Experimental Investigation on the Bioprotective Role of Trehalose on Glutamine Solutions by Infrared Spectroscopy
Abstract
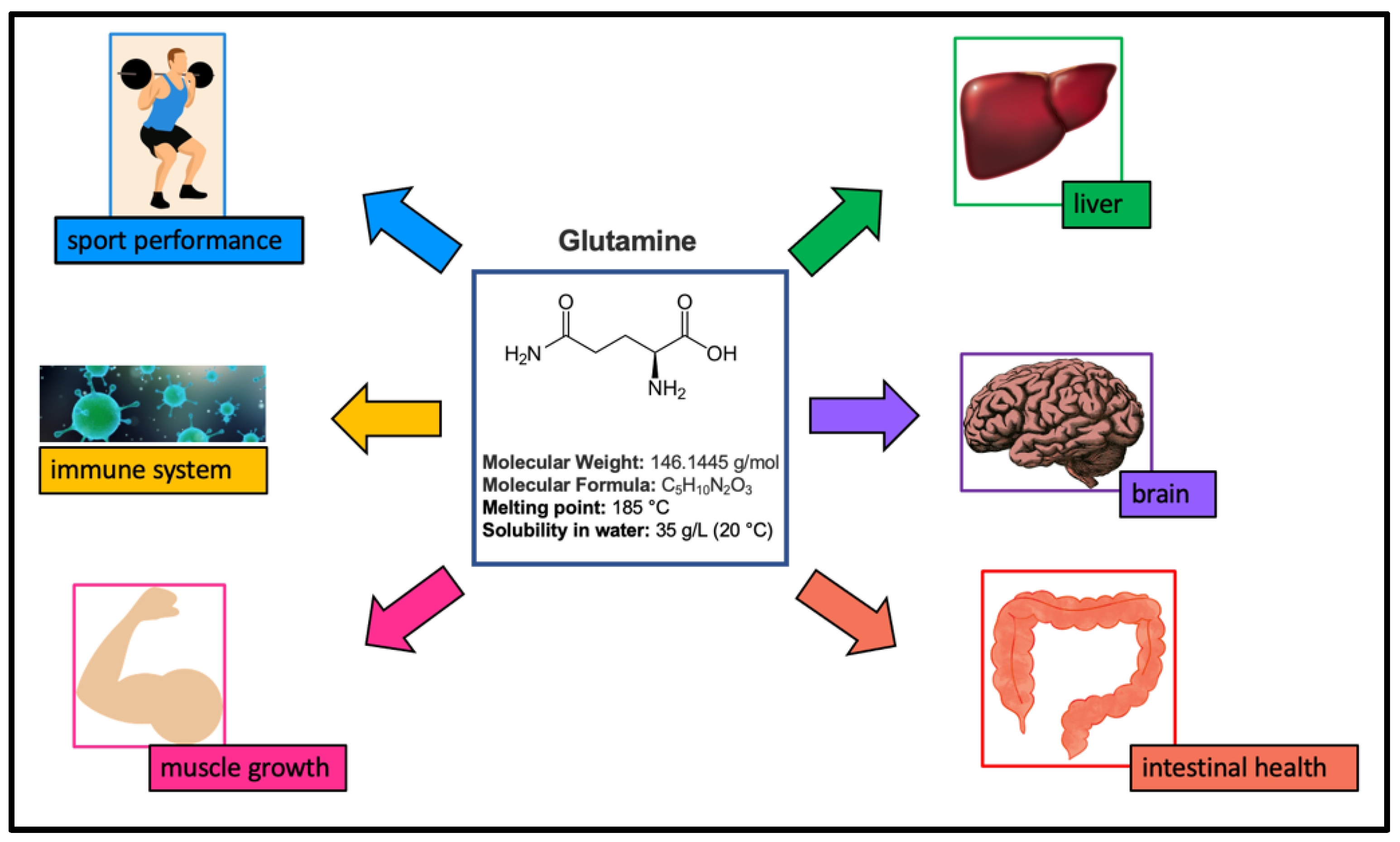
:1. Introduction
2. Materials and Experimental Set-Up
3. Methods
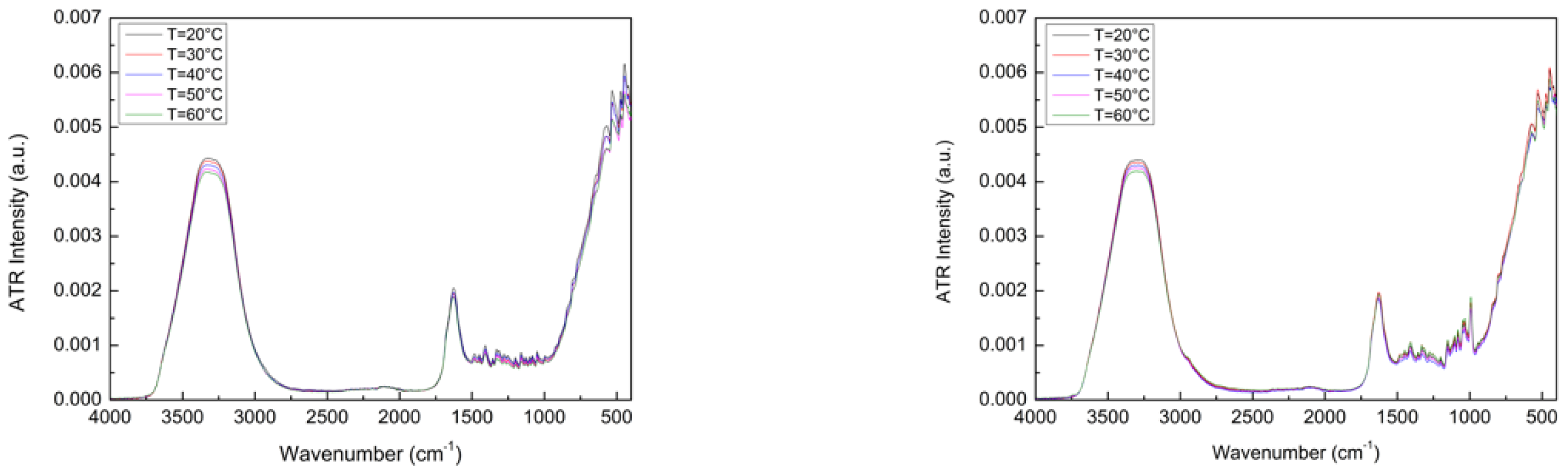
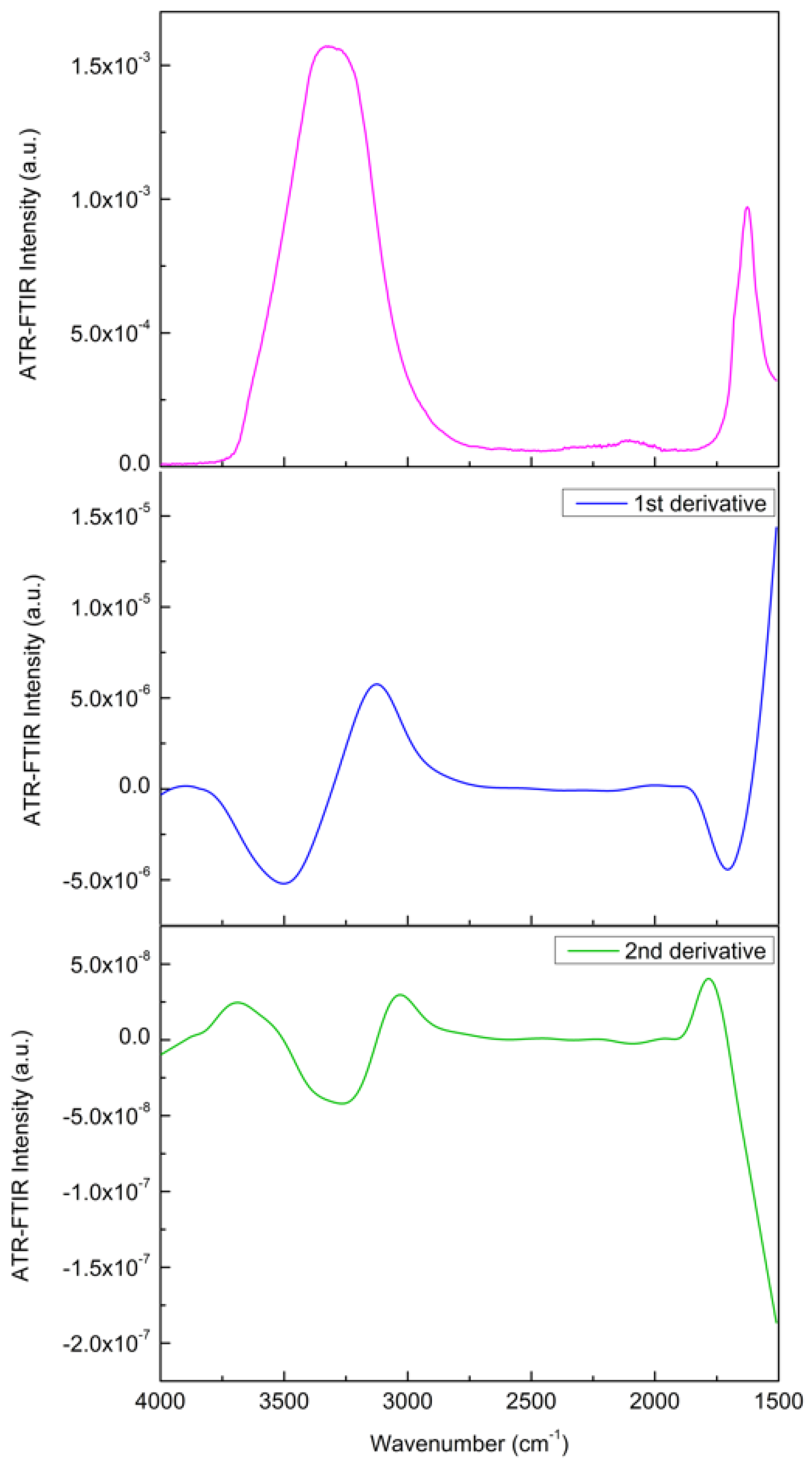
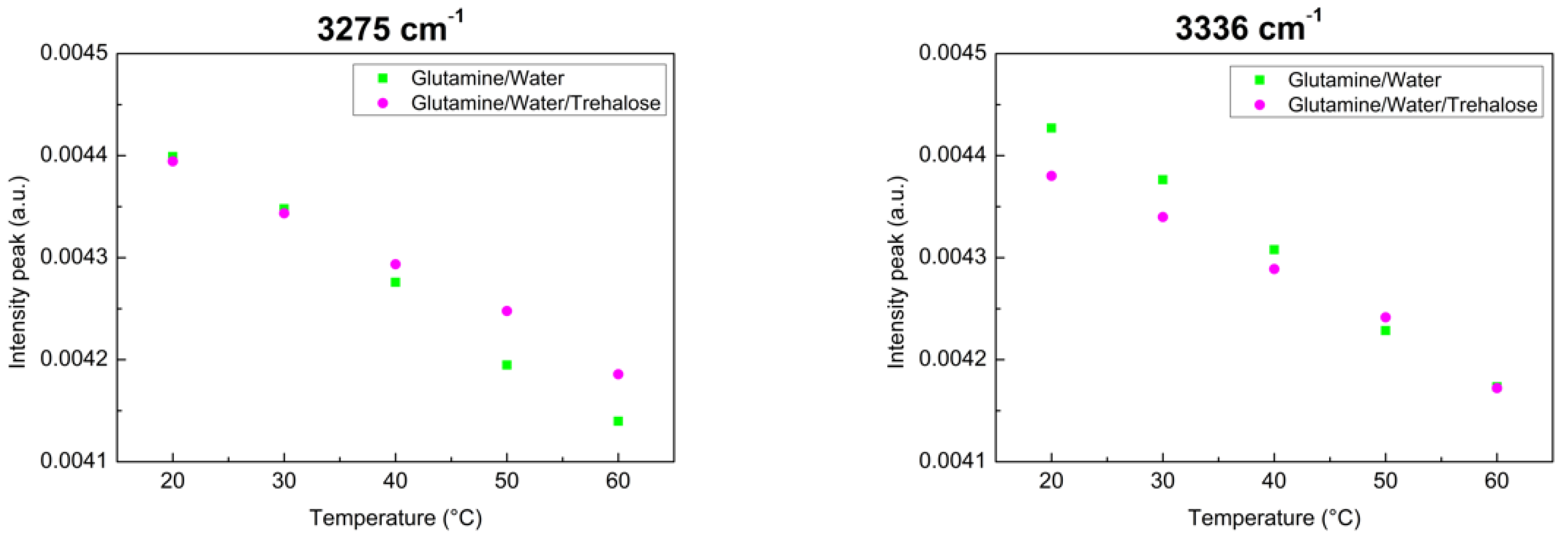
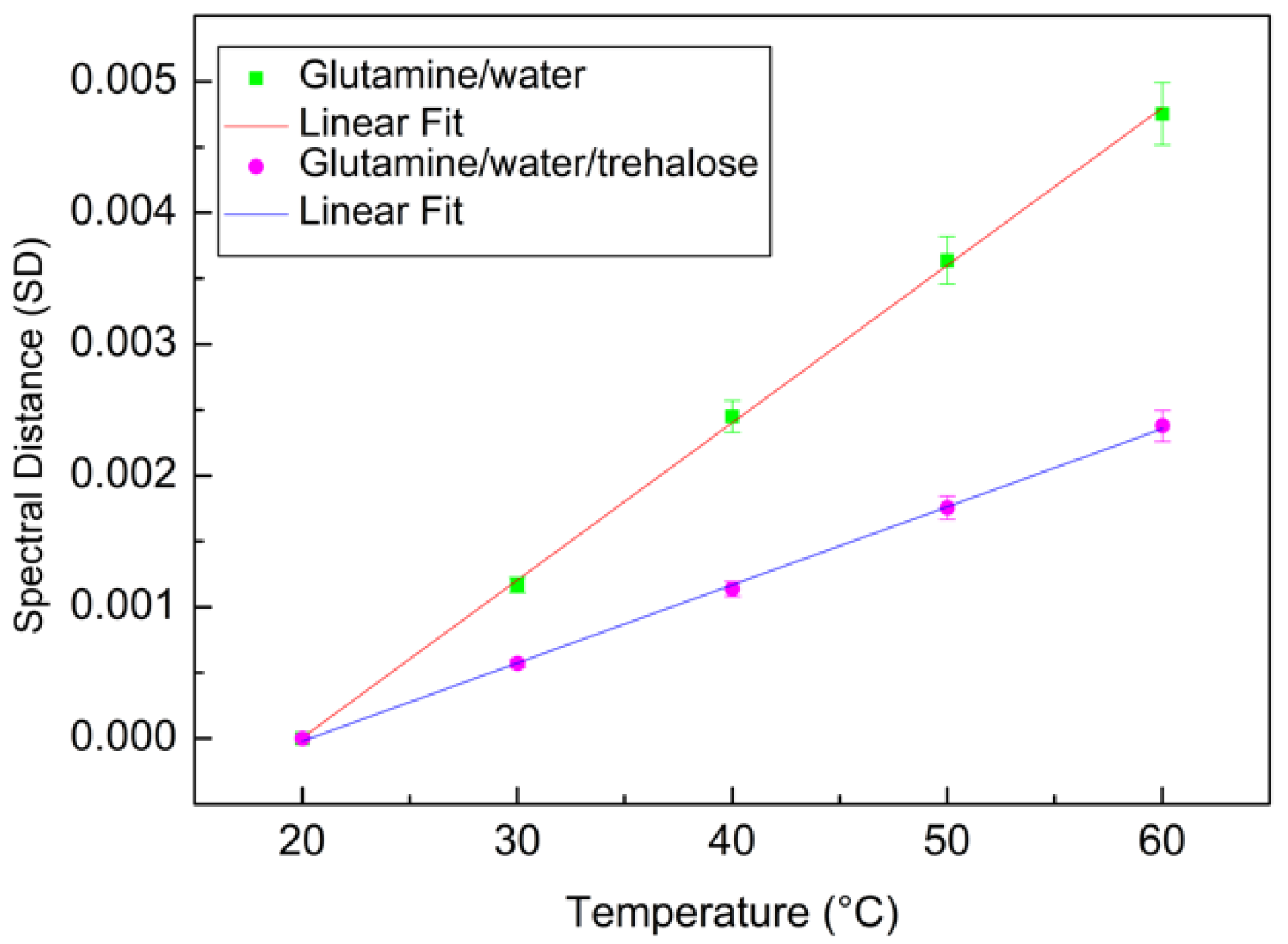
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stumvoll, M.; Perriello, G.; Meyer, C.; Gerich, J. Role of glutamine in human carbohydrate metabolism in kidney and other tissues. Kidney Int. 1999, 55, 778–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Front. Chem. 2014, 2, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedia-Mehta, N.; Finlay, D.K. Competition for nutrients and its role in controlling immune responses. Nat. Commun. 2019, 10, 2123. [Google Scholar] [CrossRef] [PubMed]
- Festuccia, W.; Wasinski, F.; Gregnani, M.F.; Ornellas, F.H.; Bacurau, A.V.N.; Câmara, N.O.; Araujo, R.C.; Bacurau, R.F. Lymphocyte Glucose and Glutamine Metabolism as Targets of the Anti-Inflammatory and Immunomodulatory Effects of Exercise. Mediat. Inflam. 2014, 2014, 326803. [Google Scholar] [CrossRef] [Green Version]
- Newsholme, P. Why Is L-Glutamine Metabolism Important to Cells of the Immune System in Health, Postinjury, Surgery or Infection? J. Nutr. 2001, 131 (Suppl. 9), 2515S–2522S. [Google Scholar] [CrossRef]
- Ardawi, M.S.M.; Newsholme, E.A. Glutamine metabolism in lymphocytes of the rat. Biochem. J. 1983, 212, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Curi, R.; Williams, J.F.; Newsholme, E.A. Formation of ketone bodies by resting lymphocytes. Int. J. Biochem. 1989, 21, 1133–1136. [Google Scholar] [CrossRef]
- Rohde, T.; Maclean, D.A.; Pedersen, B.K. Glutamine, lymphocyte proliferation and cytokine production. Scand J. Immun. 1996, 44, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Procopio, J.; Lima, M.M.; Pithon-Curi, T.C.; Curi, R. Glutamine and glutamate—Their central role in cell metabolism and function. Cell Biochem. Funct. 2003, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.F.; Procopio, J.; Pithon-Curi, T.C. Glutamine-dependent changes in gene expression and protein activity. Cell Biochem. Funct. 2005, 23, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Szondy, Z.; Newsholme, E.A. The effect of various concentrations of nucleobases, nucleosides or glutamine on the incorporation of [3H]thymidine into DNA in rat mesenteric-lymph-node lymphocytes stimulated by phytohaemagglutinin. Biochem. J. 1990, 270, 437–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernerman, J. Clinical use of glutamine supplementation. J. Nutr. 2008, 138, 2040S–2044S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooney, G.; Curi, R.; Mitchelson, A.; Newsholme, P.; Simpson, M.; Newsholme, E.A. Activities of some key enzymes of carbohydrate, ketone body, adenosine and glutamine metabolism in liver, and brown and white adipose tissues of the rat. Biochem. Biophys. Res. Commun. 1986, 138, 687–692. [Google Scholar] [CrossRef]
- Parry-Billings, M.; Dimitriadis, G.; Leighton, B.; Bond, J.; Bevan, S.; Opara, E.; Newsholme, E. Effects of hyperthyroidism and hypothyroidism on glutamine metabolism by skeletal muscle of the rat. Biochem. J. 1990, 272, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Carvalho-Peixoto, J.; Alves, R.C.; Cameron, L.-C. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Appl. Phys. Nutr. Metab. 2007, 32, 1186–1190. [Google Scholar] [CrossRef]
- De Oliveira, D.C.; da Silva Lima, F.; Sartori, T.; Santos, A.C.A.; Rogero, M.M.; Fock, R.A. Glutamine metabolism and its effects on immune response: Molecular mechanism and gene expression. Nutrire 2016, 41, 14. [Google Scholar] [CrossRef] [Green Version]
- Newsholme, P.; Lima, M.; Procopio, J.; Pithon-Curi, T.; Doi, S.; Bazotte, R.; Curi, R. Glutamine and glutamate as vital metabolites. Braz. J. Med. Biol. Res. 2003, 36, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Roth, E.; Oehler, R.; Manhart, N.; Exner, R.; Wessner, B.; Strasser, E.-M.; Spittler, A. Regulative potential of glutamine—Relation to glutathione metabolism. Nutrition 2002, 18, 217–221. [Google Scholar] [CrossRef]
- Roth, E. Nonnutritive effects of glutamine. J. Nutr. 2008, 138, 2025S–2031S. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boelens, P.G.; Nijveldt, R.J.; Houdijk, A.P.J.; Meijer, S.; van Leeuwen, P.A.M. Glutamine Alimentation in Catabolic State. J. Nutrition. 2001, 131, 2569S–2577S. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.L.; Li, X.L.; Xi, P.B.; Zhang, J.; Wu, G.; Zhu, W.Y. L-Glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids 2013, 45, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tang, Z.; Chen, H.; Ren, Z.; Ding, Q.; Liang, K.; Sun, Z. Mutual interaction between gut microbiota and protein/amino acid metabolism for host mucosal immunity and health. Anim. Nutr. 2021, 7, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Senf, S.M.; Howard, T.M.; Ahn, B.; Ferreira, L.F.; Judge, A.R. Loss of the inducible Hsp70 delays the inflammatory response to skeletal muscle injury and severely impairs muscle regeneration. PLoS ONE 2013, 8, e62687. [Google Scholar] [CrossRef] [Green Version]
- Senf, S.M. Skeletal muscle heat shock protein 70: Diverse functions and therapeutic potential for wasting disorders. Front. Physiol. 2013, 4, 00330. [Google Scholar] [CrossRef] [Green Version]
- Sottile, M.L.; Nadin, S.B. Heat shock proteins and DNA repair mechanisms: An updated overview. Cell Stress Chaperones 2018, 23, 303–315. [Google Scholar] [CrossRef]
- van Noort, J.M.; Bugiani, M.; Amor, S. Heat Shock Proteins: Old and Novel Roles in Neurodegenerative Diseases in the Central Nervous System. CNS Neurol. Disord. Drug Targets 2017, 16, 244–256. [Google Scholar] [CrossRef]
- Ramezani, A.A.; Rayyani, E.; Bahreini, M.; Mansoori, A. The effect of glutamine supplementation on athletic performance, body composition, and immune function: A systematic review and a meta-analysis of clinical trials. Clin. Nutr. 2019, 38, 1076–1091. [Google Scholar] [CrossRef]
- Coqueiro, A.Y.; Rogero, M.M.; Tirapegui, J. Glutamine as an Anti-Fatigue Amino Acid in Sports Nutrition. Nutrients 2019, 11, 863. [Google Scholar] [CrossRef] [Green Version]
- Labow, B.I.; Souba, W.W.; Abcouwer, S.F. Mechanisms governing the expression of the enzymes of glutamine metabolism—Glutaminase and glutamine synthetase. J. Nutr. 2001, 131, 2467S–2474S. [Google Scholar] [CrossRef] [Green Version]
- Berg, A.; Norberg, Å.; Martling, C.-R.; Gamrin, L.; Rooyackers, O.; Wernerman, J. Glutamine kinetics during intravenous glutamine supplementation in ICU patients on continuous renal replacement therapy. Intensiv. Care Med. 2007, 33, 660–666. [Google Scholar] [CrossRef]
- Eagle, H.; Oyama, V.I.; Levy, M.; Horton, C.L.; Fleischman, R. The growth response of mammalian cells in tissue culture to L-glutamine and L-glutamic acid. J. Biol. Chem. 1956, 218, 607–616. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, X.; Wang, S.; Zhu, Y.; Mu, D. Effects of temperature and additives on stability and spectrum of a therapeutic fibroblast growth factor. DARU J. Pharm. Sci. 2011, 19, 138–144. [Google Scholar]
- Santos, M.I.; Gerbino, E.; Tymczyszyn, E.; Gomez-Zavaglia, A. Applications of Infrared and Raman Spectroscopies to Probiotic Investigation. Foods 2015, 4, 283–305. [Google Scholar] [CrossRef] [Green Version]
- Kozak, S.; Lercher, L.; Karanth, M.N.; Meijers, R.; Carlomagno, T.; Boivin, S. Optimization of protein samples for NMR using thermal shift assays. J. Biomol. NMR 2016, 64, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Xu, S.; Yue, Y.; Wang, X. Thermal behavior of materials in laser-assisted extreme manufacturing: Raman-based novel characterization 2020. Int. J. Extrem. Manuf. 2020, 2, 032004. [Google Scholar] [CrossRef]
- Magazù, S.; Calabrò, E.; Caccamo, M.T. Experimental study of thermal restraint in bio-protectant disaccharides by FTIR spectroscopy. Open Biotechn. J. 2018, 12, 123–133. [Google Scholar] [CrossRef]
- de Almeida, F.S.; de Andrade Silva, C.A.; Lima, S.M.; Suarez, Y.R.; da Cunha Andrade, L.H. Use of Fourier transform infrared spectroscopy to monitor sugars in the beer mashing process. Food Chem. 2018, 263, 112–118. [Google Scholar] [CrossRef]
- Wright, W.W.; Guffanti, G.T.; Vanderkooi, J.M. Protein in Sugar Films and in Glycerol/Water as Examined by Infrared Spectroscopy and by the Fluorescence and Phosphorescence of Tryptophan. Biophys. J. 2003, 85, 1980–1995. [Google Scholar] [CrossRef] [Green Version]
- Caccamo, M.T.; Magazù, S. Tagging the oligomer-to-polymer crossover on EG and PEGs by infrared and Raman spectroscopies and by wavelet cross-correlation spectral analysis. Vib. Spectrosc. 2016, 85, 222–227. [Google Scholar] [CrossRef]
- Green, J.L.; Angell, C.A. Phase relations and vitrification in saccharide-water solutions and the trehalose anomaly. J. Phys. Chem. 1989, 93, 2880–2882. [Google Scholar] [CrossRef]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The role of vitrification in anhydrobiosis. Ann. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Oliver, A.E.; Tsvetkova, N.; Wolkers, W.; Tablin, F. The trehalose myth revisited: Introduction to a symposium on stabilization of cells in the dry state. Cryobiology 2001, 43, 89–105. [Google Scholar] [CrossRef]
- Donnamaria, M.C.; Howard, E.I.; Grigera, J.R. Interaction of water with α,α-trehalose in solution: Molecular dynamics simulation approach. J. Chem. Soc. Faraday Trans. 1994, 90, 2731–2735. [Google Scholar] [CrossRef]
- Ball, C.D.; Hardt, D.T.; Duddles, W.J. The influence of sugars on the formation of sulfhydryl groups in heat denaturation and heat coagulationof egg albumin. J. Biol. Chem. 1943, 151, 163–169. [Google Scholar] [CrossRef]
- Magazù, S.; Migliardo, F.; Affouard, F.; Descamps, M.; Telling, M.T.F. Study of the Relaxational and Vibrational Dynamics of Bioprotectant Glass-Forming Mixtures by Neutron Scattering and Molecular Dynamics Simulation. J. Chem. Phys. 2010, 132, 184512. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Caccamo, M.T.; Magazuù, S.; Kiselev, M.A. Colloidal stability of liposomes. AIMS Mat. Sci. 2019, 6, 200–213. [Google Scholar] [CrossRef]
- Fenimore, P.W.; Frauenfelder, H.; Magazù, S.; McMahon, B.H.; Mezei, F.; Migliardo, F.; Young, R.D.; Stroe, I. Concepts and problems in protein dynamics. Chem. Phys. 2013, 424, 2–6. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Gugliandolo, C.; Zammuto, V.; Magazù, S. Thermal properties of an exopolysaccharide produced by a marine thermotolerant Bacillus licheniformis by ATR-FTIR spectroscopy. Int. J. Biol. Macrom. 2020, 145, 77–83. [Google Scholar] [CrossRef]
- Maisano, G.; Majolino, D.; Migliardo, P.; Venuto, S.; Aliotta, F.; Magazù, S. Sound Velocity and Hydration Phenomena in Aqueous Polymeric Solutions. Mol. Phys. 1993, 78, 421. [Google Scholar] [CrossRef]
- Tsai, S.R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B 2017, 170, 197–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timchenko, S.D.; Dement’ev, V.A. Classification of IR spectra of organic compounds by the methods of multidimensional statistics. J. Struct. Chem. 1993, 34, 83–88. [Google Scholar] [CrossRef]
- Hemen, D.; Lalita, L.; Nisha, C.; Purvi, K.; Bhakti, D.; Sudhir, N. Surface Modification of Polyester Fabric by Non- Thermal Plasma Treatment and Its Effect on Coloration Using Natural Dye. J. Pol. Mat. 2013, 30, 291–304. [Google Scholar]
- Schwanninger, M.; Rodrigues, J.C.; Fackler, K.A. Review of Band Assignments in near Infrared Spectra of Wood and Wood Components. J. Near Infrared Spectrosc. 2011, 19, 287–308. [Google Scholar] [CrossRef]
- Tatulian, S.A. FTIR Analysis of Proteins and Protein-Membrane Interactions. Methods Mol. Biol. 2019, 2003, 281–325. [Google Scholar]
- Shai, Y. ATR-FTIR studies in pore forming and membrane induced fusion peptides. Biochim. Biophys Acta 2013, 1828, 2306–2313. [Google Scholar] [CrossRef] [Green Version]
- Vigano, C.; Manciu, L.; Buyse, F.; Goormaghtigh, E.; Ruysschaert, J.M. Attenuated total reflection IR spectroscopy as a tool to investigate the structure, orientation and tertiary structure changes in peptides and membrane proteins. Biopolymes 2000, 55, 373–380. [Google Scholar] [CrossRef]
- Fahmy, K. Application of ATR-FTIR spectroscopy for studies of biomolecular interactions. Recent Res. Dev. Chem. 2001, 2, 1–17. [Google Scholar]
- Wharton, C.W. Infrared spectroscopy of enzyme reaction intermediates. Nat. Prod. Rep. 2000, 17, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.; Zscherp, C. What vibrations tell us about proteins? Quart. Rev. Biophys 2002, 35, 369–430. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J. Drawing an elephant with four complex parameters. Am. J. Phys. 2010, 78, 648. [Google Scholar] [CrossRef]
- Pols, L.C.W. Real-time recognition of spoken words. IEEE Trans. Comp. 1971, 20, 972–978. [Google Scholar] [CrossRef]
- Pols, L.C.W.; Van Der Kamp, L.J.; Plomp, R. Perceptual and Physical Space of Vowel Sounds. J. Acoust. Soc. Am. 1969, 46, 458–467. [Google Scholar] [CrossRef]
- Konukoglu, E.; Glocker, B.; Criminisi, A.; Pohl, K.M. WESD—Weighted Spectral Distance for Measuring Shape Dissimilarity. IEEE Trans. Pattern Anal. Mach. Intell. 2013, 35, 2284–2297. [Google Scholar] [CrossRef] [Green Version]
- Hilda, D.; Noël, R.; Hardeberg, J. A Comprehensive Evaluation of Spectral Distance Functions and Metrics for Hyperspectral Image Processing. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 3224–3234. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, X.; Tong, A.; Wang, B.; Wang, J. An Automatic Baseline Correction Method Based on the Penalized Least Squares Method. Sensors 2020, 20, 2015. [Google Scholar] [CrossRef] [Green Version]
- Liland, K.; Almøy, T.; Bjørn-Helge, M. Optimal Choice of Baseline Correction for Multivariate Calibration of Spectra. Appl. Spectrosc. 2010, 64, 1007–1016. [Google Scholar] [CrossRef]
- Li, H.; Nozaki, T. Wavelet cross-correlation analysis and its application to a plane turbulent jet. JSME Int. J. Ser. B Fluids Therm. Eng. 1997, 40, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Grinsted, A.; Moore, J.C.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process. Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef]
- Torrence, C.; Compo, G. A Practical Guide to Wavelet Analysis. Bull. Am. Meteorol. Soc. 1998, 78, 61–78. [Google Scholar] [CrossRef] [Green Version]
- Caccamo, M.T.; Calabrò, E.; Cannuli, A.; Magazù, S. Wavelet Study of Meteorological Data Collected by Arduino-Weather Station: Impact on Solar Energy Collection Technology. In MATEC Web of Conferences; EDP Sciences: Ulis, France, 2016; Volume 55, p. 02004. [Google Scholar]
- Ramsey, J.B. The Contribution of Wavelets to the Analysis of Economic and Financial Data. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 1999, 357, 2593–2606. [Google Scholar] [CrossRef]
- Georgieva, V.; Plamen, P.; Zlatareva, D. Medical image processing based on multidimensional wavelet transforms—Advantages and trends. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2021; Volume 2333, p. 020001. [Google Scholar] [CrossRef]
- Addison, P.S. Wavelet transforms and the ECG: A review. Physiolog. Meas. 2005, 26, R155–R199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bnou, K.; Raghay, S.; Hakim, A. A wavelet denoising approach based on unsupervised learning model. EURASIP J. Adv. Signal Process. 2020, 2020, 36. [Google Scholar] [CrossRef]
- Giuffrida, S.; Cottone, G.; Librizzi, F.; Cordone, L. Coupling between the thermal evolution of the heme pocket and the external matrix structure in trehalose coated carboxymyoglobin. J. Phys. Chem. B 2003, 107, 13211–13217. [Google Scholar] [CrossRef]
- Rieppo, L.; Saarakkala, S.; Närhi, T.; Helminen, H.J.; Jurvelin, J.S.; Rieppo, J. Application of second derivative spectroscopy for increasing molecular specificity of Fourier transform infrared spectroscopic imaging of articular cartilage. Osteoart. Cartil. 2012, 20, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Fahelelbom, K.M.S.; Saleh, A.; Mansour, R.; Sayed, S. First derivative ATR-FTIR spectroscopic method as a green tool for the quantitative determination of diclofenac sodium tablets. F1000Research 2020, 9, 176. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caccamo, M.T.; Magazù, S. Experimental Investigation on the Bioprotective Role of Trehalose on Glutamine Solutions by Infrared Spectroscopy. Materials 2022, 15, 4329. https://doi.org/10.3390/ma15124329
Caccamo MT, Magazù S. Experimental Investigation on the Bioprotective Role of Trehalose on Glutamine Solutions by Infrared Spectroscopy. Materials. 2022; 15(12):4329. https://doi.org/10.3390/ma15124329
Chicago/Turabian StyleCaccamo, Maria Teresa, and Salvatore Magazù. 2022. "Experimental Investigation on the Bioprotective Role of Trehalose on Glutamine Solutions by Infrared Spectroscopy" Materials 15, no. 12: 4329. https://doi.org/10.3390/ma15124329
APA StyleCaccamo, M. T., & Magazù, S. (2022). Experimental Investigation on the Bioprotective Role of Trehalose on Glutamine Solutions by Infrared Spectroscopy. Materials, 15(12), 4329. https://doi.org/10.3390/ma15124329






