Enhanced Reactivity and Compound Mechanism of Mg/B Composite Powders Prepared by Cryomilling
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Synthesis and Characterization of Mg/B Composite Powders
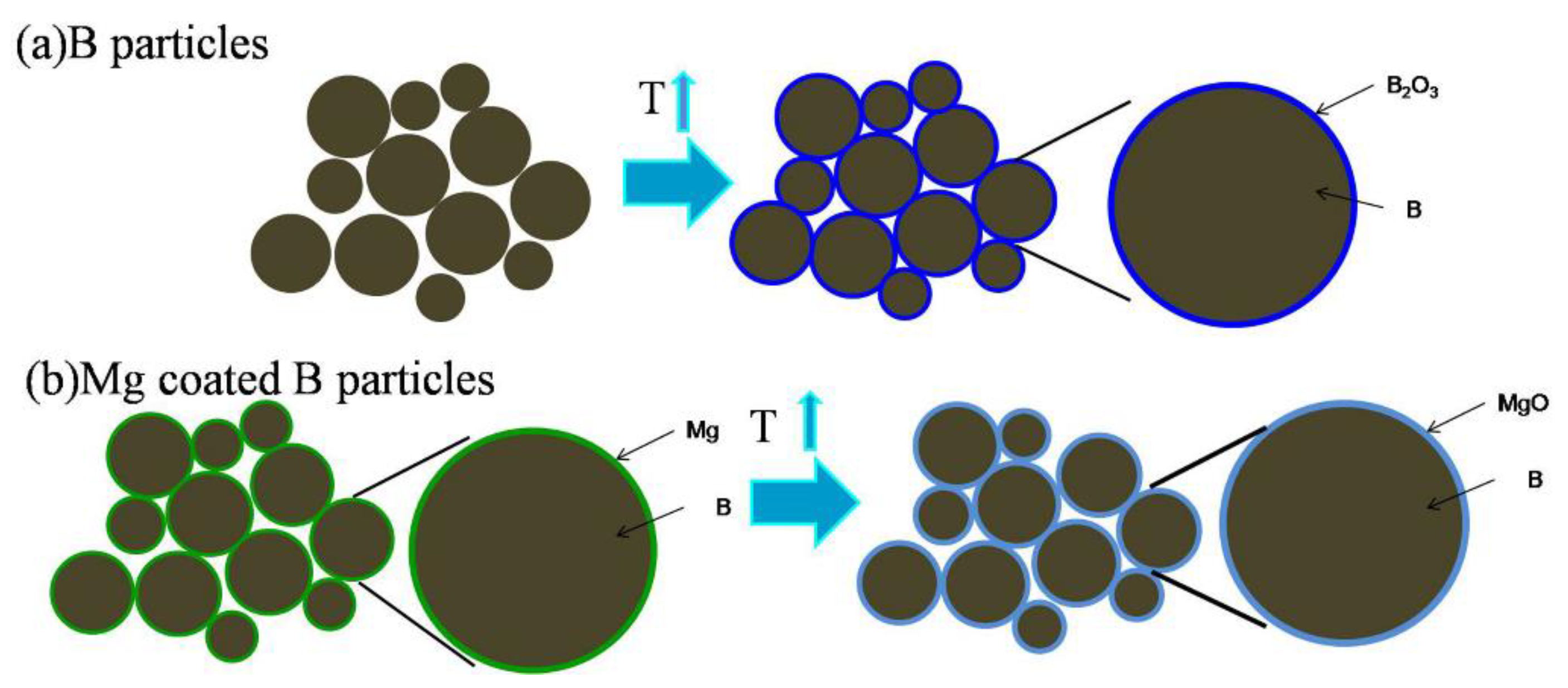
3.2. Microstructure and Compound Mechanism of Mg/B Composite Powders
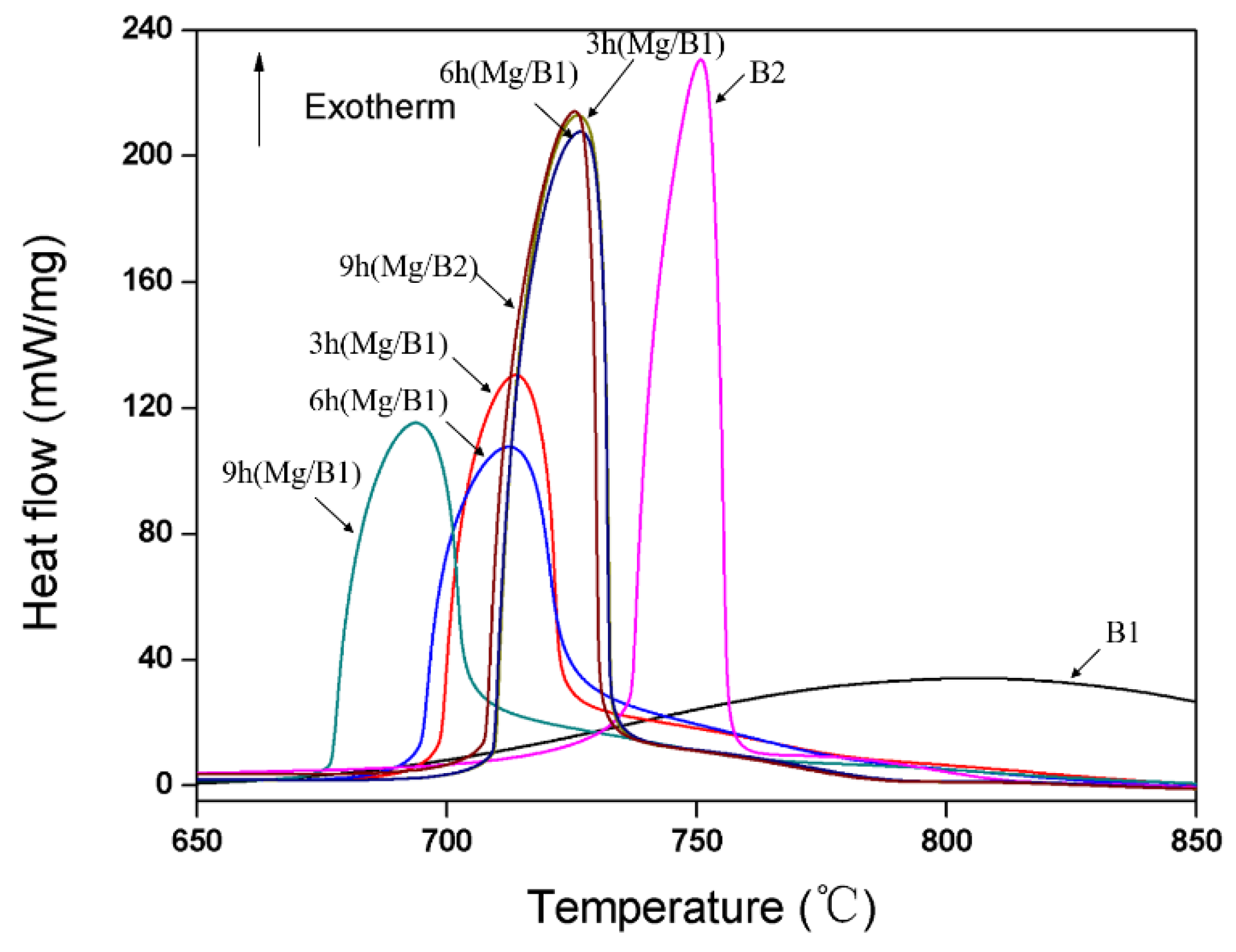
3.3. Enhanced Reactivity of Mg/B Composite Powders
4. Conclusions
- (1)
- Impurities Fe, N, and O were introduced into Mg/B composite powders via cryomilling. No obvious impacts on energy density since Fe is an energy material. The study of cryomilling shows that O and N can be removed after the degassing. The Fe content increases with the increase in cryomilling time, consequently cryomilling time should be limited;
- (2)
- By adding magnesium and cryomilling, the reaction temperature between B and O is improved by more than 90 °C for Mg/B composite powder(particle size 3.125 μm) and 24 °C for Mg/B composite powder(particle size 2.111 μm). The exothermic peak for Mg/B composite powder(particle size 3.125 μm) composite powder is higher and sharper than its starting B powder. The reaction temperature decreases with the decrease in particle size.
- (3)
- The combustion behavior of Mg/B composite powder is improved by cryomilling. In particular, the 9 h cryomilling Mg/B composite powder(particle size 3.125 μm) composite powder shows an extremely good combustion behavior with a 13,237 J/g release heat and relatively low exothermic peak temperature at 693.8 °C;
- (4)
- Comparing the physical properties of Mg/B composite powder(particle size 3.125 μm) and Mg/B composite powder(particle size 2.111 μm), the results indicate that the activity content of Mg on the surface plays a dominant role in the improvement of reactivity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonald, B.; Rice, J. Solid fuel ramjet fuel optimization for maximum thrust to drag ratio and impulse density subject to geometric restraints on missile outer mold line. Aerosp. Sci. Technol. 2018, 75, 47–57. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Chai, Z.; Dong, Z.; Hu, C.; Yu, X. Mixing and heat release characteristics in the combustor of solid-fuel rocket scramjet based on DES. Aerosp. Sci. Technol. 2019, 94, 105391. [Google Scholar] [CrossRef]
- Chintersingh, K.-L.; Schoenitz, M.; Dreizin, E.L. Combustion of boron and boron–iron composite particles in different oxidizers. Combust. Flame 2018, 192, 44–58. [Google Scholar] [CrossRef]
- Solomon, Y.; Grinstein, D.; Natan, B. Active boron dispersion and ignition in gel droplets. Int. J. Energetic Mater. Chem. Propuls. 2016, 15, 197–213. [Google Scholar] [CrossRef]
- Liu, L.-L.; He, G.-Q.; Wang, Y.-H.; Hu, S.-Q.; Liu, Y.-M. Factors affecting the primary combustion products of boron-based fuel-rich propellants. J. Propuls. Power 2016, 33, 333–337. [Google Scholar] [CrossRef]
- Liang, D.; Liu, J.; Zhou, Y.; Zhou, J. Ignition and combustion characteristics of amorphous boron and coated boron particles in oxygen jet. Combust. Flame 2017, 185, 292–300. [Google Scholar] [CrossRef]
- Keerthi, V.; Nie, H.; Pisharath, S.; Hng, H.H. Combustion characteristics of fluoropolymer coated boron powders. Combust. Sci. Technol. 2022, 194, 1183–1198. [Google Scholar] [CrossRef]
- Yu, D.; Zhuo, J.; Yao, Q. Review on ignition and combustion characteristics of boron particles. J. Solid Rocket Technol. 2017, 40, 573–582. [Google Scholar]
- Chen, B.; Liu, J.; Liang, D.; Li, H.; Zhou, J. Effect of oxidant coating boron particle on the ignition and combustion characteristics of boron-based propellant. Chin. J. Energy Mater. 2016, 24, 774–780. [Google Scholar]
- Xu, S.; Chen, Y.; Chen, X.; Wu, D.; Liu, D. Combustion heat of the Al/B powder and its application in metallized explosives in underwater explosions. Combust. Expl. Shock 2016, 52, 342–349. [Google Scholar] [CrossRef]
- Hedman, T.D.; Demko, A.R.; Kalman, J. Enhanced ignition of milled boron polytetrafluoroethylene mixtures. Combust. Flame 2018, 198, 112–119. [Google Scholar] [CrossRef]
- Connell, T.L.; Risha, G.A.; Yetter, R.A.; Roberts, C.W.; Young, G. Boron and polytetrafluoroethylene as a fuel composition for hybrid rocket applications. J. Propuls. Power 2015, 31, 373–385. [Google Scholar] [CrossRef]
- Liang, D.; Liu, J.; Li, H.; Zhou, Y.; Zhou, J. Improving effect of boron carbide on the combustion and thermal oxidation characteristics of amorphous boron. J. Therm. Anal. Calorim. 2017, 128, 1771–1782. [Google Scholar] [CrossRef]
- Liu, X.; Schoenitz, M.; Dreizin, E.L. Boron-based reactive materials with high concentrations of iodine as a biocidal additive. Chem. Eng. J. 2017, 325, 495–501. [Google Scholar] [CrossRef]
- Huang, S.; Deng, S.; Jiang, Y.; Zheng, X. Experimental effective metal oxides to enhance boron combustion. Combust. Flame 2019, 205, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Chen, X.; Xu, H.; Han, A.; Ye, M.; Pan, G. Preparation and properties of boron based nano-B/NiO thermite. Propel. Explos. Pyrotech. 2015, 40, 873–879. [Google Scholar] [CrossRef]
- Liu, J.-Z.; Xi, J.-F.; Yang, W.-J.; Hu, Y.-R.; Zhang, Y.-W.; Wang, Y.; Zhou, J. Effect of magnesium on the burning characteristics of boron particles. Acta Astronaut. 2014, 96, 89–96. [Google Scholar] [CrossRef]
- Liu, L.-L.; Liu, P.-J.; He, G.-Q. Ignition and combustion characteristics of compound of magnesium and boron. J. Therm. Anal. Calorim. 2015, 121, 1205–1212. [Google Scholar] [CrossRef]
- Liang, D.; Xiao, R.; Li, H.; Liu, J. Heterogeneous decomposition and oxidation during combustion of magnesium diboride particles. Acta Astronaut. 2018, 153, 159–165. [Google Scholar] [CrossRef]
- Li, J.; Xiong, Y.; Wang, X.; Yan, S.; Yang, C.; He, W.; Chen, J.; Wang, S.; Zhang, X.; Dai, S. Microstructure and tensile properties of bulk nanostructured aluminum/graphene composites prepared via cryomilling. Mater. Sci. Eng. A 2015, 626, 400–405. [Google Scholar] [CrossRef]
- Lau, M.L.; Lavernia, E.J. Microstructural evolution and oxidation behavior of nanocrystalline 316-stainless steel coatings produced by high-velocity oxygen fuel spraying. Mat. Sci. Eng. A 1999, 272, 222–229. [Google Scholar] [CrossRef]
- Zheng, B.; Ertorer, O.; Li, Y.; Zhou, Y.; Mathaudhu, S.N.; Tsao, C.Y.; Lavernia, E.J. High strength, nano-structured Mg–Al–Zn alloy. Mat. Sci. Eng. A 2011, 528, 2180–2191. [Google Scholar] [CrossRef]
- Wen, H.; Zhao, Y.; Zhang, Z.; Ertorer, O.; Dong, S.; Lavernia, E.J. The influence of oxygen and nitrogen contamination on the densification behavior of cryomilled copper powders during spark plasma sintering. J. Mater. Sci. 2011, 46, 3006–3012. [Google Scholar] [CrossRef] [Green Version]
- Kaisar, K.H.; Hofmeister, C.; Pedigo, A.; Giri, A.K.; Sohn, Y.; Cho, K.C.; van den Bergh, M.; Majumdar, B.S. Tensile properties and microstructure of a cryomilled nanograined Al-Mg alloy near the AA5083 composition. Mater. Sci. Eng. A 2017, 705, 239–248. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, B.; Li, P.; Chen, D.; Guo, H.; Liu, Z.; Chu, M. Interface behavior and oxidation consolidation mechanism of titanium-bearing iron sand particles with ball-milling pretreatment. Powder Technol. 2022, 396, 366–377. [Google Scholar] [CrossRef]
- Mohamed, F.A. Correlation between the minimum grain size obtainable by ball milling and lattice strain. Mater. Sci. Eng. A 2019, 752, 15–17. [Google Scholar] [CrossRef]
- Liang, D.; Liu, J.; Chen, B.; Zhou, J.; Cen, K. Improvement in energy release properties of boron-based propellant by oxidant coating. Thermochim. Acta 2016, 638, 58–68. [Google Scholar] [CrossRef]
- Liang, D.; Liu, J.; Xiao, J.; Xi, J.; Wang, Y.; Zhang, Y.; Zhou, J. Energy release properties of amorphous boron and boron-based propellant primary combustion products. Acta Astronaut 2015, 112, 182–191. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, H.; Jiao, Q.; Schoenitz, M.; Dreizin, E.L. Oxidation, ignition and combustion behaviors of differently prepared boron-magnesium composites. Combust. Flame 2020, 221, 11–19. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.H.; Deshmukh, P.R.; Hyun, H.S.; Sohn, Y.; Shin, W.G. Ignition study of facile spray drying prepared microspheres of nickel coated boron nanoparticles using a shock tube. J. Alloys Compd. 2022, 910, 164678. [Google Scholar] [CrossRef]


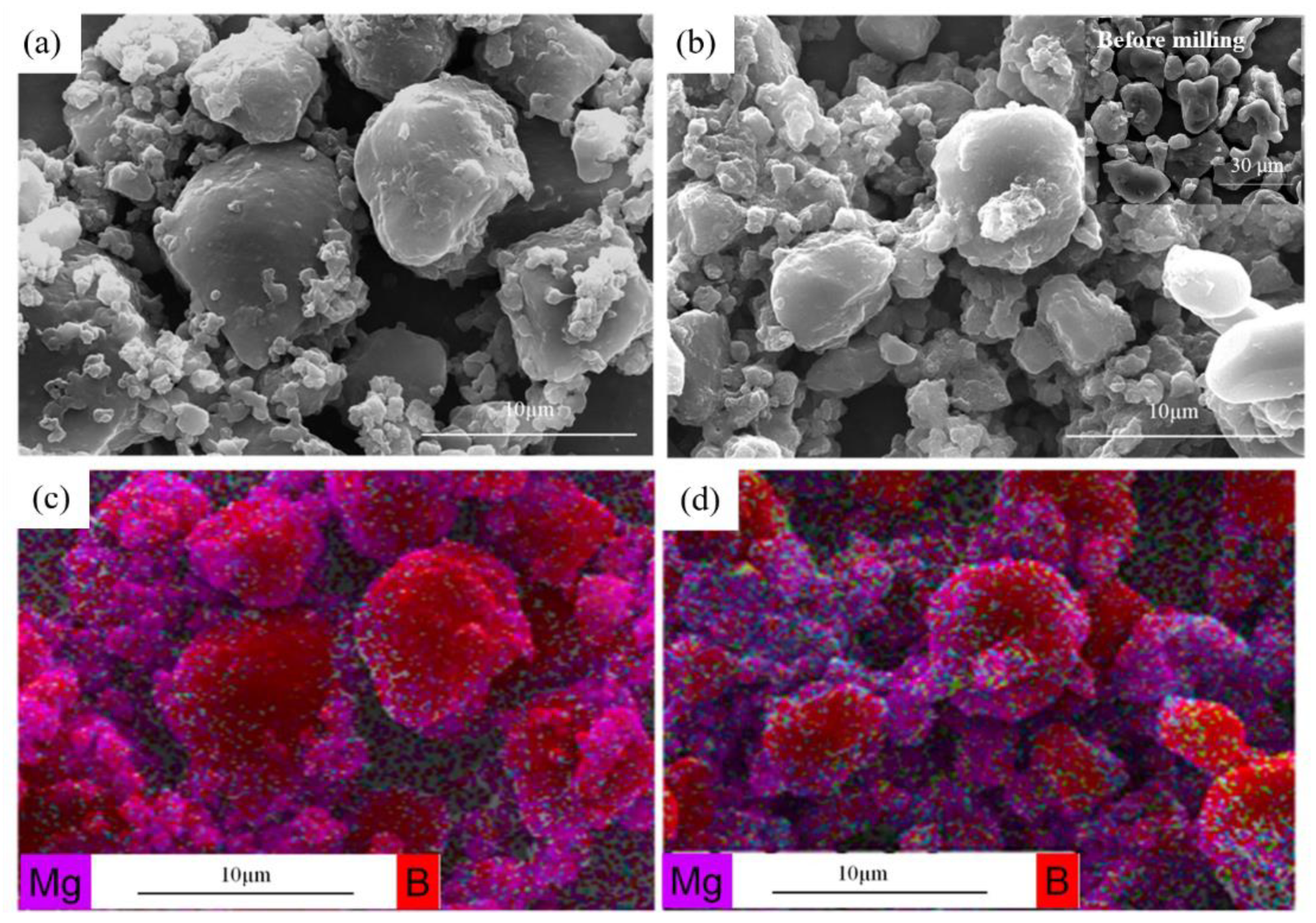
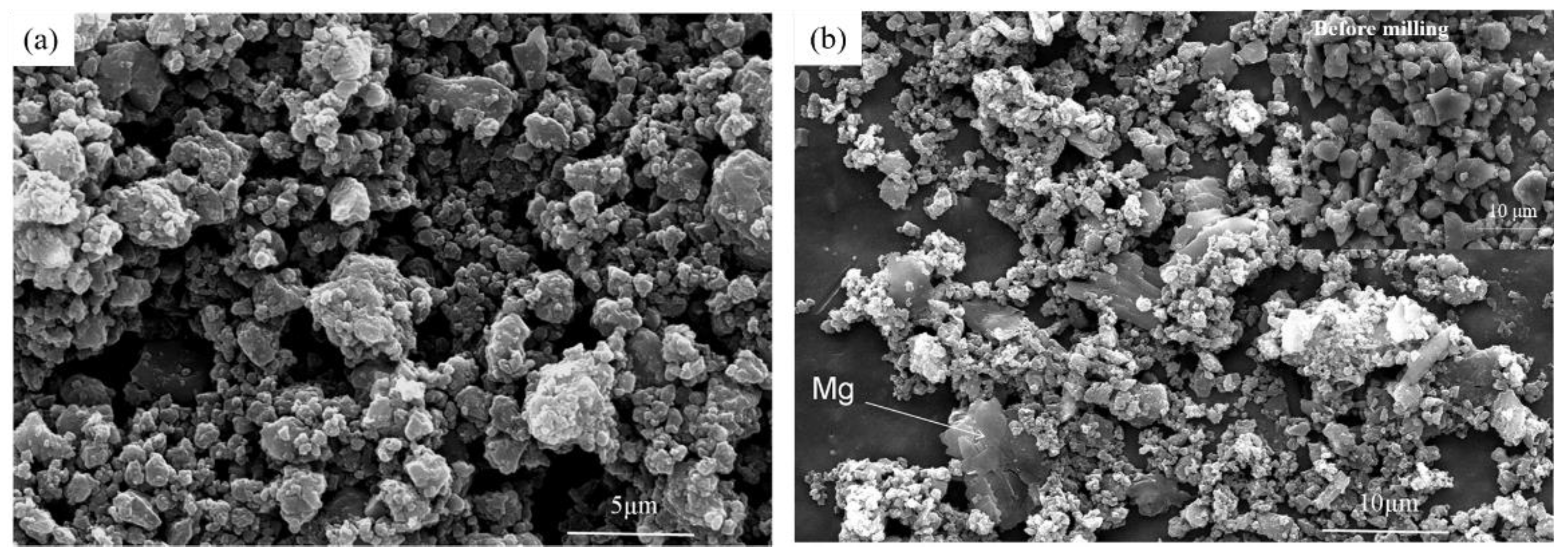
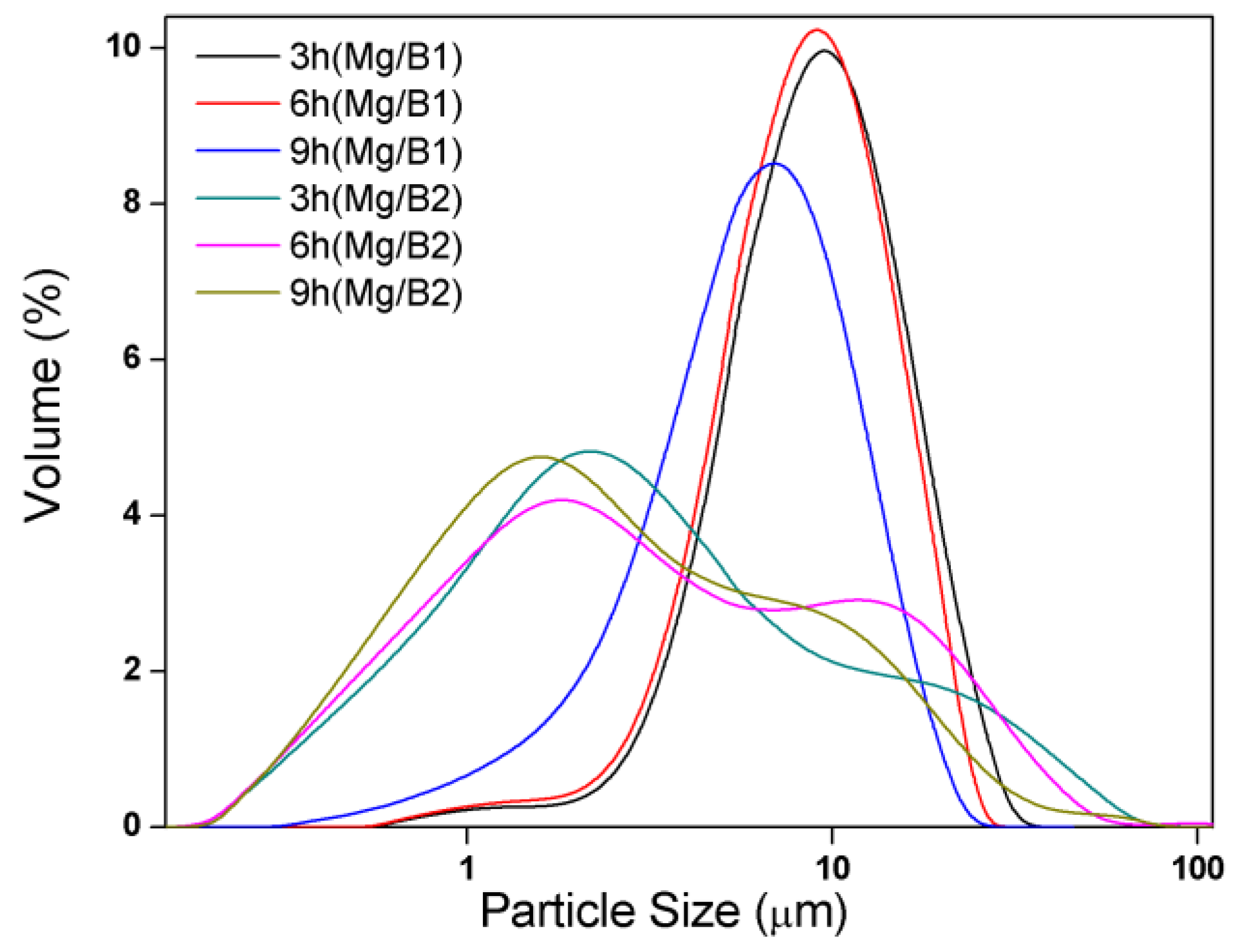
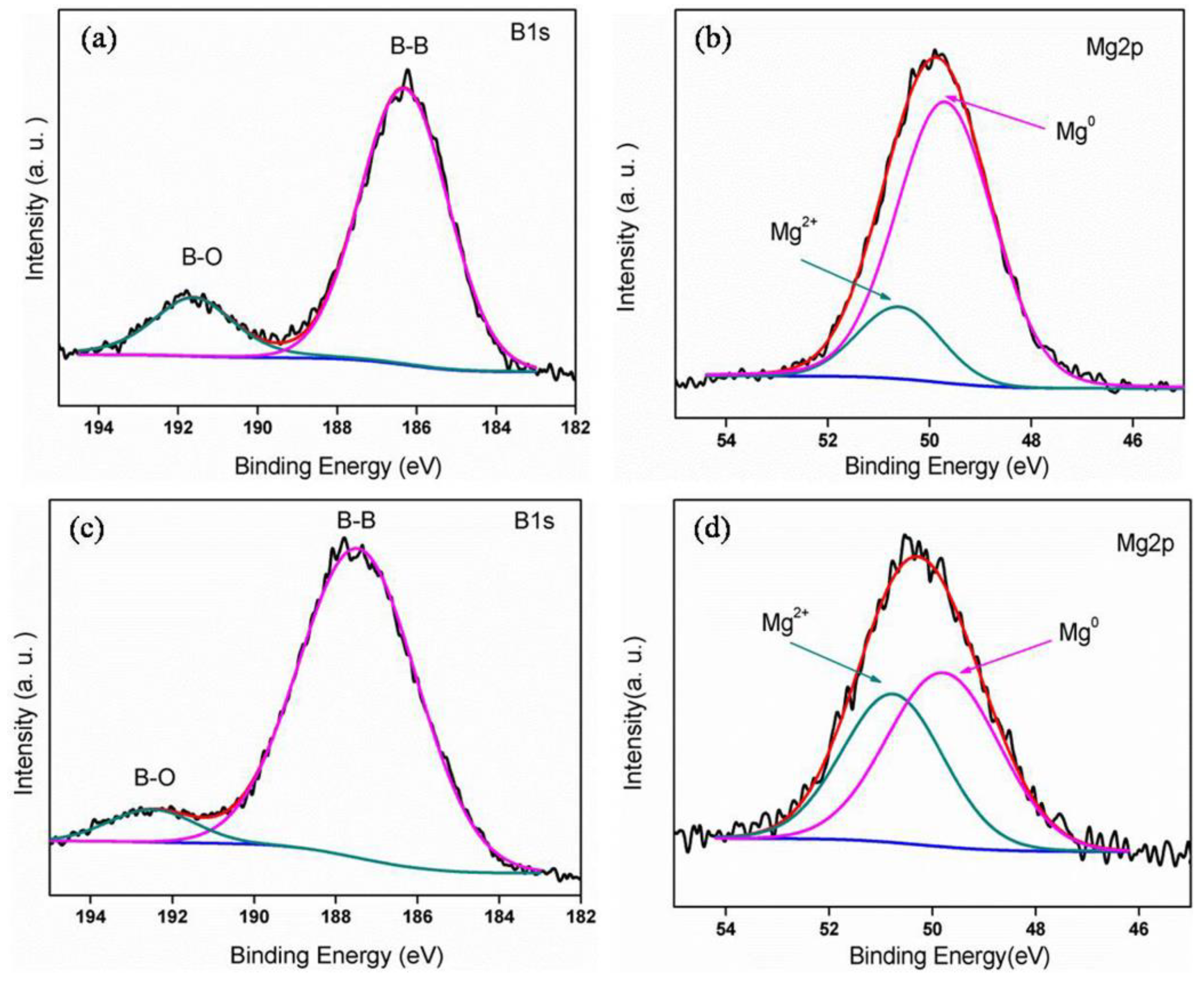
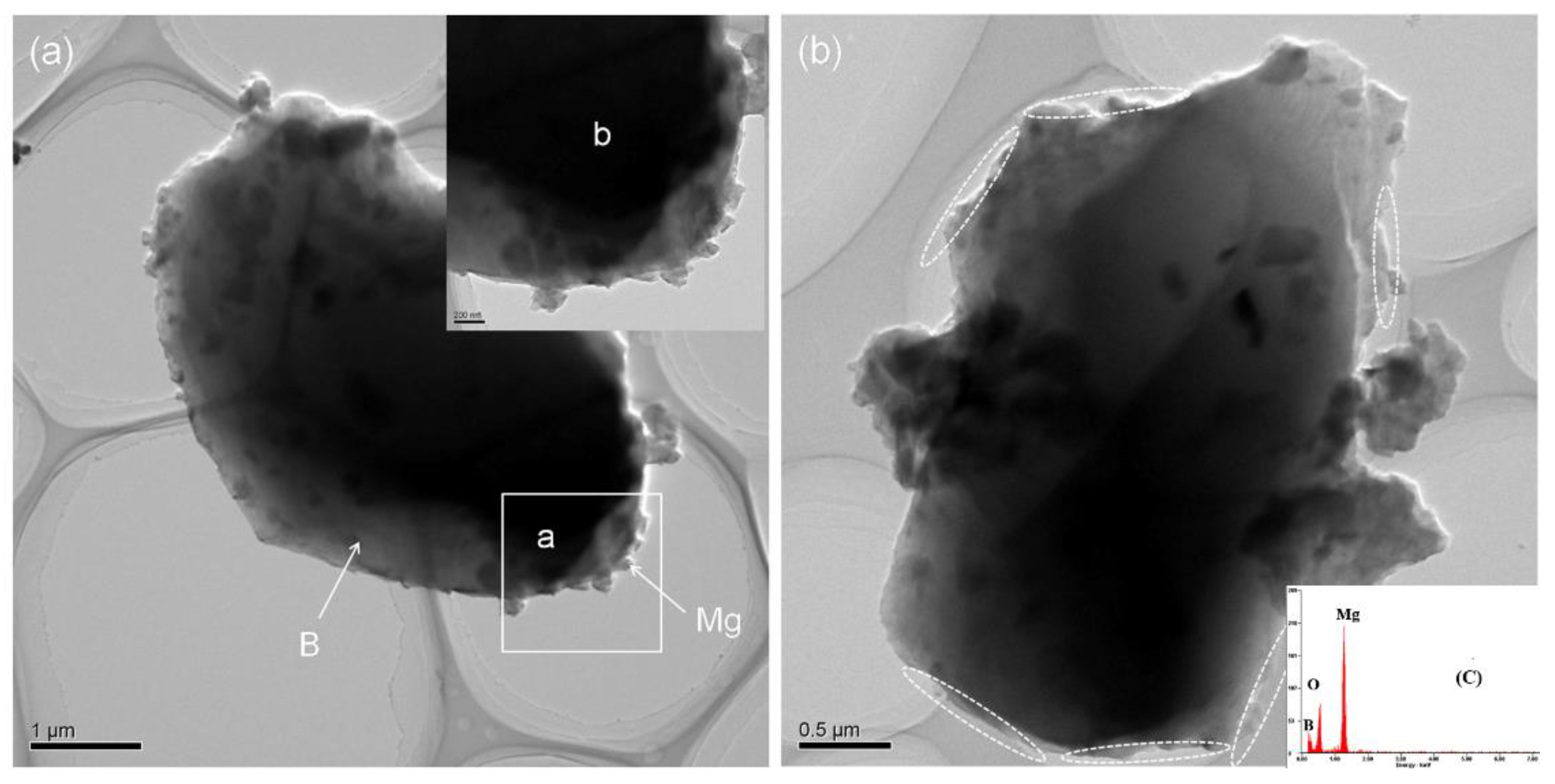



| Content [wt.%] | 3 h | 6 h | 9 h |
|---|---|---|---|
| Mg | 9.32 | 9.26 | 9.63 |
| Fe | 0.21 | 0.30 | 0.42 |
| O | 3.01 | 3.04 | 3.29 |
| N | 1.80 | 1.93 | 1.97 |
| B | 85.66 | 85.47 | 84.69 |
| Content [wt.%] | 3 h | 6 h | 9 h |
|---|---|---|---|
| Mg | 9.87 | 9.56 | 9.46 |
| Fe | 0.21 | 0.21 | 0.32 |
| O | 2.37 | 2.48 | 2.66 |
| N | 1.37 | 1.54 | 1.63 |
| B | 86.08 | 86.01 | 85.77 |
| Cryomilling Time (h) | 3 | 6 | 9 |
|---|---|---|---|
| B (Mg/B1) | 86.08 | 85.41 | 84.37 |
| Mg (Mg/B1) | 88.38 | 87.65 | 89.59 |
| B (Mg/B2) | 92.90 | 93.07 | 92.86 |
| Mg (Mg/B2) | 60.09 | 54.38 | 55.87 |
| Sample | Temperature (°C) | Heat Release (J/g) |
|---|---|---|
| B1 | 806.5 | 15,325 |
| Mg/B1 cryomilled for 3 h | 713.7 | 12,830 |
| Mg/B1 cryomilled for 6 h | 712.6 | 13,555 |
| Mg/B1 cryomilled for 9 h | 693.8 | 13,237 |
| B2 | 750 | 10,079 |
| Mg/B2 cryomilled for 3 h | 726.0 | 12,601 |
| Mg/B2 cryomilled for 6 h | 726.7 | 11,305 |
| Mg/B2 cryomilled for 9 h | 726.2 | 11,198 |
| Method | Materials | Temperature (°C) | Heat Release (J/g) |
|---|---|---|---|
| Cryomilling | Mg/B | 693.8 | 13,237 |
| Sintering | MgB2/B | 810 | 15,600 |
| Milling | MgB2/B | 750 | 8500 |
| Ultrasonic dispersion | HMX/B | 607 | 9110 |
| Laser ignition | Mg-Al/B | 749 | 7993 |
| Cryomilling Time (h) | 0 | 3 | 6 | 9 |
|---|---|---|---|---|
| Mg/B1 composite powders | 0.05 | 0.69 | 0.83 | 1.12 |
| Mg/B2 composite powders | 0.05 | 0.09 | 0.15 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, Q.; Tang, L.; Chen, F. Enhanced Reactivity and Compound Mechanism of Mg/B Composite Powders Prepared by Cryomilling. Materials 2022, 15, 4618. https://doi.org/10.3390/ma15134618
Zhang C, Wang Q, Tang L, Chen F. Enhanced Reactivity and Compound Mechanism of Mg/B Composite Powders Prepared by Cryomilling. Materials. 2022; 15(13):4618. https://doi.org/10.3390/ma15134618
Chicago/Turabian StyleZhang, Chi, Qin Wang, Liying Tang, and Fei Chen. 2022. "Enhanced Reactivity and Compound Mechanism of Mg/B Composite Powders Prepared by Cryomilling" Materials 15, no. 13: 4618. https://doi.org/10.3390/ma15134618
APA StyleZhang, C., Wang, Q., Tang, L., & Chen, F. (2022). Enhanced Reactivity and Compound Mechanism of Mg/B Composite Powders Prepared by Cryomilling. Materials, 15(13), 4618. https://doi.org/10.3390/ma15134618




