Phase Equilibrium and Microstructure Examinations of Eutectic Fe-C-Mn-B Alloys
Abstract
1. Introduction
2. Materials and Methods
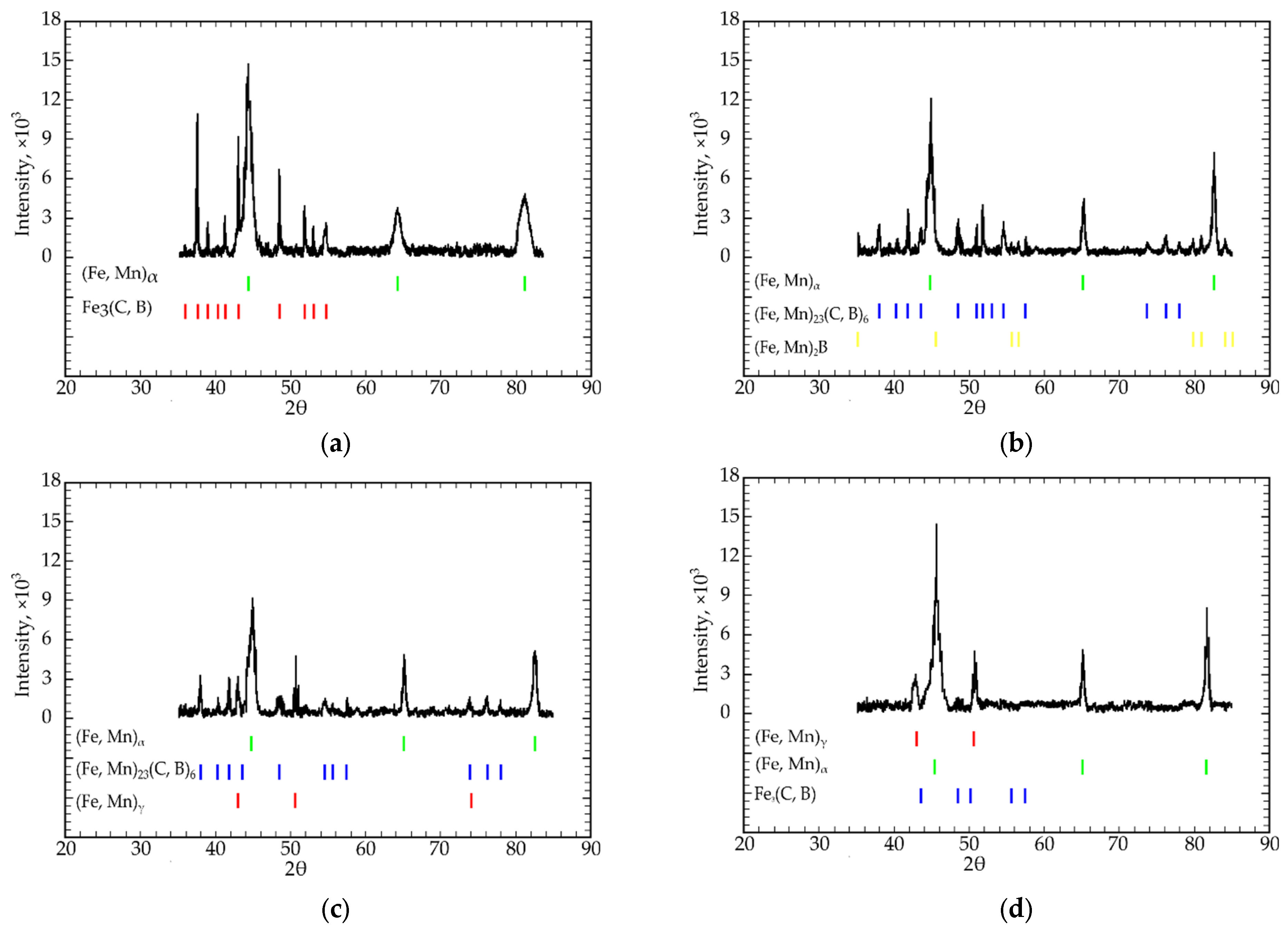
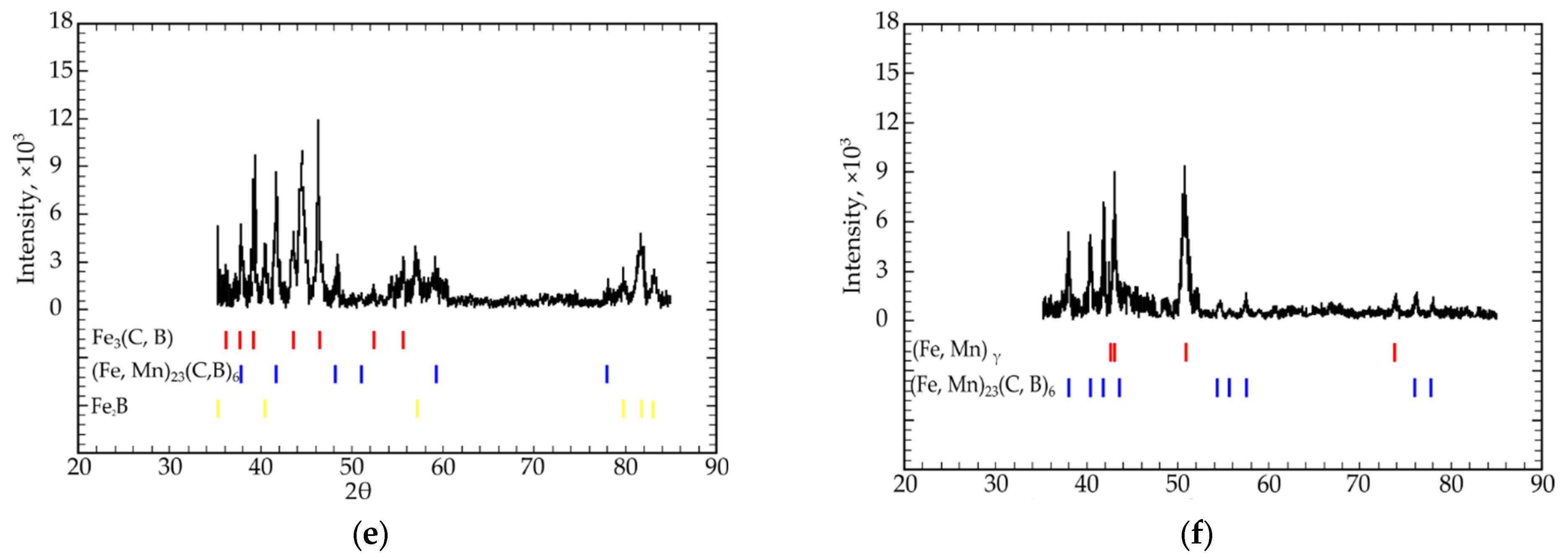
3. Results and Discussion
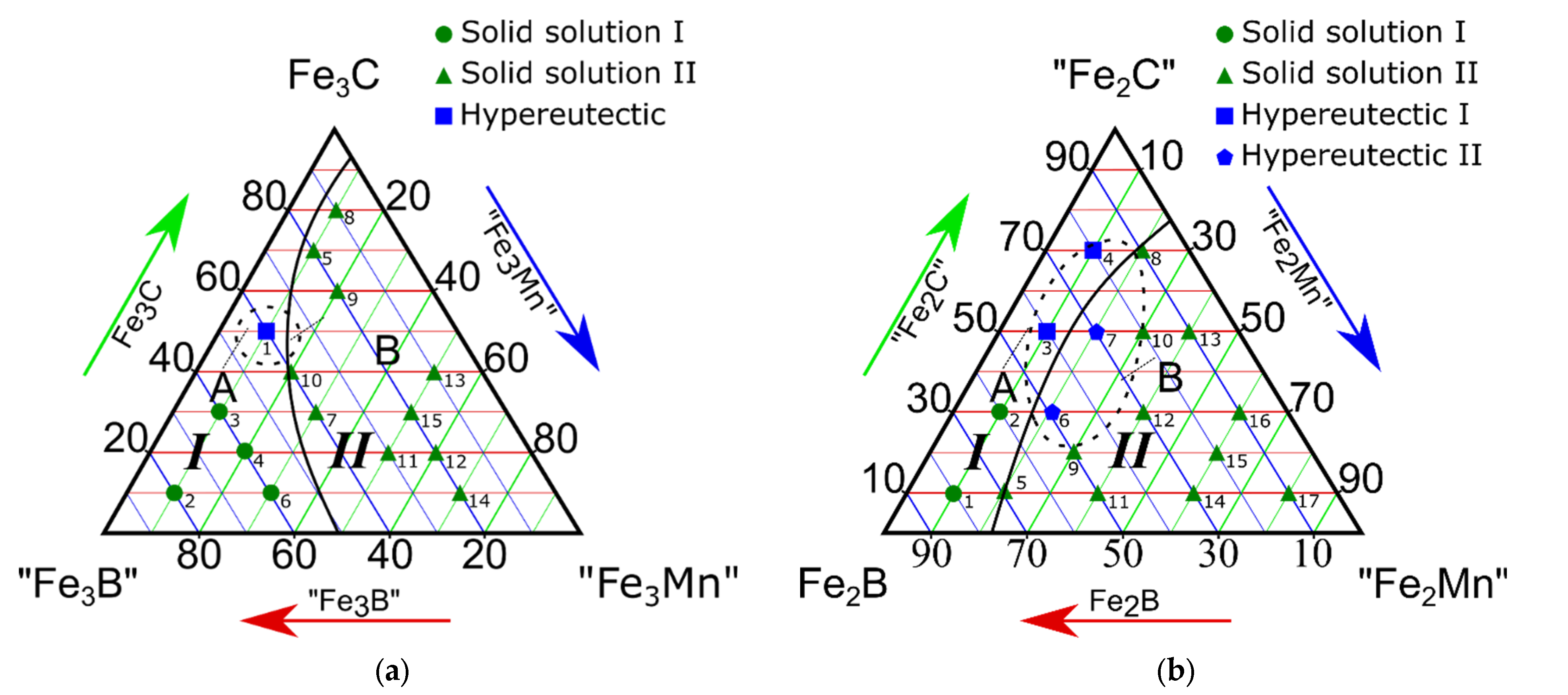
3.1. Study on the Isothermal Section of the “Fe3B”-Fe3C-“Fe3Mn” Pseudo-Ternary System
3.2. Study on the Isothermal Section of the Fe2B-“Fe2C”-“Fe2Mn” Pseudo-Ternary System
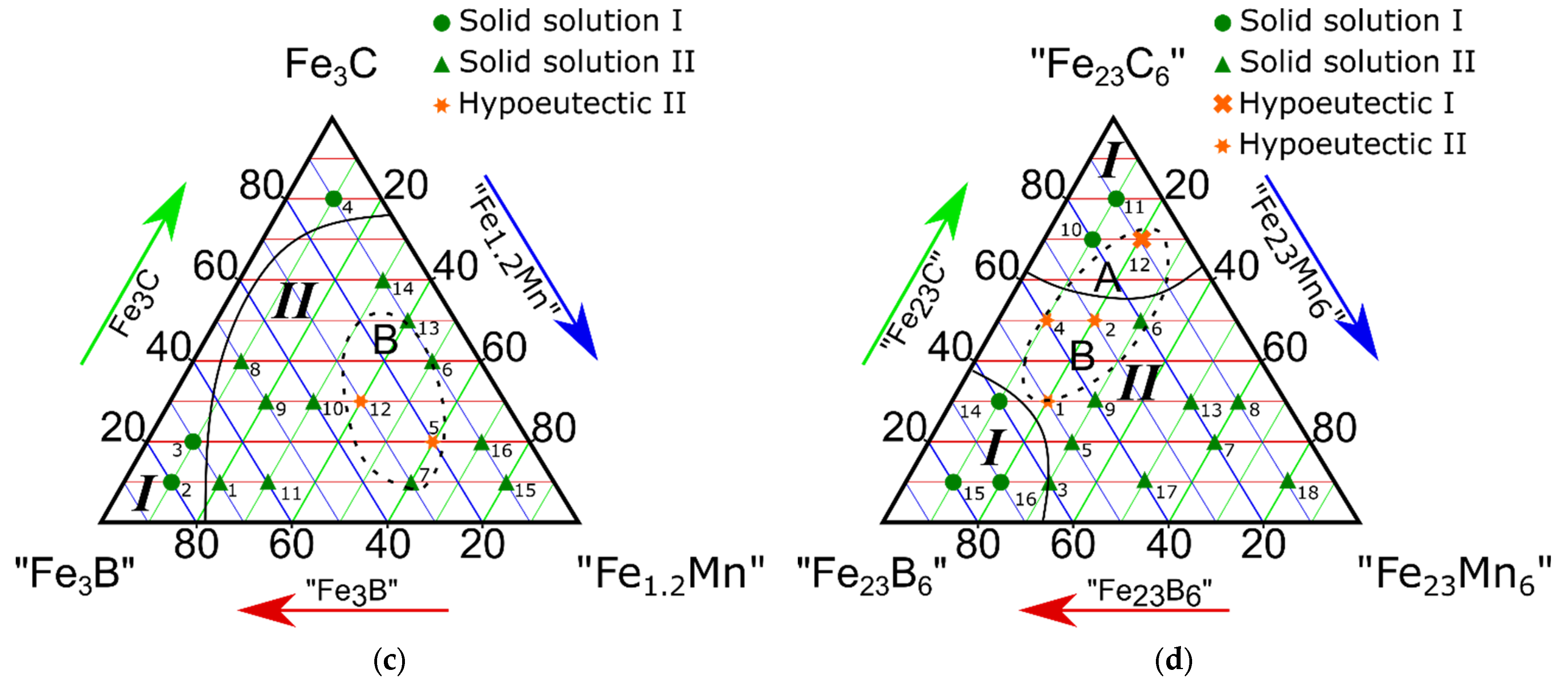
3.3. Study on the Isothermal Section of the “Fe3B”-Fe3C-“Fe1.2Mn” Pseudo-Ternary System
3.4. Study on the Isothermal Section of the “Fe23B6”-“Fe23C6”-“Fe23Mn” Pseudo-Ternary System
4. Conclusions
- Fe-C-B type eutectic alloys were formed with a manganese content of 1.6–7.6 wt.%. The concentration of the other components was 85.1–92.5 wt.% for iron, 2.6–7.0 wt.% for carbon, and 0.2–3.5 wt.% for boron;
- Fe-C-Mn type eutectic alloys were formed with a boron content of 0.6–2.5 wt.%. The concentrations of the other alloying elements were 73.3–92. 5 wt.% for iron, 3.1–23.8 wt.% for manganese, and 0.6–6.4 wt.% for carbon;
- All four isothermal sections consisted of two different phase regions composed of ternary Fe-C-B (region I) and Fe-C-Mn (region II) systems (Figure 2a–c). Region I consisted of a combination of the following phases: Fe3(C, B) + (Fe, Mn)α + (Fe, Mn)γ, and region II consisted of (Fe, Mn)γ + (Fe, Mn)α + (Fe, Mn)23(C, B)6;
- The alloys of the “Fe3B”-Fe3C-“Fe1.2Mn” isothermal section at 66.6 at.% for iron contained no α-phase. This result was only observed when the iron content was higher than 75 at.%;
- In the eutectic region with the highest Fe concentration (79; 75 at.%), the (Fe, Mn)γ, (Fe, Mn)α, Fe3(C, B), and (Fe, Mn)23(C, B)6 phases were in equilibrium. When the iron concentration reduced to 66.6 at.% (the “Fe3B”-Fe3C-“Fe1.2Mn” isothermal section), the alloys presented no γ-α transition in the Fe-based solid solution. In this case, Fe3(C, B) + (Fe, Mn)γ or (Fe, Mn)23(C, B)6 + (Fe, Mn)γ were in equilibrium;
- The eutectic regions in the isothermal sections of the “Fe3B”-Fe3C-“Fe3Mn”, Fe2B-“Fe2C”-“Fe2Mn”, and “Fe23B6”-“Fe23C6”-“Fe23Mn” pseudo-ternary systems were found in both phase regions. The “Fe3B”-Fe3C-“Fe1.2Mn” system featured a eutectic Fe-C-Mn structure in region II only. Basically, the Fe-C-B type eutectic was formed when the boron and carbon contents were high, whereas the Fe-C-Mn type eutectic was found in the region with a high Mn concentration.
- It was experimentally proved that in the Fe-C-Mn-B quaternary system there are two separate eutectic regions—Fe-C-B and Fe-C-Mn—and they have the same concentrations, as it is in respective ternary systems. The knowledge of this is fundamental in the development of new eutectic alloys based on the quaternary Fe-C-Mn-B system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddique, S.; Imran, M.; Wycisk, E.; Emmelmann, C.; Walther, F. Influence of process-induced microstructure and imperfections on mechanical properties of AlSi12 processed by selective laser melting. J. Mater. Process. Technol. 2015, 221, 205–213. [Google Scholar] [CrossRef]
- Siddique, S.; Imran, M.; Wycisk, E.; Emmelmann, C.; Walther, F. Fatigue Assessment of Laser Additive Manufactured AlSi12 Eutectic Alloy in the Very High Cycle Fatigue (VHCF) Range up to 1E9 cycles. Mater. Today Proc. 2016, 3, 2853–2860. [Google Scholar] [CrossRef]
- Albu, M.; Krisper, R.; Lammer, J.; Kothleitner, G.; Fiocchi, J.; Bassani, P. Microstructure evolution during in-situ heating of AlSi10Mg alloy powders and additive manufactured parts. Addit. Manuf. 2020, 36, 101605. [Google Scholar] [CrossRef]
- Shakil, S.; Hadadzadeh, A.; Amirkhiz, B.S.; Pirgazi, H.; Mohammadi, M.; Haghshenas, M. Additive manufactured versus cast AlSi10Mg alloy: Microstructure and micromechanics. Results Mater. 2021, 10, 100178. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, Q.; Du, Y.; Zhang, Y.; Bai, X.; Zhang, Y.; Zhang, H.; Zhang, C.; Yuan, Y. Investigation of the effect of torch tilt and external magnetic field on arc during overlapping deposition of wire arc additive manufacturing. Rapid Prototyp. J. 2020, 27, 24–36. [Google Scholar] [CrossRef]
- Carranza, R.M.; Robinson, J.; Ashton, I.; Fox, P.; Sutcliffe, C.; Patterson, E. A novel device for in-situ force measurements during laser powder bed fusion (L-PBF). Rapid Prototyp. J. 2021, 27, 1423–1431. [Google Scholar] [CrossRef]
- Duan, W.; Han, J.; Xia, Q.; Wang, K.; Wu, M.; Song, D. Investigation on the relationship between bending angle of the overhanging surface and overhanging surface quality printed using selective laser melting. Rapid Prototyp. J. 2021, 27, 1573–1579. [Google Scholar] [CrossRef]
- Requena, G.; Bugelnig, K.; Sket, F.; Milenkovic, S.; Rödler, G.; Weisheit, A.; Gussone, J.; Haubrich, J.; Barriobero-Vila, P.; Pusztai, T.; et al. Ultrafine Fe-Fe2Ti eutectics by directed energy deposition: Insights into microstructure formation based on experimental techniques and phase field modelling. Addit. Manuf. 2020, 33, 101133. [Google Scholar] [CrossRef]
- Gussone, J.; Bugelnig, K.; Barriobero-Vila, P.; da Silva, J.C.; Hecht, U.; Dresbach, C.; Sket, F.; Cloetens, P.; Stark, A.; Schell, N.; et al. Ultrafine eutectic Ti-Fe-based alloys processed by additive manufacturing—A new candidate for high temperature applications. Appl. Mater. Today 2020, 20, 100767. [Google Scholar] [CrossRef]
- Schmelzer, J.; Rittinghaus, S.-K.; Gruber, K.; Veit, P.; Weisheit, A.; Krüger, M. Printability and microstructural evolution of a near-eutectic three-phase V-based alloy. Addit. Manuf. 2020, 34, 101208. [Google Scholar] [CrossRef]
- Joseph, J.; Imran, M.; Hodgson, P.; Barnett, M.; Fabijanic, D. Towards the large-scale production and strength prediction of near-eutectic AlxCoCrFeNi2.1 alloys by additive manufacturing. Manuf. Lett. 2020, 25, 16–20. [Google Scholar] [CrossRef]
- Dong, B.; Wang, Z.; Pan, Z.; Muránsky, O.; Shen, C.; Reid, M.; Wu, B.; Chen, X.; Li, H. On the development of pseudo-eutectic AlCoCrFeNi2.1 high entropy alloy using Powder-bed Arc Additive Manufacturing (PAAM) process. Mater. Sci. Eng. A 2020, 802, 140639. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, F.; Huang, Z.; Jia, M.; Chen, G.; Ye, Y.; Lin, Y.; Liu, W.; Chen, B.; Shen, Q.; et al. Additive manufacturing of functionally graded materials: A review. Mater. Sci. Eng. A 2019, 764, 138209. [Google Scholar] [CrossRef]
- Yadav, A.; Srivastav, A.; Singh, A.; Mushtaque, M.; Khan, S.; Kumar, H.; Arora, P. Investigation on the materials used in additive manufacturing: A study. Mater. Today Proc. 2021, 43, 154–157. [Google Scholar] [CrossRef]
- Kumar, M.B.; Sathiya, P. Methods and materials for additive manufacturing: A critical review on advancements and challenges. Thin-Walled Struct. 2020, 159, 107228. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Ansari, M.; Jabari, E.; Toyserkani, E. Opportunities and challenges in additive manufacturing of functionally graded metallic materials via powder-fed laser directed energy deposition: A review. J. Mater. Process. Technol. 2021, 294, 117117. [Google Scholar] [CrossRef]
- Li, N.; Huang, S.; Zhang, G.; Qin, R.; Liu, W.; Xiong, H.; Shi, G.; Blackburn, J. Progress in additive manufacturing on new materials: A review. J. Mater. Sci. Technol. 2018, 35, 242–269. [Google Scholar] [CrossRef]
- Qin, R.; Zhang, X.; Guo, S.; Sun, B.; Tang, S.; Li, W. Laser cladding of high Co–Ni secondary hardening steel on 18Cr2Ni4WA steel. Surf. Coat. Technol. 2016, 285, 242–248. [Google Scholar] [CrossRef]
- Ettefagh, A.H.; Guo, S.; Raush, J. Corrosion performance of additively manufactured stainless steel parts: A review. Addit. Manuf. 2020, 37, 101689. [Google Scholar] [CrossRef]
- Bajaj, P.; Hariharan, A.; Kini, A.; Kürnsteiner, P.; Raabe, D.; Jägle, E.A. Steels in additive manufacturing: A review of their microstructure and properties. Mater. Sci. Eng. A 2020, 772, 138633. [Google Scholar] [CrossRef]
- Xue, L.; Chen, J.; Wang, S.-H. Freeform Laser Consolidated H13 and CPM 9V Tool Steels. Mettalogr. Microstruct. Anal. 2013, 2, 67–78. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, F.; Wu, S. Improvement of pitting corrosion resistance of wire arc additive manufactured duplex stainless steel through post-manufacturing heat-treatment. Mater. Charact. 2020, 171, 110743. [Google Scholar] [CrossRef]
- Dobrzański, L.A. Basics of Material Science: Metal Science; WNT: Warsaw, Poland, 2002. [Google Scholar]
- Lenik, K.; Pashechko, M. Development of a New Family of Wear-Resistant Eutectic Alloys. In Works of the Institute of Machine Design and Operation; Publishing House of the Wroclaw University of Technology: Wroclaw, Poland, 2002; pp. 196–201. [Google Scholar]
- Lenik, K. Anti-Wear Eutectic Coatings of the Fe-C-Mn-B System. In The National Academy of Science of Ukraine; Institute on Problems of Materials Science: Kyiv, Ukraine, 2004. [Google Scholar]
- Kindrachuk, M.V.; Dushek, Y.Y.; Luchka, M.V. The Local Character of the Stress-Strained State of a Composite Loaded by Friction Forces. Poroshkovaya Metallurgia 1994, 9–10, 56–61. [Google Scholar]
- Volchenko, D.A.; Kindrachuk, M.V.; Nikipchuk, S.V.; Nasirova, M.M.; Klochko, N.B.; Yurchuk, A.O. Electrothermomechanical Friction of Wet Elements of Automotive Disc-shoe Brakes. J. Nano-Electron. Phys. 2020, 12, 06020-1–06020-8. [Google Scholar] [CrossRef]
- Chernets, M.; Kindrachuk, M.; Kornienko, A.; Yurchuk, A. Experimental Estimation of Wear Resistance of Polyamide Composites, Reinforced by Carbon and Glass Fibres Used in Metal-Polymer Gearings. Acta Mech. Autom. 2020, 14, 206–210. [Google Scholar] [CrossRef]
- Paszeczko, M.; Lenik, K.; Paszeczko, L. Increased durability of machine parts by plasma deposition using eutectic alloys of Fe-C-Mn-B. In II International Scientific Conference “New Technologies of Treatment and Hardening of Power Plant Elements”; Zaporizhzhia-Alushta: Zaporizhzhia, Ukraine, 2002; pp. 77–78. [Google Scholar]
- Paszeczko, M.; Lenik, K.; Szewczuk, J. Increasing wear resistance of digger disk of root-harvesting machine KS-6B by deposition of eutectic coating of Fe-C-Mn-B-Si-Cr system. Probl. Tribol. 2003, 2, 154–157. (In Ukrainian) [Google Scholar]
- Pashechko, M.; Gorecki, T. Surface layer models and self-organisation of the surface with abrasive wear. In Zagadnienia Dydaktyczne w Środowisku Systemów Technologicznych; LTN: Lublin, Poland, 2003; pp. 157–162. (In Polish) [Google Scholar]
- Paszeczko, M.; Lenik, K. Improving wear resistance of machine elements by plasma surface-welding using eutectic alloys of Fe-C-Mn-B system. Enginbuild. Gerald. 2002, 1, 127–131. (In Ukrainian) [Google Scholar]
- Paszeczko, M.; Popławskij, O. Influence of surface atoms segregation on tribological properties of alloys. Probl. Tribol. 2001, 4, 115–125. [Google Scholar]
- Pashechko, M.; Józwik, J.; Dziedzic, K.; Karolus, M.; Usydus, I. Surface Hardening of HS6-5-2 Quick-Cutting Steel in the Course of Chemical Thermal Treatment. Mater. Sci. 2017, 52, 834–840. [Google Scholar] [CrossRef]
- Pashechko, M.; Lenik, K. Segregation of atoms of the eutectic alloys Fe-Mn-C-B-Si-Ni-Cr at friction wear. Wear 2009, 267, 1301–1304. [Google Scholar] [CrossRef]
- Sil’Man, G.I. Phase Diagram of Alloys of the Fe-C-Mn System and Some Structural Effects in this System. Part 1. Interphase Distribution of Manganese. Met. Sci. Heat Treat. 2005, 47, 48–52. [Google Scholar] [CrossRef]
- Pashechko, M.I.; Dziedzic, K.; Barszcz, M. Study of the Structure and Properties of Wear-Resistant Eutectic Fe–Mn–C–B–Si–Ni–Cr Coatings. Powder Metall. Met. Ceram. 2013, 52, 469–476. [Google Scholar] [CrossRef]
- Pashechko, M.I. Wear resistance of eutectic coatings of the Fe–Mn–C–B system alloyed with Si, Ni, and Cr. Mater. Sci. 2011, 46, 695–701. [Google Scholar] [CrossRef]
- Paszeczko, M.; Dziedzic, K.; Barszcz, M. Study of Coatings Obtained from Alloy Fe-Mn-C-B-Si-Ni-Cr. Adv. Sci. Technol. Res. J. 2016, 10, 194–198. [Google Scholar] [CrossRef][Green Version]
- Pashechko, M.I.; Montusiewicz, J. Evaluation of the Wear Resistance of Eutectic Coatings of the Fe–Mn–C–B System Alloyed by Si, Ni, and Cr Using Multi-Criteria Analysis. Mater. Sci. 2012, 47, 813–821. [Google Scholar] [CrossRef]
- Cherepova, T.S.; Dmitriev, G.P.; Pryadko, T.V.; Kindrachuk, M.V.; Tisov, O.V.; Dukhota, O.I.; Yurchuk, A.O.; Gerasymova, O.V. Dependence of properties of cobalt alloy HTN-62 on additional alloying. Funct. Mater. 2020, 27. [Google Scholar] [CrossRef]
- Barszcz, M.; Paszeczko, M.; Lenik, K. Self-Organization of Friction Surface of Fe-Mn-C-B Coating with Increased Resistance to Abrasion. Arch. Metall. Mater. 2015, 60, 2651–2656. [Google Scholar] [CrossRef]
- Paszeczko, M.; Lenik, K.; Dziedzic, K.; Barszcz, M. Investigation of Changing of Chemical and Phase Composition of the Friction Surface Fe-Mn-C-B-Si-Ni-Cr coating depend on depth. Key Eng. Mater. 2018, 759, 65–68. [Google Scholar] [CrossRef]
- Holubets, V.M.; Pashechko, M.I.; Dzedzic, K.; Borc, J.; Tisov, A.V. Frictional strength of electric spark coatings from powder wires under friction without lubrication. J. Frict. Wear. 2020, 41, 443–446. [Google Scholar] [CrossRef]
- Barszcz, M.; Pashechko, M.; Dziedzic, K.; Jozwik, J. Study on the Self-Organization of an Fe-Mn-C-B Coating during Friction with Surface-Active Lubricant. Materials 2020, 13, 3025. [Google Scholar] [CrossRef] [PubMed]
- Holubets, V.; Pashechko, M.; Borc, J.; Tisov, O.; Shpuliar, Y. Wear Resistance of Electrospark-Deposited Coatings in Dry Sliding Friction Conditions. Powder Metall. Met. Ceram. 2021, 60, 90–96. [Google Scholar] [CrossRef]
- Barszcz, M. Wpływ Samoorganizacji Powierzchni Podczas Tarcia Powłok ze Stopu Eutektycznego Fe-C-Mn-B na Trwałość Wybranych Elementów Maszyn. Ph.D. Thesis, Politechnika Lubelksa, Lublin, Poland, 2015. Available online: http://bc.pollub.pl/dlibra/publication/12090/edition/11046?language=pl (accessed on 15 January 2022). (In Polish).
- Dziedzic, K. Badania Struktur Wtórnych Powstających Pod Czas Zużycia Ciernego Powłok ze Stopu Eutektycznego Fe-C-Mn-B. Ph.D. Thesis, Politechnika Lubelksa, Lublin, Poland, 2013. Available online: http://www.bc.pollub.pl/dlibra/publication/9518/edition/9037#description (accessed on 15 January 2022). (In Polish).
- Pashechko, M.I.; Dziedzic, K.; Mendyk, E.; Jozwik, J. Chemical and Phase Composition of the Friction Surfaces Fe–Mn–C–B–Si–Ni–Cr Hardfacing Coatings. J. Tribol. 2018, 140, 021302. [Google Scholar] [CrossRef]

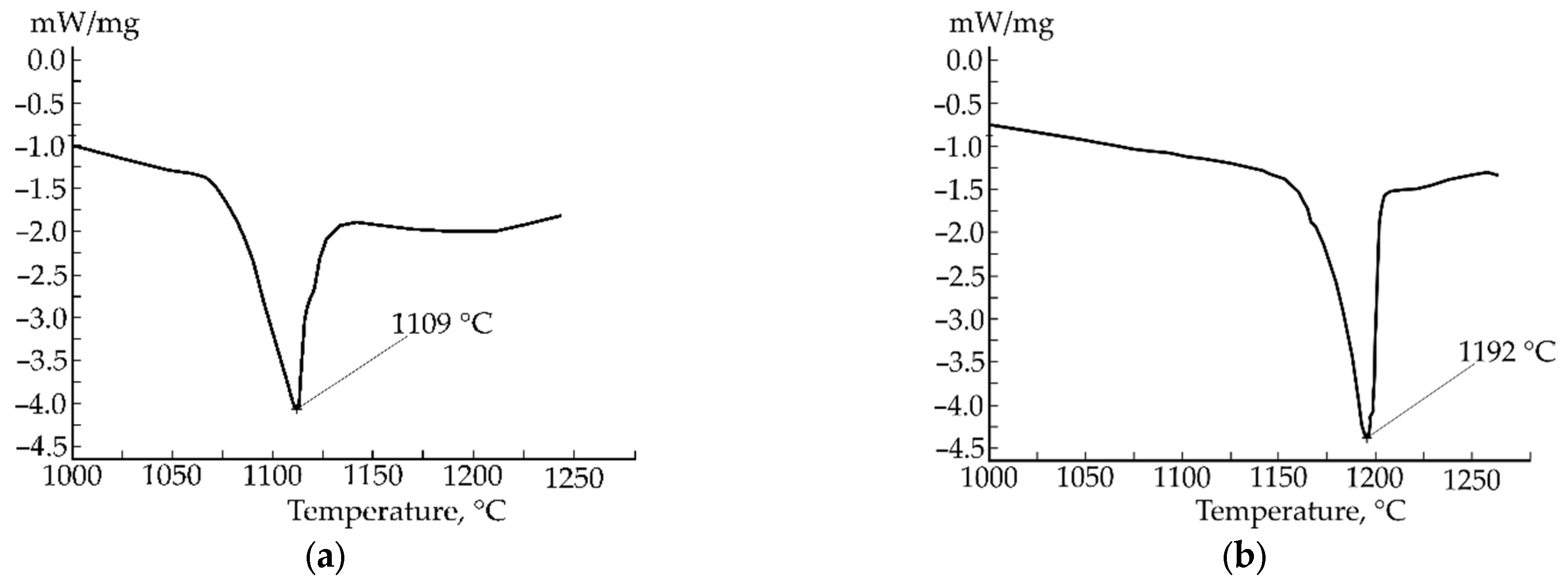



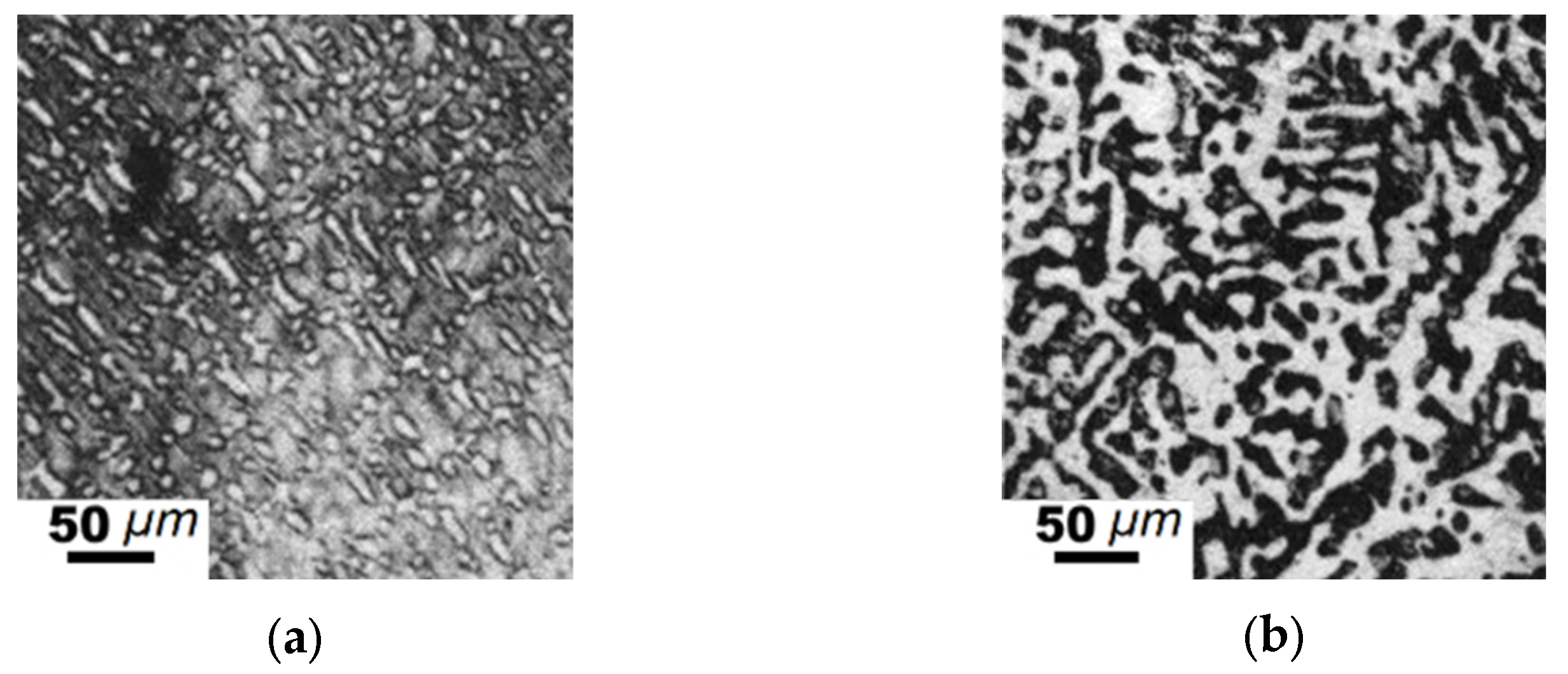
| Element | Fe-C-Mn wt.% | Fe-C-B wt.% | ||
|---|---|---|---|---|
| Min | Max | Min | Max | |
| Fe | 73.3 | 92.5 | 85.1 | 92.5 |
| C | 0.6 | 6.4 | 2.6 | 7.0 |
| Mn | 3.1 | 23.8 | 1.6 | 7.6 |
| B | 0.6 | 2.5 | 0.2 | 3.5 |
| Component Contents, Molar Fractions | Phase Composition | Alloy Type | Phase Region (Figure 2a) | |||
|---|---|---|---|---|---|---|
| # | “Fe3B” | Fe3C | “Fe3Mn” | |||
| 1 | 40 | 50 | 10 | (Fe, Mn)γ + (Fe, Mn)α + Fe3(C, B) | Hypereutectic alloy | I |
| 2 | 80 | 10 | 10 | (Fe, Mn)α + Fe3(C, B) | Solid solution | |
| 3 | 60 | 30 | 10 | |||
| 4 | 60 | 20 | 20 | |||
| 5 | 20 | 70 | 10 | (Fe, Mn)α + Fe3(C, B) | ||
| 6 | 60 | 10 | 30 | |||
| 7 | 40 | 30 | 30 | |||
| 8 | 10 | 80 | 10 | (Fe, Mn)α + (Fe, Mn)23(C, B)6 + (Fe, Mn)2B | Hypereutectic | II |
| 9 | 20 | 60 | 20 | |||
| 10 | 40 | 40 | 20 | (Fe, Mn)α + (Fe, Mn)γ + (Fe, Mn)23(C, B)6 | Solid solution | |
| 11 | 30 | 20 | 50 | |||
| 12 | 20 | 20 | 60 | |||
| 13 | 10 | 40 | 50 | (Fe, Mn)γ + (Fe, Mn)α + (Fe, Mn)23(C, B)6 | ||
| 14 | 20 | 10 | 70 | |||
| 15 | 20 | 30 | 50 | (Fe, Mn)γ + (Fe, Mn)23(C, B)6 | ||
| Component Contents, Molar Fractions | Phase Composition | Alloy Type | Phase Region (Figure 2b) | |||
|---|---|---|---|---|---|---|
| # | Fe2B | “Fe2C” | “Fe2Mn” | |||
| 1 | 80 | 10 | 10 | Feα + Fe3(C; B) | Solid solution | I |
| 2 | 60 | 30 | 10 | |||
| 3 | 40 | 50 | 10 | Hypereutectic alloy | ||
| 4 | 20 | 70 | 20 | |||
| 5 | 70 | 10 | 20 | Solid solution | II | |
| 9 | 50 | 20 | 30 | Fe3(C, B) + (Fe, Mn)23(C, B)6 + Fe2B | ||
| 6 | 50 | 30 | 20 | Hypereutectic alloy | ||
| 7 | 30 | 50 | 20 | (Fe, Mn)γ + (Fe, Mn)23(C, B)6 | ||
| 10 | 20 | 50 | 30 | (Fe, Mn)γ + (Fe, Mn)23(C, B)6 | Solid solution | |
| 11 | 50 | 10 | 40 | |||
| 12 | 30 | 30 | 40 | |||
| 13 | 10 | 50 | 40 | |||
| 15 | 20 | 20 | 60 | |||
| 14 | 30 | 10 | 60 | (Fe, Mn)γ + (Fe, Mn)23(C, B)6 | ||
| 16 | 10 | 30 | 60 | |||
| 17 | 10 | 10 | 60 | |||
| 8 | 10 | 70 | 20 | (Fe, Mn)γ + | ||
| Component Contents, Molar Fractions | Phase Composition | Alloy Type | Phase Region (Figure 2c) | |||
|---|---|---|---|---|---|---|
| # | “Fe3B” | Fe3C | “Fe1.2Mn” | |||
| 2 | 80 | 10 | 10 | (Fe, Mn)γ + Fe3(C, B) | Solid solution | I |
| 3 | 70 | 20 | 10 | Fe3(C, B) | ||
| 4 | 10 | 80 | 10 | |||
| 1 | 70 | 10 | 20 | (Fe, Mn)23(C, B)6 + Fe3(C, B) | Solid solution | II |
| 5 | 20 | 20 | 30 | (Fe, Mn)γ + (Fe, Mn)23(C, B)6 | Hypoeutectic | |
| 12 | 30 | 30 | 40 | |||
| 6 | 10 | 40 | 50 | Solid solution | ||
| 7 | 30 | 10 | 60 | |||
| 8 | 50 | 40 | 10 | |||
| 9 | 50 | 30 | 20 | |||
| 10 | 40 | 30 | 30 | |||
| 11 | 60 | 10 | 30 | |||
| 13 | 10 | 50 | 40 | |||
| 14 | 10 | 60 | 30 | (Fe, Mn)γ + (Fe, Mn)23(C, B)6 + | ||
| 15 | 10 | 10 | 80 | |||
| 16 | 10 | 20 | 70 | (Fe, Mn)γ + (Fe, Mn)23 (C, B)6 | ||
| Component Contents, Molar Fractions | Phase Composition | Alloy Type | Phase Region (Figure 2d) | |||
|---|---|---|---|---|---|---|
| # | “Fe23B6” | “Fe23C6” | “Fe23Mn6” | |||
| 1 | 50 | 30 | 20 | (Fe, Mn)α + (Fe, Mn)23(C, B)6 + Fe3(C, B) | Hypoeutectic | II |
| 2 | 30 | 50 | 20 | Feα + (Fe, Mn)23(C, B)6 | ||
| 4 | 40 | 50 | 10 | (Fe, Mn)23(C, B)6 | ||
| 3 | 60 | 10 | 30 | (Fe, Mn)γ + (Fe, Mn)α + Fe3(C, B) + (Fe, Mn)23(C, B)6 | Solid solution | |
| 5 | 40 | 20 | 30 | (Fe, Mn)γ + (Fe, Mn)23(C, B)6 | ||
| 6 | 20 | 50 | 30 | |||
| 7 | 20 | 20 | 60 | (Fe, Mn)γ + (Fe, Mn)23(C, B)6 | ||
| 8 | 10 | 30 | 60 | |||
| 13 | 20 | 30 | 50 | (Fe, Mn)γ + (Fe, Mn)α + Fe3(C, B) | ||
| 9 | 40 | 30 | 30 | (Fe, Mn)γ + (Fe, Mn)23(C, B)6 | Solid solution | I |
| 17 | 40 | 10 | 50 | |||
| 18 | 10 | 10 | 80 | |||
| 10 | 20 | 70 | 10 | (Fe, Mn)α + (Fe, Mn) γ + (Fe, Mn)23(C, B)6 | ||
| 11 | 10 | 80 | 10 | Feα + Fe3(C, B) | ||
| 12 | 10 | 70 | 20 | (Fe, Mn)α + (Fe, Mn)γ + Fe3(C, B) | Hypoeutectic | |
| 14 | 60 | 30 | 10 | (Fe, Mn)γ + (Fe, Mn)23(C, B)6 + Fe3(C, B) | ||
| 15 | 80 | 10 | 10 | |||
| 16 | 70 | 10 | 20 | |||
| Pseudo-Ternary Section | Content of Elements | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | C | Mn | B | ||||||
| min | max | min | max | min | max | min | Max | ||
| System | Fe-C-Mn-B | 73.3 65.3 | 92.5 * 79.3 ** | 0.6 2.8 | 7.0 25.0 | 1.6 1.2 | 23.8 21.5 | 0.2 1.1 | 3.5 18.4 |
| Eutectic | Fe-C-Mn | 73.3 65.3 | 92.5 79.3 | 0.6 2.8 | 6.4 23.4 | 3.1 2.7 | 23.8 21.5 | 0.6 2.9 | 2.5 18.4 |
| Eutectic | Fe-C-B | 85.1 66.6 | 92.5 79.3 | 2.6 10.5 | 7.0 25.0 | 1.6 1.2 | 7.6 6.2 | 0.2 1.1 | 3.5 17.7 |
| Section | Fe2B-“Fe2C”-“Fe2Mn” | 81.2 66.6 | 89.2 | 2.0 7.7 | 7.0 25.0 | 2.2 1.7 | 13.1 11.0 | 0.9 4.0 | 4.5 8.4 |
| Eutectic | Fe-C-Mn | 81.2 66.6 | 86.3 | 2.0 7.7 | 6.4 23 | 6.0 4.7 | 13.1 11.0 | 0.9 4.0 | 4.6 18.4 |
| Eutectic | Fe-C-B | 85.1 66.6 | 89.2 | 3.0 11.0 | 7.0 25.0 | 2.2 1.7 | 7.6 6.0 | 0.9 4.0 | 4.4 17.7 |
| Intersection | “Fe3B”-Fe3C-“Fe3Mn” | 87.1– 67.8– | 92.5 75.0 | 2.8 11.0 | 3.7 13.9 | 1.6 1.2 | 5.7 4.5 | 1.9 8.5 | 3.5 14.3 |
| Eutectic | Fe-C-Mn | 90.1 75.0 | 91.2 | 2.8 11.0 | 3.5 13.7 | 3.3 2.8 | 4.8 4.0 | 1.9 8.5 | 2.4 10.0 |
| Eutectic | Fe-C-B | 87.1 67.8 | 92.5 75.0 | 2.8 11.0 | 3.7 13.9 | 1.6 1.2 | 5.7 4.5 | 2.0 8.5 | 3.5 14.3 |
| Intersection | “Fe3B”-Fe3C-“Fe1,2Mn” | 73.3 – | 84.9 – | 0.6 – | 0.4 – | 10.2 – | 23.8 – | 0.6 – | 2.3 – |
| Eutectic | Fe-C-Mn | 65.3 – | 71.1 – | 2.8 | 15.4 – | 8.7 – | 21.5 – | 2.9 – | 10.4 – |
| Intersection | “Fe23B6”-“Fe23C6”-“Fe23Mn6” | 89.7 79.3 | 92.5 | 1.4 5.4 | 3.9 15.5 | 3.1 2.7 | 6.9 6.2 | 0.2 1.1 | 2.5 10.8 |
| Eutectic | Fe-C-Mn | 89.7 79.3 | 92.5 | 1.4 5.4 | 2.9 10.9 | 3.1 2.7 | 6.9 6.2 | 0.7 3.6 | 2.5 10.8 |
| Eutectic | Fe-C-B | 89.7 79.3 | 92.5 | 2.8 10.7 | 3.9 15.5 | 3.1 2.7 | 6.7 6.1 | 0.2 1.1 | 1.5 6.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashechko, M.; Tisov, O. Phase Equilibrium and Microstructure Examinations of Eutectic Fe-C-Mn-B Alloys. Materials 2022, 15, 4393. https://doi.org/10.3390/ma15134393
Pashechko M, Tisov O. Phase Equilibrium and Microstructure Examinations of Eutectic Fe-C-Mn-B Alloys. Materials. 2022; 15(13):4393. https://doi.org/10.3390/ma15134393
Chicago/Turabian StylePashechko, Mykhaylo, and Oleksandr Tisov. 2022. "Phase Equilibrium and Microstructure Examinations of Eutectic Fe-C-Mn-B Alloys" Materials 15, no. 13: 4393. https://doi.org/10.3390/ma15134393
APA StylePashechko, M., & Tisov, O. (2022). Phase Equilibrium and Microstructure Examinations of Eutectic Fe-C-Mn-B Alloys. Materials, 15(13), 4393. https://doi.org/10.3390/ma15134393






