aligned boron nitride/silicone rubber composites. Mater. Des. 2019, 165, 107580. [Google Scholar] [CrossRef]
- Adamczyk, W.P.; Pawlak, S.; Ostrowski, Z. Determination of thermal conductivity of CFRP composite materials using unconventional laser flash technique. Measurement 2018, 124, 147–155. [Google Scholar] [CrossRef]
- Adamczyk, W.; Białecki, R.; Orlande, H.R.B.; Ostrowski, Z. Nondestructive, real time technique for in-plane heat diffusivity measurements. Int. J. Heat Mass Transf. 2020, 154, 119659. [Google Scholar] [CrossRef]
- Knupp, D.C. Integral transform technique for the direct identification of thermal conductivity and thermal capacity in heterogeneous media. Int. J. Heat Mass Transf. 2021, 171, 121112. [Google Scholar] [CrossRef]
- Wu, H.Y.; Cheng, P. An experimental study of convective heat transfer in silicon microchannels with different surface conditions. Int. J. Heat Mass Transf. 2003, 46, 2547–2556. [Google Scholar] [CrossRef]
- Han, J.; Kim, S.-J.; Lee, Y.-K.; Hur, D.-H. Chemical cleaning of magnetite deposits on the flow mini-channels of a printed ciruit heat exchanger in an EEDTA-based solution. Materials 2022, 15, 1471. [Google Scholar] [CrossRef]
- Rosa, P.; Karayiannis, T.G.; Collins, M.W. Single-phase heat transfer in microchannels: The importance of scaling effects. Appl. Therm. Eng. 2009, 29, 3447–3468. [Google Scholar] [CrossRef] [Green Version]
- Sikora, M. Flow structure investigations during Novec refrigerant condensation in minichannels. Materials 2021, 14, 6889. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Bohdal, T.; Formela, K. Experimental study of HFE 7000 refrigerant condensation in horizontal pipe minichannels. Materials 2021, 14, 6886. [Google Scholar] [CrossRef] [PubMed]
- Moharana, M.K.; Agarwal, G.; Khandekar, S. Axial conduction in single-phase simultaneously developing flow in a rectangular mini-channel array. Int. J. Therm. Sci. 2011, 50, 1001–1012. [Google Scholar] [CrossRef]
- Hejcik, J.; Jicha, M. Single phase heat transfer in minichannels. EPJ Web Conf. 2014, 67, 02034. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, S.E.; Ranjbar, A.A.; Hosseini, M.J. Experimental and numerical investigation of circular minichannel heat sinks with various hydraulic diameter for electronic cooling application. Microelectron. Reliab. 2017, 73, 97–105. [Google Scholar] [CrossRef]
- Ghasemi, S.E.; Ranjbar, A.A. Cooling performance analysis of water-cooled heat sinks with circular and rectangular minichannels using finite element method. Iran. J. Chem. Chem. Eng. 2018, 37, 231–239. [Google Scholar]
- Soudagar, M.E.M.; Kalam, M.A.; Sajid, M.U.; Afzal, A.; Banapurmath, N.R.; Akram, N.; Mane, S.D.; Saleel, C.A. Thermal analyses of minichannels and use of mathematical and numerical models. Numer. Heat Transf. Part A Appl. 2020, 77, 497–537. [Google Scholar] [CrossRef]
- Wilk, J. Heat/mass transfer analogy in the case of convective fluid flow through minichannels. Int. J. Therm. Sci. 2020, 156, 106467. [Google Scholar] [CrossRef]
- Wilk, J. A review of measurement of the mass transfer in minichannels using the limiting current technique. Exp. Therm. Fluid Sci. 2014, 57, 242–249. [Google Scholar] [CrossRef]
- Sara, O.N.; Ergu, Ő.B.; Arzutug, M.E.; Yapıcı, S. Experimental study of laminar forced convective mass transfer and pressure drop in microtubes. Int. J. Therm. Sci. 2009, 48, 1894–1900. [Google Scholar] [CrossRef]
- Ergu, Ő.B.; Sara, O.N.; Yapıcı, S.; Arzutug, M.E. Pressure drop and point mass transfer in a rectangular microchannel. Int. Commun. Heat Mass Transf. 2009, 36, 618–623. [Google Scholar] [CrossRef]
- Wilk, J. Experimental investigation of convective mass/heat transfer in short minichannel at low Reynolds numbers. Exp. Therm. Fluid Sci. 2009, 33, 267–272. [Google Scholar] [CrossRef]
- Wilk, J. Convective mass/heat transfer in the entrance region of the short circular minichannel. Exp. Therm. Fluid Sci. 2012, 38, 107–114. [Google Scholar] [CrossRef]
- Kumar, K.A.; Sarma, G.V.S.; Ramesh, K.V. Mass transfer at the confining wall of a minichannel. Mater. Today: Proc. 2020, 27, 524–538. [Google Scholar]
- Yarin, L.P.; Mosyak, A.; Hetsroni, G. Fluid Flow, Heat Transfer and Boiling in Micro-Cannels; Springer: Berlin/Heidelberg, Germany, 2009; pp. 121–127. [Google Scholar]
- Altay, I.; Yapıcı, S.; Demirkiran, N. Usability of alloys with different composition as electrode for reduction of ferricyanide ion. SN Appl. Sci. 2022, 4, 129. [Google Scholar] [CrossRef]
- Chilton, T.H.; Colburn, A.P. Mass transfer (absorption) coefficients prediction from data on heat transfer and fluid friction. Ind. Eng. Chem. 1934, 26, 1183–1187. [Google Scholar] [CrossRef]
- Gnielinski, V. On heat transfer in tubes. Int. J. Heat Mass Transf. 2013, 63, 134–140. [Google Scholar] [CrossRef]
- Tso, C.P.; Mahulikar, S.P. Experimental verification of the role of Brinkman number in microchannels using local parameters. Int. J. Heat Mass Transf. 2000, 43, 1837–1849. [Google Scholar] [CrossRef]
- Hetsroni, G.; Mosyak, A.; Pogrebnyak, E.; Yarin, L.P. Heat transfer in micro-channels: Comparison of experiments with theory and numerical results. Int. J. Heat Mass Transf. 2005, 48, 5580–5601. [Google Scholar] [CrossRef]
- Celata, G.P.; Cumo, M.; Marconi, V.; McPhail, S.J.; Zummo, G. Microtube liquid single-phase heat transfer in laminar flow. Int. J. Heat Mass Transf. 2006, 49, 3538–3546. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Garimella, S.; Li, D.; Colin, S.; King, M.R. Heat Transfer and Fluid Flow in Minichannels and Microchannels; Elsevier: Oxford, UK, 2006; pp. 87–136. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilk, J.; Grosicki, S.; Smusz, R. Mass/Heat Transfer Analogy Method in the Research on Convective Fluid Flow through a System of Long Square Mini-Channels. Materials 2022, 15, 4617. https://doi.org/10.3390/ma15134617
Wilk J, Grosicki S, Smusz R. Mass/Heat Transfer Analogy Method in the Research on Convective Fluid Flow through a System of Long Square Mini-Channels. Materials. 2022; 15(13):4617. https://doi.org/10.3390/ma15134617
Chicago/Turabian StyleWilk, Joanna, Sebastian Grosicki, and Robert Smusz. 2022. "Mass/Heat Transfer Analogy Method in the Research on Convective Fluid Flow through a System of Long Square Mini-Channels" Materials 15, no. 13: 4617. https://doi.org/10.3390/ma15134617
APA StyleWilk, J., Grosicki, S., & Smusz, R. (2022). Mass/Heat Transfer Analogy Method in the Research on Convective Fluid Flow through a System of Long Square Mini-Channels. Materials, 15(13), 4617. https://doi.org/10.3390/ma15134617





