Effect of Calcium Carbide Residue on Strength Development along with Mechanisms of Cement-Stabilized Dredged Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixture Design and Experimental Procedure
2.3. Testing Methods
2.3.1. Unconfined Compressive Strength Test
2.3.2. pH and Electrical Conductivity Tests
2.3.3. Microstructural Analyses
3. Results and Discussion
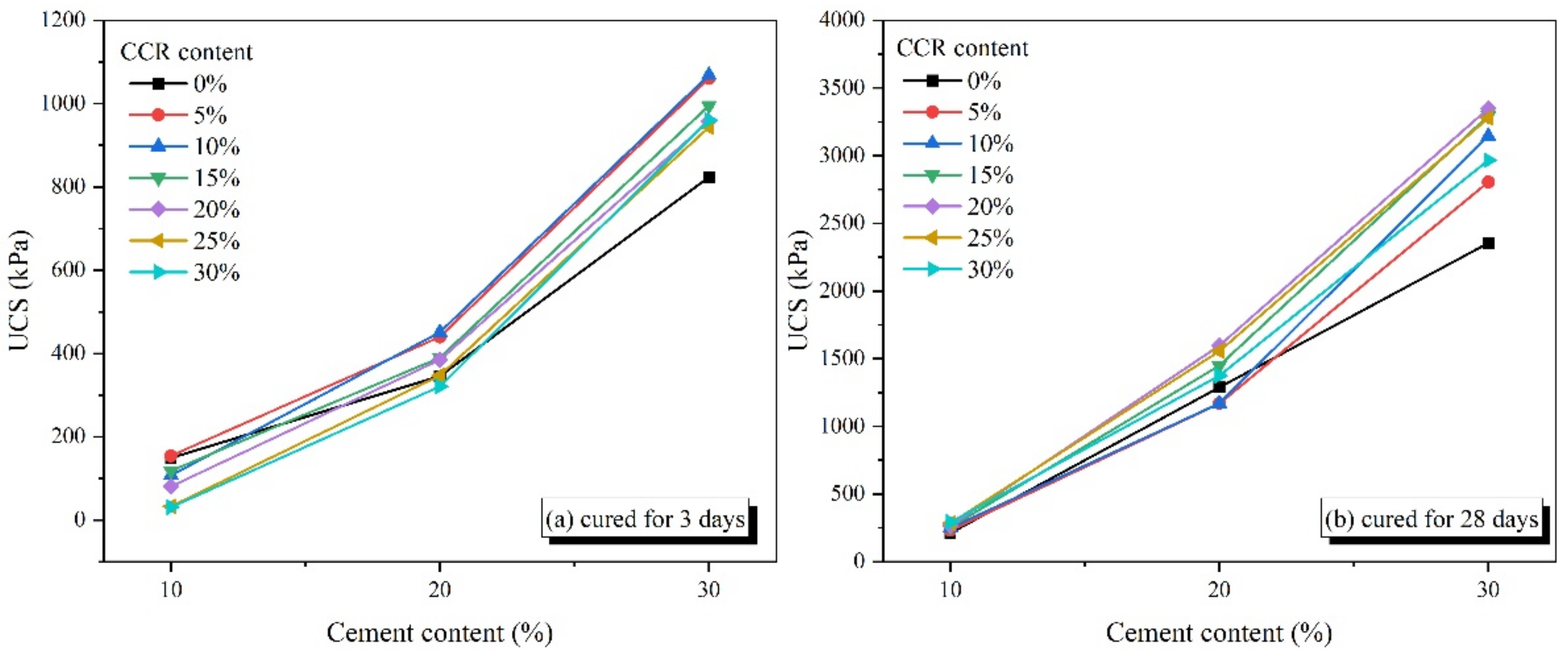
3.1. Unconfined Compressive Strength
3.1.1. Effect of Cement Content
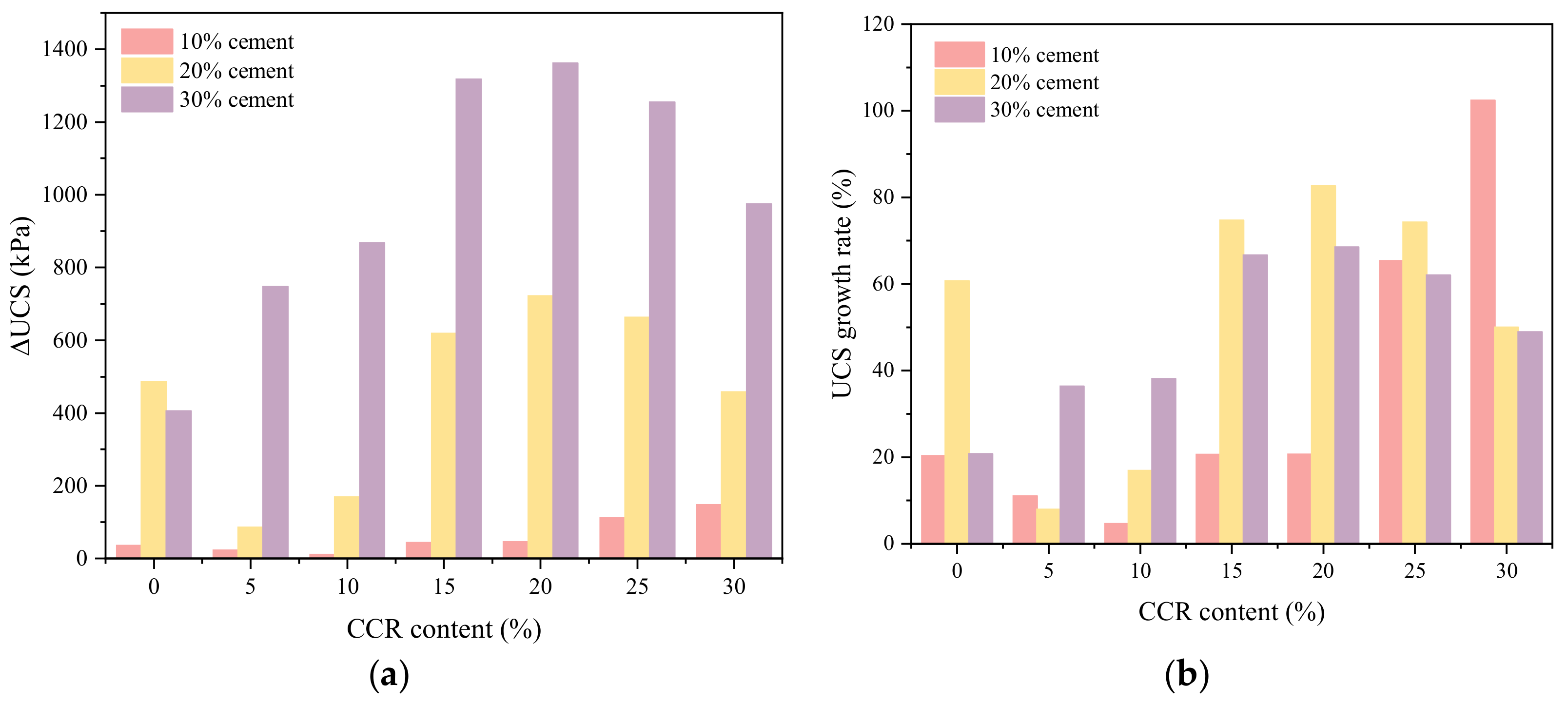
3.1.2. Effect of CCR Content
3.1.3. Effect of Curing Age
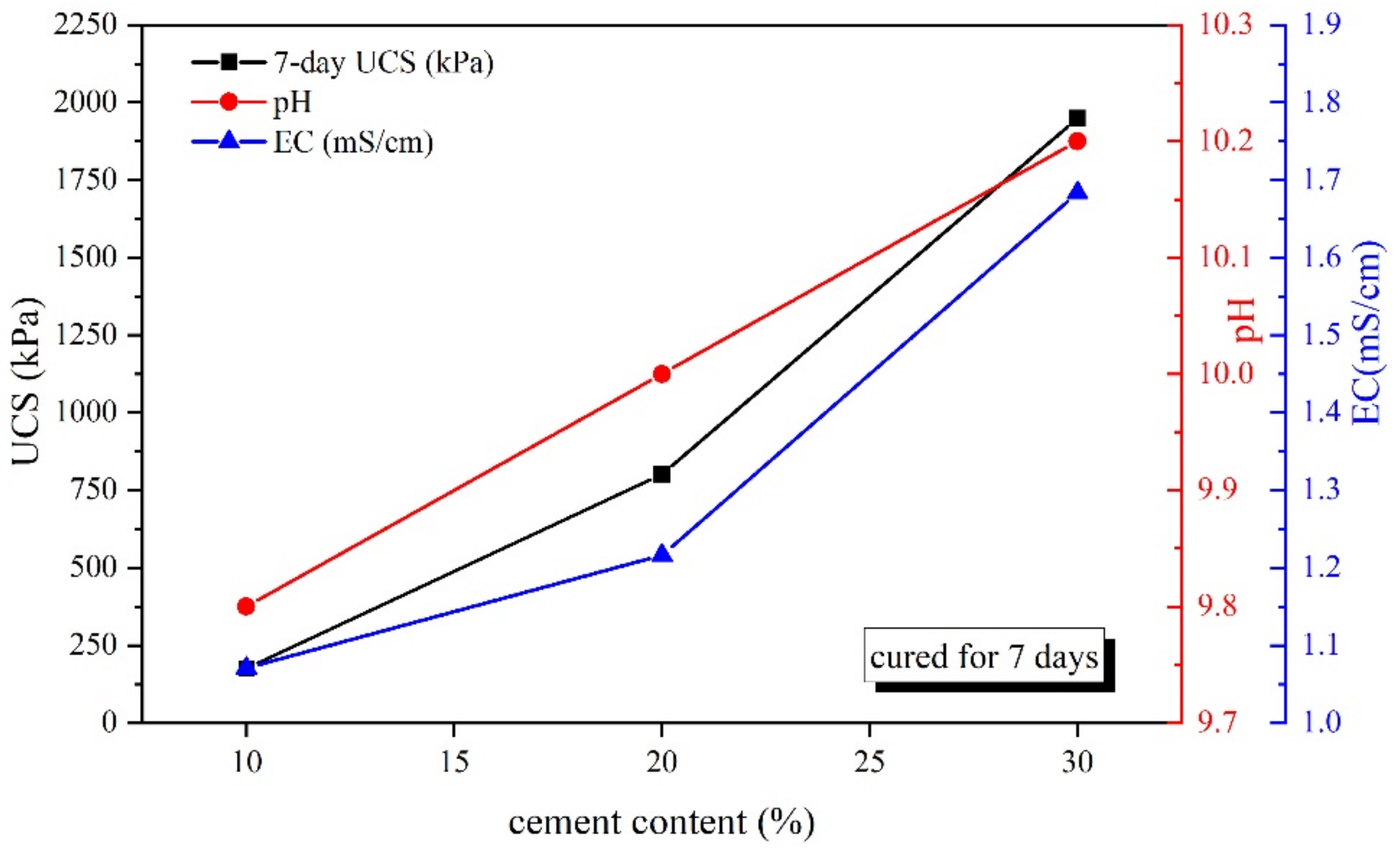
3.2. pH and Electric Conductivity
3.3. SEM Analysis
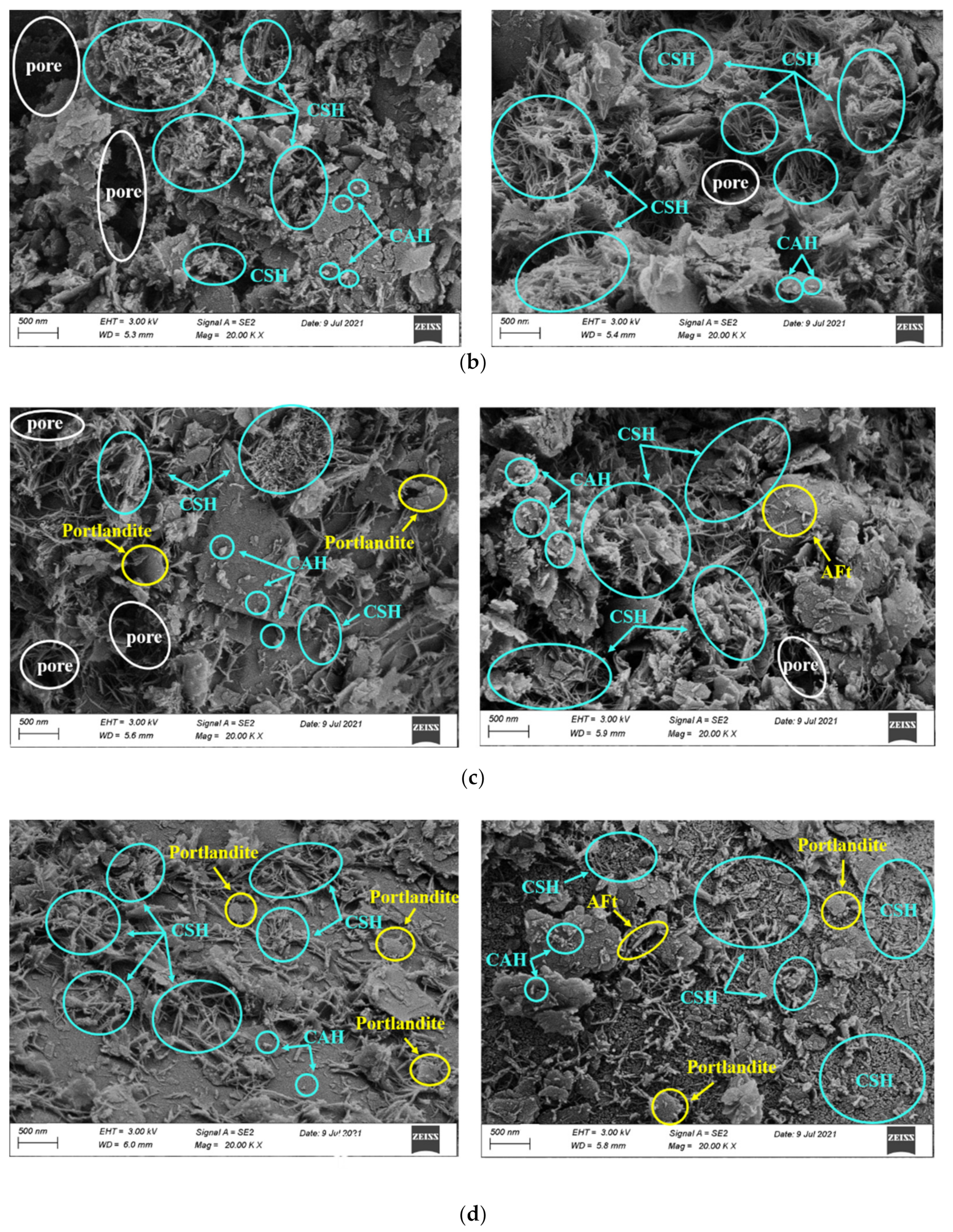
3.3.1. Effect of CCR Content
3.3.2. Effect of Curing Time
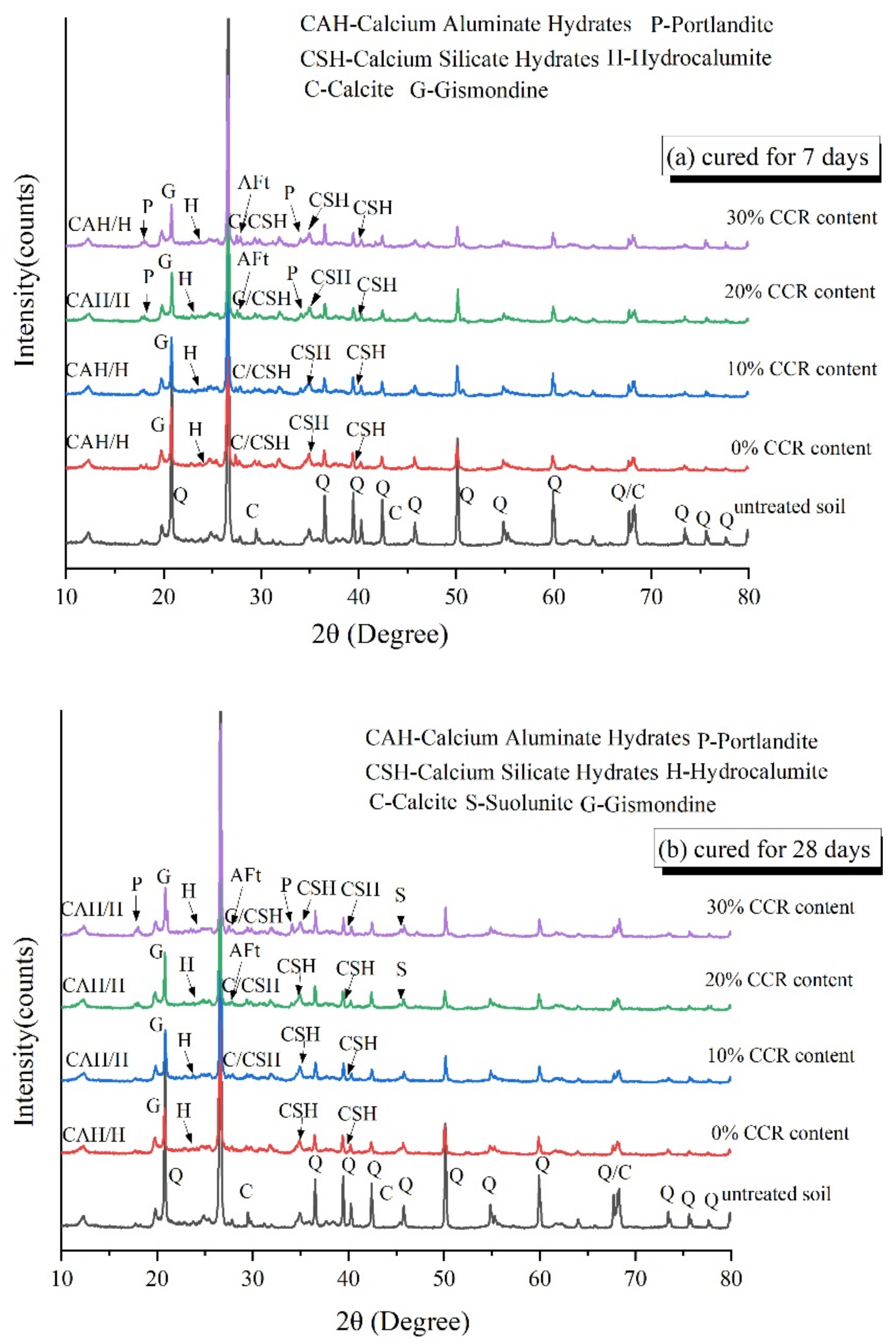
3.4. XRD Analysis
3.5. Carbon Footprint Evaluation
3.6. Solidification Mechanism
4. Conclusions
- CCR improved the UCS of CPDS significantly, and the maximum strength of CPDS was 1.42 times that of cemented dredged sludge cured for 28 days. However, the UCS then decreased as CCR content increased continuously, suggesting that excessive CCR may have a detrimental effect on the strength development of CPDS.
- On the basis of the strength difference (ΔUCS) and strength growth rate (UCSgr) of CPDS, it was recommended that the usage of 20% cement with 20% CCR to solidify dredged sludge is the most effective to develop the long-term strength.
- An alkaline environment promotes the development of CPDS strength, and the strength increased initially and then decreased with the growth of pH. Overall, the pH value corresponding to UCS of 9.9–10.5 may be suitable for evaluating the strength gain of CPDS.
- The SEM and XRD results indicated that the addition of CCR promoted the formation of hydration products and the formation of denser structure in CPDS. However, the excessive amount of CCR would lead to the precipitation of Ca(OH)2 and the change of CSH morphology, resulting in a risk of strength loss.
- The emission CO2-e of CPDS (20% cement content with the addition of 20% CCR) was 19.11% lower than that of cemented dredged sludge at UCS of 800 kPa, showing that the carbon footprint was significantly reduced with the addition of CCR. Under the optimal CCR admixture, CPDS can not only improve the mechanical properties but also reduce the cement consumption and CO2 emissions, thus achieving sustainable soil improvement from both engineering and environmental perspectives.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Zentar, R.; Abriak, N.E. Temperature-accelerated strength development in stabilized marine soils as road construction materials. J. Mater. Civ. Eng. 2016, 29, 04016281. [Google Scholar] [CrossRef]
- Song, F.; Gu, L.; Zhu, N.; Yuan, H. Leaching behavior of heavy metals from sewage sludge solidified by cement-based binders. Chemosphere 2013, 92, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Song, C.; Xue, L.; Chen, B. Effectiveness of waste steel slag powder on the strength development and associated micro-mechanisms of cement-stabilized dredged sludge. Constr. Build. Mater. 2020, 240, 117975. [Google Scholar] [CrossRef]
- Lang, L.; Liu, N.; Chen, B. Strength development of solidified dredged sludge containing humic acid with cement, lime and nano-sio2. Constr. Build. Mater. 2020, 230, 116971. [Google Scholar] [CrossRef]
- Wang, D.; Korkiala-Tanttu, L. 1-d compressibility behaviour of cement-lime stabilized soft clays. Eur. J. Environ. Civ. Eng. 2020, 24, 1013–1031. [Google Scholar] [CrossRef]
- Chiu, C.F.; Zhu, W.; Zhang, C.L. Yielding and shear behaviour of cement-treated dredged materials. Eng. Geol. 2009, 103, 1–12. [Google Scholar] [CrossRef]
- Han, J.; Liu, D.; Guan, Y.; Chen, Y.; Li, T.; Jia, D.; Yan, F.; Jia, P.; Zhao, Y. Study on shear behavior and damage constitutive model of tendon-grout interface. Constr. Build. Mater. 2022, 320, 126223. [Google Scholar] [CrossRef]
- Cheng, Q.; Xiao, H.; Liu, Y.; Wang, W.; Jia, L. Primary yielding locus of cement-stabilized marine clay and its applications. Mar. Georesources Geotechnol. 2019, 37, 488–505. [Google Scholar] [CrossRef]
- Kang, G.O.; Tsuchida, T.; Kim, Y.S. Strength and stiffness of cement-treated marine dredged clay at various curing stages. Constr. Build. Mater. 2017, 132, 71–84. [Google Scholar] [CrossRef]
- Yao, K.; Chen, Q.; Ho, J.; Xiao, H.; Lee, F.H. Strain-dependent shear stiffness of cement-treated marine clay. J. Mater. Civ. Eng. 2018, 30, 04018255. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.Q.; Jiang, Y.J.; Sun, M.M.; Chen, E.J.; Lee, F.H. Effect of in situ water content variation on the spatial variation of strength of deep cement-mixed clay. Géotechnique 2019, 69, 391–405. [Google Scholar] [CrossRef] [Green Version]
- Giannakopoulou, P.P.; Rogkala, A.; Lampropoulou, P.; Kalpogiannaki, M.; Petrounias, P. Evaluation of cement performance using industrial byproducts such as nano mgo and fly ash from greece. Appl. Sci. 2021, 11, 11601. [Google Scholar] [CrossRef]
- Tzevelekou, T.; Lampropoulou, P.; Giannakopoulou, P.; Rogkala, A.; Koutsovitis, P.; Koukouzas, N.; Petrounias, P. Valorization of slags produced by smelting of metallurgical dusts and lateritic ore fines in manufacturing of slag cements. Appl. Sci. 2020, 10, 4670. [Google Scholar] [CrossRef]
- Yi, Y.; Liska, M.; Unluer, C.; Al-Tabbaa, A. Carbonating magnesia for soil stabilization. Can. Geotech. J. 2013, 50, 899–905. [Google Scholar] [CrossRef]
- Gu, K.; Jin, F.; Al-Tabbaa, A.; Shi, B.; Liu, C.; Gao, L. Incorporation of reactive magnesia and quicklime in sustainable binders for soil stabilisation. Eng. Geol. 2015, 195, 53–62. [Google Scholar] [CrossRef]
- Li, W.; Lang, L.; Lin, Z.; Wang, Z.; Zhang, F. Characteristics of dry shrinkage and temperature shrinkage of cement-stabilized steel slag. Constr. Build. Mater. 2017, 134, 540–548. [Google Scholar] [CrossRef]
- Li, W.; Lang, L.; Wang, D.; Wu, Y.; Li, F. Investigation on the dynamic shear modulus and damping ratio of steel slag sand mixtures. Constr. Build. Mater. 2018, 162, 170–180. [Google Scholar] [CrossRef]
- Mohamadi, M.; Choobbasti, A.J. Stabilization of sandy soil using microfine cement and nanosilica grout. Arab. J. Geosci. 2021, 14, 1617. [Google Scholar] [CrossRef]
- Puppala, A.; Hoyos, L.; Potturi, A. Resilient moduli response of moderately cement-treated reclaimed asphalt pavement aggregates. J. Mater. Civ. Eng. 2011, 23, 990–998. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, G.; Liu, S. Reclaimed lignin-stabilized silty soil: Undrained shear strength, atterberg limits, and microstructure characteristics. J. Mater. Civ. Eng. 2018, 30, 04018277. [Google Scholar] [CrossRef]
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and carbon emissions. Mater. Struct. 2014, 47, 1055–1065. [Google Scholar] [CrossRef]
- Davis Steven, J.; Lewis Nathan, S.; Shaner, M.; Aggarwal, S.; Arent, D.; Azevedo Inês, L.; Benson Sally, M.; Bradley, T.; Brouwer, J.; Chiang, Y.-M.; et al. Net-zero emissions energy systems. Science 2018, 360, eaas9793. [Google Scholar] [CrossRef] [Green Version]
- Horpibulsuk, S.; Rachan, R.; Suddeepong, A. Assessment of strength development in blended cement admixed bangkok clay. Constr. Build. Mater. 2011, 25, 1521–1531. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Arulrajah, A.; Disfani, M.M.; Horpibulsuk, S.; Bo, M.W.; Darmawan, S. Effects of industrial by-product based geopolymers on the strength development of a soft soil. Soils Found. 2018, 58, 716–728. [Google Scholar] [CrossRef]
- Cristelo, N.; Fernández-Jiménez, A.; Castro, F.; Fernandes, L.; Tavares, P. Sustainable alkaline activation of fly ash, aluminium anodising sludge and glass powder blends with a recycled alkaline cleaning solution. Constr. Build. Mater. 2019, 204, 609–620. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R.; Liu, C.; Liu, H.; Lu, C. Co2 capture by carbide slag from chlor-alkali plant in calcination/carbonation cycles. Int. J. Greenh. Gas Control 2012, 9, 117–123. [Google Scholar] [CrossRef]
- Krammart, P.; Tangtermsirikul, S. Properties of cement made by partially replacing cement raw materials with municipal solid waste ashes and calcium carbide waste. Constr. Build. Mater. 2004, 18, 579–583. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, H.; Yu, T.; Yuan, P.; Deng, L.; Zhang, B. Utilization of calcium carbide residue as solid alkali for preparing fly ash-based geopolymers: Dependence of compressive strength and microstructure on calcium carbide residue, water content and curing temperature. Materials 2022, 15, 973. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cao, J.; Wang, Z.; Chen, H.; Gong, X. Discovery of impurities existing state in carbide slag by chemical dissociation. Int. J. Miner. Process. 2014, 130, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.; Park, S.; Yoon, H.N.; Jang, J.G.; Kim, S.H.; Lee, H.K. Utilization of calcium carbide residue using granulated blast furnace slag. Materials 2019, 12, 3511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suksiripattanapong, C.; Phetchuay, C.; Suebsuk, J.; Phoo-ngernkham, T.; Arulrajah, A. Water treatment sludge–calcium carbide residue geopolymers as nonbearing masonry units. J. Mater. Civ. Eng. 2017, 29, 04017095. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Munsrakest, V.; Udomchai, A.; Chinkulkijniwat, A.; Arulrajah, A. Strength of sustainable non-bearing masonry units manufactured from calcium carbide residue and fly ash. Constr. Build. Mater. 2014, 71, 210–215. [Google Scholar] [CrossRef]
- Latifi, N.; Vahedifard, F.; Ghazanfari, E.; Safuan, A.R.A. Sustainable usage of calcium carbide residue for stabilization of clays. J. Mater. Civ. Eng. 2018, 30, 04018099. [Google Scholar] [CrossRef]
- Yi, Y.; Gu, L.; Liu, S.; Puppala, A. Carbide slag-activated ground granulated blastfurnace slag for soft clay stabilization. CaGeJ 2014, 52, 150113073645001. [Google Scholar] [CrossRef]
- He, J.; Shi, X.K.; Li, Z.X.; Zhang, L.; Feng, X.Y.; Zhou, L.R. Strength properties of dredged soil at high water content treated with soda residue, carbide slag, and ground granulated blast furnace slag. Constr. Build. Mater. 2020, 242, 118126. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Unconfined Compressive Strength Index of Chemical-Grouted Soils; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM. Standard Test Method for pH of Soils; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Xiao, Z.-Y.; Xu, W. Assessment of strength development in cemented coastal silt admixed granite powder. Constr. Build. Mater. 2019, 206, 470–482. [Google Scholar] [CrossRef]
- Phetchuay, C.; Horpibulsuk, S.; Suksiripattanapong, C.; Chinkulkijniwat, A.; Arulrajah, A.; Disfani, M.M. Calcium carbide residue: Alkaline activator for clay–fly ash geopolymer. Constr. Build. Mater. 2014, 69, 285–294. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S.P. Effect of synthesis parameters on compressive strength of fly ash-slag blended geopolymer. Constr. Build. Mater. 2018, 170, 225–234. [Google Scholar] [CrossRef]
- Huo, W.; Zhu, Z.; Chen, W.; Zhang, J.; Kang, Z.; Pu, S.; Wan, Y. Effect of synthesis parameters on the development of unconfined compressive strength of recycled waste concrete powder-based geopolymers. Constr. Build. Mater. 2021, 292, 123264. [Google Scholar] [CrossRef]
- Ahmari, S.; Ren, X.; Toufigh, V.; Zhang, L. Production of geopolymeric binder from blended waste concrete powder and fly ash. Constr. Build. Mater. 2012, 35, 718–729. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Mechanical and microstructural properties of heat cured alkali-activated slag mortars. Mater. Des. 2012, 35, 374–383. [Google Scholar] [CrossRef]
- Taylor, H.F. Cement Chemistry; Thomas Telford: London, UK, 1997; Volume 2. [Google Scholar]
- Ramachandran, V.S.; Paroli, R.M.; Beaudoin, J.J.; Delgado, A.H. Handbook of Thermal Analysis of Construction Materials; William Andrew: Norwich, NY, USA, 2002. [Google Scholar]
- Sun, J.; Zhang, Z.; Zhuang, S.; He, W. Hydration properties and microstructure characteristics of alkali–activated steel slag. Constr. Build. Mater. 2020, 241, 118141. [Google Scholar] [CrossRef]
- Chew, S.H.; Kamruzzaman, A.H.M.; Lee, F.H. Physicochemical and engineering behavior of cement treated clays. J. Geotech. Geoenviron. Eng. 2004, 130, 696–706. [Google Scholar] [CrossRef]
- Cong, P.; Mei, L. Using silica fume for improvement of fly ash/slag based geopolymer activated with calcium carbide residue and gypsum. Constr. Build. Mater. 2021, 275, 122171. [Google Scholar] [CrossRef]
- Duan, X.; Xia, J.; Yang, J. Influence of coal gangue fine aggregate on microstructure of cement mortar and its action mechanism. Jianzhu Cailiao Xuebao J. Build. Mater. 2014, 17, 700–705. [Google Scholar] [CrossRef]
- Ma, C.; Qin, Z.; Zhuang, Y.; Chen, L.; Chen, B. Influence of sodium silicate and promoters on unconfined compressive strength of portland cement-stabilized clay. Soils Found. 2015, 55, 1222–1232. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Wang, X.Q.; Su, Y.; Li, Z.X.; Shi, X.K. Shear strength of stabilized clay treated with soda residue and ground granulated blast furnace slag. J. Mater. Civ. Eng. 2019, 31, 06018029. [Google Scholar] [CrossRef]
- Nidzam, R.M.; Kinuthia, J.M. Sustainable soil stabilisation with blastfurnace slag—A review. Proc. Inst. Civ. Eng. -Constr. Mater. 2010, 163, 157–165. [Google Scholar] [CrossRef]
- Phair, J.W.; Van Deventer, J.S.J.; Smith, J.D. Mechanism of polysialation in the incorporation of zirconia into fly ash-based geopolymers. Ind. Eng. Chem. Res. 2000, 39, 2925–2934. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, N.; Mou, G.; Liao, L. Crystal structure refinement of suolunite and its significance to the cement techniques. Chin. Sci. Bull. 1999, 44, 2125–2130. [Google Scholar] [CrossRef]
- Phetchuay, C.; Horpibulsuk, S.; Arulrajah, A.; Suksiripattanapong, C.; Udomchai, A. Strength development in soft marine clay stabilized by fly ash and calcium carbide residue based geopolymer. Appl. Clay Sci. 2016, 127–128, 134–142. [Google Scholar] [CrossRef]
- Lang, L.; Chen, B.; Li, N. Utilization of lime/carbide slag-activated ground granulated blast-furnace slag for dredged sludge stabilization. Mar. Georesour. Geotechnol. 2021, 39, 659–669. [Google Scholar] [CrossRef]
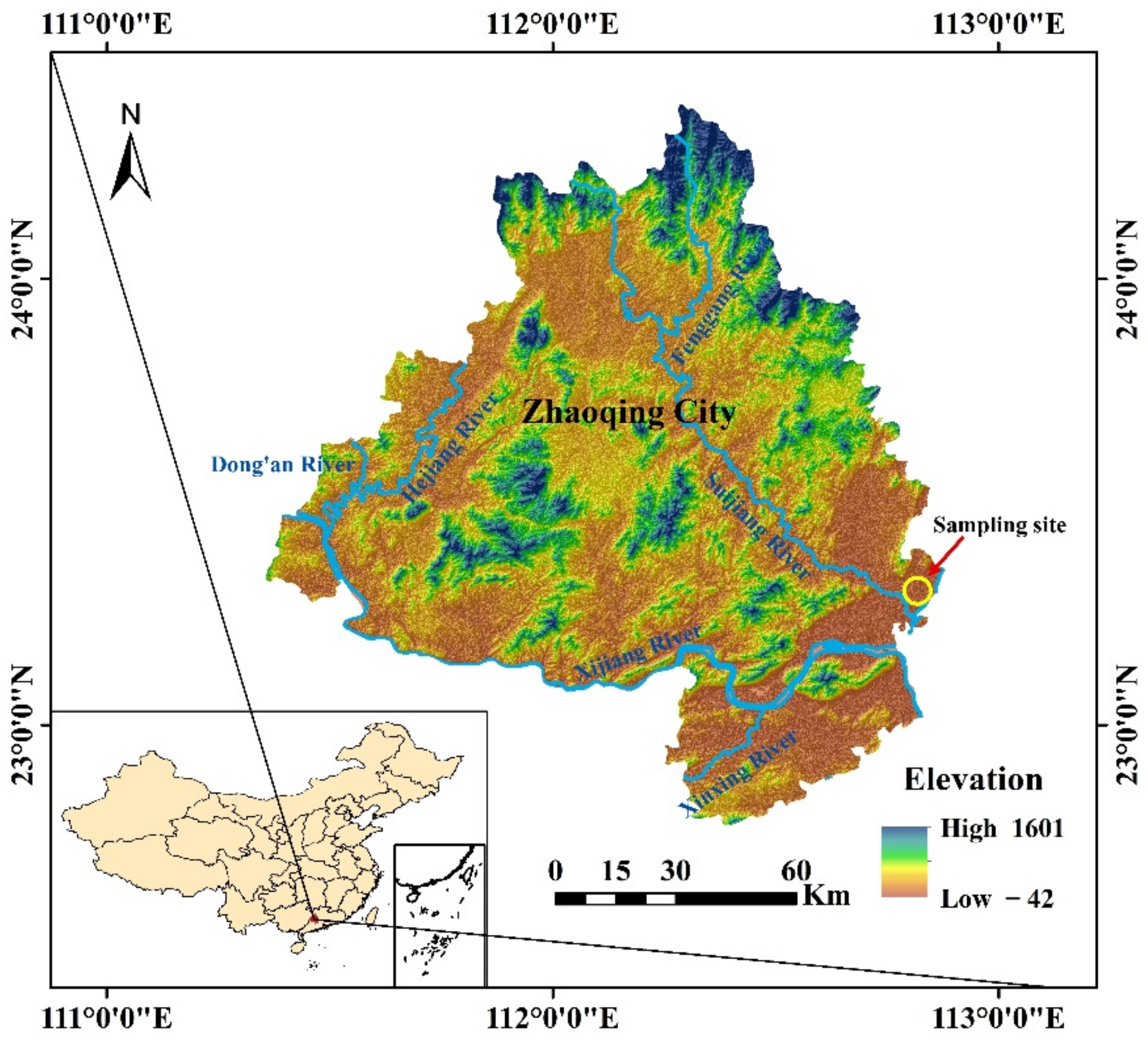
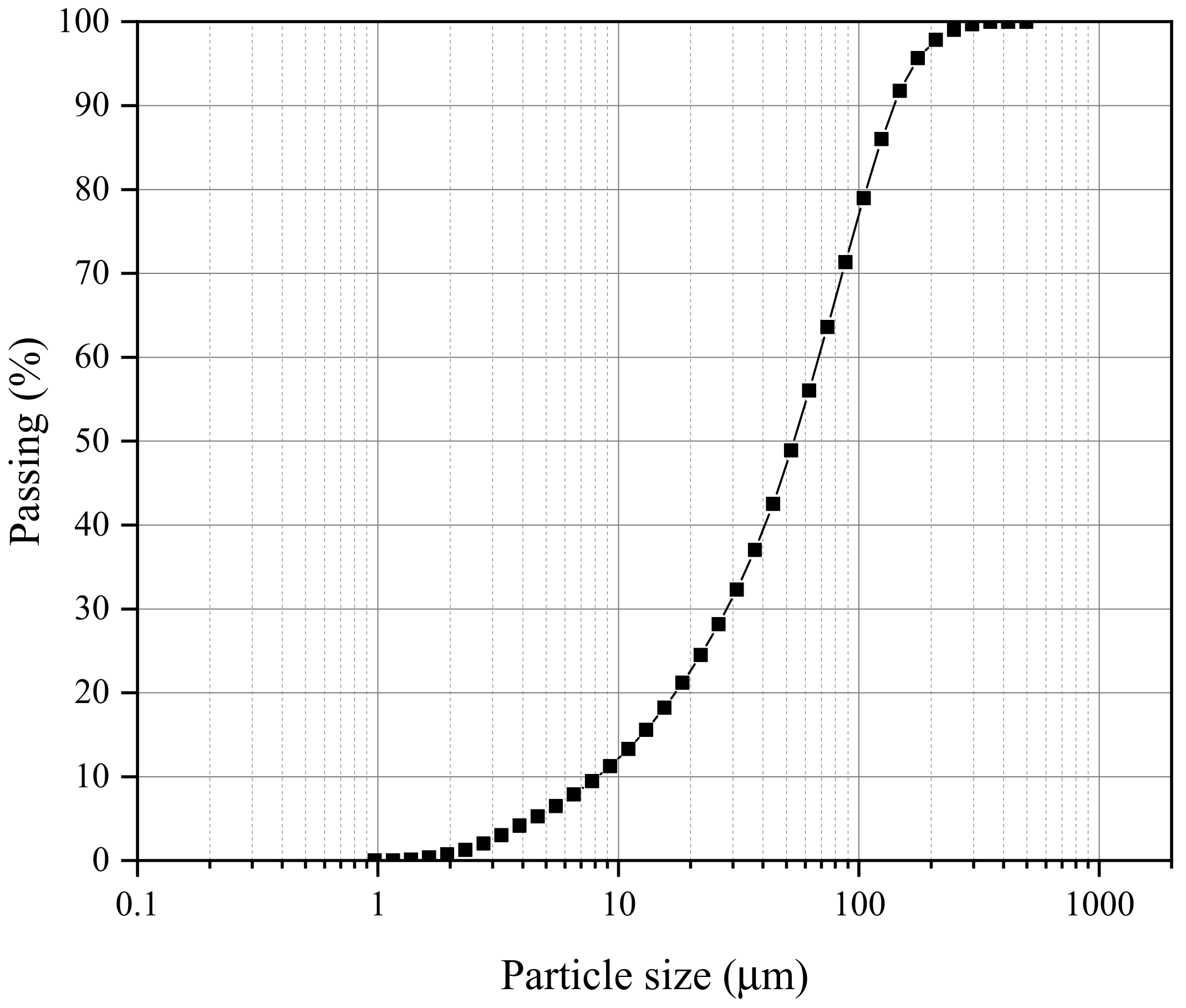


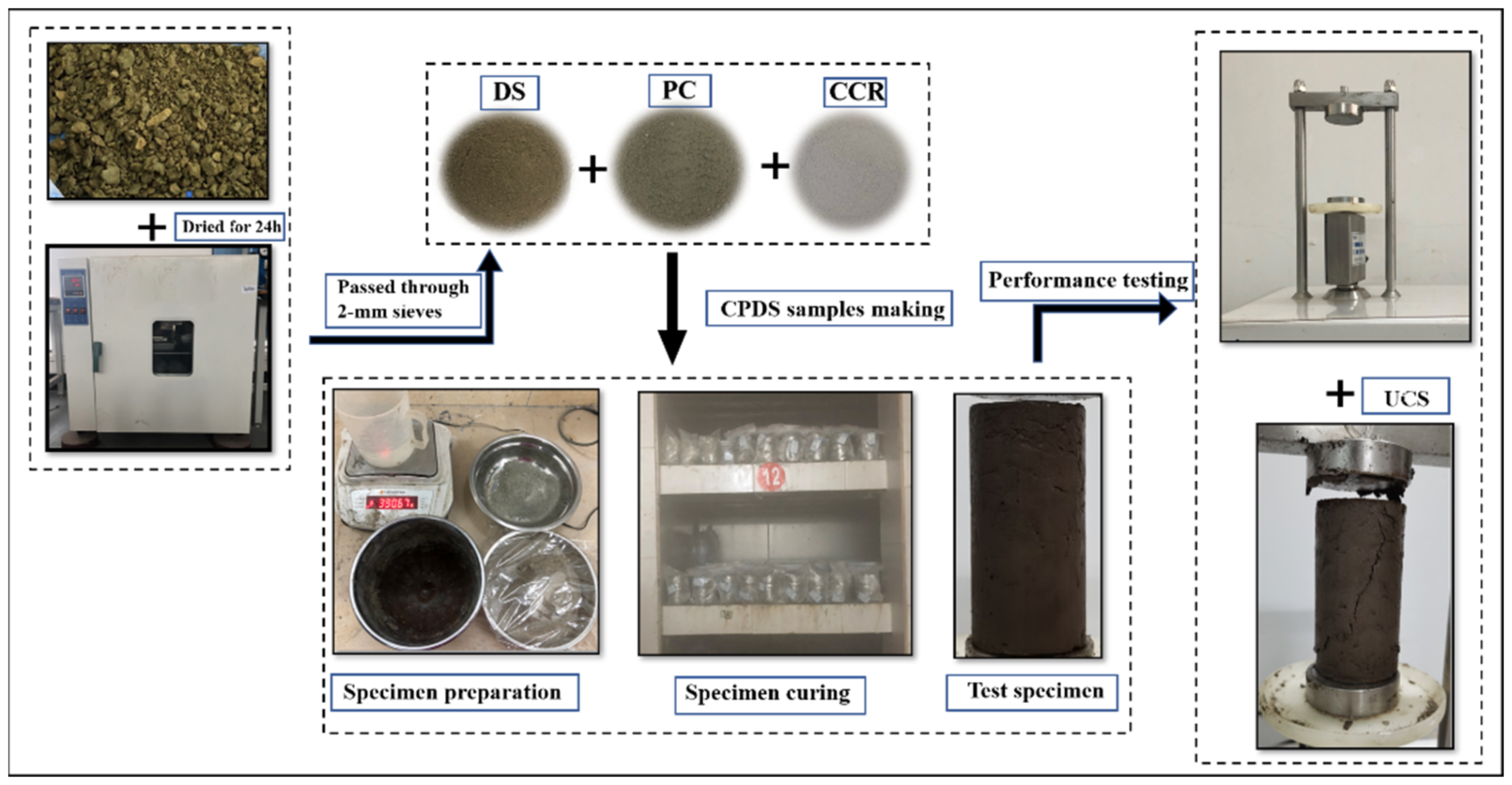








| Properties | Values |
|---|---|
| Initial water content, % | 60.0 |
| Liquid limit, % | 50.0 |
| Plastic limit, % | 27.6 |
| Plasticity index, % | 22.4 |
| Dry density, g/cm3 | 1.01 |
| pH | 7.20 |
| Electrical conductivity (EC), mS/cm | 0.0391 |
| Oxide (%) | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | Na2O | K2O | Others |
|---|---|---|---|---|---|---|---|---|---|
| Dredged sludge | 0.602 | 57.276 | 27.417 | 8.555 | 1.609 | 0.329 | 0.360 | 2.387 | 1.464 |
| CCR | 94.297 | 2.654 | 0.836 | 0.262 | 0.159 | 0.446 | 1.045 | - | 0.301 |
| Portland cement | 59.450 | 20.940 | 6.661 | 2.718 | 4.225 | 4.208 | 0.189 | 0.924 | 0.686 |
| Sample | PC Content | CCR Content | Sample | PC Content | CCR Content | Sample | PC Content | CCR Content |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | |||
| P10C0 | 10 | 0 | P20C0 | 20 | 0 | P30C0 | 30 | 0 |
| P10C5 | 10 | 5 | P20C5 | 20 | 5 | P30C5 | 30 | 5 |
| P10C10 | 10 | 10 | P20C10 | 20 | 10 | P30C10 | 30 | 10 |
| P10C15 | 10 | 15 | P20C15 | 20 | 15 | P30C15 | 30 | 15 |
| P10C20 | 10 | 20 | P20C20 | 20 | 20 | P30C20 | 30 | 20 |
| P10C25 | 10 | 25 | P20C25 | 20 | 25 | P30C25 | 30 | 25 |
| P10C30 | 10 | 30 | P20C30 | 20 | 30 | P30C30 | 30 | 30 |
| Sample | SIR | Sample | SIR | Sample | SIR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 Days | 7 Days | 28 Days | 3 Days | 7 Days | 28 Days | 3 Days | 7 Days | 28 Days | |||
| P10C0 | 1.00 | 1.00 | 1.00 | P20C0 | 1.00 | 1.00 | 1.00 | P30C0 | 1.00 | 1.00 | 1.00 |
| P10C5 | 1.03 | 1.18 | 1.09 | P20C5 | 1.27 | 1.35 | 0.91 | P30C5 | 1.29 | 1.06 | 1.19 |
| P10C10 | 0.72 | 1.36 | 1.18 | P20C10 | 1.30 | 1.25 | 0.91 | P30C10 | 1.30 | 1.17 | 1.34 |
| P10C15 | 0.79 | 1.21 | 1.21 | P20C15 | 1.13 | 1.03 | 1.12 | P30C15 | 1.21 | 1.01 | 1.40 |
| P10C20 | 0.54 | 1.26 | 1.26 | P20C20 | 1.11 | 1.09 | 1.24 | P30C20 | 1.16 | 1.02 | 1.42 |
| P10C25 | 0.22 | 0.98 | 1.34 | P20C25 | 1.00 | 1.11 | 1.21 | P30C25 | 1.15 | 1.04 | 1.39 |
| P10C30 | 0.21 | 0.82 | 1.38 | P20C30 | 0.93 | 1.14 | 1.07 | P30C30 | 1.17 | 1.02 | 1.26 |
| Emission | CPDS | Cemented Dredged Sludge | |||
|---|---|---|---|---|---|
| P20C15 | P20C20 | P20C25 | P20C30 | P20C0 | |
| Cement (kg CO2-e/ton) | 95.09 | 86.23 | 88.44 | 100.11 | 106.78 |
| CCR (kg CO2-e/ton) | 0.12 | 0.14 | 0.18 | 0.24 | / |
| Total (kg CO2-e/ton) | 95.20 | 86.37 | 88.62 | 100.35 | 106.78 |
| Reduction (%) | 10.84 | 19.11 | 17.01 | 6.02 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Niu, F.; Ren, L.; Jiao, C.; Jiang, H.; Yao, X. Effect of Calcium Carbide Residue on Strength Development along with Mechanisms of Cement-Stabilized Dredged Sludge. Materials 2022, 15, 4453. https://doi.org/10.3390/ma15134453
Zhu X, Niu F, Ren L, Jiao C, Jiang H, Yao X. Effect of Calcium Carbide Residue on Strength Development along with Mechanisms of Cement-Stabilized Dredged Sludge. Materials. 2022; 15(13):4453. https://doi.org/10.3390/ma15134453
Chicago/Turabian StyleZhu, Xinyi, Fujun Niu, Lu Ren, Chenglong Jiao, Haiqiang Jiang, and Xiaoyue Yao. 2022. "Effect of Calcium Carbide Residue on Strength Development along with Mechanisms of Cement-Stabilized Dredged Sludge" Materials 15, no. 13: 4453. https://doi.org/10.3390/ma15134453
APA StyleZhu, X., Niu, F., Ren, L., Jiao, C., Jiang, H., & Yao, X. (2022). Effect of Calcium Carbide Residue on Strength Development along with Mechanisms of Cement-Stabilized Dredged Sludge. Materials, 15(13), 4453. https://doi.org/10.3390/ma15134453






