Preparation and Properties of Magnesium Phosphate Cement-Based Fire Retardant Coating for Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
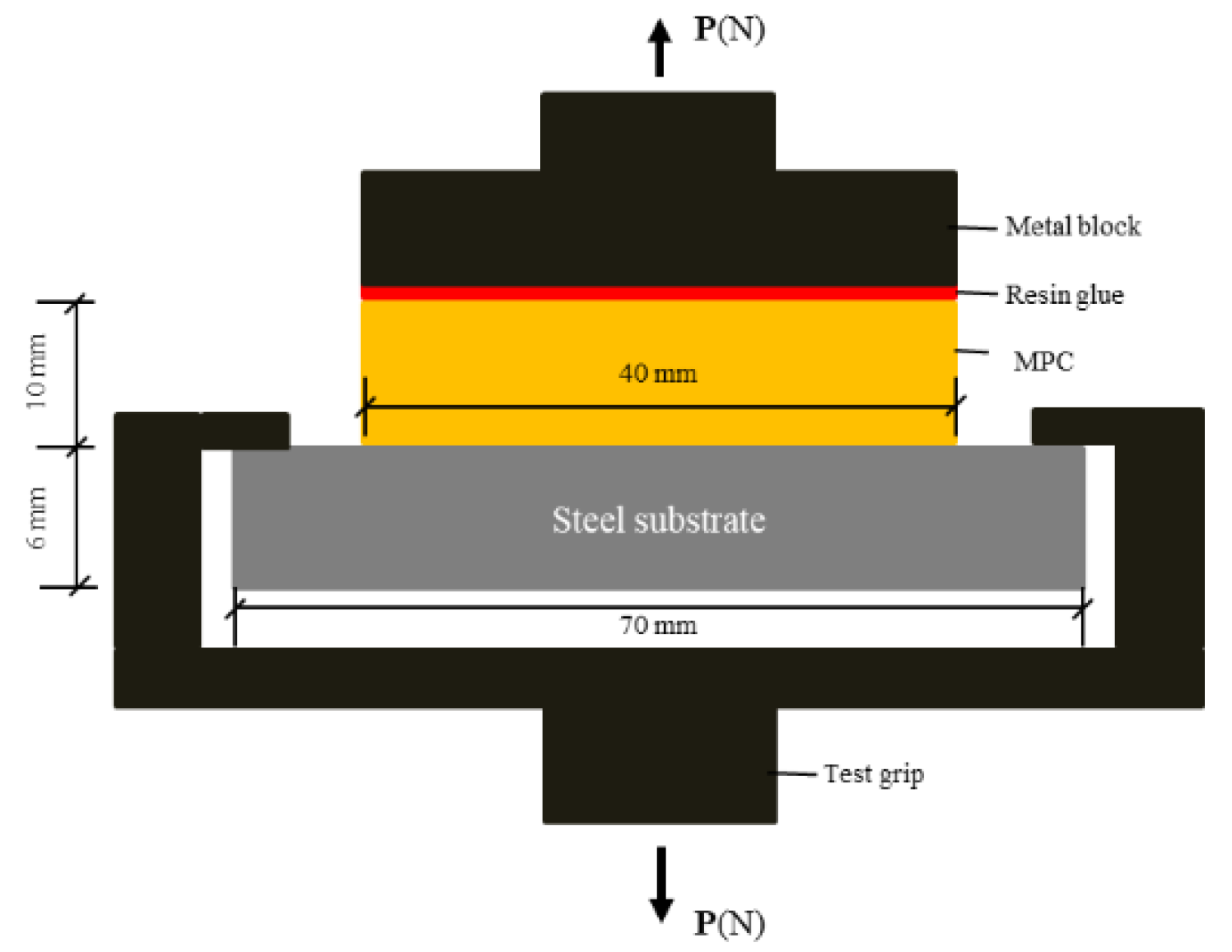
2.2. Sample Preparation
2.3. Test Methods
2.3.1. Fire Test
2.3.2. Physical-Mechanical Properties of the Coating
2.3.3. X-ray Diffraction
2.3.4. Scanning Electron Microscopy
2.3.5. Electrochemical Measurement
3. Results and Discussion
3.1. Basic Properties of MPC Paste Coating
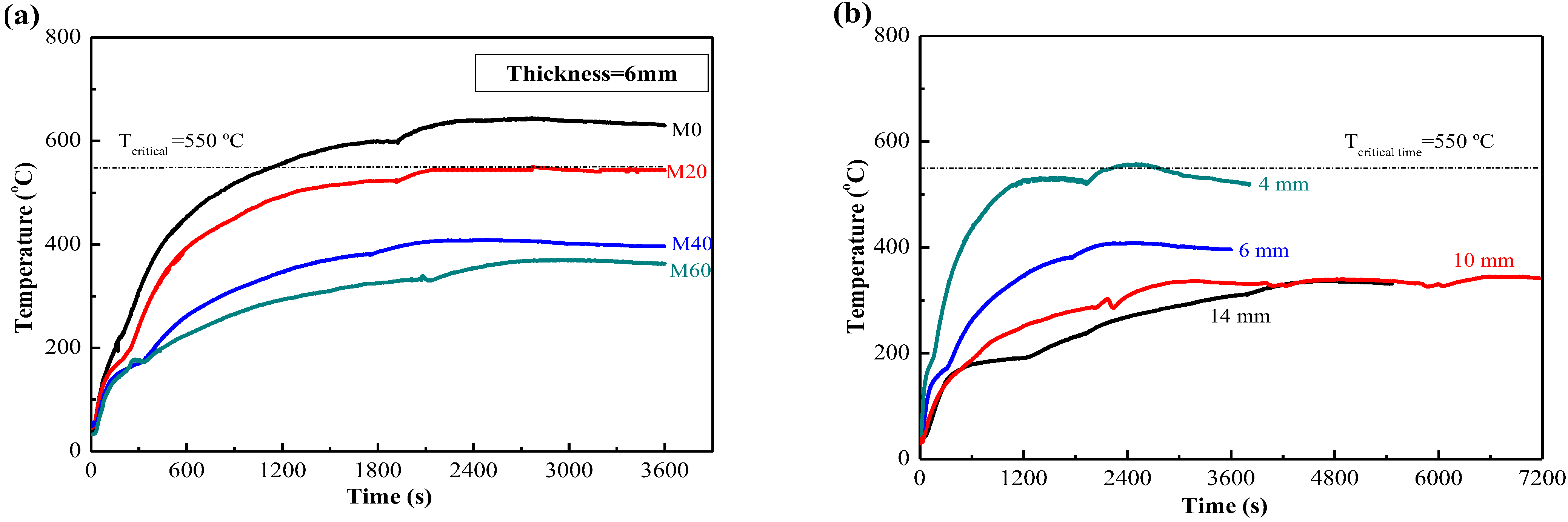
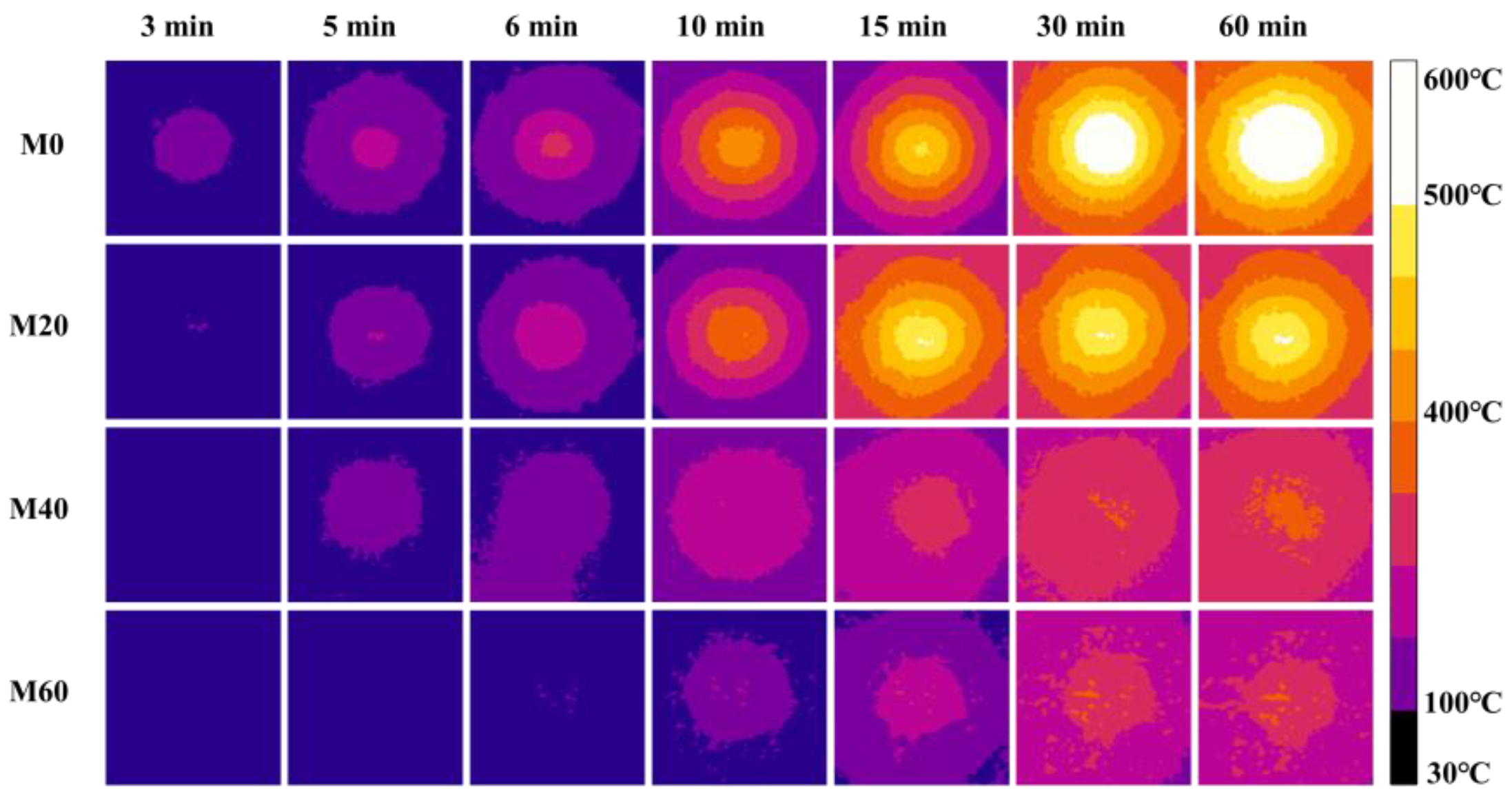
3.2. Fireproof Performance of MPC Paste Coating
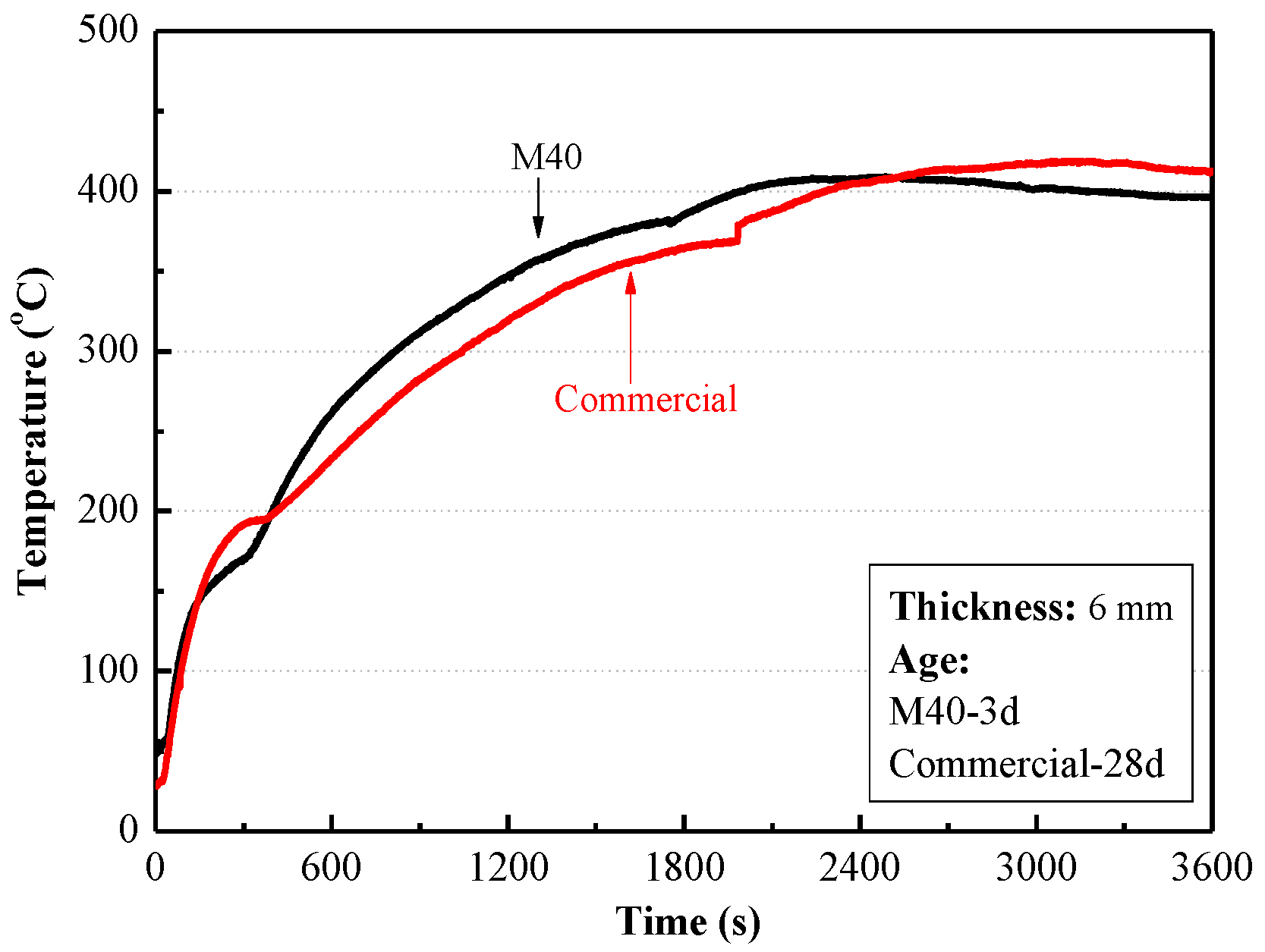
3.3. Fireproof Performance of MPC Mortar Coating with EV
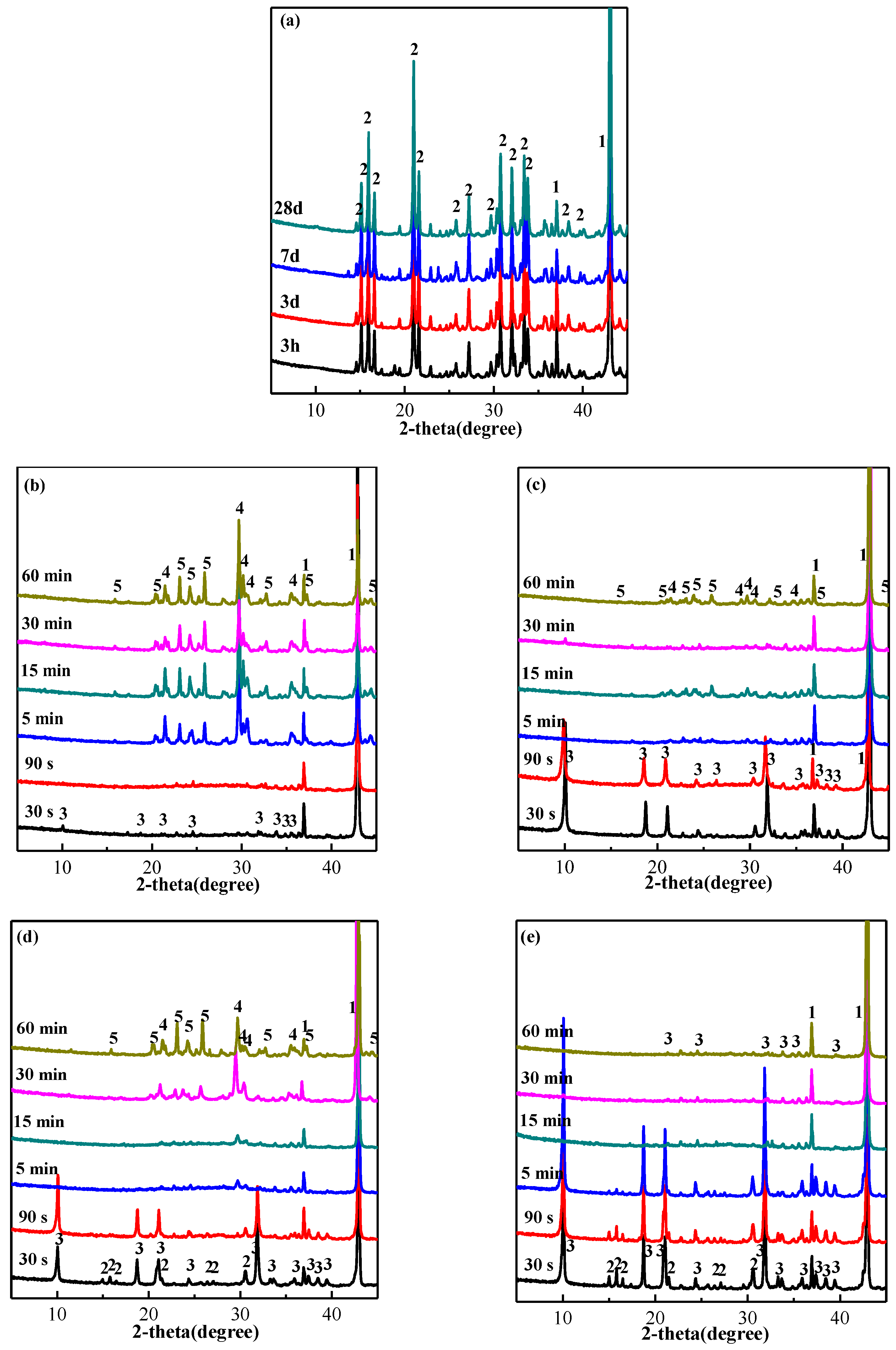
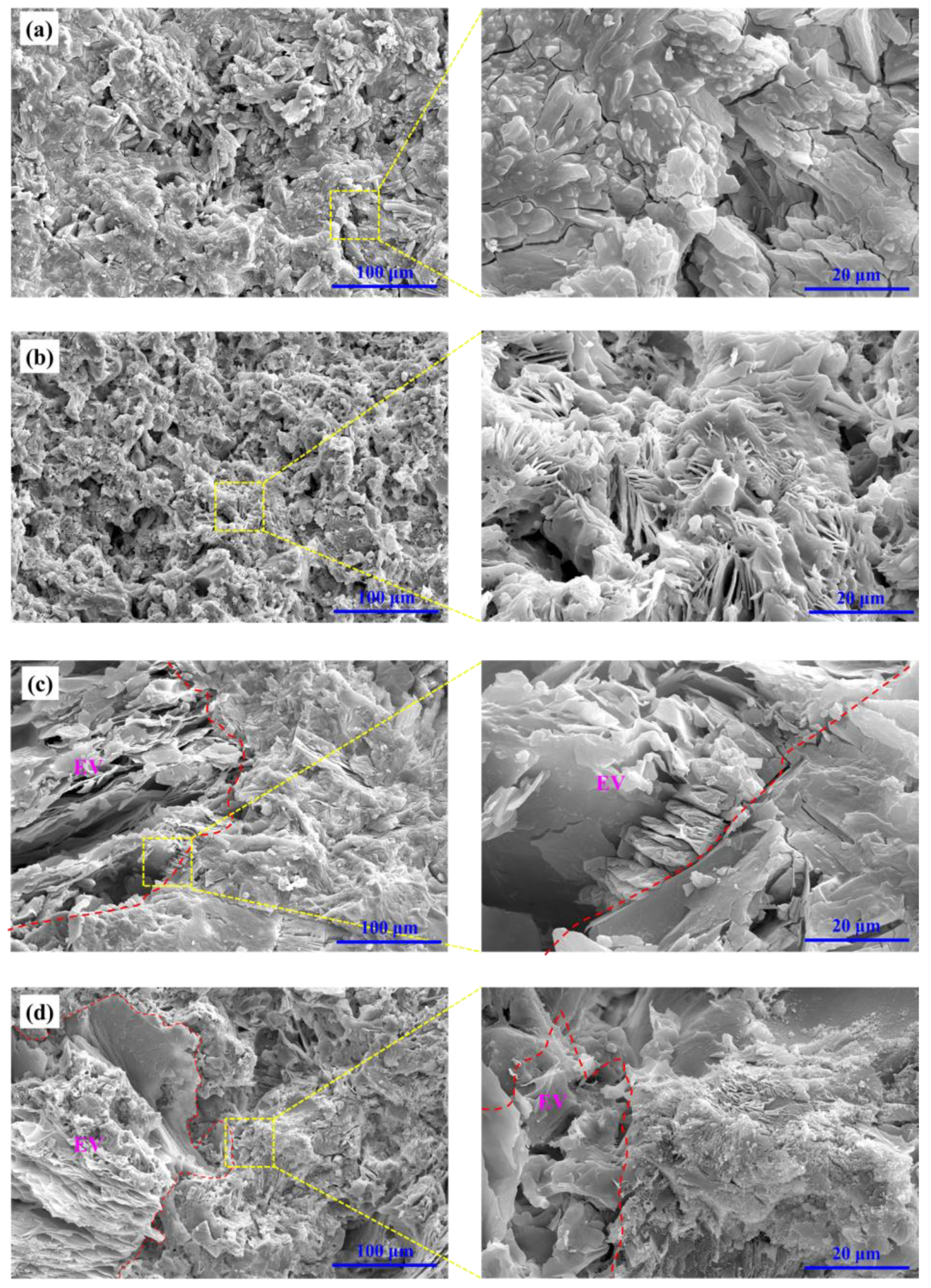
3.4. Phase Evolution and Microstructure Change of MPC Coating after Fire Test
3.5. Discussion
4. Conclusions
- (1)
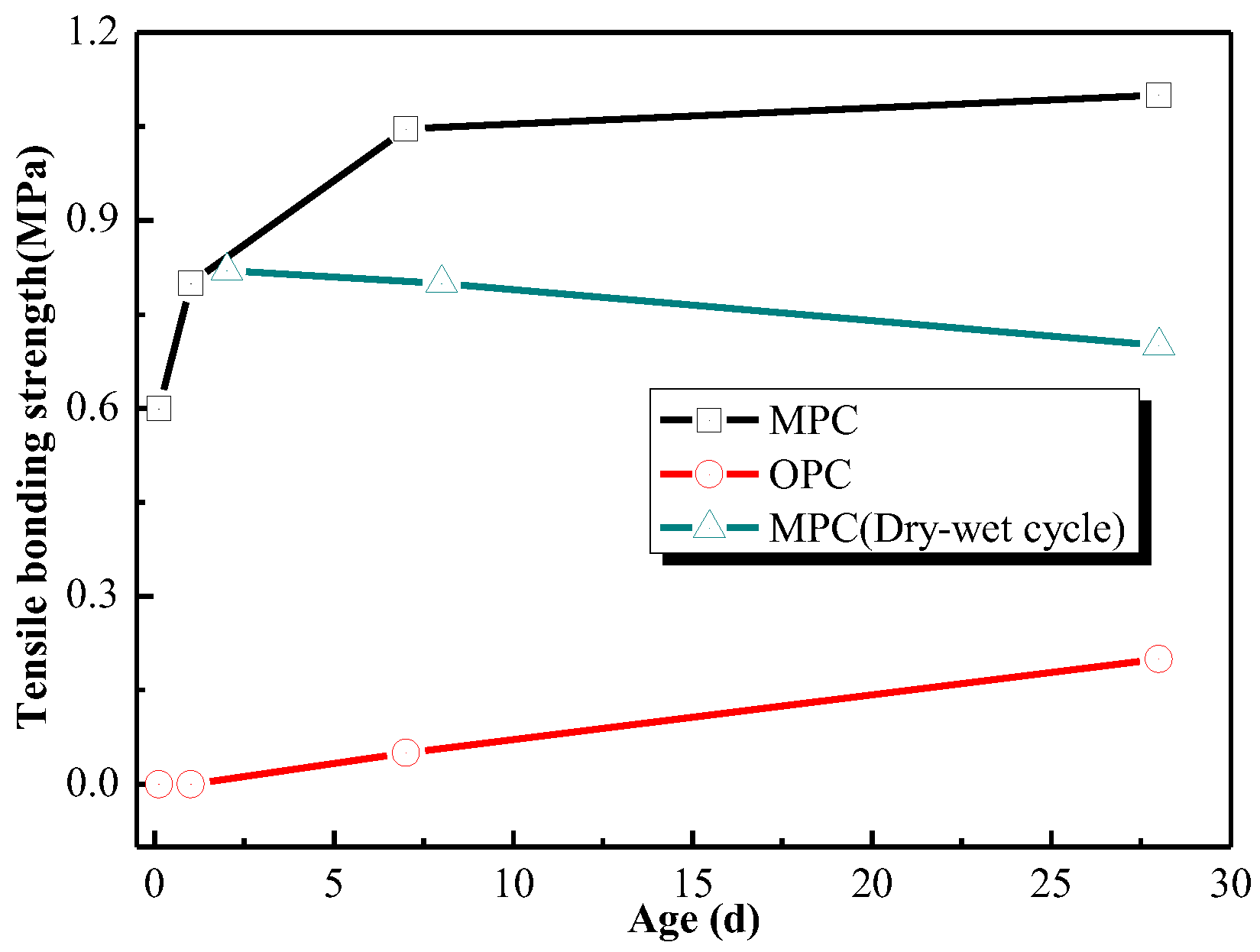
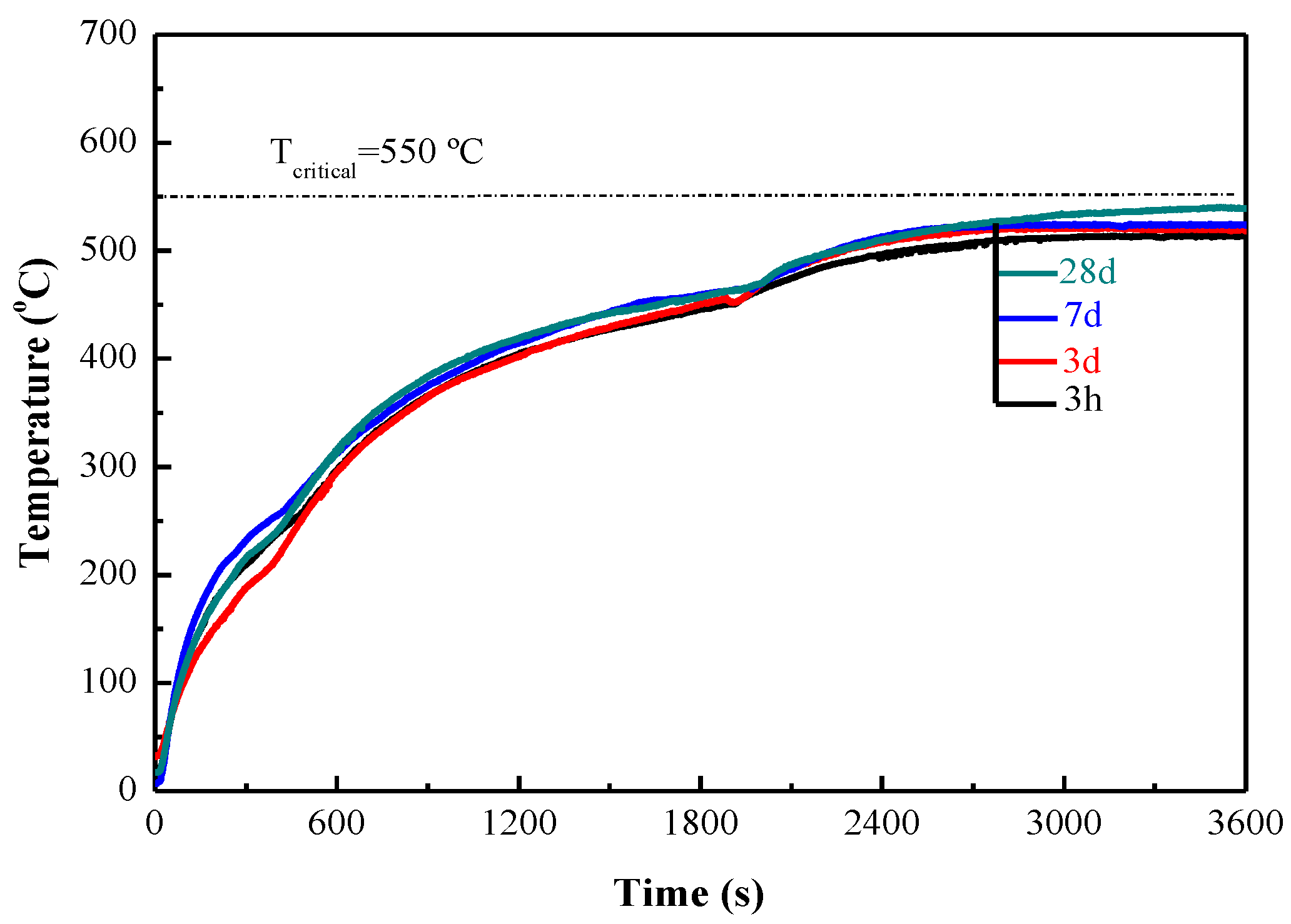
- NH4H2PO4-based MPC can be used as the binder of inorganic fire-retardant coating for steel. The surface drying time of coating was less than 30 min and the fireproof performance of coating took effect as early as 3 h. The tensile bonding strength between MPC coating and steel was 0.6 MPa at 3 h and 1.10 MPa at 28 days, far higher than that required by Chinese standard. It illustrated that the MPC coating not only set rapidly, but also bound tightly to steel.
- (2)
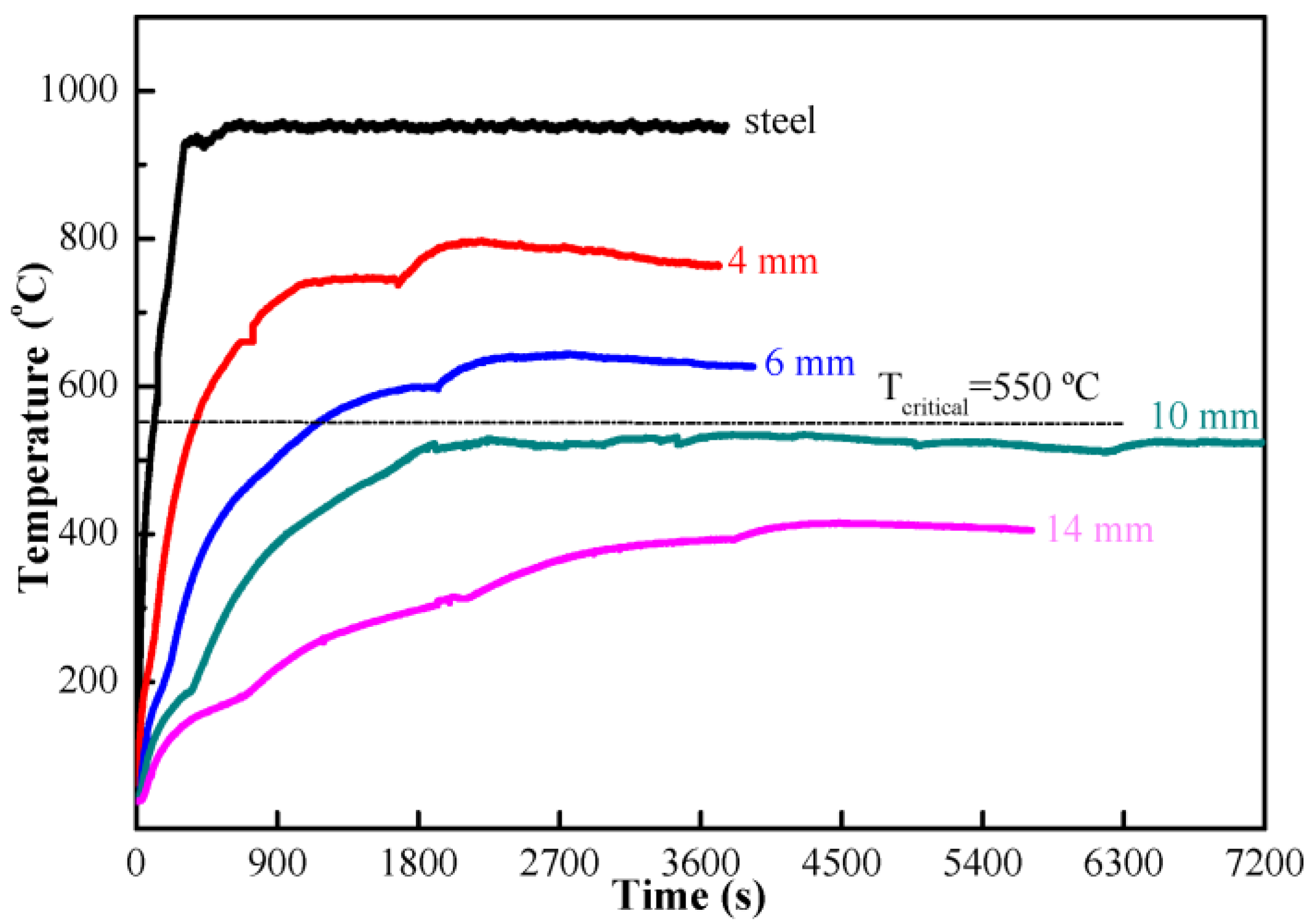
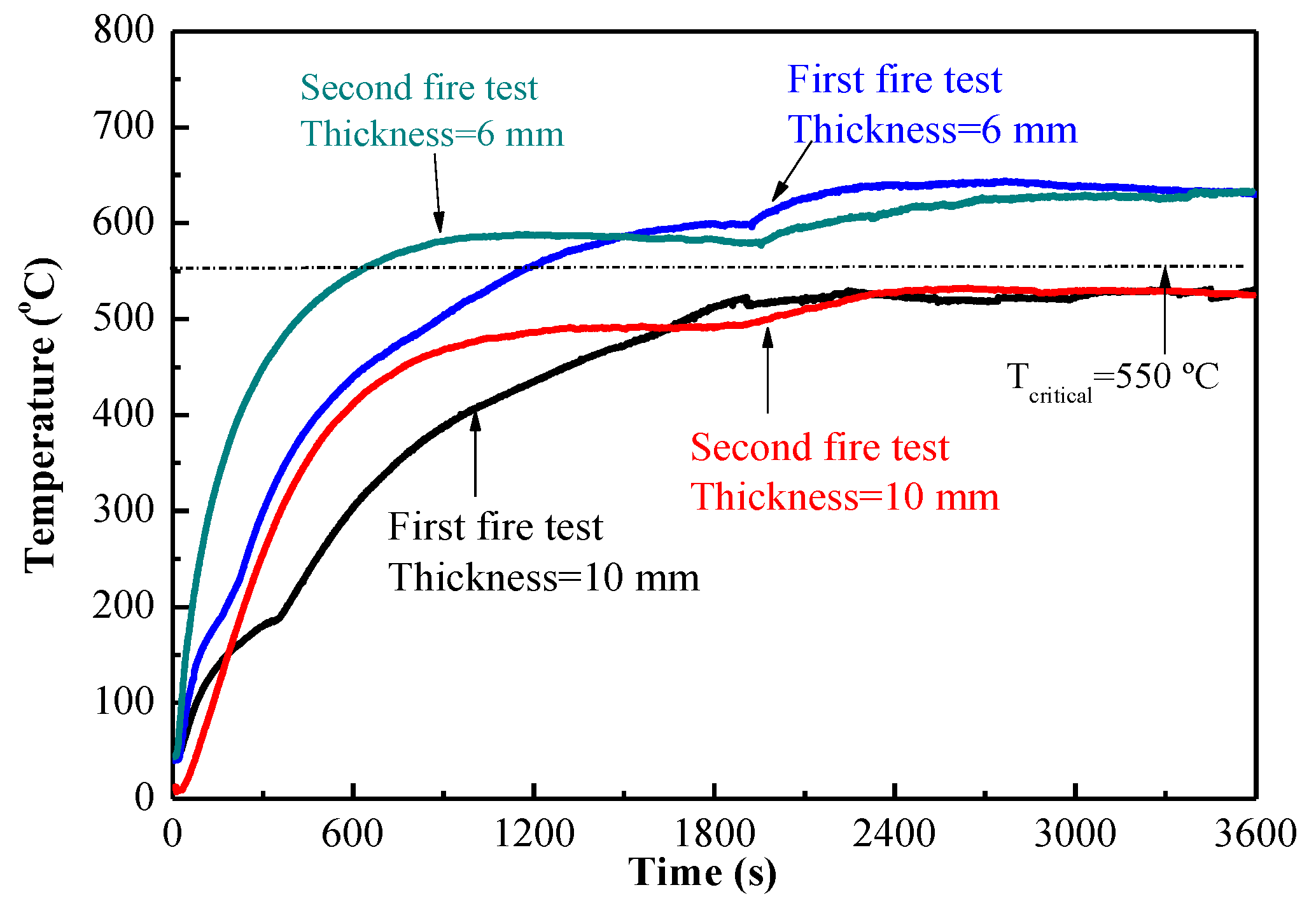
- The fireproof performance of MPC paste coating met the requirement, the maximum temperature being less than 550 °C after firing for 2 h, as long as the thickness was up to 10 mm, while the thickness of MPC mortar coating decreased to 4 mm when adding 40% expanded vermiculite (EV) as a lightweight aggregate. MPC coating with and without lightweight aggregate can be used in different conditions.
- (3)
- Dehydration and decomposition of the reacted products in MPC coating during the fire test are good for the fireproof performance of MPC coatings. The slight ceramic formation of MPC coating during the fire test would compensate for the decrease of strength and shrinkage of volume. The fireproof performance of MPC coating in the second fire test was similar to that in the first fire test. MPC coating may be even potential to use for permanent fireproof conditions.
- (4)
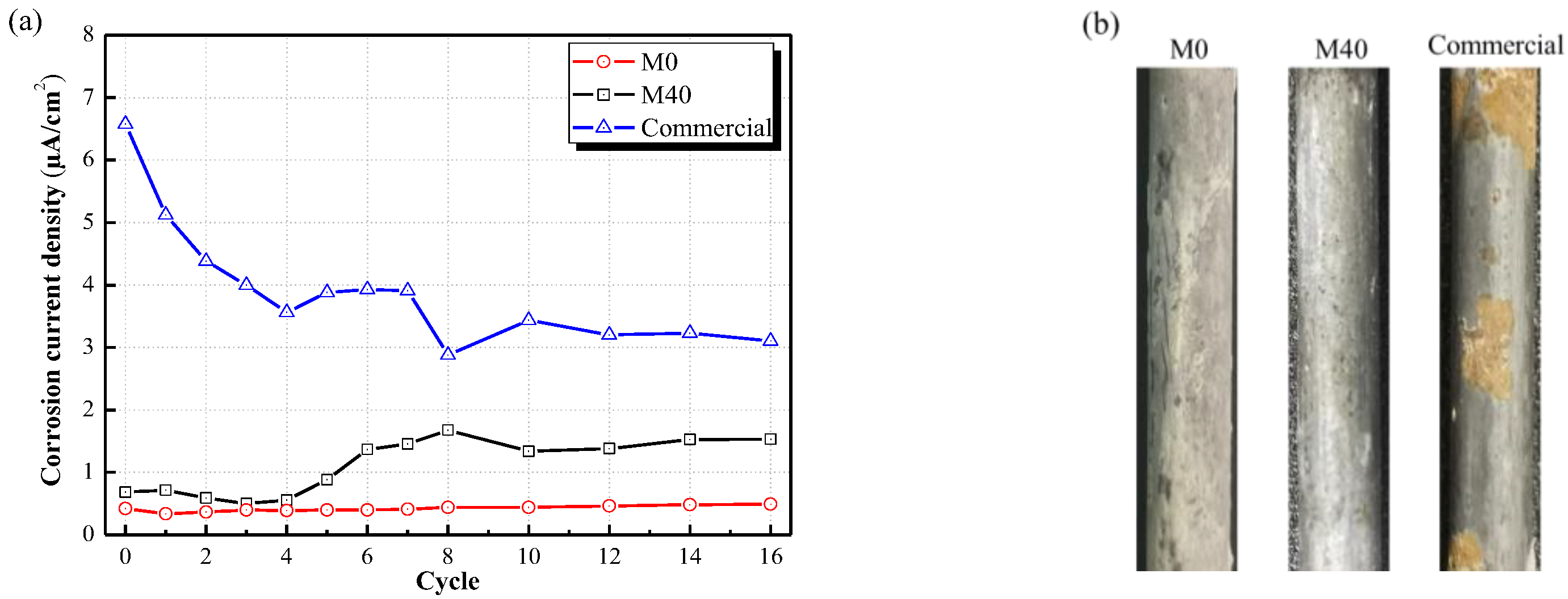
- Considering the comprehensive performance but not the only fireproof performance of MPC coatings, including high tensile bonding strength with steel, rapid hardening, excellent corrosion resistance to steel and permanent fireproof performance, MPC coating for steel exhibits more advantages. Further research or improvement on this coating is necessary.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ISO-834; Fire Resistance Tests—Elements of Building Construction. International Organization for Standardization: Geneva, Switzerland, 1999.
- Piquer, A.; Hernandez-Figueirido, D. Protected steel columns vs partially encased columns: Fire resistance and economic considerations. J. Constr. Steel. Res. 2016, 124, 47–56. [Google Scholar] [CrossRef]
- Li, G.Q.; Wang, P.J. Advanced Analysis and Design for Fire Safety of Steel Structures; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Skowrónski, W. Fire Safety of Metal Structures: Theory and Design Criteria; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2001. [Google Scholar]
- Puri, R.G.; Khanna, A.S. Intumescent coatings: A review on recent progress. J. Coat. Technol. Res. 2017, 14, 1–20. [Google Scholar] [CrossRef]
- Lucherini, A.; Maluk, C. Intumescent coatings used for the fire-safe design of steel structures: A review. J. Constr. Steel. Res. 2019, 162, 105712. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, H. A novel blowing agent polyelectrolyte for fabricating intumescent multilayer coating that retards fire on cotton fabric. J. Appl. Polym. Sci. 2018, 135, 46583. [Google Scholar] [CrossRef]
- Carabba, L. Alkali activated lightweight mortars for passive fire protection: A preliminary study. Constr. Build. Mater. 2019, 195, 75–84. [Google Scholar] [CrossRef]
- Well, E.; Mc Swigan, B. Melamine Phosphates (MP) and Polyphosphate in Flame-retardant Coatings: Old Products with New Potential. J. Coat. Technol. Res. 1994, 66, 75–82. [Google Scholar]
- Clive, A. Developments in intumescent coating. Polym. Paint Color J. 1997, 187, 16. [Google Scholar]
- Ding, Z.; Dong, B.; Xing, F.; Han, N.; Li, Z. Cementing mechanism of potassium phosphate based magnesium phosphate cement. J. Ceram. Int. 2012, 38, 6281–6288. [Google Scholar] [CrossRef]
- Montle, J.F.; Mayhan, K.G. The role of magnesium oxychloride as a fire-resistive material. Fire Technol. 1974, 10, 201–210. [Google Scholar] [CrossRef]
- Rashad, A.M. Vermiculite as a construction material—A short guide for Civil Engineer. Constr. Build. Mater. 2016, 125, 53–62. [Google Scholar] [CrossRef]
- Qin, J.H.; Qian, J.S.; Dai, X.B.; You, C.; Ma, H.Y.; Li, Z. Effect of water content on microstructure and properties of magnesium potassium phosphate cement pastes with different magnesia-to-phosphate ratios. J. Am. Ceram. Soc. 2021, 104, 2799–2819. [Google Scholar] [CrossRef]
- Wang, A.J.; Zhang, J.; Li, J.M.; Ma, A.B.; Liu, L.T. Effect of liquid-to-solid ratios on the properties of magnesium phosphate chemically bonded ceramics. J. Mater. Sci. Eng. C 2013, 33, 2508–2512. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.H.; Qian, J.S.; Li, Z.; You, C.; Dai, X.B. Mechanical properties of basalt fiber reinforced magnesium phosphate cement composites. Constr. Build. Mater. 2018, 188, 946–955. [Google Scholar] [CrossRef]
- You, C.; Qian, J.S.; Qin, J.H.; Wang, H.T. Effect of early hydration temperature on hydration product and strength development of magnesium phosphate cement (MPC). Cem. Concr. Res. 2015, 78, 179–189. [Google Scholar] [CrossRef]
- Wang, A.J.; Yuan, Z.L.; Zhang, J.; Liu, L.T.; Li, J.M.; Liu, Z. Effect of raw material ratios on the compressive strength of magnesium potassium phosphate chemically bonded ceramics. J. Mater. Sci. Eng. C 2013, 33, 5058. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.H.; Qian, J.S.; You, C.; Fan, Y.R. Bond behavior and interfacial micro-characteristics of magnesium phosphate cement onto old concrete substrate. Constr. Build. Mater. 2018, 167, 166–176. [Google Scholar] [CrossRef]
- Gao, X.J.; Zhang, A.L.; Li, S.X.; Sun, B.C.; Zhang, L.C. The resistance to high temperature of magnesia phosphate cement paste containing wollastonite. Mater. Struct. 2016, 49, 3423–3434. [Google Scholar] [CrossRef]
- Li, Z.H. Fire resistance of magnesium phosphate cement. J. Food Agric. Environ. 2013, 11, 2542–2546. [Google Scholar]
- Gardner, L.J.; Lejeune, V.; Corkhill, C.L.; Bernal, S.A.; Provis, J.L.; Stennett, M.C.; Hyatt, N.C. Evolution of phase assemblage of blended magnesium potassium phosphate cement binders at 200 °C and 1000 °C. J. Adv. Appl. Ceram. 2015, 114, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shi, T.F.; Chen, B.; Li, Y.Q. Performance of magnesium phosphate cement at elevated temperatures. Constr. Build. Mater. 2015, 91, 126–132. [Google Scholar] [CrossRef]
- Tang, H.; Qian, J.S.; Ji, Z.W.; Dai, X.B. The protective effect of magnesium phosphate cement on steel corrosion. Constr. Build. Mater. 2020, 255, 119422. [Google Scholar] [CrossRef]
- Wagh, A.S. Chemically Bonded Phosphate Ceramic Coatings, Chemically Bonded Phosphate Ceramics, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 203–231. [Google Scholar]
- Torrico, D.D.; Jirangrat, W. A novel emulsion coating and its effects on internal quality and shelf life of eggs during room temperature storage. Int. J. Food Sci. Tech. 2010, 45, 2241–2249. [Google Scholar] [CrossRef]
- GB/T 700-2006; Carbon Structure Steels. Standards Press of China: Beijing, China, 2006.
- ASTM C191-13; Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2018.
- GB/T 13477.5-2002; Sealing Materials for Building Test Methods. Part 5: Determination of Surface Drying Time. Standards Press of China: Beijing, China, 2002.
- GB/T 24586-2009; Determination of Apparent Density, True Density and Porosity of Iron Ore. Standards Press of China: Standards Press of China, 2009.
- GB 14907-2018; Fire Resistive Coating for Steel Structure. Standards Press of China: Beijing, China, 2018.
- Yang, H.L.; Fu, M.J.; Qian, J.S. Effect of Fe2O3 on the Immobilization of High-Level Waste with Magnesium Potassium Phosphate Ceramic. Sci. Technol. Nucl. Ins. 2019, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]


| Composition | Magnesia (MgO, M) | 62.5% (wt) |
| Ammonium dihydrogen phosphate (NH4H2PO4, P) | 31.3% (wt) | |
| Borax (Na2B4O7·10H2O, B) | 6.2% (wt) | |
| Water-cement ratio (W/C) | 0.20 | |
| Setting time (min) | 26.0 | |
| 3 h compressive strength (MPa) | 25.2 | |
| 1 day compressive strength (MPa) | 60.9 | |
| 7 days compressive strength (MPa) | 64.5 | |
| 7 days flexural strength (MPa) | 7.2 | |
| Age | Compressive Strength (MPa) | Tensile Bonding Strength (MPa) | Surface Drying Time (min) | Bulk Density (g/cm3) | Thermal Conductivity (W/(m·K) | |
|---|---|---|---|---|---|---|
| Commercial | 28 days | 0.4 | 0.06 | 750 | 0.71 | 0.15 |
| MPC mortar with 40% EV | 3 h | 1.1 | 0.11 | 25.5 | 0.79 | 0.26 |
| 28 days | 2.6 | 0.24 |
| Type of Coating | MPC Paste Coating | MPC Mortar Coating with EV (mass) | Commercial Coating | ||
|---|---|---|---|---|---|
| 20% EV | 40% EV | 60% EV | |||
| Surface drying time (min) | 23.3 | 28.1 | 25.5 | 26.9 | 750 |
| Thermal conductivity (W/(m·K)) | 2.11 | 0.41 | 0.26 | 0.18 | 0.15 |
| Bulk density (g/cm3) (Dry) | 2.16 | 1.42 | 0.78 | 0.61 | 0.71 |
| 3 h Compressive strength (MPa) | 25.2 | 2.10 | 1.10 | 0.63 | n.d. |
| 3 h Tensile bonding strength (MPa) | 0.60 | 0.42 | 0.11 | 0.06 | n.d. |
| 28 d Compressive strength (MPa) | 67.2 | 5.62 | 2.40 | 1.30 | 0.4 |
| 28 d Tensile bonding strength (MPa) | 1.11 | 0.82 | 0.24 | 0.12 | 0.08 |
| Minimum thickness of coating (mm) | 10 | 6 | 4 | <4 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; Qian, J.; Qin, J.; Yue, Y.; Zhao, Y.; Jia, X. Preparation and Properties of Magnesium Phosphate Cement-Based Fire Retardant Coating for Steel. Materials 2022, 15, 4134. https://doi.org/10.3390/ma15124134
Dai X, Qian J, Qin J, Yue Y, Zhao Y, Jia X. Preparation and Properties of Magnesium Phosphate Cement-Based Fire Retardant Coating for Steel. Materials. 2022; 15(12):4134. https://doi.org/10.3390/ma15124134
Chicago/Turabian StyleDai, Xiaobing, Jueshi Qian, Jihui Qin, Yanfei Yue, Yushan Zhao, and Xingwen Jia. 2022. "Preparation and Properties of Magnesium Phosphate Cement-Based Fire Retardant Coating for Steel" Materials 15, no. 12: 4134. https://doi.org/10.3390/ma15124134
APA StyleDai, X., Qian, J., Qin, J., Yue, Y., Zhao, Y., & Jia, X. (2022). Preparation and Properties of Magnesium Phosphate Cement-Based Fire Retardant Coating for Steel. Materials, 15(12), 4134. https://doi.org/10.3390/ma15124134







