Superhydrophobic Modification of Biomass Cuttlebone Applied to Oil Spill Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
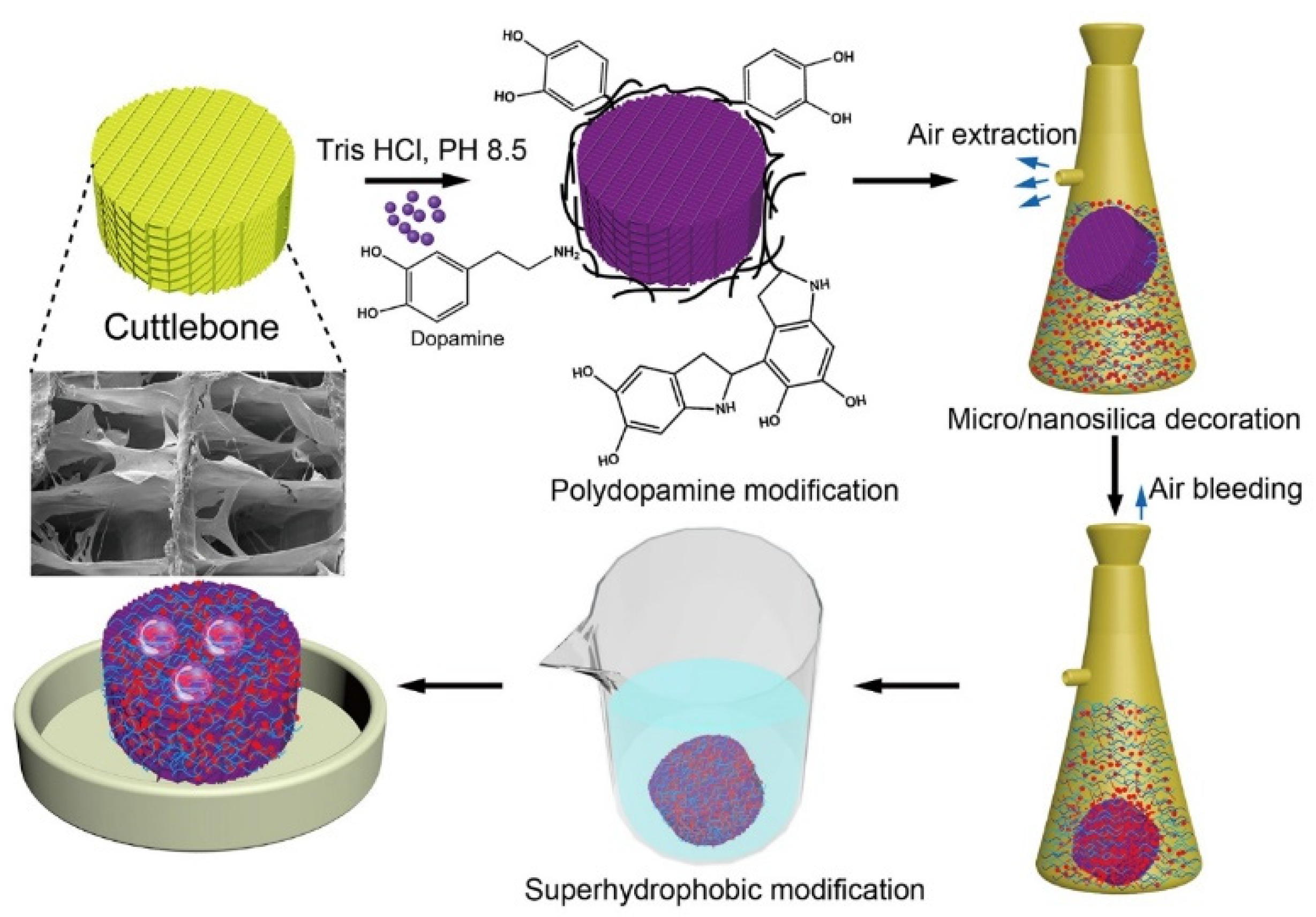
2.2. Preparation of Polydopamine-Modified Cuttlebone
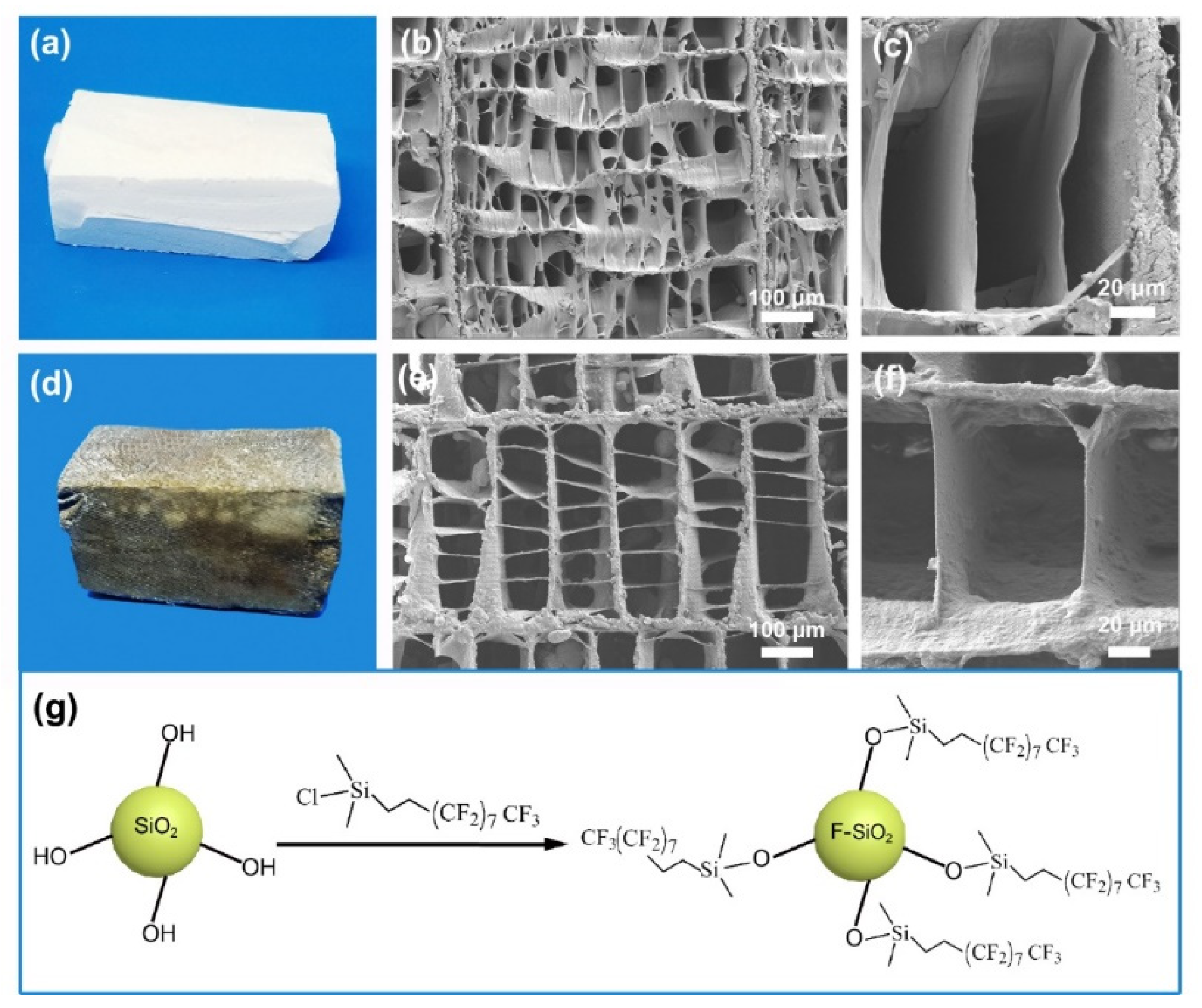
2.3. Preparation of Superhydrophobic and Oleophilic Cuttlebone
2.4. Characterization
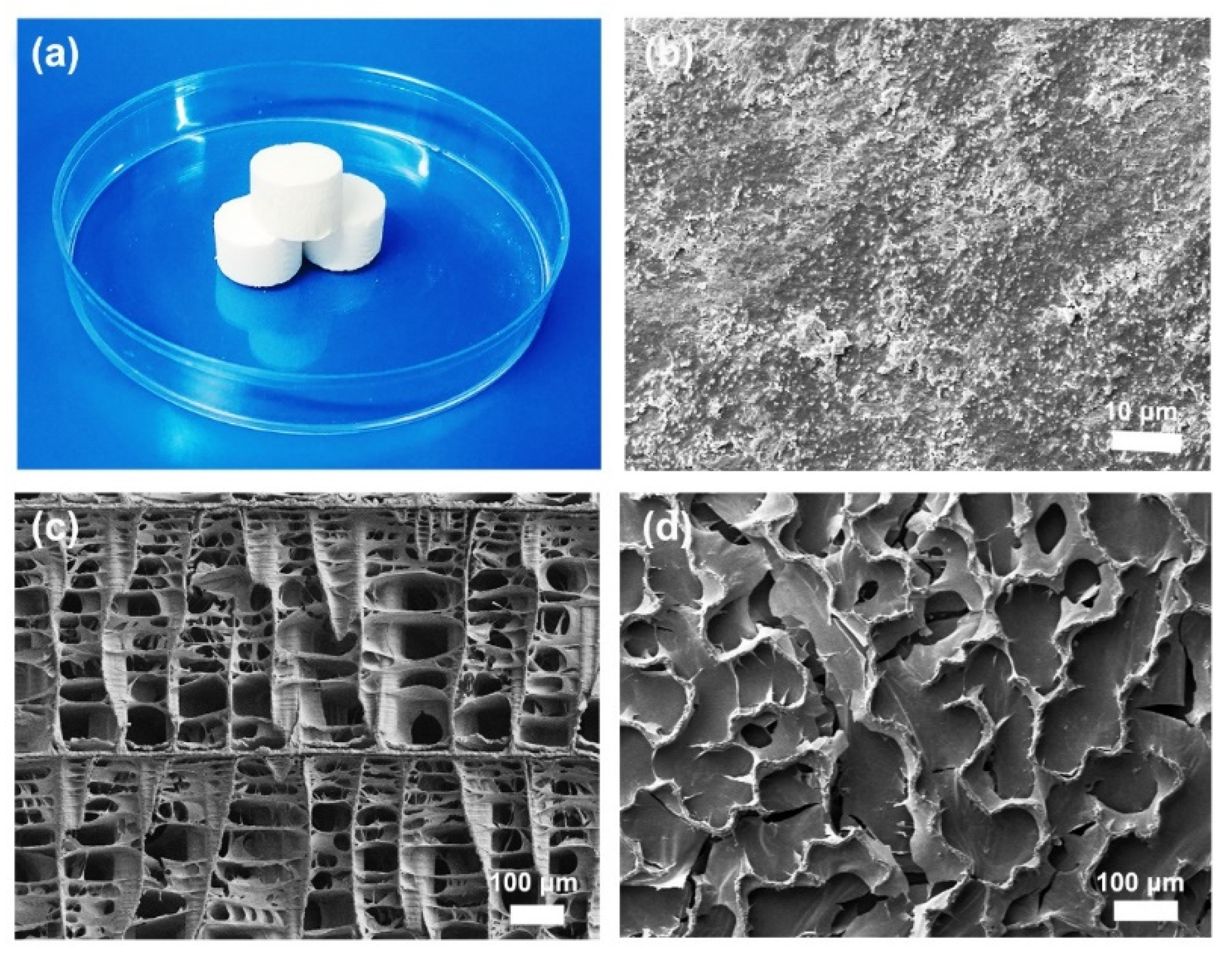
2.4.1. Scanning Electron Microscopy (SEM) Analysis
2.4.2. Fourier Infrared Spectroscopy (FTIR) Analysis
2.4.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.4.4. Static Contact Angle (CA) Analysis
2.4.5. Oil Absorption Capacity Analysis
2.4.6. Oil–Water Separation Efficiency Analysis
3. Results and Discussion
3.1. Morphology and Composition Characterization
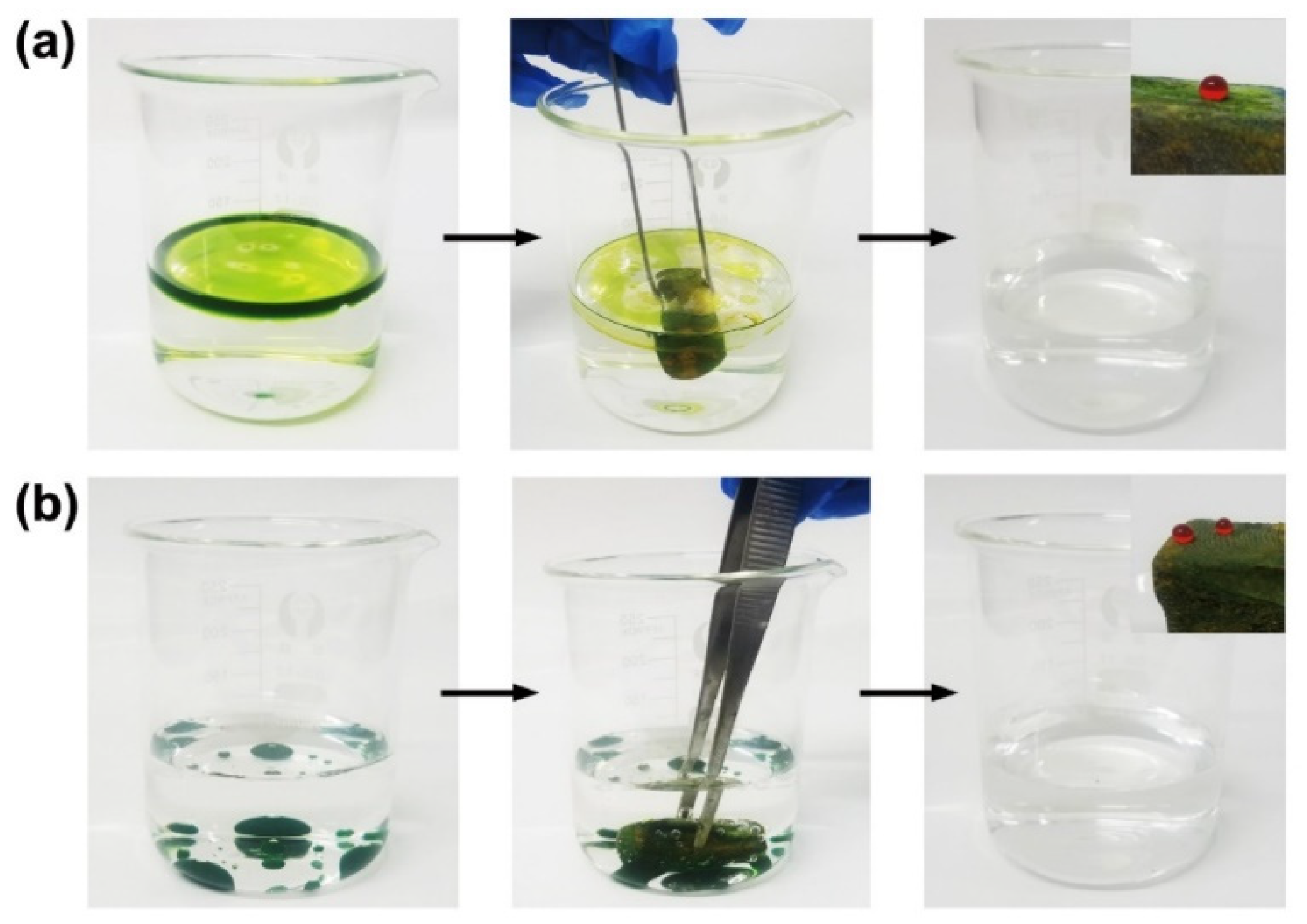
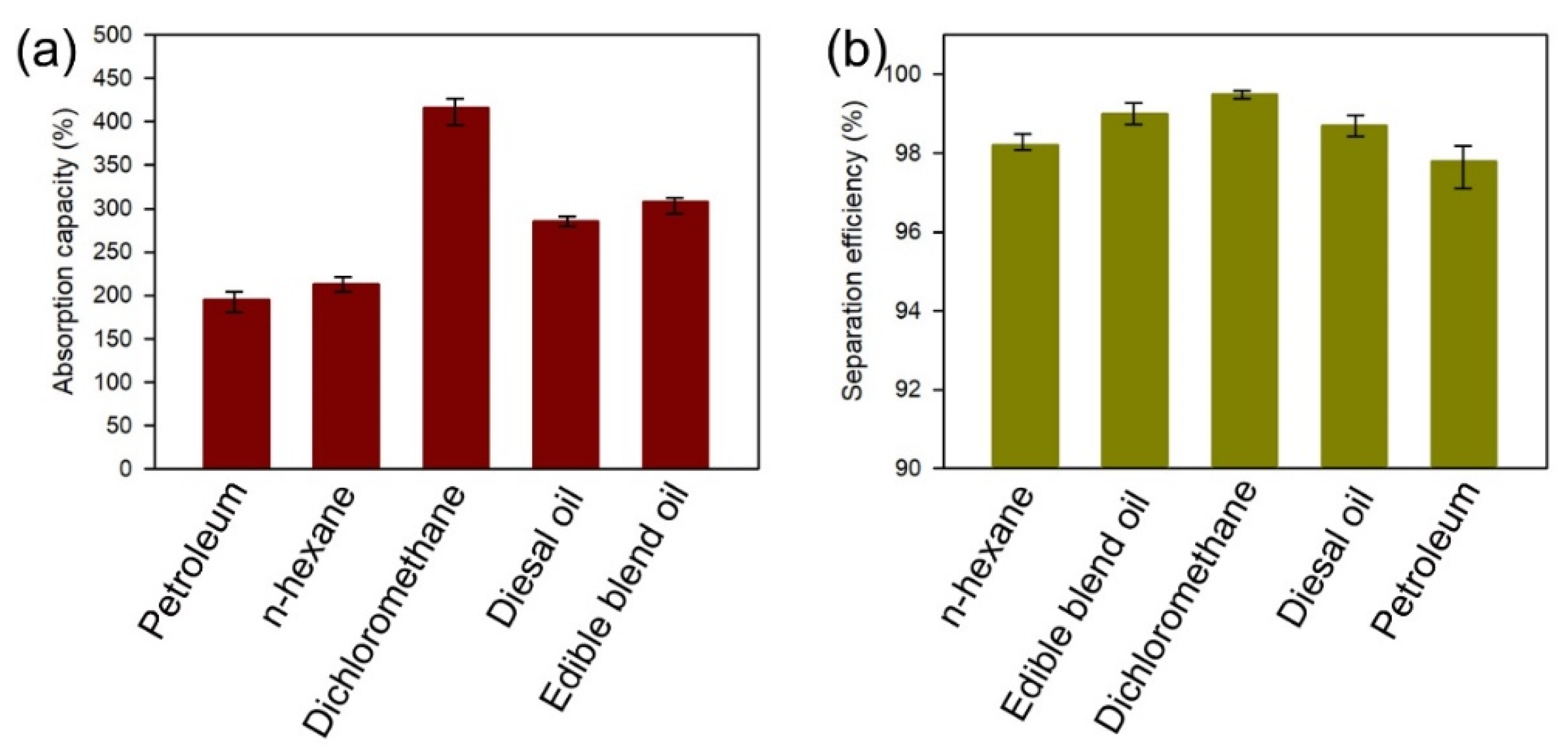
3.2. Oil–Water Separation Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruan, C.; Ai, K.; Li, X.; Lu, L. A superhydrophobic sponge with excellent absorbency and flame retardancy. Angew. Chem. Int. Ed. 2014, 53, 5556–5560. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Sun, D.; Chen, S.; Shanmugam, L.; Xiang, Y.; Yang, J. Robust microcapsules with durable superhydrophobicity and superoleophilicity for efficient oil-water separation. ACS Appl. Mater. Interfaces 2020, 12, 57547–57559. [Google Scholar] [CrossRef]
- Joerg, L. Environmental nanotechnology: Nanomaterials clean up. Nat. Nanotechnol. 2008, 3, 320–321. [Google Scholar]
- Mark, S. Oil spill: Deep wounds. Nature 2011, 472, 152–154. [Google Scholar]
- Ge, J.; Ye, Y.-D.; Yao, H.-B.; Zhu, X.; Wang, X.; Wu, L.; Wang, J.-L.; Ding, H.; Yong, N.; He, L.-H.; et al. Pumping through porous hydrophobic/oleophilic materials: An alternative technology for oil spill remediation. Angew. Chem. Int. Ed. 2014, 126, 3686–3690. [Google Scholar] [CrossRef]
- Kang, L.; Li, J.; Zeng, J.; Gao, W.; Xu, J.; Cheng, Z.; Chen, K.; Wang, B. A water solvent-assisted condensation polymerization strategy of superhydrophobic lignocellulosic fibers for efficient oil/water separation. J. Mater. Chem. A 2019, 7, 16447–16457. [Google Scholar] [CrossRef]
- Calcagnile, P.; Fragouli, D.; Bayer, I.S.; Anyfantis, G.C.; Martiradonna, L.; Cozzoli, P.D.; Cingolani, R.; Athanassiou, A. Magnetically driven floating foams for the removal of oil contaminants from water. ACS Nano 2012, 6, 5413–5419. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Liu, X.; Liu, H.; Li, N.; Liu, C.; Schubert, D.W.; Shen, C. Facile fabrication of superhydrophobic and eco-friendly poly(lactic acid) foam for oil-water separation via skin peeling. ACS Appl. Mater. Interfaces 2019, 11, 14362–14367. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Yuan, H.; Su, M.; Shao, C.; Liu, C.; Guo, Z.; Shen, C.; Liu, X. Simple fabrication of superhydrophobic PLA with honeycomb-like structures for high-efficiency oil-water separation. Chin. Chem. Lett. 2020, 31, 365–368. [Google Scholar] [CrossRef]
- Qiu, L.; Sun, Y.; Guo, Z. Designing novel superwetting surfaces for high-efficiency oil–water separation: Design principles, opportunities, trends and challenges. J. Mater. Chem. A 2020, 8, 16831–16853. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Jiao, Y.; Sun, Z.; Yang, X.; Cheng, Z.; Bai, Q.; Zhang, Y.; Wang, K.; Shao, L. Constructing scalable superhydrophobic membranes for ultrafast water-oil separation. ACS Nano 2021, 15, 3500–3508. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Bai, L.; Li, J.; Huang, L.; Sun, H.; Gao, X. Robust preparation of flexibly super-hydrophobic carbon fiber membrane by electrospinning for efficient oil-water separation in harsh environments. Carbon 2021, 182, 11–22. [Google Scholar] [CrossRef]
- Kang, L.; Wang, B.; Zeng, J.; Cheng, Z.; Li, J.; Xu, J.; Gao, W.; Chen, K. Degradable dual superlyophobic lignocellulosic fibers for high-efficiency oil/water separation. Green Chem. 2020, 22, 504–512. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, S.; Lin, L.; Chen, L.; Liu, M.; Feng, L.; Jiang, L. A novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil/water separation. Adv. Mater. 2011, 23, 4270–4273. [Google Scholar] [CrossRef]
- Cheng, Q.; Liu, C.; Liu, S. Fabrication of a robust superhydrophobic polyurethane sponge for oil–water separation. Surf. Eng. 2018, 35, 403–410. [Google Scholar] [CrossRef]
- Bo, D.; Huimin, G.; Meng, H.; Lina, Z. Hydrophobic modification on surface of chitin sponges for highly effective separation of oil. ACS Appl. Mater. Interfaces 2014, 6, 19933–19942. [Google Scholar]
- Arbatan, T.; Fang, X.; Shen, W. Superhydrophobic and oleophilic calcium carbonate powder as a selective oil sorbent with potential use in oil spill clean-ups. Chem. Eng. J. 2011, 166, 787–791. [Google Scholar] [CrossRef]
- Checa, A.G.; Cartwright, J.H.; Sanchez-Almazo, I.; Andrade, J.P.; Ruiz-Raya, F. The cuttlefish sepia officinalis (Sepiidae, Cephalopoda) constructs cuttlebone from a liquid-crystal precursor. Sci. Rep. 2015, 5, 11513. [Google Scholar] [CrossRef] [Green Version]
- Palaveniene, A.; Harkavenko, V.; Kharchenko, V.; Daugela, P.; Pranskunas, M.; Juodzbalys, G.; Babenko, N.; Liesiene, J. Cuttlebone as a marine-derived material for preparing bone grafts. Mar. Biotechnol. 2018, 20, 363–374. [Google Scholar] [CrossRef]
- Knoller, A.; Runcevski, T.; Dinnebier, R.E.; Bill, J.; Burghard, Z. Cuttlebone-like V2O5 nanofibre scaffolds -advances in structuring cellular solids. Sci. Rep. 2017, 7, 42951. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhao, Y.; Bing, Y.; Li, Y.; Gan, N.; Guo, Z.; Peng, Z.; Zhu, Y. Biotemplated syntheses of macroporous materials for bone tissue engineering scaffolds and experiments in vitro and vivo. ACS Appl. Mater. Interfaces 2013, 5, 5557–5562. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Wesarg, F.; Hessler, N.; Muller, F.A.; Kralisch, D.; Fischer, D. Loading of bacterial nanocellulose hydrogels with proteins using a high-speed technique. Carbohydr. Polym. 2014, 106, 410–413. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, X.; Batchelor, W. Cellulose nanofibre aerogel filter with tuneable pore structure for oil/water separation and recovery. RSC Adv. 2016, 6, 21435–21438. [Google Scholar] [CrossRef]
- Druel, L.; Kenkel, A.; Baudron, V.; Buwalda, S.; Budtova, T. Cellulose aerogel microparticles via emulsion-coagulation technique. Biomacromolecules 2020, 21, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. Hydrogels prepared from cross-linked nanofibrillated cellulose. ACS Sustain. Chem. Eng. 2014, 2, 772–780. [Google Scholar] [CrossRef]
- McKee, J.R.; Hietala, S.; Seitsonen, J.; Laine, J.; Kontturi, E.; Ikkala, O. Thermoresponsive nanocellulose hydrogels with tunable mechanical properties. ACS Macro Lett. 2014, 3, 266–270. [Google Scholar] [CrossRef]
- Syverud, K.; Pettersen, S.R.; Draget, K.; Chinga-Carrasco, G. Controlling the elastic modulus of cellulose nanofibril hydrogels-scaffolds with potential in tissue engineering. Cellulose 2014, 22, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cheng, F.; Grenman, H.; Spoljaric, S.; Seppala, J.; Eriksson, J.E.; Willfor, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; He, J.; Wu, J.; Li, W.; Zhang, H.; Wang, H.; Tu, J.; Zhou, Y.; Dong, Y.; et al. Natural and sustainable superhydrophobic nanochitin aerogels for collecting methane bubbles from underwater. ACS Sustain. Chem. Eng. 2021, 9, 5000–5009. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Chen, G.; Chen, T.; Song, T.; Wu, J.; Shi, C.; He, M.; Tian, J. Engineering biocompatible hydrogels from bicomponent natural nanofibers for anticancer drug delivery. J. Agric. Food Chem. 2018, 66, 935–942. [Google Scholar] [CrossRef]
- Rocha, J.H.; Lemos, A.F.; Agathopoulos, S.; Valerio, P.; Kannan, S.; Oktar, F.N.; Ferreira, J.M. Scaffolds for bone restoration from cuttlefish. Bone 2005, 37, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, A.R.; Kalyani, D. Naturally derived porous hydroxyapatitepolymer biocomposite of cuttlebone and eggshell for dental and orthopedic applications. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 471–478. [Google Scholar]
- Culverwell, E.; Wimbush, S.C.; Hall, S.R. Biotemplated synthesis of an ordered macroporous superconductor with high critical current density using a cuttlebone template. Chem. Commun. 2008, 9, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Si, L.; Liu, Y.; Guan, G.; Wu, D.; Wang, Z.; Hao, X. Ultrastable coaxial cable-like superhydrophobic mesh with self-adaption effect: Facile synthesis and oil/water separation application. J. Mater. Chem. A 2016, 4, 8080–8090. [Google Scholar] [CrossRef]
- Cao, C.; Ge, M.; Huang, J.; Li, S.; Deng, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. Robust fluorine-free superhydrophobic PDMS–ormosil@fabrics for highly effective self-cleaning and efficient oil–water separation. J. Mater. Chem. A 2016, 4, 12179–12187. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, D.; An, W.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A robust and cost-effective superhydrophobic graphene foam for efficient oil and organic solvent recovery. Small 2015, 11, 5222–5229. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Salgado, C.L.; Song, W.; Mano, J.F. Combinatorial on-chip study of miniaturized 3D porous scaffolds using a patterned superhydrophobic platform. Small 2013, 9, 768–778. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, S.; Qing, Y.; Luo, S.; Liu, M. A versatile and efficient method to fabricate durable superhydrophobic surfaces on wood, lignocellulosic fiber, glass, and metal substrates. J. Mater. Chem. A 2016, 4, 14111–14121. [Google Scholar] [CrossRef]
- Florek, M.; Fornal, E.; Gómez-Romero, P.; Zieba, E.; Paszkowicz, W.; Lekki, J.; Nowak, J.; Kuczumow, A. Complementary microstructural and chemical analyses of sepia officinalis endoskeleton. Mater. Sci. Eng. C 2009, 29, 1220–1226. [Google Scholar] [CrossRef]
- Ni, M.; Ratner, B.D. Differentiation of calcium carbonate polymorphs by surface analysis technique—An XPS and TOF-SIMS study. Surf. Interface Anal. 2008, 40, 1356–1361. [Google Scholar] [CrossRef] [Green Version]
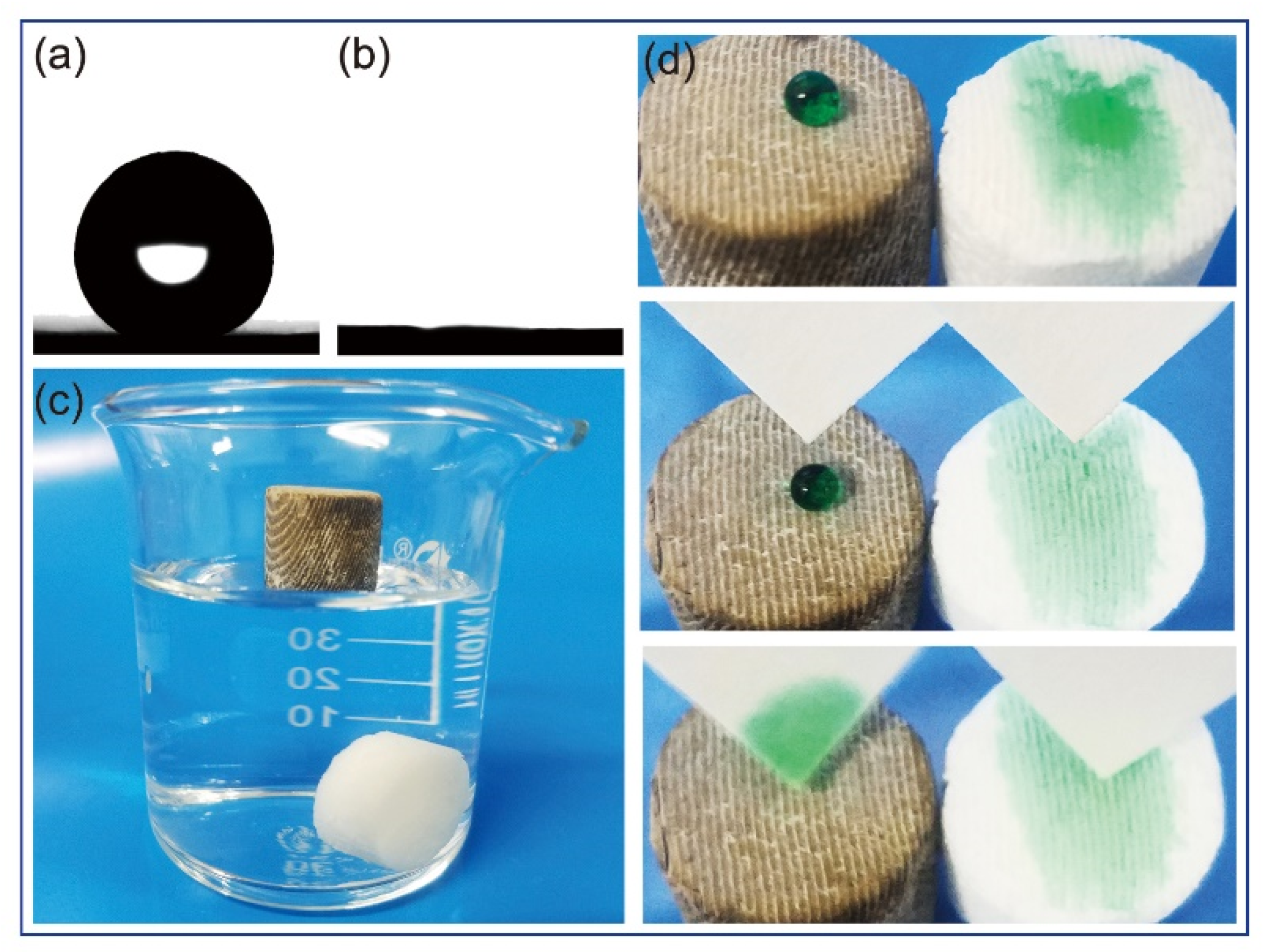

| Samples | Contact Angle |
|---|---|
| Superhydrophobic cuttlebone | 152° |
| After absorbing gasoline | 150° |
| After absorbing chloroform | 151° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Che, P.; Zhang, H.; Zhang, Y.; Wu, J.; Li, W.; He, J.; Ma, Z.; Li, T.; Dong, Y.; et al. Superhydrophobic Modification of Biomass Cuttlebone Applied to Oil Spill Remediation. Materials 2022, 15, 4401. https://doi.org/10.3390/ma15134401
Xu J, Che P, Zhang H, Zhang Y, Wu J, Li W, He J, Ma Z, Li T, Dong Y, et al. Superhydrophobic Modification of Biomass Cuttlebone Applied to Oil Spill Remediation. Materials. 2022; 15(13):4401. https://doi.org/10.3390/ma15134401
Chicago/Turabian StyleXu, Junfei, Pengchao Che, Hailong Zhang, Yuliang Zhang, Jun Wu, Weiqi Li, Jizhong He, Zhihui Ma, Tengfei Li, Yunyuan Dong, and et al. 2022. "Superhydrophobic Modification of Biomass Cuttlebone Applied to Oil Spill Remediation" Materials 15, no. 13: 4401. https://doi.org/10.3390/ma15134401
APA StyleXu, J., Che, P., Zhang, H., Zhang, Y., Wu, J., Li, W., He, J., Ma, Z., Li, T., Dong, Y., Yu, J., & Tong, R. (2022). Superhydrophobic Modification of Biomass Cuttlebone Applied to Oil Spill Remediation. Materials, 15(13), 4401. https://doi.org/10.3390/ma15134401






