Nanoswimmers Based on Capped Janus Nanospheres
Abstract
:1. Introduction
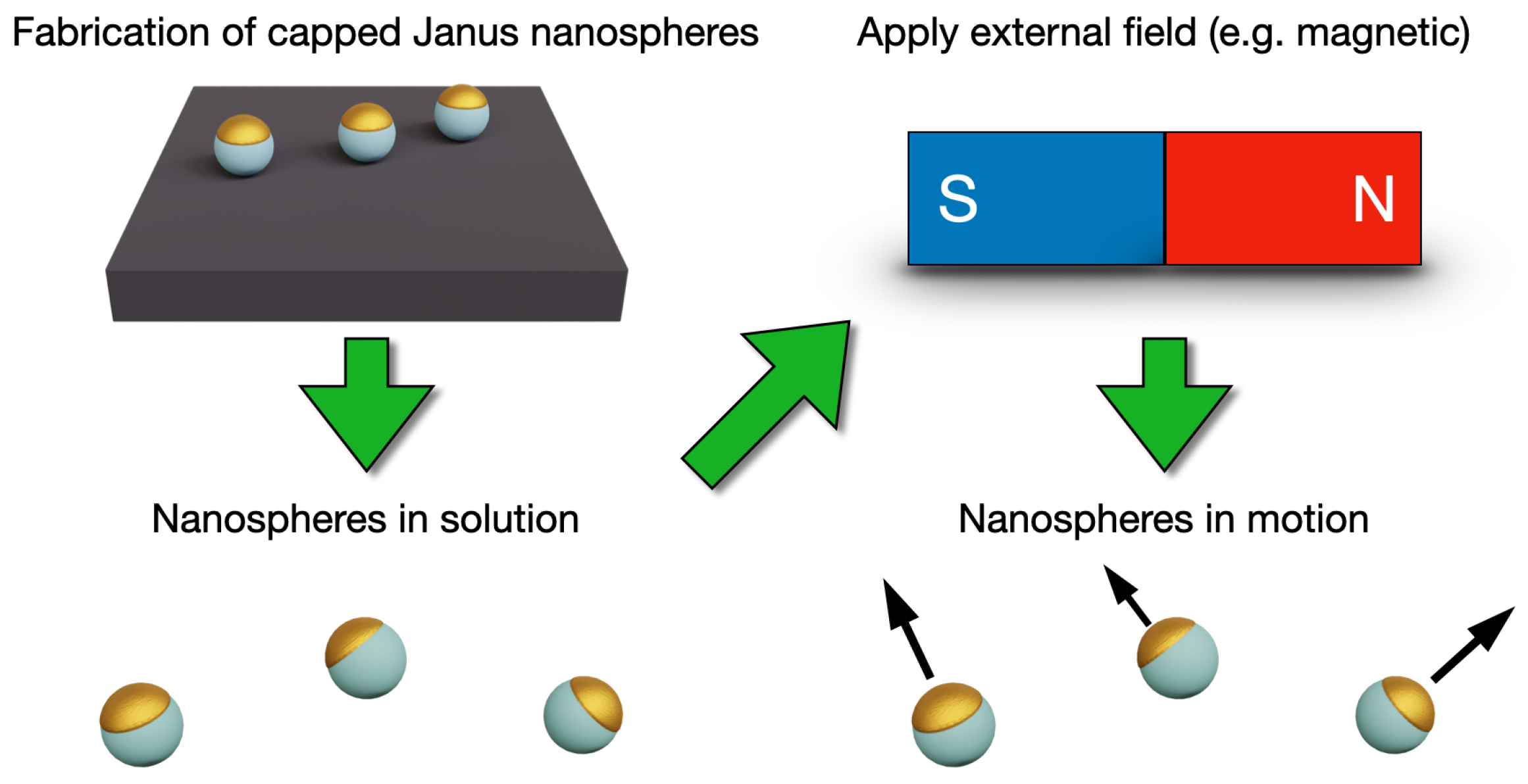
2. Nanoswimmers
3. Classification and Fabrication of Capped Janus Particles
3.1. Capped Janus Nanoparticles
3.2. Synthesis of Capped Janus Nanospheres
4. Capped Janus Particles as Nanoswimmers
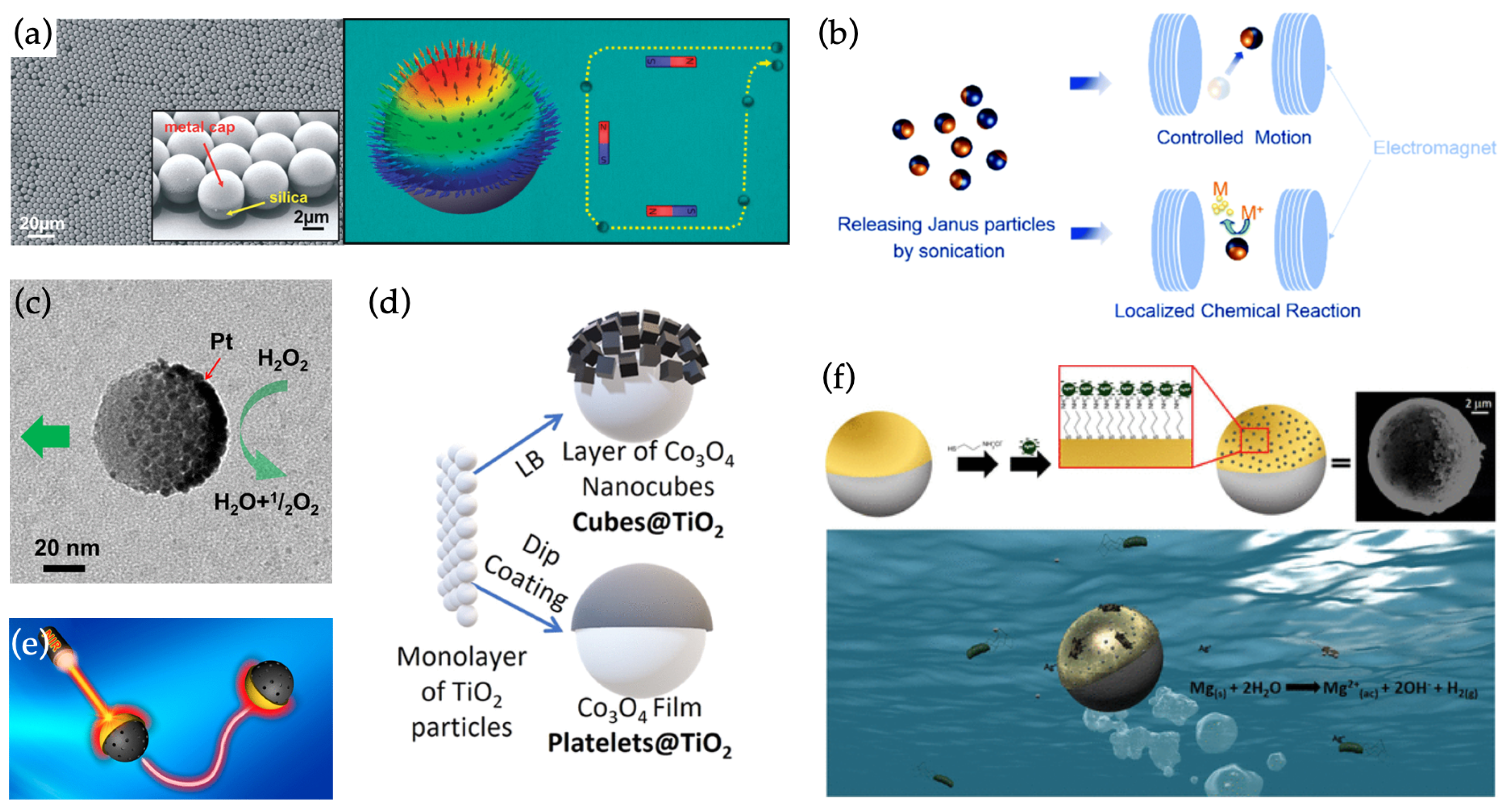
4.1. Non-Hybrid Swimmers Based on Capped Janus Nanospheres
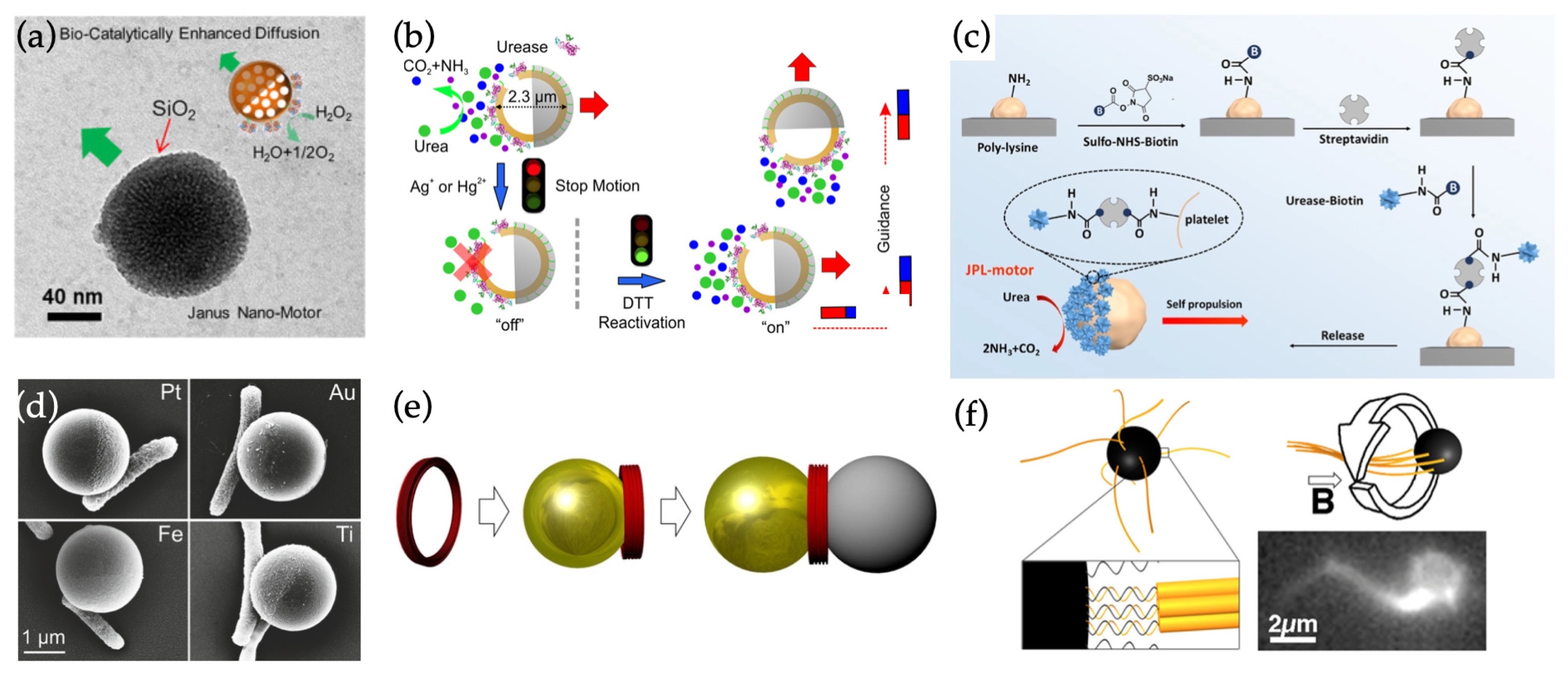
4.2. Hybrid Swimmers
4.2.1. Hybrid Swimmers Based on Capped Janus Nanospheres
4.2.2. Hybrid Swimmers Based on Nanospheres and DNA Nanostructures
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lattuada, M.; Hatton, T.A. Synthesis, properties and applications of Janus nanoparticles. Nano Today 2011, 6, 286–308. [Google Scholar] [CrossRef]
- Naito, M.; Yokoyama, T.; Hosokawa, K.; Nogi, K. Nanoparticle Technology Handbook; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2018. [Google Scholar]
- Linko, V.; Zhang, H.; Nonappa; Kostiainen, M.A.; Ikkala, O. From precision colloidal hybrid materials to advanced functional assemblies. Acc. Chem. Res. 2022, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Golestanian, R.; Liverpool, T.B.; Ajdari, A. Designing phoretic micro- and nano-swimmers. New J. Phys. 2007, 9, 126. [Google Scholar] [CrossRef]
- Sánchez, S.; Soler, L.; Katuri, J. Chemically powered micro- and nanomotors. Angew. Chem. Int. Ed. 2015, 54, 1414–1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Duan, W.; Ahmed, S.; Sen, A.; Mallouk, T.E. From one to many: Dynamic assembly and collective behavior of self-propelled colloidal motors. Acc. Chem. Res. 2015, 48, 1938–1946. [Google Scholar] [CrossRef]
- Gao, W.; Wang, J. Synthetic micro/nanomotors in drug delivery. Nanoscale 2014, 6, 10486–10494. [Google Scholar] [CrossRef] [Green Version]
- Wang, J. Nanomachines: Fundamentals and Applications; John Wiley & Sons Inc.: Weinheim, Germany, 2013. [Google Scholar]
- Peng, F.; Tu, Y.; Wilson, D.A. Micro/nanomotors towards in vivo application: Cell, tissue and biofluid. Chem. Soc. Rev. 2017, 46, 5289–5310. [Google Scholar] [CrossRef]
- Purcell, E.M. Life at low Reynolds number. Am. J. Phys. 1977, 45, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Duan, W.; Ahmed, S.; Mallouk, T.E.; Sen, A. Small power: Autonomous nano- and micromotors propelled by self-generated gradients. Nano Today 2013, 8, 531–554. [Google Scholar] [CrossRef]
- Einstein, A. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann. Phys. 1905, 322, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Katuri, J.; Ma, X.; Stanton, M.M.; Sánchez, S. Designing micro- and nanoswimmers for specific applications. Acc. Chem. Res. 2017, 50, 2–11. [Google Scholar] [CrossRef]
- Wang, W.; Mallouk, T.E. A practical guide to analyzing and reporting the movement of nanoscale swimmers. ACS Nano 2021, 15, 15446–15460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, Q.; Duan, H.; Song, J.; Yang, H. Janus nanoparticles: From fabrication to (bio)applications. ACS Nano 2021, 15, 6147–6191. [Google Scholar] [CrossRef]
- Su, H.; Hurd Price, C.A.; Jing, L.; Tian, Q.; Liu, J.; Qian, K. Janus particles: Design, preparation, and biomedical applications. Mater. Today Bio 2019, 4, 100033. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Marschelke, C.; Synytska, A. Hybrid Janus particles: Challenges and opportunities for the design of active functional interfaces and surfaces. ACS Appl. Mater. Interfaces 2019, 11, 9643–9671. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Frueh, J.; Hu, N.; Liu, L.; Gai, M.; He, Q. Guidable thermophoretic janus micromotors containing gold nanocolorifiers for infrared laser assisted tissue welding. Adv. Sci. 2016, 3, 1600206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmar, J.; Vilela, D.; Villa, K.; Wang, J.; Sánchez, S. Micro- and nanomotors as active environmental microcleaners and sensors. J. Am. Chem. Soc. 2018, 140, 9317–9331. [Google Scholar] [CrossRef]
- Seeman, N.C.; Sleiman, H.F. DNA nanotechnology. Nat. Rev. Mater. 2018, 3, 17068. [Google Scholar] [CrossRef]
- Nummelin, S.; Kommeri, J.; Kostiainen, M.A.; Linko, V. Evolution of structural DNA nanotechnology. Adv. Mater. 2018, 30, 1703721. [Google Scholar] [CrossRef] [Green Version]
- Safdar, M.; Khan, S.U.; Jänis, J. Progress toward catalytic micro- and nanomotors for biomedical and environmental applications. Adv. Mater. 2018, 30, 1703660. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.R.; Yoshinaga, N.; Sano, M. Active motion of a Janus particle by self-thermophoresis in a defocused laser beam. Phys. Rev. Lett. 2010, 105, 268302. [Google Scholar] [CrossRef] [PubMed]
- Baraban, L.; Streubel, R.; Makarov, D.; Han, L.; Karnaushenko, D.; Schmidt, O.G.; Cuniberti, G. Fuel-free locomotion of Janus motors: Magnetically induced thermophoresis. ACS Nano 2013, 7, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mou, F.; Gong, H.; Luo, M.; Guan, J. Light-driven micro/nanomotors: From fundamentals to applications. Chem. Soc. Rev. 2017, 46, 6905–6926. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Z.; Hoop, M.; Mushtaq, F.; Siringil, E.; Hu, C.; Nelson, B.J.; Pané, S. Recent developments in magnetically driven micro- and nanorobots. Appl. Mater. Today 2017, 9, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Paxton, W.F.; Kistler, K.C.; Olmeda, C.C.; Sen, A.; St. Angelo, S.K.; Cao, Y.; Mallouk, T.E.; Lammert, P.E.; Crespi, V.H. Catalytic nanomotors: Autonomous movement of striped nanorods. J. Am. Chem. Soc. 2004, 126, 13424–13431. [Google Scholar] [CrossRef]
- Sánchez, S.; Solovev, A.A.; Mei, Y.; Schmidt, O.G. Dynamics of biocatalytic microengines mediated by variable friction control. J. Am. Chem. Soc. 2010, 132, 13144–13145. [Google Scholar] [CrossRef]
- Luo, M.; Li, S.; Wan, J.; Yang, C.; Chen, B.; Guan, J. Enhanced propulsion of urease-powered micromotors by multilayered assembly of ureases on Janus magnetic microparticles. Langmuir 2020. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, G.; Pumera, M. Crucial role of surfactants in bubble-propelled microengines. J. Phys. Chem. C 2014, 118, 5268–5274. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Lin, X.; Si, T.; He, Q. Gold-nanoshell-functionalized polymer nanoswimmer for photomechanical poration of single-cell membrane. J. Am. Chem. Soc. 2019, 141, 6601–6608. [Google Scholar] [CrossRef]
- Ahmed, D.; Baasch, T.; Jang, B.; Pane, S.; Dual, J.; Nelson, B.J. Artificial swimmers propelled by acoustically activated flagella. Nano Lett. 2016, 16, 4968–4974. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huang, F.; Ren, C.; Liu, J.; Yang, L.; Chen, S.; Chang, J.; Yang, C.; Wang, W.; Zhang, C.; et al. Enhanced radiosensitization by gold nanoparticles with acid-triggered aggregation in cancer radiotherapy. Adv. Sci. 2019, 6, 1801806. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, S.; Lee, J.H.; Jeong, Y.Y.; Jon, S. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo X-ray computed tomography imaging. J. Am. Chem. Soc. 2007, 129, 7661–7665. [Google Scholar] [CrossRef] [PubMed]
- Seferos, D.S.; Giljohann, D.A.; Hill, H.D.; Prigodich, A.E.; Mirkin, C.A. Nano-flares: Probes for transfection and mRNA detection in living cells. J. Am. Chem. Soc. 2007, 129, 15477–15479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Cao, G. Nanostructures and Nanomaterials: Synthesis, Properties and Applications; World Scientific Publishing Company: Singapore, 2004. [Google Scholar]
- Loget, G.; Kuhn, A. Bulk synthesis of Janus objects and asymmetric patchy particles. J. Mater. Chem. 2012, 22, 15457–15474. [Google Scholar] [CrossRef]
- Wibberley, L. The Mouse on the Moon; William Morrow & Co.: New York, NY, USA, 1962. [Google Scholar]
- Zhang, L.; Chen, Y.; Li, Z.; Li, L.; Saint-Cricq, P.; Li, C.; Lin, J.; Wang, C.; Su, Z.; Zink, J.I. Tailored synthesis of octopus-type Janus nanoparticles for synergistic actively-targeted and chemo-photothermal therapy. Angew. Chem. Int. Ed. 2016, 55, 2118–2121. [Google Scholar] [CrossRef]
- Feng, L.; Dreyfus, R.; Sha, R.; Seeman, N.C.; Chaikin, P.M. DNA patchy particles. Adv. Mater. 2013, 25, 2779–2783. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Pacheco, M.; Maria-Hormigos, R.; Escarpa, A. Perspectives on Janus micromotors: Materials and applications. Appl. Mater. Today 2017, 9, 407–418. [Google Scholar] [CrossRef]
- Shao, J.; Xuan, M.; Zhang, H.; Lin, X.; Wu, Z.; He, Q. Chemotaxis-guided hybrid neutrophil micromotors for targeted drug transport. Angew. Chem. Int. Ed. 2017, 56, 12935–12939. [Google Scholar] [CrossRef]
- Berger, S.; Synytska, A.; Ionov, L.; Eichhorn, K.J.; Stamm, M. Stimuli-responsive bicomponent polymer janus particles by “grafting from”/“grafting to” approaches. Macromolecules 2008, 41, 9669–9676. [Google Scholar] [CrossRef]
- Vilela, D.; Stanton, M.M.; Parmar, J.; Sánchez, S. Microbots Decorated with Silver Nanoparticles Kill Bacteria in Aqueous Media. ACS Appl. Mater. Interfaces 2017, 9, 22093–22100. [Google Scholar] [CrossRef]
- Perro, A.; Meunier, F.; Schmitt, V.; Ravaine, S. Production of large quantities of “Janus” nanoparticles using wax-in-water emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 57–62. [Google Scholar] [CrossRef]
- Deng, R.; Liang, F.; Qu, X.; Wang, Q.; Zhu, J.; Yang, Z. Diblock copolymer based Janus nanoparticles. Macromolecules 2015, 48, 750–755. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, F.; Gong, H.; Wei, F.; Zhuang, J.; Karshalev, E.; de Ávila, B.E.F.; Huang, C.; Zhou, Z.; Li, Z.; et al. Enzyme-powered Janus platelet cell robots for active and targeted drug delivery. Sci. Robot. 2020, 5, eaba6137. [Google Scholar] [CrossRef]
- Baraban, L.; Makarov, D.; Streubel, R.; Mönch, I.; Grimm, D.; Sánchez, S.; Schmidt, O.G. Catalytic Janus motors on microfluidic chip: Deterministic motion for targeted cargo deliver. ACS Nano 2012, 6, 3383–3389. [Google Scholar] [CrossRef]
- Chen, X.Z.; Shamsudhin, N.; Hoop, M.; Pieters, R.; Siringil, E.; Sakar, M.S.; Nelson, B.J.; Pané, S. Magnetoelectric micromachines with wirelessly controlled navigation and functionality. Mater. Horiz. 2016, 3, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Wittmann, M.; Ali, A.; Gemming, T.; Stavale, F.; Simmchen, J. Semiconductor-based microswimmers: Attention to detail matters. J. Phys. Chem. Lett. 2021, 12, 9651–9656. [Google Scholar] [CrossRef]
- Xuan, M.; Wu, Z.; Shao, J.; Dai, L.; Si, T.; He, Q. Near infrared light-powered Janus mesoporous silica nanoparticle motors. J. Am. Chem. Soc. 2016, 138, 6492–6497. [Google Scholar] [CrossRef]
- Ma, X.; Hahn, K.; Sánchez, S. Catalytic mesoporous Janus nanomotors for active cargo delivery. J. Am. Chem. Soc. 2015, 137, 4976–4979. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, Q.; Gao, W.; Pei, A.; Ren, B. Highly Efficient Light-Driven TiO2–Au Janus Micromotors. ACS Nano 2016, 10, 839–844. [Google Scholar] [CrossRef] [Green Version]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Wang, L.; Popescu, M.N.; Stavale, F.; Ali, A.; Gemming, T.; Simmchen, J. Cu@TiO2 Janus microswimmers with a versatile motion mechanism. Soft Matter 2018, 14, 6969–6973. [Google Scholar] [CrossRef]
- Wang, L.; Kaeppler, A.; Fischer, D.; Simmchen, J. Photocatalytic TiO2 micromotors for removal of microplastics and suspended matter. ACS Appl. Mater. Interfaces 2019, 11, 32937–32944. [Google Scholar] [CrossRef] [PubMed]
- Harraq, A.A.; Choudhury, B.D.; Bharti, B. Field-induced assembly and propulsion of colloids. Langmuir 2022, 38, 3001–3016. [Google Scholar] [CrossRef]
- Madden, I.P.; Wang, L.; Simmchen, J.; Luijten, E. Hydrodynamically controlled self-organization in mixtures of active and passive colloids. Small 2022, 18, 2107023. [Google Scholar] [CrossRef] [PubMed]
- Maric, T.; Nasir, M.Z.M.; Webster, R.D.; Pumera, M. Tailoring metal/TiO2 interface to influence motion of light-activated Janus micromotors. Adv. Funct. Mater. 2020, 30, 1908614. [Google Scholar] [CrossRef]
- Vutukuri, H.R.; Lisicki, M.; Lauga, E.; Vermant, J. Light-switchable propulsion of active particles with reversible interactions. Nat. Commun. 2020, 11, 2628. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, H.; Tang, J.; Wang, W. Photochemically powered AgCl Janus micromotors as a model system to understand ionic self-diffusiophoresis. Langmuir 2018, 34, 3289–3295. [Google Scholar] [CrossRef] [PubMed]
- Bastos-Arrieta, J.; Bauer, C.; Eychmüller, A.; Simmchen, J. Galvanic replacement induced electromotive force to propel Janus micromotors. J. Chem. Phys. 2019, 150, 144902. [Google Scholar] [CrossRef]
- Feuerstein, L.; Biermann, C.G.; Xiao, Z.; Holm, C.; Simmchen, J. Highly efficient active colloids driven by galvanic exchange reactions. J. Am. Chem. Soc. 2021, 143, 17015–17022. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, W.; Zhou, C.; Liu, Q.; Gu, J.; Ye, H.; Li, M.; Wang, W.; Ma, X. Phoretic liquid metal micro/nanomotors as intelligent filler for targeted microwelding. Adv. Mater. 2019, 31, 1905067. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Gao, C.; Wang, D.; He, Q. Bubble-propelled Janus gallium/zinc micromotors for the active treatment of bacterial infections. Angew. Chem. Int. Ed. 2021, 60, 8750–8754. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Meng, S.; Zheng, T.; Liu, Y.; Ma, X.; Feng, H. Alkaline-driven liquid metal Janus micromotor with a coating material-dependent propulsion mechanism. ACS Appl. Mater. Interfaces 2021, 13, 35897–35904. [Google Scholar] [CrossRef] [PubMed]
- Martel, S. Bacterial microsystems and microrobots. Biomed. Microdevices 2012, 14, 1033–1045. [Google Scholar] [CrossRef]
- Xu, W.; Qin, H.; Tian, H.; Liu, L.; Gao, J.; Peng, F.; Tu, Y. Biohybrid micro/nanomotors for biomedical applications. Appl. Mater. Today 2022, 27, 101482. [Google Scholar] [CrossRef]
- Stanton, M.M.; Simmchen, J.; Ma, X.; Miguel-López, A.; Sánchez, S. Biohybrid Janus motors driven by Escherichia coli. Adv. Mater. Interfaces 2016, 3, 1500505. [Google Scholar] [CrossRef]
- Mestre, R.; Patiño, T.; Sánchez, S. Biohybrid robotics: From the nanoscale to the macroscale. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1703. [Google Scholar] [CrossRef]
- Ma, X.; Hortelão, A.C.; Patiño, T.; Sánchez, S. Enzyme catalysis to power micro/manomachines. ACS Nano 2016, 10, 9111–9122. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Gao, Y.; Xu, L.; Hong, W.; She, Y.; Yang, G. Enzyme-driven micro/nanomotors: Recent advances and biomedical applications. Int. J. Biol. Macromol. 2021, 167, 457–469. [Google Scholar] [CrossRef]
- Ma, X.; Sánchez, S. Bio-catalytic mesoporous Janus nano-motors powered by catalase enzyme. Tetrahedron 2017, 73, 4883–4886. [Google Scholar] [CrossRef]
- Ma, X.; Wang, X.; Hahn, K.; Sánchez, S. Motion control of urea-powered biocompatible hollow microcapsules. ACS Nano 2016, 10, 3597–3605. [Google Scholar] [CrossRef] [PubMed]
- Heerwig, A.; Schubel, M.; Schirmer, C.; Herms, A.; Cichos, F.; Mertig, M. DNA origami ring structures as construction element of self-thermophoretic swimmers. Phys. Stat. Solidi A 2019, 216, 1800775. [Google Scholar] [CrossRef]
- Maier, A.M.; Weig, C.; Oswald, P.; Frey, E.; Fischer, P.; Liedl, T. Magnetic propulsion of microswimmers with DNA-based flagellar bundles. Nano Lett. 2016, 16, 906–910. [Google Scholar] [CrossRef] [Green Version]
- Xuan, M.; Shao, J.; Gao, C.; Wang, W.; Dai, L.; He, Q. Self-propelled nanomotors for thermomechanically percolating cell membranes. Angew. Chem. Int. Ed. 2018, 57, 12463–12467. [Google Scholar] [CrossRef] [PubMed]
- Bastos-Arrieta, J.; Revilla-Guarinos, A.; Uspal, W.E.; Simmchen, J. Bacterial Biohybrid Microswimmers. Front. Robot. AI 2018, 5, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darnton, N.; Turner, L.; Breuer, K.; Berg, H.C. Moving fluid with bacterial carpets. Biophys. J. 2004, 86, 1863–1870. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, R.W.; Sitti, M. Bio-hybrid cell-based actuators for microsystems. Small 2014, 10, 3831–3851. [Google Scholar] [CrossRef]
- Park, S.J.; Park, S.H.; Cho, S.; Kim, D.M.; Lee, Y.; Ko, S.Y.; Hong, Y.; Choy, H.E.; Min, J.J.; Park, J.O.; et al. New paradigm for tumor theranostic methodology using bacteria-based microrobot. Sci. Rep. 2013, 3, 3394. [Google Scholar] [CrossRef] [Green Version]
- Behkam, B.; Sitti, M. Effect of quantity and configuration of attached bacteria on bacterial propulsion of microbeads. Appl. Phys. Lett. 2008, 93, 223901. [Google Scholar] [CrossRef]
- Pacheco, M.; Jurado-Sánchez, B.; Escarpa, A. Transition metal dichalcogenide-based Janus micromotors for on-the-fly Salmonella detection. Microchim. Acta 2022, 189, 194. [Google Scholar] [CrossRef]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, S.; Fan, C.; Gothelf, K.V.; Li, J.; Lin, C.; Liu, L.; Liu, N.; Nijenhuis, M.A.; Saccà, B.; Simmel, F.C.; et al. DNA origami. Nat. Rev. Methods Primers 2021, 1, 13. [Google Scholar] [CrossRef]
- Nummelin, S.; Shen, B.; Piskunen, P.; Liu, Q.; Kostiainen, M.A.; Linko, V. Robotic DNA nanostructures. ACS Synth. Biol. 2020, 9, 1923–1940. [Google Scholar] [CrossRef] [PubMed]
- Herms, A.; Günther, K.; Sperling, E.; Heerwig, A.; Kick, A.; Cichos, F.; Mertig, M. Concept, synthesis, and structural characterization of DNA origami based self-thermophoretic nanoswimmers. Phys. Stat. Solidi A 2017, 214, 1600957. [Google Scholar] [CrossRef]
- Pauer, C.; Venczel, A.; Dass, M.; Liedl, T.; Tavacoli, J. Propulsion of magnetic beads asymmetrically covered with DNA origami appendages. Adv. Mater. Technol. 2022, 7, 2200450. [Google Scholar] [CrossRef]
- Fu, J.; An, D.; Song, Y.; Wang, C.; Qiu, M.; Zhang, H. Janus nanoparticles for cellular delivery chemotherapy: Recent advances and challenges. Coord. Chem. Rev. 2020, 422, 213467. [Google Scholar] [CrossRef]
- Xu, Y.; Bian, Q.; Wang, R.; Gao, J. Micro/nanorobots for precise drug delivery via targeted transport and triggered release: A review. Int. J. Pharm. 2022, 616, 121551. [Google Scholar] [CrossRef]
- Szymański, J.; Patkowski, A.; Wilk, A.; Garstecki, P.; Holyst, R. Diffusion and viscosity in a crowded environment: From nano- to macroscale. J. Phys. Chem. B 2006, 110, 25593–25597. [Google Scholar] [CrossRef]
- Kalwarczyk, T.; Ziebacz, N.; Bielejewska, A.; Zaboklicka, E.; Koynov, K.; Szymanski, J.; Wilk, A.; Patkowski, A.; Gapinski, J.; Butt, H.J.; et al. Comparative analysis of viscosity of complex liquids and cytoplasm of mammalian cells at the nanoscale. Nano Lett. 2011, 11, 2157–2163. [Google Scholar] [CrossRef]
- Ohnuma, A.; Cho, E.C.; Camargo, P.H.C.; Au, L.; Ohtani, B.; Xia, Y. A facile synthesis of asymmetric hybrid colloidal particles. J. Am. Chem. Soc. 2009, 131, 1352–1353. [Google Scholar] [CrossRef] [Green Version]
- Lane, L.A.; Qian, X.; Smith, A.M.; Nie, S. Physical chemistry of nanomedicine: Understanding the complex behaviors of nanoparticles in vivo. Annu. Rev. Phys. Chem. 2015, 66, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Liu, H.; Chen, Y.; Mao, C. DNA nanotubes as combinatorial vehicles for cellular delivery. Biomacromolecules 2008, 9, 3039–3043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Fan, C.; Pei, H.; Shi, J.; Huang, Q. Smart drug delivery nanocarriers with self-assembled DNA nanostructures. Adv. Mater. 2013, 25, 4386–4396. [Google Scholar] [CrossRef] [PubMed]
- Gossai, N.P.; Naumann, J.A.; Li, N.S.; Zamora, E.A.; Gordon, D.J.; Piccirilli, J.A.; Gordon, P.M. Drug conjugated nanoparticles activated by cancer cell specific mRNA. Oncotarget 2016, 7, 38243–38256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, Q.; Wei, X.; Su, F.; Liu, Y.; Youngbull, C.; Johnson, R.; Lindsay, S.; Yan, H.; Meldrum, D. Stability of DNA origami nanoarrays in cell lysate. Nano Lett. 2011, 11, 1477–1482. [Google Scholar] [CrossRef] [Green Version]
- Seferos, D.S.; Giljohann, D.A.; Rosi, N.L.; Mirkin, C.A. Locked nucleic acid–nanoparticle conjugates. ChemBioChem 2007, 8, 1230–1232. [Google Scholar] [CrossRef]
- Keller, A.; Linko, V. Challenges and perspectives of DNA nanostructures in biomedicine. Angew. Chem. Int. Ed. 2020, 59, 15818–15833. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA nanotechnology-enabled drug delivery systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef]
- Ijäs, H.; Shen, B.; Heuer-Jungemann, A.; Keller, A.; Kostiainen, M.A.; Liedl, T.; Ihalainen, J.A.; Linko, V. Unraveling the interaction between doxorubicin and DNA origami nanostructures for customizable chemotherapeutic drug release. Nucleic Acids Res. 2021, 49, 3048–3062. [Google Scholar] [CrossRef]
- Piskunen, P.; Latham, R.; West, C.E.; Castronovo, M.; Linko, V. Integrating CRISPR/Cas systems with programmable DNA nanostructures for delivery and beyond. iScience 2022, 25, 104389. [Google Scholar] [CrossRef] [PubMed]

| Swimmer Type | Composition | Mechanism | Application |
|---|---|---|---|
| Non-Hybrid Swimmers | |||
| Multilayer magnetic/catalytic swimmer [49] | Co/Pt multilayer on SiO | HO catalysis and magnetical steering | Studying swimming behavior |
| Magnetoresponsive swimmer [50] | CoFeO and BaTiO on SiO | Charge generation via interaction of magnetostrictive and piezoelectric layers | Remote controlling/triggering electrochemical reactions |
| Catalytic swimmer [53] | Pt on mesoporous SiO | Pt catalyzed HO breakdown | Drug delivery |
| Fuel-free photocatalytic swimmer [54] | Au coated TiO | UV-light driven self-electrophoresis | Illumination controlled propulsion |
| Photochemically driven swimmer [56] | Cu on TiO | UV light or HO fuel depending on solution conditions | Studying swimming and interactions between passive and active colloids |
| Photochemically driven swimmers [60] | Various metals on TiO | UV light and HO fuel | Studying effects of coating material on swimmer propulsion |
| Two-directional photoresponsive swimmer [61] | Au-capped TiO | Switch in reaction site (Au to TiO) based on used light wavelength (UV to green) | Reversible propulsion direction |
| Nanocube and platelet-coated swimmers [51] | CoO nanocubes or platelets on TiO | UV-light-driven photocatalytic/self-electrophoretic propulsion | Studying effects of coating morphology on swimming |
| Photochemically driven polymer-cored swimmer [62] | AgCl on PMMA | UV-and-visible-light-driven decomposition of AgCl to Ag | Studying ionic self-diffusiophoresis |
| Photothermal swimmer [52] | Au on SiO | Light-induced thermal gradient between cap and uncoated half | Fuel-free light-controlled propulsion |
| Magnetothermal swimmer [24] | Permalloy on SiO | Asymmetric heating of particle with AC magnetic field, steering with DC magnetic field | Magnetic steering and propulsion |
| Multilayered antibacterial swimmer [45] | Ag on Au on Fe on Mg | Mg-based propulsion, magnetic guidance and collection, bacterial adhesion and Ag release | Killing and collecting bacteria in water |
| Photocatalytic magnetic swimmer [57] | Ni and Au on TiO | UV-driven propulsion, magnetic reclaiming | Herding, aggregating and collecting passive colloidal species in solution |
| Galvanic exchange swimmers [63,64] | Metal coating on SiO | Galvanic exchange of partial coating with more noble metal in solution induces an electromotive force | Capping synthesis and material exchange, switching of propulsion mechanism |
| Liquid metal alloy swimmers [65,66,67] | Capped liquid metal core | Self-diffusiophoresis (non-metallic) or self-electrophoresis (metal) depending on cap material | Propulsion in alkaline environments, biocompatible and bactericidal swimmers, microwelding |
| Hybrid Swimmers | |||
| Catalase swimmer [74] | SiO-capped catalase-modified mesoporous SiO | HO breakdown on catalase-coated side | Biocatalytic propulsion, drug delivery |
| Hollow-cored urease swimmer [75] | Urease-coated mesoporous SiO with SiO, Fe or Au capping | Propulsion via urea decomposition, magnetic steering (with Fe cap), cargo space within particle | Controlled enzymatic swimming, delivery of large-particle cargo |
| Cell-membrane-coated swimmer [78] | Au-capped SiO with macrophage cell membrane on exposed SiO | Thermophoretic propulsion, cloaking and cell-specific targeting due to cell membrane coating | Immunological cloaking, cancer cell targeting, assisted cell membrane penetration |
| Capped platelet cells [48] | Urease-capped platelet cells | Urea-fueled propulsion, retained cell functionality | Harnessing of cells as nanoswimmers |
| E. coli-based swimmer [70] | E. coli attached to metal-capped polystyrene | Random tumble motion of E. coli, magnetic steering (with Fe coatings) | Fuel-free random or directed propulsion, drug delivery |
| Biosensor swimmer [84] | MoS or WS inside polymer shell partially coated with metals and fluorescent affinity peptide | Catalytic propulsion, magnetic steering and collecting, peptide release and light emission upon encountering target endotoxin | On/off-type species-selective biosensor for bacteria detection |
| DNA-Based Swimmers | |||
| Thermophoretic DNA origami swimmers [76,88] | Custom DNA origami structures on Au particles | Propulsion via asymmetric heating during illumination | Platform for thermophoretic swimmers with adjustable behavior |
| Magnetic swimmers with DNA origami tails [77,89] | DNA origami flagella conjugated to Au-capped magnetic beads | Flagella-mediated propulsion during magnetic rotation of beads | Custom engineering of movement behavior |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piskunen, P.; Huusela, M.; Linko, V. Nanoswimmers Based on Capped Janus Nanospheres. Materials 2022, 15, 4442. https://doi.org/10.3390/ma15134442
Piskunen P, Huusela M, Linko V. Nanoswimmers Based on Capped Janus Nanospheres. Materials. 2022; 15(13):4442. https://doi.org/10.3390/ma15134442
Chicago/Turabian StylePiskunen, Petteri, Martina Huusela, and Veikko Linko. 2022. "Nanoswimmers Based on Capped Janus Nanospheres" Materials 15, no. 13: 4442. https://doi.org/10.3390/ma15134442
APA StylePiskunen, P., Huusela, M., & Linko, V. (2022). Nanoswimmers Based on Capped Janus Nanospheres. Materials, 15(13), 4442. https://doi.org/10.3390/ma15134442







