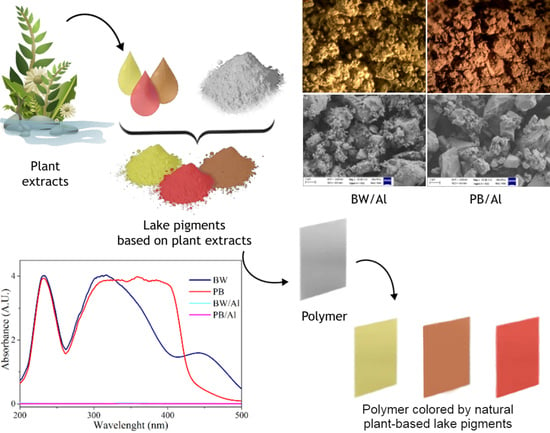
Structure and Stability Characterization of Natural Lake Pigments Made from Plant Extracts and Their Potential Application in Polymer Composites for Packaging Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Raw Materials
2.2. Extraction of Dyes from Weld, Persian Berries, and Brazilwood
2.3. Lake Pigment Preparation Method
2.3.1. Lake Pigments from Weld, Persian Berries, and Brazilwood with Alum (RE/Al, JP/Al, BW/Al)
2.3.2. Lake Pigment from Weld with Tin (RE/Sn)
2.4. Preparation of Polymer Composites
2.5. Characterization Techniques
2.5.1. Chromatographic Analysis
2.5.2. Thermogravimetric Analysis
2.5.3. Chemical Resistance Characteristic
2.5.4. UV-Vis Spectroscopy
2.5.5. Optical Microscopy
2.5.6. Scanning Electron Microscopy (SEM)
2.5.7. Colorimetric Measurements
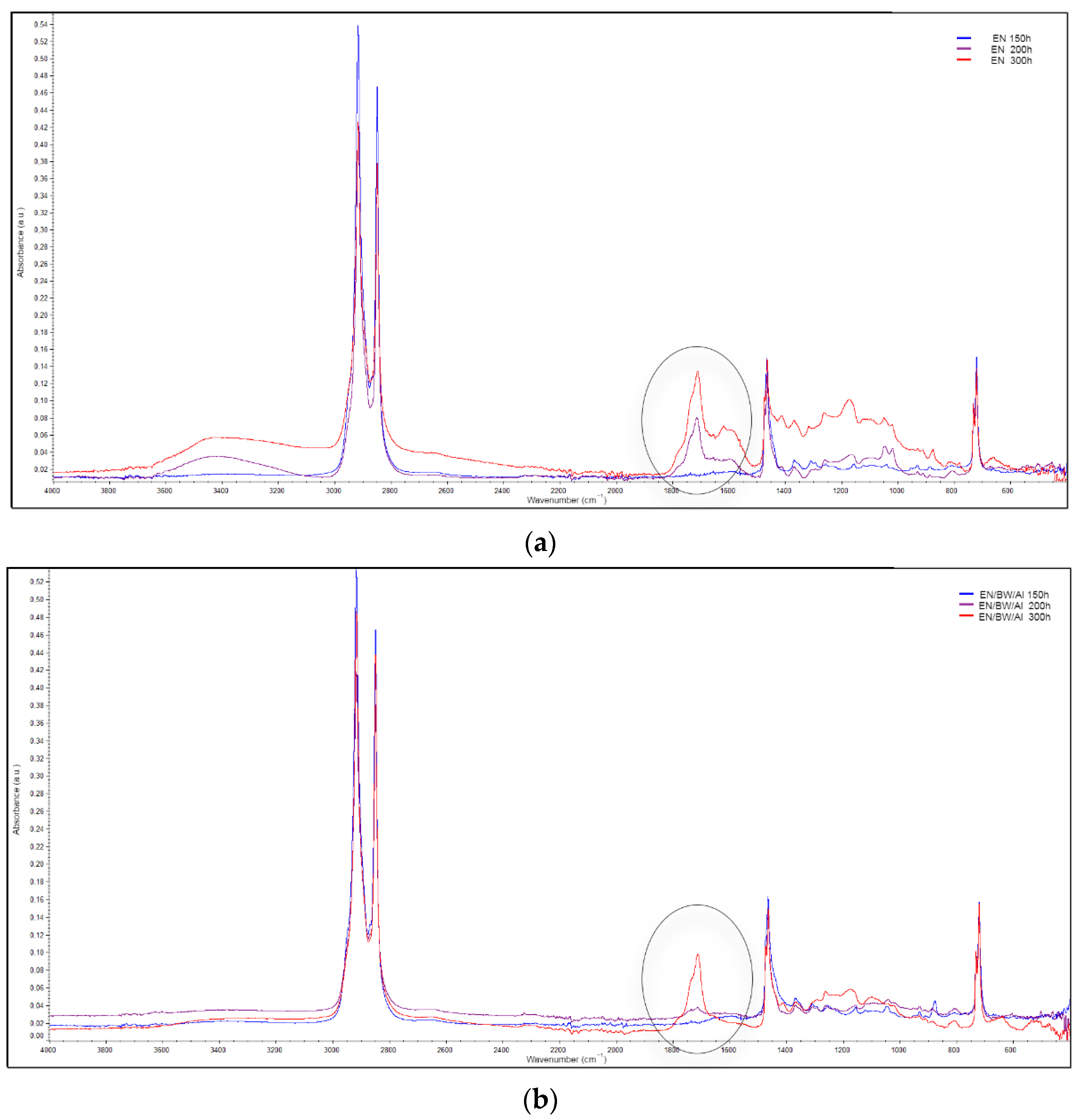
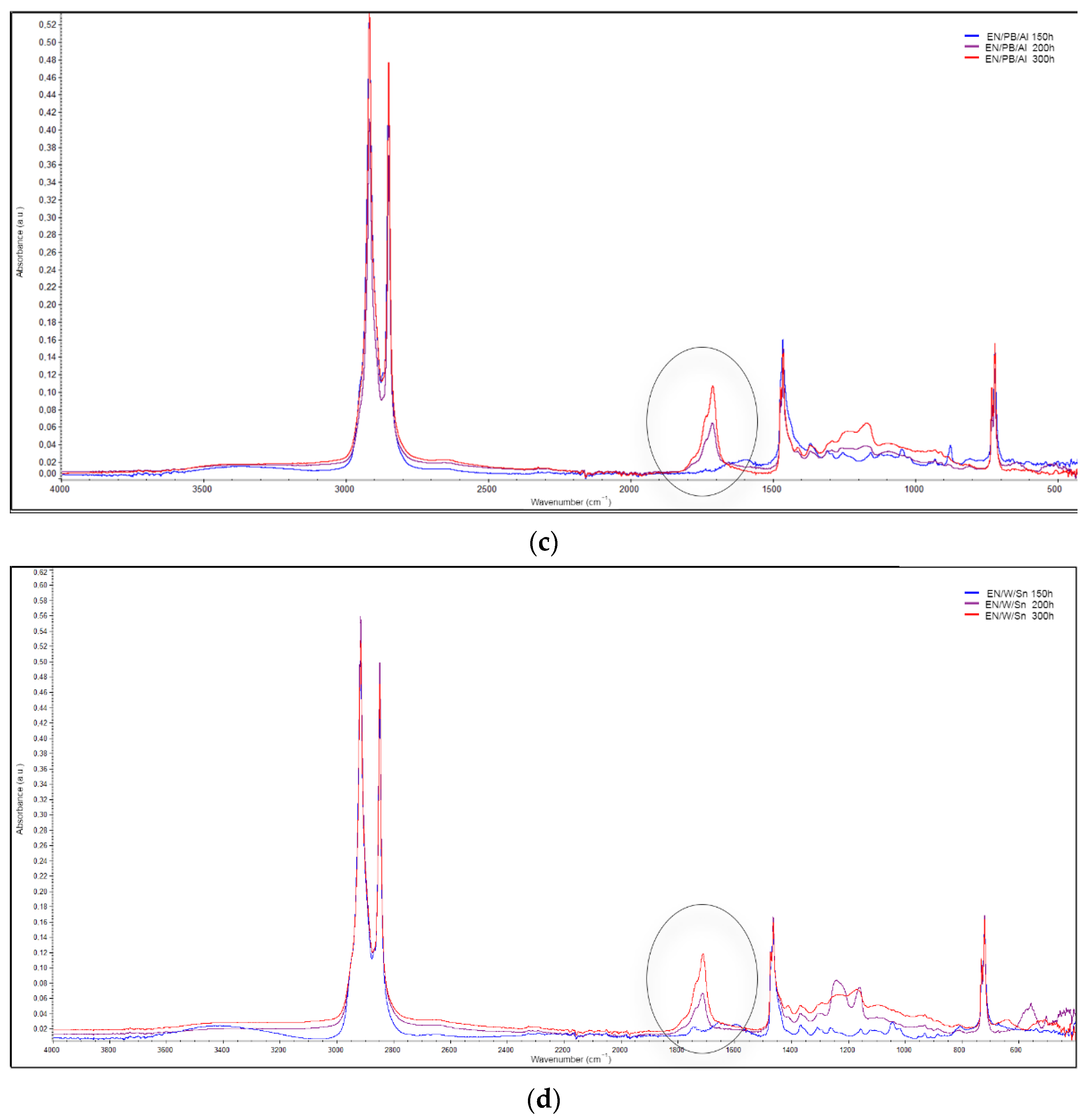
2.5.8. Carbonyl Index Determination (FTIR Measurements)
2.5.9. Mechanical Properties of EN Composites
2.5.10. Accelerated UV Aging and Aging Factor Determination
3. Results and Discussion
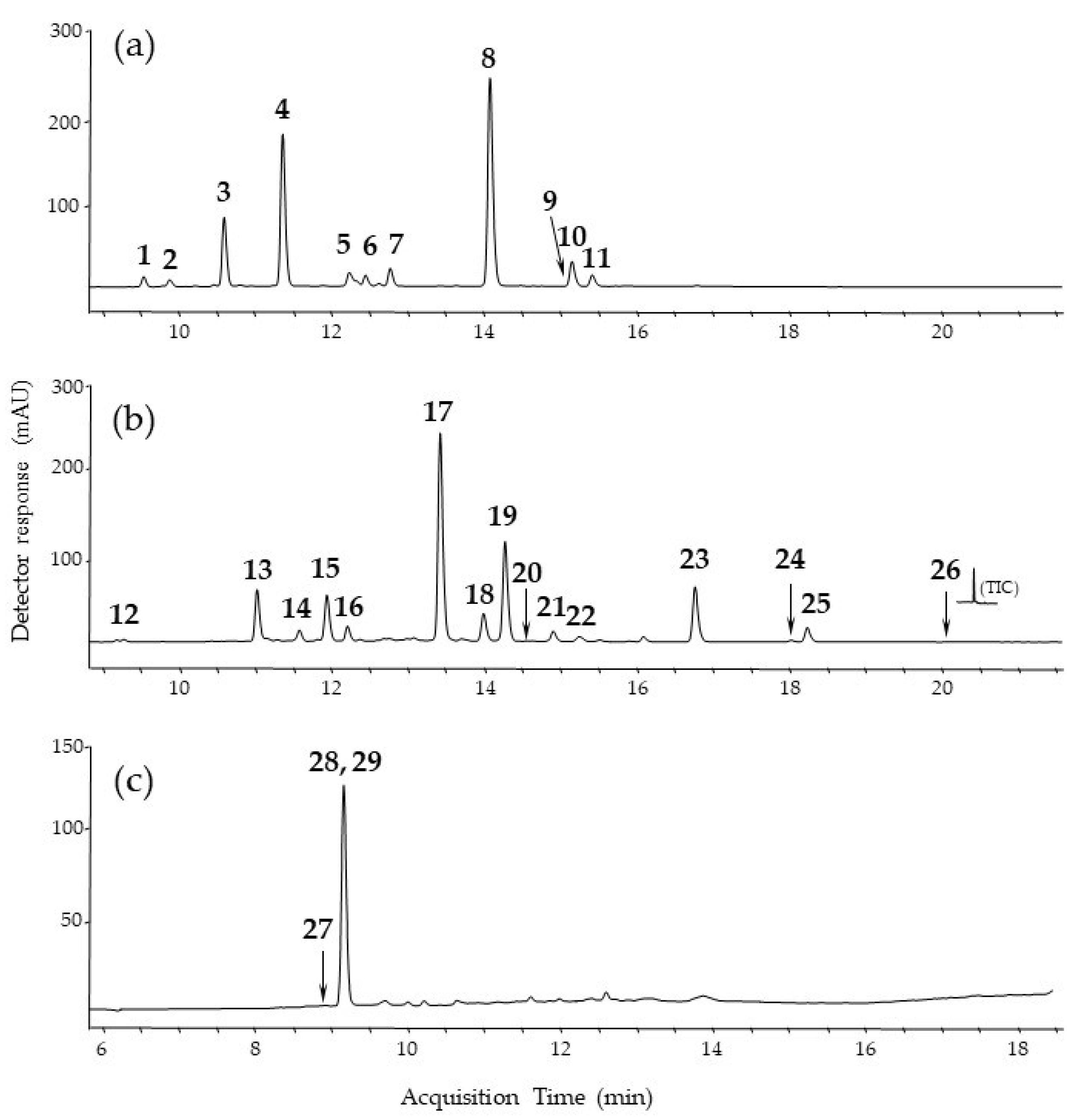
3.1. Characterization of Plant Extracts by Liquid Chromatography-Mass Spectrometry
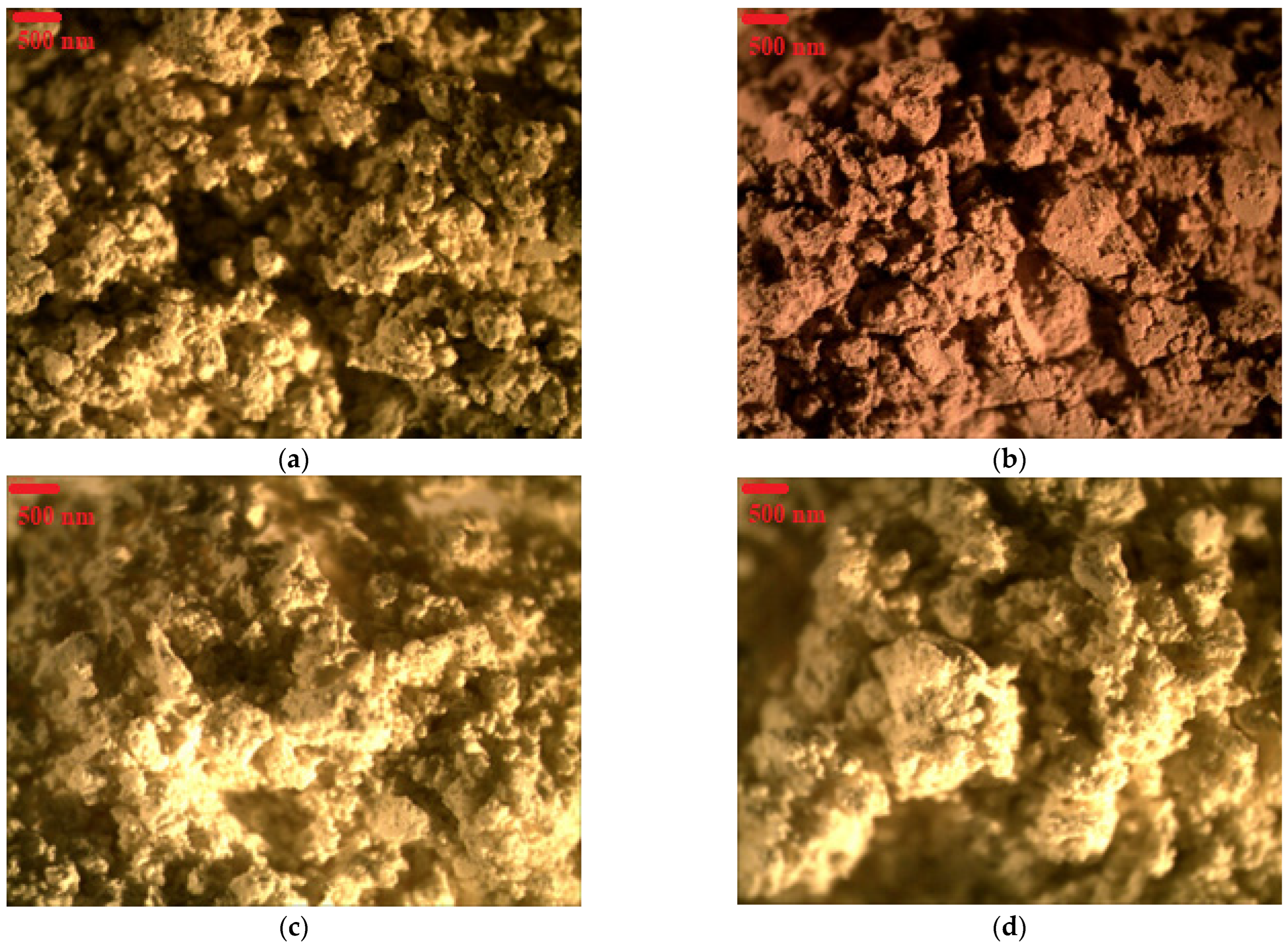
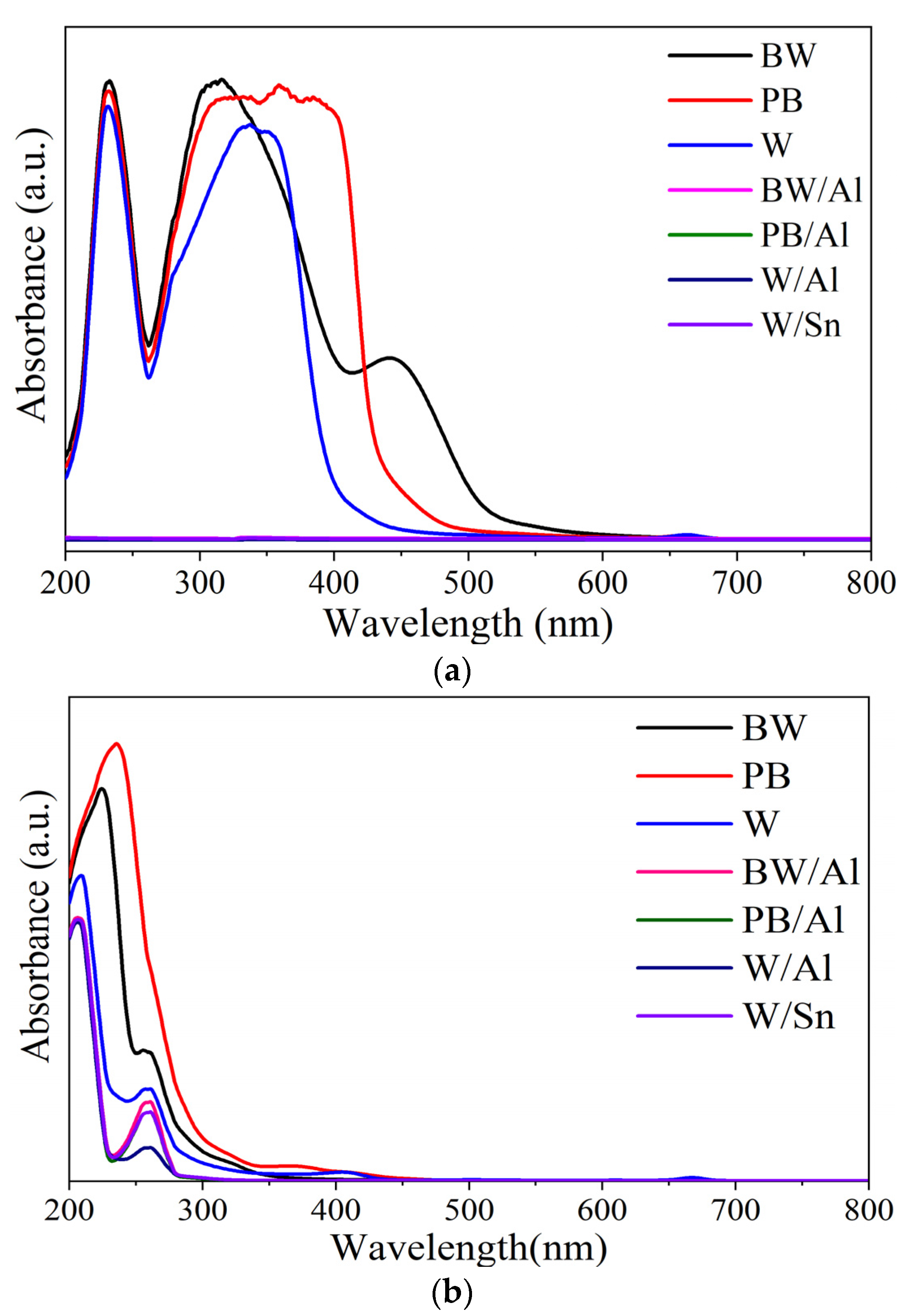
3.2. Structural Characterization of Lake Pigments
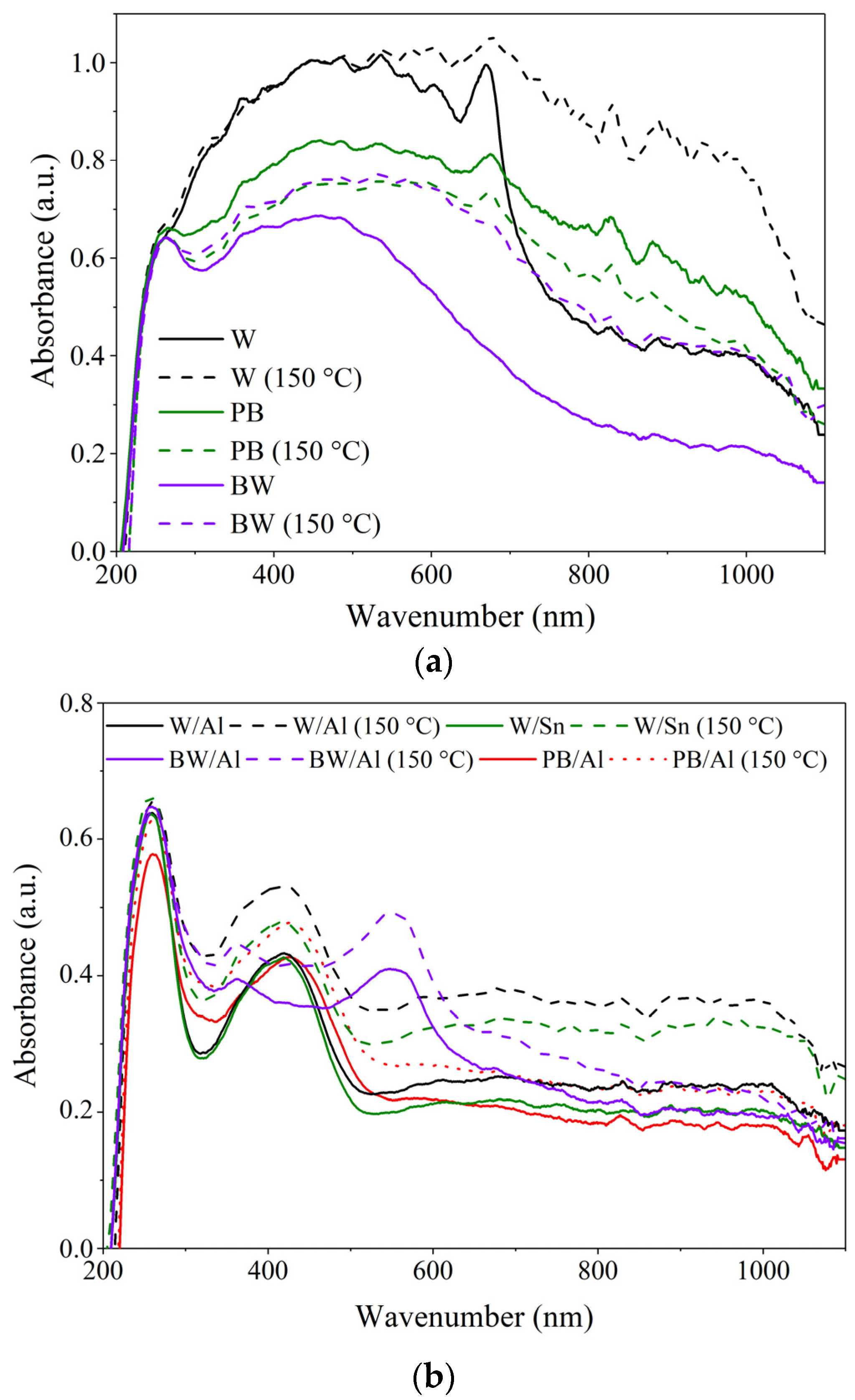
3.3. Chemical Resistance of Lake Pigments
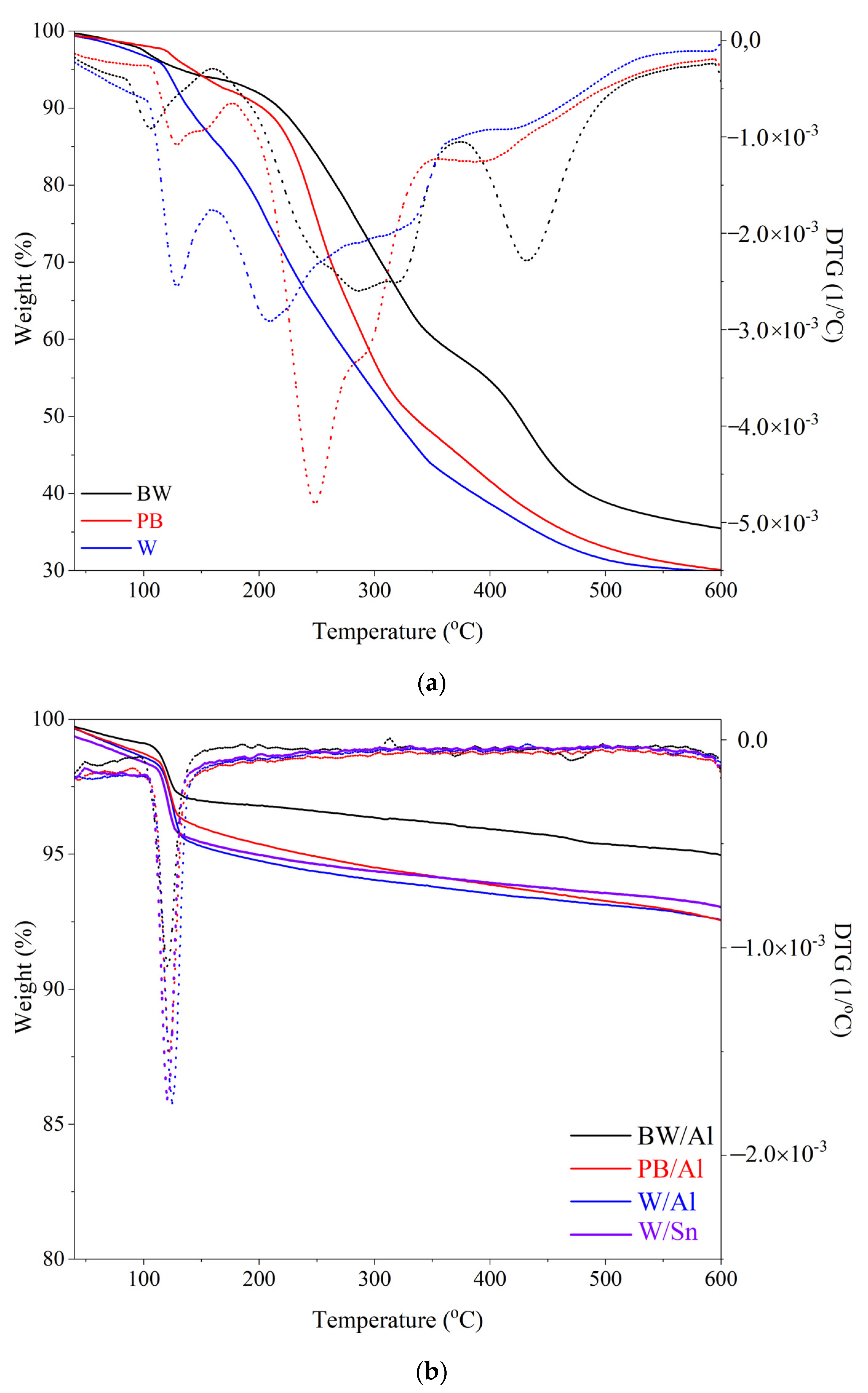
3.4. Thermal Stability of Lake Pigments
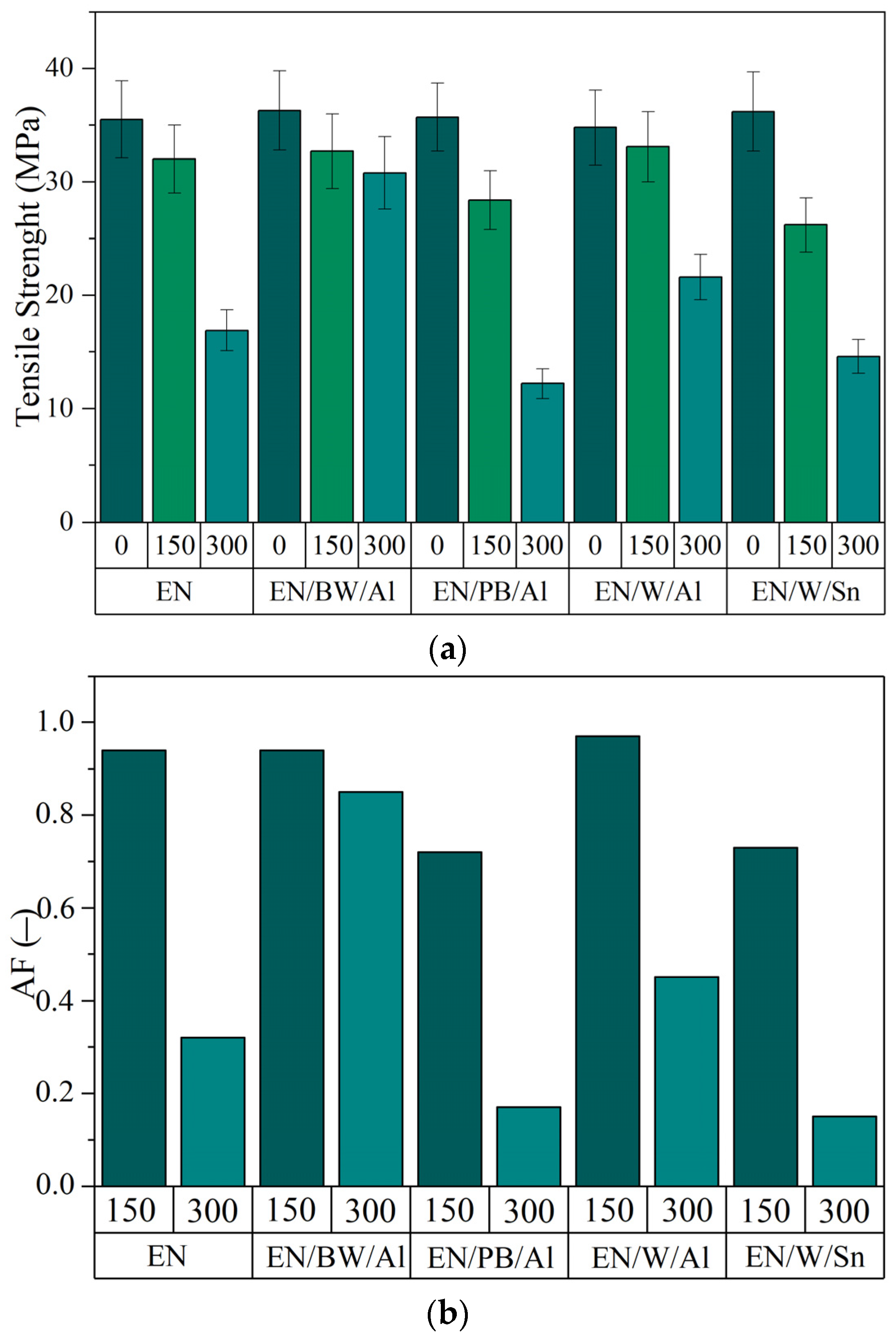
3.5. Application of Lake Pigments in Plastic
4. Conclusions
- ▪
- The lake pigments precipitated on tin and aluminum showed different color performance, depending on the natural dye and inorganic support.
- ▪
- Importantly, the prepared lake pigments exhibited improved thermal and chemical stability compared to the raw natural dyes.
- ▪
- The application of the studied lake pigments in ethylene-norbornene (EN) copolymer materials led to colorful products with improved resistance to long-term UV radiation.
- ▪
- In most cases, the EN composites containing natural lake pigments exhibited lower reductions in their mechanical parameters after 300 h of UV exposure compared to the neat EN copolymer. The enhanced UV resistance of the colored composites was also confirmed by the lower content of C=O groups generated on their surfaces during aging.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Fleischmann, C.; Lievenbrück, M.; Ritter, H. Polymers and dyes: Developments and applications. Polymers 2015, 7, 717–746. [Google Scholar] [CrossRef] [Green Version]
- Herbst, W.; Hunger, K. Industrial Organic Pigments: Production, Properties, Applications; John Wiley& Sons: Weinheim, Germany, 2006; pp. 1–659. [Google Scholar]
- Pfaff, G. Inorganic Pigments; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2017; pp. 1–329. [Google Scholar]
- Wang, X.; Mu, B.; Hui, A.; Wang, Q.; Wang, A. Low-cost bismuth yellow hybrid pigments derived from attapulgite. Dye. Pigm. 2018, 149, 521–530. [Google Scholar] [CrossRef]
- Kang, N.J.; Wang, D.Y.; Kutlu, B.; Zhao, P.C.; Leuteritz, A.; Wagenknecht, U.; Heinrich, G. Anew approach to reducing the flammability of layered double hydroxide (LDH)-based polymer composites: Preparation and characterization of dye structure-intercalated LDH and its effect on the flammability of polypropylene-grafted maleic ahydride/d-LDH composites. ACS Appl. Mater. Interfaces 2013, 5, 8991–8997. [Google Scholar] [CrossRef] [PubMed]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Zaborski, M. New organic-inorganic hybrids as multifunctional additives to improve ethylene-norbornene (EN) composite stability. Polym. Degrad. Stab. 2019, 160, 110–119. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Rybiński, P.; Maniukiewicz, W.; Zaborski, M. Aluminum-magnesium hydroxycarbonate/azo dye hybrids as novel multifunctional colorants for elastomer composites. Polymers 2018, 11, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoodi, A.; Ghodrati, S.; Khorasani, M. High-strength, low-permeable, and light-protective nanocomposite films based on a hybrid nanopigment and biodegradable PLA for food packaging applications. ACS Omega 2019, 4, 14947–14954. [Google Scholar] [CrossRef] [Green Version]
- Hajibeygi, M.; Omidi-Ghallemohamadi, M. One-step synthesized azo-dye modified Mg-Al-LDH reinforced biobased semi-aromatic polyamide containing naphthalene ring; study on thermal stability and optical properties. J. Polym. Res. 2017, 24, 61–71. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Krysztafkiewicz, A.; Jesionowski, T. Adsorption of organic dyes on the aminosilane modified TiO2 surface. Dye. Pigm. 2004, 62, 121–130. [Google Scholar] [CrossRef]
- Jesionowski, T.; Binkowski, S.; Krysztafkiewicz, A. Adsorption of the selected organic dyes on the functionalized surface of precipitated silica via emulsion route. Dye. Pigm. 2005, 65, 267–279. [Google Scholar] [CrossRef]
- Fournier, F.; de Viguerie, L.; Balme, S.; Janot, J.M.; Walter, P.; Jaber, M. Physico-chemical characterization of lake pigments based on montmorillonite and carminic acid. Appl. Clay Sci. 2016, 130, 12–17. [Google Scholar] [CrossRef]
- Wiyantoko, B.; Kurniawati, P.; Purbaningtias, T.E.; Fatimah, I. Synthesis and characterization of hydrotalcite at different Mg/Al molar ratios. Procedia Chem. 2015, 17, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Li, D.; Zhang, W.; Pu, M.; Evans, D.G.; Duan, X. Preparation of an anionic azopigment-pillared layered double hydroxide and the thermo-and photostability of the resulting intercalated material. J. Solid State Chem. 2004, 177, 4597–4604. [Google Scholar] [CrossRef]
- Li, D.; Qian, L.; Feng, Y.; Feng, J.; Tang, P.; Yang, L. Co-intercalation of acid red 337 and a UV absorbent into layered double hydroxides: Enhancement of photostability. ACS Appl. Mater. Interfaces 2014, 6, 20603–20611. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mu, B.; Wang, X.; Wang, A. Recent researches on natural pigments stabilized by clay minerals: A review. Dye. Pigm. 2021, 190, 109322–109333. [Google Scholar] [CrossRef]
- Costa, F.R.; Leuteritz, A.; Wagenknecht, U.; Jehnichen, D.; Häußler, L.; Heinrich, G. Intercalation of Mg-Al layered double hydroxide by anionic surfactants: Preparation and characterization. Appl. Clay Sci. 2008, 38, 153–164. [Google Scholar] [CrossRef]
- Christie, R.M.; Mackay, J.L. Metal salt azo pigments. Color. Technol. 2008, 124, 133–144. [Google Scholar] [CrossRef]
- Alam, S.M.M.; Islam, S.; Akter, S. Reviewing the sustainability of natural dyes. Adv. Res. Text Eng. 2020, 5, 1050. [Google Scholar]
- Deveoglu, O.; Cakmakcı, E.; Taskopru, T.; Torgan, E.; Karadag, R. Identification by RP-HPLC-DAD, FTIR, TGA and FESEM-EDAX of natural pigments prepared from Datisca cannabina L. Dye. Pigm. 2012, 94, 437–442. [Google Scholar] [CrossRef]
- Zhou, Q.; Rather, L.J.; Ali, A.; Wang, W.; Zhang, Y.; Haque, Q.M.R.; Li, Q. Environmental friendly bioactive finishing of wool textiles using the tannin-rich extracts of Chinese tallow (Sapiumsebiferum L.) waste/fallen leaves. Dye. Pigm. 2020, 176, 108230. [Google Scholar] [CrossRef]
- Mahmud-Ali, A.; Fitz-Binder, C.; Bechtold, T. Aluminium based dye lakes from plant extracts for textile coloration. Dye. Pigm. 2012, 94, 533–540. [Google Scholar] [CrossRef]
- Beltran, V.; Marchetti, A.; De Meyer, S.; Nuyts, G.; De Wael, K. Geranium lake pigments: The role of the synthesis on the structure and composition. Dye. Pigm. 2021, 189, 109260. [Google Scholar] [CrossRef]
- Pozzi, F.; van den Berg, K.J.; Fiedler, I.; Casadio, F. A systematic analysis of red lake pigments in French Impressionist and Post-Impressionist paintings by surface-enhanced Raman spectroscopy (SERS). J. Raman. Spectrosc. 2014, 45, 1119–1126. [Google Scholar] [CrossRef]
- Sirirak, J.; Suppharatthanya, P.; Chantha, K.; Girdthep, S.; Chayabutra, S. Eco-friendly lake pigment from sappanwood: Adsorption study and its application as natural colorant for natural rubber toy balloon. J. Met. Mater. Miner. 2021, 31, 27–37. [Google Scholar] [CrossRef]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Rybiński, P.; Maniukiewicz, W. Novel eco-friendly hybrid pigment with improved stability as a multifunctional additive for elastomer composites with reduced flammability and pH sensing properties. Dye. Pigm. 2021, 186, 108965. [Google Scholar] [CrossRef]
- Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Beyou, E.; Marzec, A. New natural organic–inorganic pH indicators: Synthesis and characterization of pro-ecological hybrid pigments based on anthraquinone dyes and mineral supports. J. Ind. Eng. Chem. 2022, 105, 446–462. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. The effect of natural additives on the composting properties of aliphatic polyesters. Polymers 2020, 12, 1856. [Google Scholar] [CrossRef]
- Maciejewska, M.; Sowińska, A.; Kucharska, J. Organic zinc salts as pro-ecological activators for sulfur vulcanization of styrene–butadiene rubber. Polymers 2019, 11, 1723. [Google Scholar] [CrossRef] [Green Version]
- Otłowska, O.; Ślebioda, M.; Wachowiak, M.; Śliwka-Kaszyńska, M. Identification and characterization of the Indian Yellow dye-stuff and its degradation products in historical oil paint tube by liquid chromatography mass spectrometry. RSC Adv. 2015, 5, 48786–48792. [Google Scholar] [CrossRef]
- Otłowska, O.; Ślebioda, M.; Wachowiak, M.; Śliwka-Kaszyńska, M. A multi-analytical approach to the characterization of natural organic dyestuffs and inorganic substrates present in the 19th-century artistic oil paints manufactured by a French art materials supplier Richard Aines. Anal. Methods 2017, 9, 94–102. [Google Scholar] [CrossRef]
- Otłowska, O.; Ślebioda, M.; Kot-Wasik, A.; Karczewski, J.; Śliwka-Kaszyńska, M. Chromatographic and spectroscopic identification and recognition of natural dyes, uncommon dyestuff components and mordants in 16th century carpet. Molecules 2018, 23, 339. [Google Scholar] [CrossRef] [Green Version]
- Śliwka-Kaszyńska, M.; Ślebioda, M.; Brillowska-Dąbrowska, A.; Mroczyńska, M.; Karczewski, J.; Marzec, A.; Rybiński, P.; Drążkowska, A. Multi-Technique Investigation of Grave Robes from 17th and 18th Century Crypts Using Combined Spectroscopic, Spectrometric Techniques, and New-Generation Sequencing. Materials 2021, 14, 3535. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, J.; Zhao, C.; Xiao, H.; Fang, X.; Zheng, J. LC-Q-TOF-MS/MS detection of food flavonoids: Principle, methodology, and applications. Crit. Rev. Food Sci. Nutr. 2021, 21, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of Flavone, Flavonol, and Flavanone Aglycones by Negative Ion Liquid Chromatography Electrospray Ion Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Lech, K.; Fornal, E. A Mass Spectrometry-Based Approach for Characterization of Red, Blue, and Purple Natural Dyes. Molecules 2020, 25, 3223. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Fernandez, J.; Silvan, B.; Entrialgo-Cadierno, R.; Villar, C.J.; Capasso, R.; Uranga, J.A.; Lombo, F.; Abalo, R. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. Pharmacother. 2021, 143, 112241. [Google Scholar] [CrossRef]
- Rodriguez-Arce, E.; Saldias, M. Antioxidant properties of flavonoid metal complexes and their potential inclusion in the development of novel strategies for the treatment against neurodegenerative diseases. Biomed. Pharmacother. 2021, 143, 112236. [Google Scholar] [CrossRef]
- Berrie, B.H.; Strumfels, Y. Change is permanent: Thoughts on the fading of cochineal-based watercolor pigments. Herit. Sci. 2017, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Moszyński, D.; Rybiński, P.; Zaborski, M. Carminic acid stabilized with aluminum-magnesium hydroxycarbonate as new colorant reducing flammability of polymer composites. Molecules 2019, 24, 560. [Google Scholar] [CrossRef] [Green Version]
- Marzec, A.; Szadkowski, B.; Kuśmierek, M.; Rogowski, J.; Maniukiewicz, W.; Rybiński, P.; Zaborski, M. Impact of organic-inorganic color additive on the properties of ethylene-norbornene copolymer. Polym. Test 2020, 82, 106290. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Fernández-Lledó, V.; Angosto, J.M. New insights into red plant pigments: More than just natural colorants. RSC Adv. 2020, 10, 24669–24682. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Deng, L.Z.; Mujumdar, A.S.; Xiao, H.W.; Zhang, Q.; Kan, Z. Evolution and modeling of colour changes of red pepper (Capsicum annuum L.) during hot air drying. J. Food Eng. 2018, 231, 101–108. [Google Scholar] [CrossRef]
- Wolski, K.; Cichosz, S.; Masek, A. Surface hydrophobisation of lignocellulosic waste for the preparation of biothermoelastoplastic composites. Eur. Polym. J. 2019, 118, 481–491. [Google Scholar] [CrossRef]
- Plota, A.; Masek, A. Plant-Origin Stabilizer as an Alternative of Natural Additive to Polymers Used in Packaging Materials. Int. J. Mol. Sci. 2021, 22, 4012. [Google Scholar] [CrossRef] [PubMed]
- Krehula, L.K.; Katančić, Z.; Siročić, A.P.; Hrnjak-Murgić, Z. Weathering of high-density polyethylene-wood plastic composites. J. Wood Chem. Technol. 2014, 34, 39–54. [Google Scholar] [CrossRef]
- Olejnik, O.; Masek, A. Bio-based packaging materials containing substances derived from coffee and tea plants. Materials 2020, 13, 5719. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Czylkowska, A.; Efenberger-Szmechtyk, M.; Nowak, A.; Strzelec, K. Anti-oxidative activity of alcohol-water extracts from field horsetail (Equisteumarvense) in elastomer vulcanizates subjected to accelerated aging processes. Materials 2020, 13, 4903. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montava-Jordà, S.; Boronat, T.; Sammon, C.; Balart, R.; Torres-Giner, S. On the use of gallic acid as a potential natural antioxidant and ultraviolet light stabilizer in cast-extruded bio-based high-density polyethylene films. Polymers 2019, 12, 31. [Google Scholar] [CrossRef] [Green Version]
- Grause, G.; Chien, M.F.; Inoue, C. Changes during the weathering of polyolefins. Polym. Degrad. Stab. 2020, 181, 109364. [Google Scholar] [CrossRef]
- Rodriguez, A.K.; Mansoor, B.; Ayoub, G.; Colin, X.; Benzerga, A.A. Effect of UV-aging on the mechanical and fracture behavior of low density polyethylene. Polym. Degrad. Stab. 2020, 180, 109185. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Weir, M.P.; Truss, R.W.; Garvey, C.J.; Nicholson, T.M.; Halley, P.J. A fundamental study on photo-oxidative degradation of linear low density polyethylene films at embrittlement. Polymer 2012, 53, 2385–2393. [Google Scholar] [CrossRef]
- Marzec, A.; Szadkowski, B. Improved aging stability of ethylene-norbornene composites filled with lawsone-based hybrid pigment. Polymers 2019, 11, 723. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Column | Poroshell EC-C18 (3.0 × 150 mm), 2.7 μm, Agilent Technologies | ||
| Column temperature | 40 °C | ||
| Injection volume | 2 µL | ||
| Flow rate | 0.4 mL min−1 | ||
| Eluents | (A) 0.1 % HCOOH in water, (B) 0.1 % HCOOH in ACN/MeOH (1:1; v/v) | ||
| Gradient program | Time, min | %A | %B |
| 0 | 90 | 10 | |
| 20 | 0 | 100 | |
| 30 | 0 | 100 | |
| UV–Vis detection | Wavelengths: 254, 350 nm | ||
| ESI MS detection | Polarity negative | ||
| Mode | Profile 50–1000 m/z Product ion 50–650 m/z | ||
| Peak No. | tR (min) | [M-H]−, (m/z) | Fragment Ions (m/z) | Elemental Composition | Proposed Identification | λmax (nm) | |
|---|---|---|---|---|---|---|---|
| weld (Reseda luteola L.) | 1 | 9.6 | 593 | 503, 575, 473, 383 | C27H30O15 | apigenin-C-diglucoside | 272, 335 |
| 2 | 9.9 | 609 | 447, 285 | C27H30O16 | luteolin-O-diglucoside | 268, 336 | |
| 3 | 10.6 | 609 | 447, 285 | C27H30O16 | luteolin-3,7′-O-diglucoside | 268, 341 | |
| 4 | 11.3 | 447 | 285, 284 | C21H20O11 | luteolin-7-O-glucoside | 268, 349 | |
| 5 | 12.2 | 447 | 285 | C21H20O11 | luteolin-O-glucoside | 268, 337 | |
| 6 | 12.2 | 431 | 311, 269, 268 | C21H20O10 | apigenin-7-O-glucoside | 266, 348 | |
| 7 | 12.4 | 461 | 341, 299, 284 | C22H22O11 | chryoseriol-O-glucoside | 266, 348 | |
| 8 | 12.8 | 447 | 285 | C21H20O11 | luteolin-4′-O-glucoside | 268, 342 | |
| 9 | 14.2 | 285 | 257, 217, 199, 175, 151 | C15H10O6 | luteolin | 255, 349 | |
| 10 | 15.3 | 269 | 225, 151, 117 | C15H10O5 | apigenin | 267, 337 | |
| 11 | 15.5 | 299 | 284, 256 | C16H12O6 | chryoseriol | 266, 347 | |
| Persian berries (Rhamni maturi) | 12 | 9.4 | 609 | 447, 285 | C27H30O16 | kaempferol-O-dihexoside | 268, 325 |
| 13 | 11.0 | 755 | 609, 463, 301 | C33H40O20 | quercetin-O-dirhamnoside-glucoside | 256, 356 | |
| 14 | 11.6 | 739 | 593, 447, 285 | C33H40O19 | kaempferol-O-dirhamnoside-glucoside | 266, 348 | |
| 15 | 11.9 | 609 | 447, 301 | C27H30O16 | quercetin-O-rhamnoside-glucoside | 256, 350 | |
| 16 | 12.2 | 447 | 301, 211, 151 | C21H20O11 | quercetin-O-rhamnoside | 257, 349 | |
| 17 | 13.4 | 769 | 623, 447, 315 | C34H42O20 | rhamnetin-O-dirhamnoside-glucoside | 257, 357 | |
| 18 | 14.0 | 301 | 232, 151, 121 | C15H10O7 | quercetin | 255, 360 | |
| 19 | 14.2 | 783 | 637, 491, 329, 314 | C35H44O20 | rhamnazin-3-O-dirhamnoside-glucoside | 256, 356 | |
| 20 | 14.5 | 637 | 329, 314, 299 | C29H34O16 | rhamnazin-O-rhamnoside-glucoside | 250, 355 | |
| 21 | 14.8 | 623 | 461, 315 | C28H32O16 | rhamnetin-3-O-rutinoside | 257, 347 | |
| 22 | 15.2 | 285 | 257, 151 | C15H10O6 | kaempferol | 266, 354 | |
| 23 | 16.7 | 315 | 300, 272, 244, 165 | C16H12O7 | rhamnetin | 256, 371 | |
| 24 | 18.0 | 299 | 284, 271, 256, 243 | C16H12O6 | rhamnocitrin | 266, 367 | |
| 25 | 18.2 | 329 | 314, 301, 299, 286, 271 | C17H14O7 | rhamnazin | 256, 371 | |
| 26 | 20.1 | 269 | 241, 225, 197 | C15H10O5 | emodin | 252, 286 | |
| Brazilwood | 27 | 8.9 | 283 | 271, 255, 229, 211 | C16H12O5 | brazilein | 290, 440 |
| 28 | 9.3 | 303 | 273, 229 | C16H16O6 | protosappanin B | 287, 255 | |
| 29 | 9.3 | 285 | 163, 135, 121 | C16H14O5 | brazilin | - |
| Sample | T05 (°C) | T10 (°C) | T20 (°C) | T50 (°C) | Char Residue 600 °C (%) |
|---|---|---|---|---|---|
| BW | 130 | 217 | 265 | 424 | 35 |
| PB | 142 | 202 | 241 | 334 | 30 |
| W | 118 | 139 | 190 | 316 | 29 |
| BW/Al | 597 | - | - | - | 95 |
| PB/Al | 239 | - | - | - | 93 |
| W/Al | 174 | - | - | - | 93 |
| W/Sn | 196 | - | - | - | 93 |
| Sample | Image | L | a* | b* |
|---|---|---|---|---|
| EN |  | 86.52 | 0.58 | 5.06 |
| EN/BW/Al |  | 47.52 | 23.87 | 9.57 |
| EN/PB/Al |  | 65.72 | 3.41 | 53.16 |
| EN/W/Al |  | 72.27 | −6.31 | 40.96 |
| EN/W/Sn |  | 74.85 | −7.31 | 38.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szadkowski, B.; Kuśmierek, M.; Śliwka-Kaszyńska, M.; Marzec, A. Structure and Stability Characterization of Natural Lake Pigments Made from Plant Extracts and Their Potential Application in Polymer Composites for Packaging Materials. Materials 2022, 15, 4608. https://doi.org/10.3390/ma15134608
Szadkowski B, Kuśmierek M, Śliwka-Kaszyńska M, Marzec A. Structure and Stability Characterization of Natural Lake Pigments Made from Plant Extracts and Their Potential Application in Polymer Composites for Packaging Materials. Materials. 2022; 15(13):4608. https://doi.org/10.3390/ma15134608
Chicago/Turabian StyleSzadkowski, Bolesław, Małgorzata Kuśmierek, Magdalena Śliwka-Kaszyńska, and Anna Marzec. 2022. "Structure and Stability Characterization of Natural Lake Pigments Made from Plant Extracts and Their Potential Application in Polymer Composites for Packaging Materials" Materials 15, no. 13: 4608. https://doi.org/10.3390/ma15134608
APA StyleSzadkowski, B., Kuśmierek, M., Śliwka-Kaszyńska, M., & Marzec, A. (2022). Structure and Stability Characterization of Natural Lake Pigments Made from Plant Extracts and Their Potential Application in Polymer Composites for Packaging Materials. Materials, 15(13), 4608. https://doi.org/10.3390/ma15134608