Role of Cr Doping on the Structure, Electronic Structure, and Electrochemical Properties of BiFeO3 Nanoparticles
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Method
2.3. Sample Characterizations
3. Results and Discussion
3.1. XRD Analysis
3.2. Morphological Analysis
3.3. UV–Vis Spectroscopy
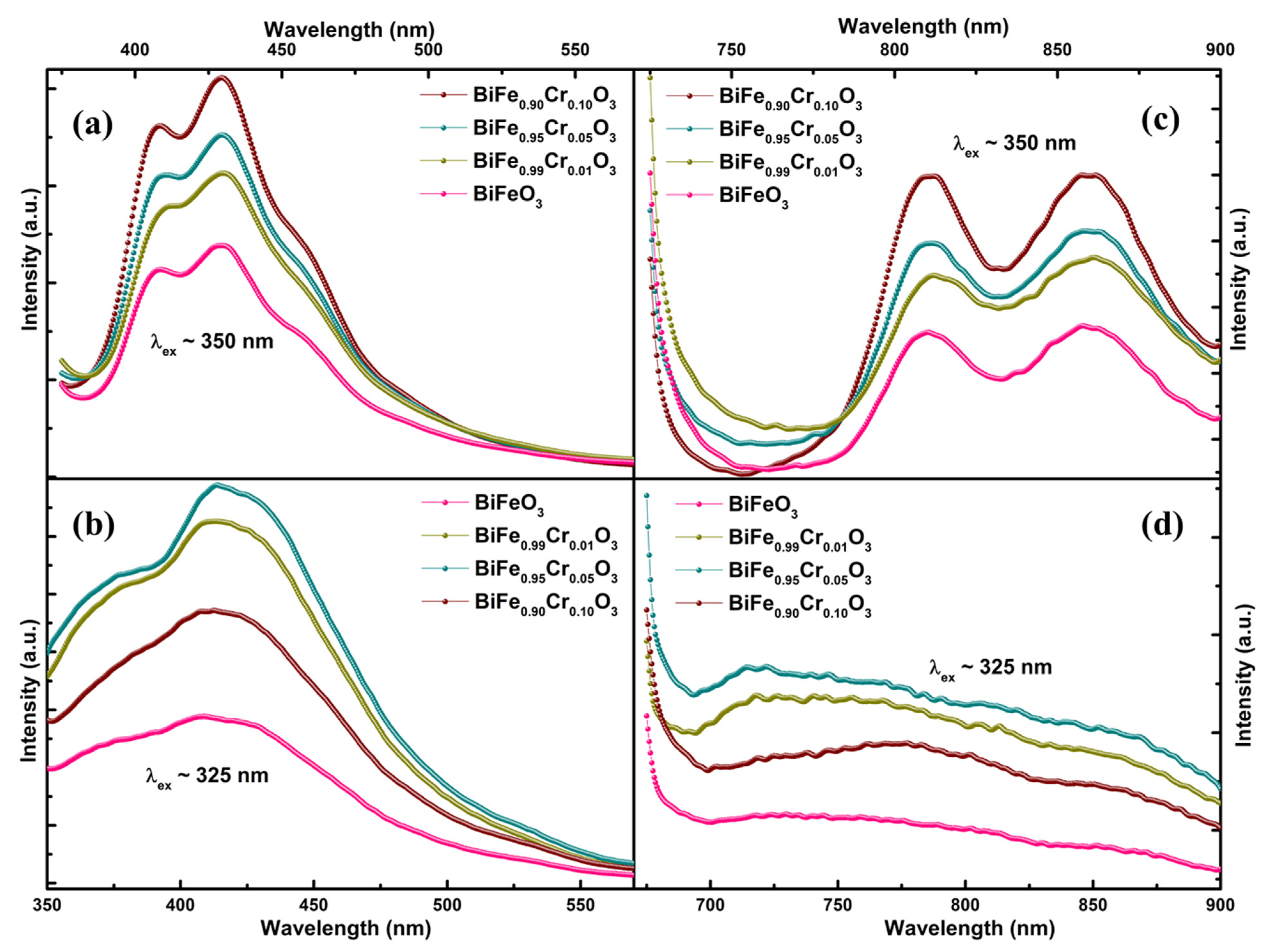
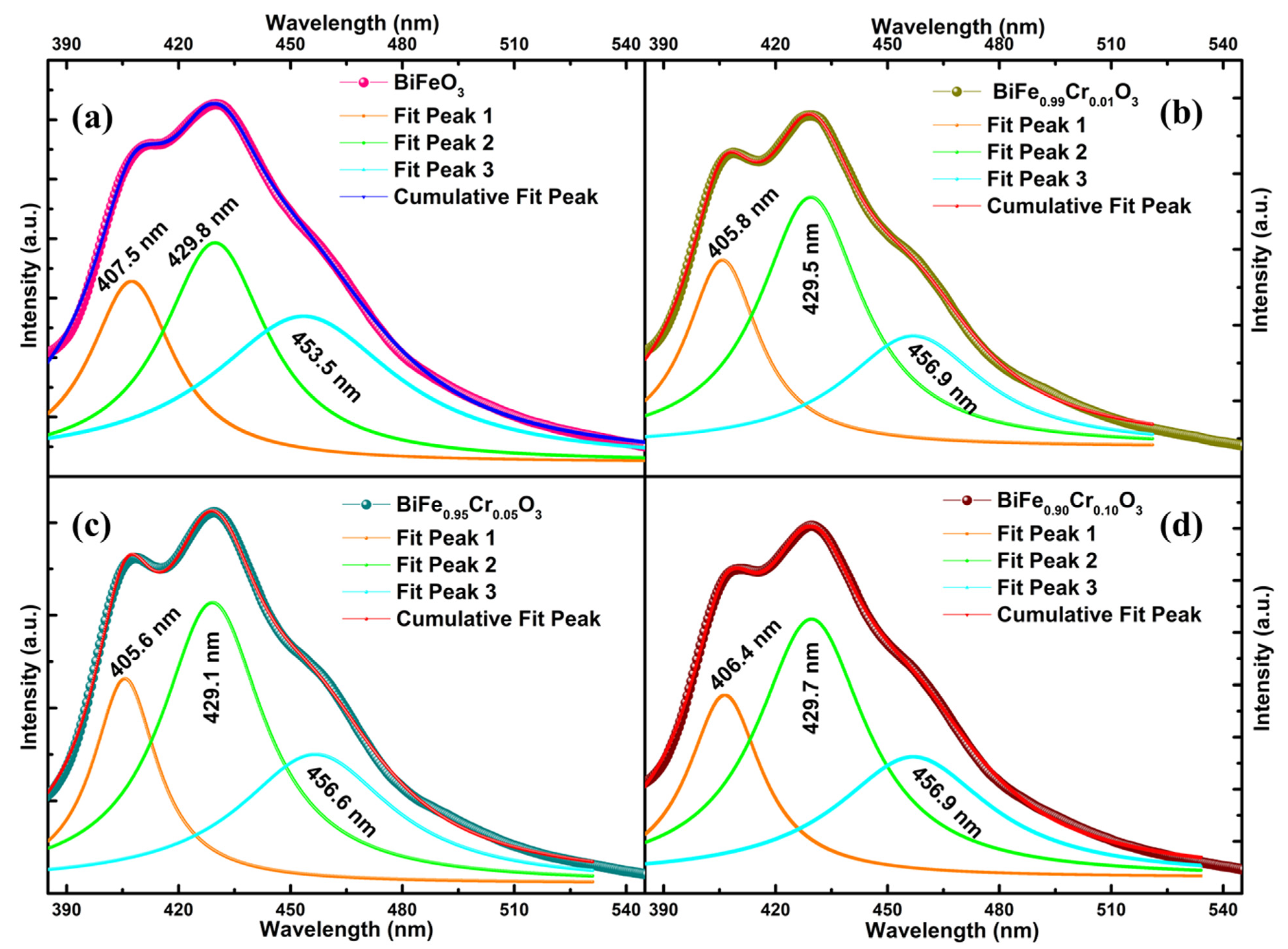
3.4. Photoluminescence (PL) Spectroscopy
3.5. Magnetization Analysis
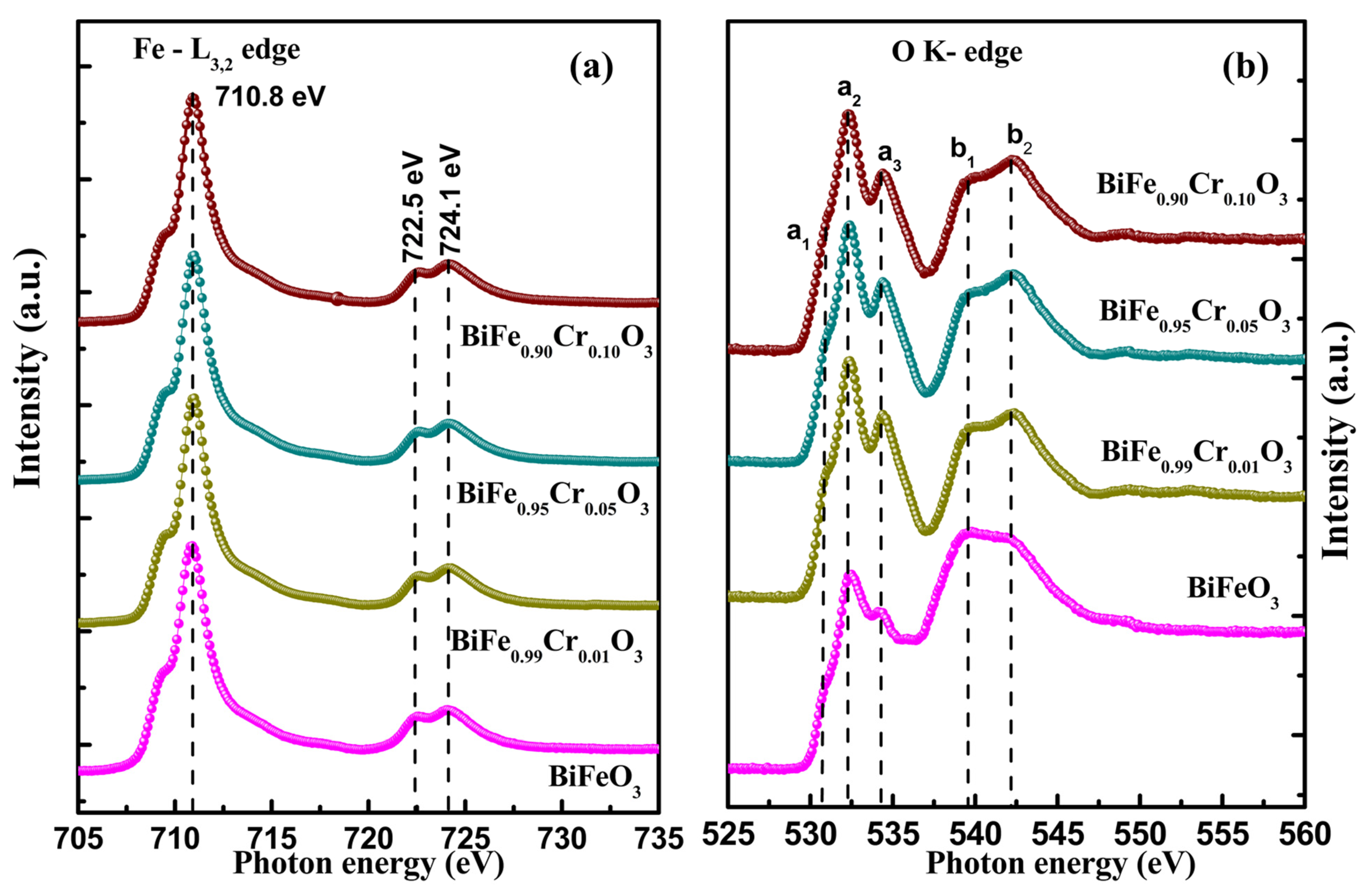
3.6. Near Edge X-Ray Absorption Spectroscopy
3.6.1. Fe L3,2 Edge
3.6.2. O K Edge
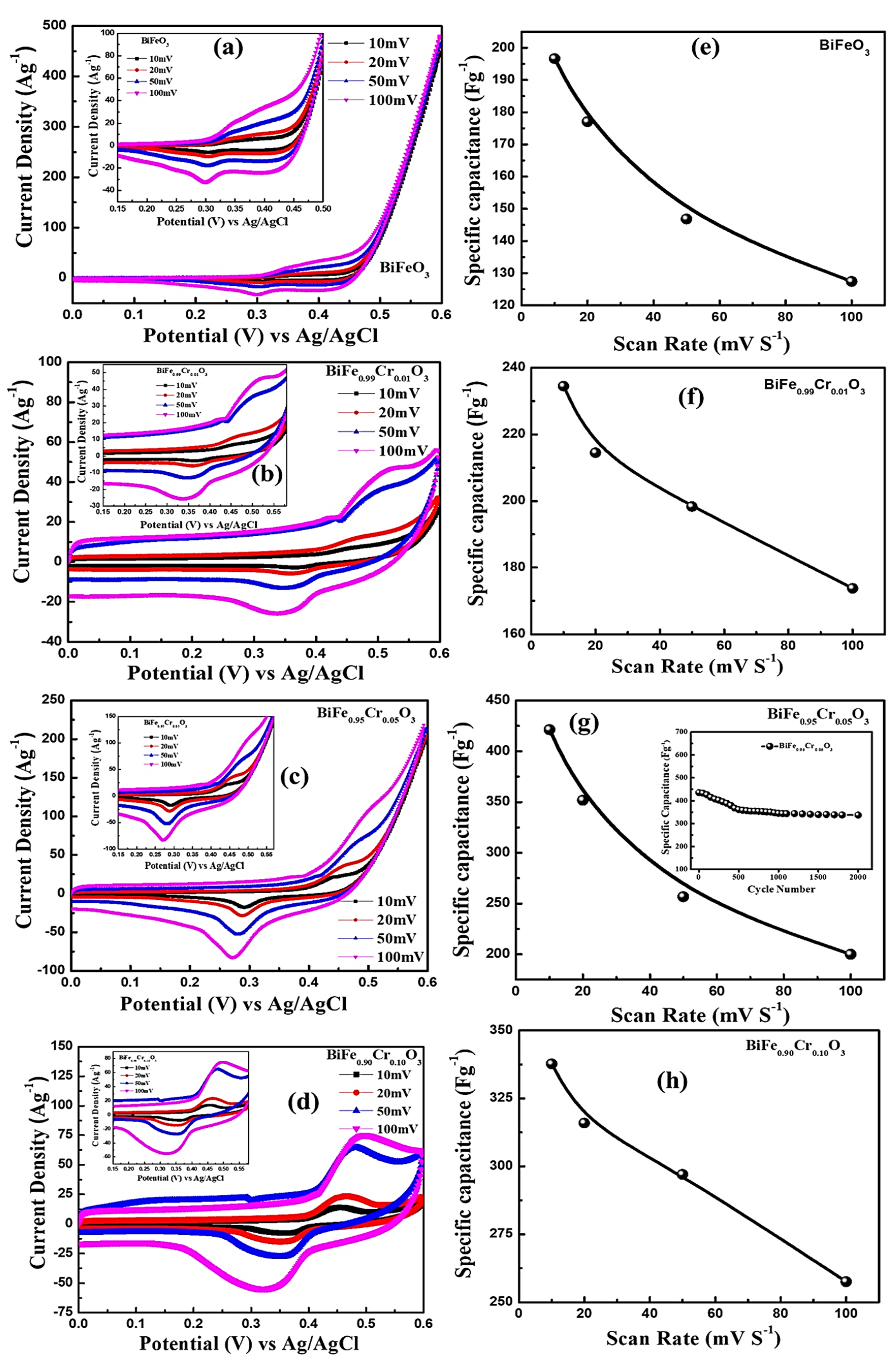
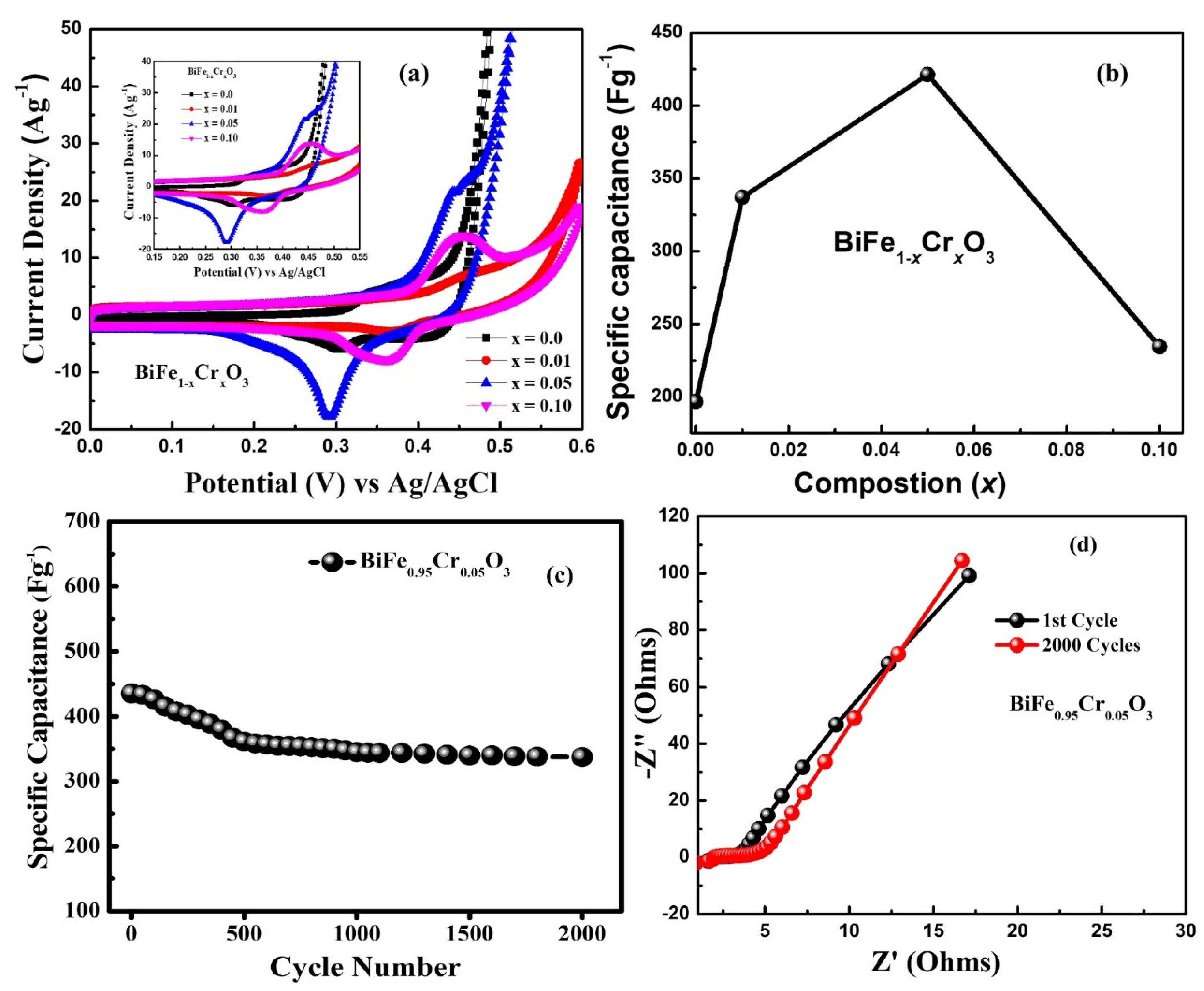
3.7. Electrochemical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haromae, H.; Pattananuwat, P. Preparation of bismuth ferrite as photo-supercapacitive electrode. IOP Conf. Ser. Mater. Sci. Eng. 2019, 600, 012005. [Google Scholar] [CrossRef]
- Lokhande, C.; Gujar, T.; Shinde, V.; Mane, R.; Han, S.-H. Electrochemical supercapacitor application of pervoskite thin films. Electrochem. Commun. 2007, 9, 1805–1809. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, G.; Almutairi, G.; Ahmed, F.; Shaalan, N.M.; Dalela, S.; Kumar, R.; Kumar, A.P.; Alvi, P.; Chae, K.; et al. Electronic structure and electrochemical properties of La-doped BiFeO3 nanoparticles. J. Electron. Spectrosc. Relat. Phenom. 2021, 253, 147138. [Google Scholar] [CrossRef]
- Kumar, V.; Soam, A.; Sahoo, P.K.; Panda, H.S. Enhancement of electrochemical properties of carbon solution doped bismuth ferrite for supercapacitor application. Mater. Today Proc. 2020, 41, 165–171. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Ray, S.S. Performance of bismuth-based materials for supercapacitor applications: A review. Mater. Today Commun. 2020, 25, 101691. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.; Li, Y.; Wang, H.; Mousavi, M.F.; Kaner, R.B. Graphene-based materials for flexible supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665. [Google Scholar] [CrossRef]
- Jadhav, V.V.; Zate, M.K.; Liu, S.; Naushad, M.; Mane, R.S.; Hui, K.N.; Han, S.-H. Mixed-phase bismuth ferrite nanoflake electrodes for supercapacitor application. Appl. Nanosci. 2015, 6, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Durai, L.; Moorthy, B.; Thomas, C.I.; Kim, D.K.; Bharathi, K.K. Electrochemical properties of BiFeO 3 nanoparticles: Anode material for sodium-ion battery application. Mater. Sci. Semicond. Process. 2017, 68, 165–171. [Google Scholar] [CrossRef]
- Shameem, A.; Devendran, P.; Siva, V.; Murugan, A.; Sasikumar, S.; Nallamuthu, N.; Hussain, S.; Bahadur, S.A. Robust one-step synthesis of bismuth molybdate nanocomposites: A promising negative electrode for high end ultracapacitors. Solid State Sci. 2020, 106, 106303. [Google Scholar] [CrossRef]
- Ma, C.-J.; Chen, Y.; Zhu, C.; Chen, Q.; Song, W.-L.; Jiao, S.; Chen, H.-S.; Fang, D. Bismuth ferrite: An abnormal perovskite with electrochemical extraction of ions from A site. J. Mater. Chem. A 2019, 7, 12176–12190. [Google Scholar] [CrossRef]
- Moitra, D.; Anand, C.; Ghosh, B.K.; Chandel, M.; Ghosh, N.N. One-Dimensional BiFeO3 Nanowire-Reduced Graphene Oxide Nanocomposite as Excellent Supercapacitor Electrode Material. ACS Appl. Energy Mater. 2018, 1, 464–474. [Google Scholar] [CrossRef]
- Yuan, P. Electrode behaviors of BiFeO3 powders: A possible application of Bi2O3 oxide in rechargeable battery. Int. J. Electrochem. Sci. 2017, 12, 3686–3696. [Google Scholar] [CrossRef]
- Khajonrit, J.; Phumying, S.; Maensiri, S. Structure and magnetic/electrochemical properties of Cu-doped BiFeO3 nanoparticles prepared by a simple solution method. Jpn. J. Appl. Phys. 2016, 55, 06GJ14. [Google Scholar] [CrossRef]
- Rana, S.; Dutta, N.; Bandyopadhyay, S.K.; Sen, P.; Himanshu, A.K. High capacitance in BiFeO3 nanorod structure. AIP Conf. Proc. 2014, 1591, 254–255. [Google Scholar] [CrossRef]
- Rajeevan, N.E.; Pradyumnan, P.P.; Kumar, R.; Shukla, D.K.; Kumar, S.; Singh, A.K.; Patnaik, S.; Arora, S.K.; Shvets, I.V. Magnetoelectric properties of BixCo2−xMnO4 (0 ≤ x ≤ 0.3). Appl. Phys. Lett. 2008, 92, 102910. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Fan, Z.; Xiao, D.; Zhu, J.; Wang, J. Multiferroic bismuth ferrite-based materials for multifunctional applications: Ceramic bulks, thin films and nanostructures. Prog. Mater. Sci. 2016, 84, 335–402. [Google Scholar] [CrossRef] [Green Version]
- Guennou, M.; Viret, M.; Kreisel, J. Bismuth-based perovskites as multiferroics. Comptes Rendus. Phys. 2015, 16, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.T.; Lu, M.H.; Wu, D.; Chen, Y.F.; Ming, N.B. Larger polarization and weak ferromagnetism in quenched BiFeO3 ceramics with a distorted rhombohedral crystal structure. Appl. Phys. Lett. 2005, 87, 262907. [Google Scholar] [CrossRef]
- Kumari, A.; Kumari, K.; Ahmed, F.; Alshoaibi, A.; Alvi, P.A.; Dalela, S.; Ahmad, M.M.; Aljawfi, R.N.; Dua, P.; Vij, A.; et al. Influence of Sm doping on structural, ferroelectric, electrical, optical and magnetic properties of BaTiO3. Vacuum 2021, 184, 109872. [Google Scholar] [CrossRef]
- Kumari, A.; Kumari, K.; Ahmed, F.; Ahmad, M.M.; Sharma, J.; Vij, A.; Kumar, S. Role of Bi-excess on structural, electrical, optical, and magnetic properties BiFeO3 nanoparticles. J. Mater. Sci. Mater. Electron. 2021, 32, 23968–23982. [Google Scholar] [CrossRef]
- Kumari, A.; Kumari, K.; Aljawfi, R.N.; Alvi, P.A.; Dalela, S.; Ahmad, M.M.; Chawla, A.K.; Kumar, R.; Vij, A.; Kumar, S. Role of La substitution on structural, optical, and multiferroic properties of BiFeO3 nanoparticles. Appl. Nanosci. 2021, 1–20. [Google Scholar] [CrossRef]
- Yang, C.-H.; Koo, T.; Jeong, Y. How to obtain magnetocapacitance effects at room temperature: The case of Mn-doped BiFeO3. Solid State Commun. 2005, 134, 299–301. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, H.; Han, J.; Ling, Y. Thermal, magnetic and photoelectrical behaviors of sillenite Bi25FeO39 microcrystals. Ceram. Int. 2020, 47, 9941–9947. [Google Scholar] [CrossRef]
- Lopes, A.M.L.; Araújo, J.P.; Ferdov, S. Room temperature synthesis of Bi25FeO39 and hydrothermal kinetic relations between sillenite- and distorted perovskite-type bismuth ferrites. Dalton Trans. 2014, 43, 18010–18016. [Google Scholar] [CrossRef]
- Casas-Cabanas, M.; Reynaud, M.; Rikarte, J.; Horbach, P.; Rodríguez-Carvajal, J. FAULTS: A program for refinement of structures with extended defects. J. Appl. Crystallogr. 2016, 49, 2259–2269. [Google Scholar] [CrossRef]
- Kumar, A.; Varshney, D. Crystal structure refinement of Bi1−xNdxFeO3 multiferroic by the Rietveld method. Ceram. Int. 2012, 38, 3935–3942. [Google Scholar] [CrossRef]
- Muneeswaran, M.; Jegatheesan, P.; Giridharan, N.V. Synthesis of nanosized BiFeO3 powders by co-precipitation method. J. Exp. Nanosci. 2013, 8, 341–346. [Google Scholar] [CrossRef]
- Arafat, S.S. Structural and magnetic properties of BiFe1−xCrxO3 synthesized samples. Chin. Phys. B 2014, 23, 066101. [Google Scholar] [CrossRef]
- Luo, B.-C.; Chen, C.-L.; Xu, Z.; Xie, Q. Effect of Cr substitution on the multiferroic properties of BiFe1−xCrxO3 compounds. Phys. Lett. A 2010, 374, 4265–4268. [Google Scholar] [CrossRef]
- Layek, S.; Saha, S.; Verma, H.C. Preparation, structural and magnetic studies on BiFe1-xCrxO3(x = 0.0, 0.05 and 0.1) multiferroic nanoparticles. AIP Adv. 2013, 3, 032140. [Google Scholar] [CrossRef] [Green Version]
- Kumari, K.; Vij, A.; Chae, K.H.; Hashim, M.; Aljawfi, R.N.; Alvi, P.A.; Kumar, S. Near-edge X-ray absorption fine structure spectroscopy and structural properties of Ni-doped CeO2 nanoparticles. Radiat. Eff. Defects Solids 2017, 172, 985–994. [Google Scholar] [CrossRef]
- Ramachandran, B.; Rao, M.S.R. Low temperature magnetocaloric effect in polycrystalline BiFeO3 ceramics. Appl. Phys. Lett. 2009, 95, 142505. [Google Scholar] [CrossRef]
- Pandey, D.K.; Modi, A.; Pandey, P.; Gaur, N.K. Variable excitation wavelength photoluminescence response and optical absorption in BiFeO3 nanostructures. J. Mater. Sci. Mater. Electron. 2017, 28, 17245–17253. [Google Scholar] [CrossRef]
- Cebela, M.; Zagorac, D.; Batalović, K.; Radakovic, J.; Stojadinović, B.; Spasojevic, V.; Hercigonja, R. BiFeO3 perovskites: A multidisciplinary approach to multiferroics. Ceram. Int. 2017, 43, 1256–1264. [Google Scholar] [CrossRef]
- Guo, B.; Deng, H.; Zhai, X.; Zhou, W.; Meng, X.; Weng, G.; Chen, S.; Yang, P.; Chu, J. Cr doping-induced structural phase transition, optical tuning and magnetic enhancement in BiFeO3 thin films. Mater. Lett. 2016, 186, 198–201. [Google Scholar] [CrossRef]
- Rhaman, M.; Matin, M.; Hakim, M.; Islam, M. Bandgap tuning of samarium and cobalt co-doped bismuth ferrite nanoparticles. Mater. Sci. Eng. B 2020, 263, 114842. [Google Scholar] [CrossRef]
- Sinha, A.K.; Bhushan, B.; Sharma, R.K.; Sen, S.; Mandal, B.P.; Meena, S.S.; Bhatt, P.; Prajapat, C.L.; Priyam, A.; Mishra, S.K.; et al. Enhanced dielectric, magnetic and optical properties of Cr-doped BiFeO3 multiferroic nanoparticles synthesized by sol-gel route. Results Phys. 2019, 13, 102299. [Google Scholar] [CrossRef]
- Yousaf, M.; Lu, Y.; Hu, E.; Wang, B.; Akhtar, M.N.; Noor, A.; Akbar, M.; Shah, M.Y.; Wang, F.; Zhu, B. Tunable magneto-optical and interfacial defects of Nd and Cr-doped bismuth ferrite nanoparticles for microwave absorber applications. J. Colloid Interface Sci. 2021, 608, 1868–1881. [Google Scholar] [CrossRef]
- Kharel, P.; Talebi, S.; Ramachandran, B.; Dixit, A.; Naik, V.M.; Sahana, M.B.; Sudakar, C.; Naik, R.; Rao, M.S.R.; Lawes, G. Structural, magnetic, and electrical studies on polycrystalline transition-metal-doped BiFeO3 thin films. J. Phys. Condens. Matter 2008, 21, 036001. [Google Scholar] [CrossRef]
- Hashim, M.; Kumar, S.; Koo, B.H.; Shirsath, S.E.; Mohammed, E.M.; Shah, J.; Kotnala, R.K.; Choi, H.K.; Chung, H.; Kumar, R. Structural, electrical and magnetic properties of Co–Cu ferrite nanoparticles. J. Alloys Compd. 2011, 518, 11–18. [Google Scholar] [CrossRef]
- Kumar, S.; Park, J.; Kim, D.; Lee, M.; Song, T.K.; Gautam, S.; Chae, K.; Kim, S.; Kim, M.-H. Electronic structure and magnetic properties of Co doped TiO2 thin films using X-ray absorption spectroscopy. Ceram. Int. 2015, 41, S370–S375. [Google Scholar] [CrossRef]
- Kumar, S.; Vats, P.; Gautam, S.; Gupta, V.; Verma, K.; Chae, K.; Hashim, M.; Choi, H. Electronic structure, magnetic and structural properties of Ni doped ZnO nanoparticles. Mater. Res. Bull. 2014, 59, 377–381. [Google Scholar] [CrossRef]
- Kumar, S.; Gautam, S.; Song, T.; Chae, K.H.; Jang, K.; Kim, S. Electronic structure study of Co doped CeO2 nanoparticles using X-ray absorption fine structure spectroscopy. J. Alloys Compd. 2014, 611, 329–334. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, J.P.; Won, S.O.; Chae, K.H.; Sharma, S.K.; Kumar, S. Introduction to X-Ray Absorption Spectroscopy and Its Applications in Material Science; Springer: Berlin/Heidelberg, Germany, 2018; pp. 497–548. [Google Scholar] [CrossRef]
- Hashim, M.; Alimuddin; Kumar, S.; Shirsath, S.; Kotnala, R.; Shah, J.; Kumar, R. Influence of Cr3+ ion on the structural, ac conductivity and magnetic properties of nanocrystalline Ni–Mg ferrite. Ceram. Int. 2013, 39, 1807–1819. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Dutta, N.; Bandyopadhyay, S.K.; Rana, S.; Sen, P.; Himanshu, A.K. Remarkably high value of Capacitance in BiFeO3 Nanorod. arXiv 2013, arXiv:1309.5690. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, L.-B.; Cai, J.-J.; Luo, Y.-C.; Kang, L. Nanoflake-like cobalt hydroxide/ordered mesoporous carbon composite for electrochemical capacitors. J. Solid State Electrochem. 2010, 14, 2065–2075. [Google Scholar] [CrossRef]
- Niu, H.; Zhou, D.; Yang, X.; Li, X.; Wang, Q.; Qu, F.Y. Towards three-dimensional hierarchical ZnO nano-fiber@Ni(OH)2nanoflake core–shell heterostructures for high performance asymmetric supercapacitors. J. Mater. Chem. A 2015, 3, 18413–18421. [Google Scholar] [CrossRef]
- Ahmed, F.; Almutairi, G.; AlOtaibi, B.; Kumar, S.; Arshi, N.; Hussain, S.G.; Umar, A.; Ahmad, N.; Aljaafari, A. Binder-Free Electrode Based on ZnO Nanorods Directly Grown on Aluminum Substrate for High Performance Supercapacitors. Nanomaterials 2020, 10, 1979. [Google Scholar] [CrossRef]
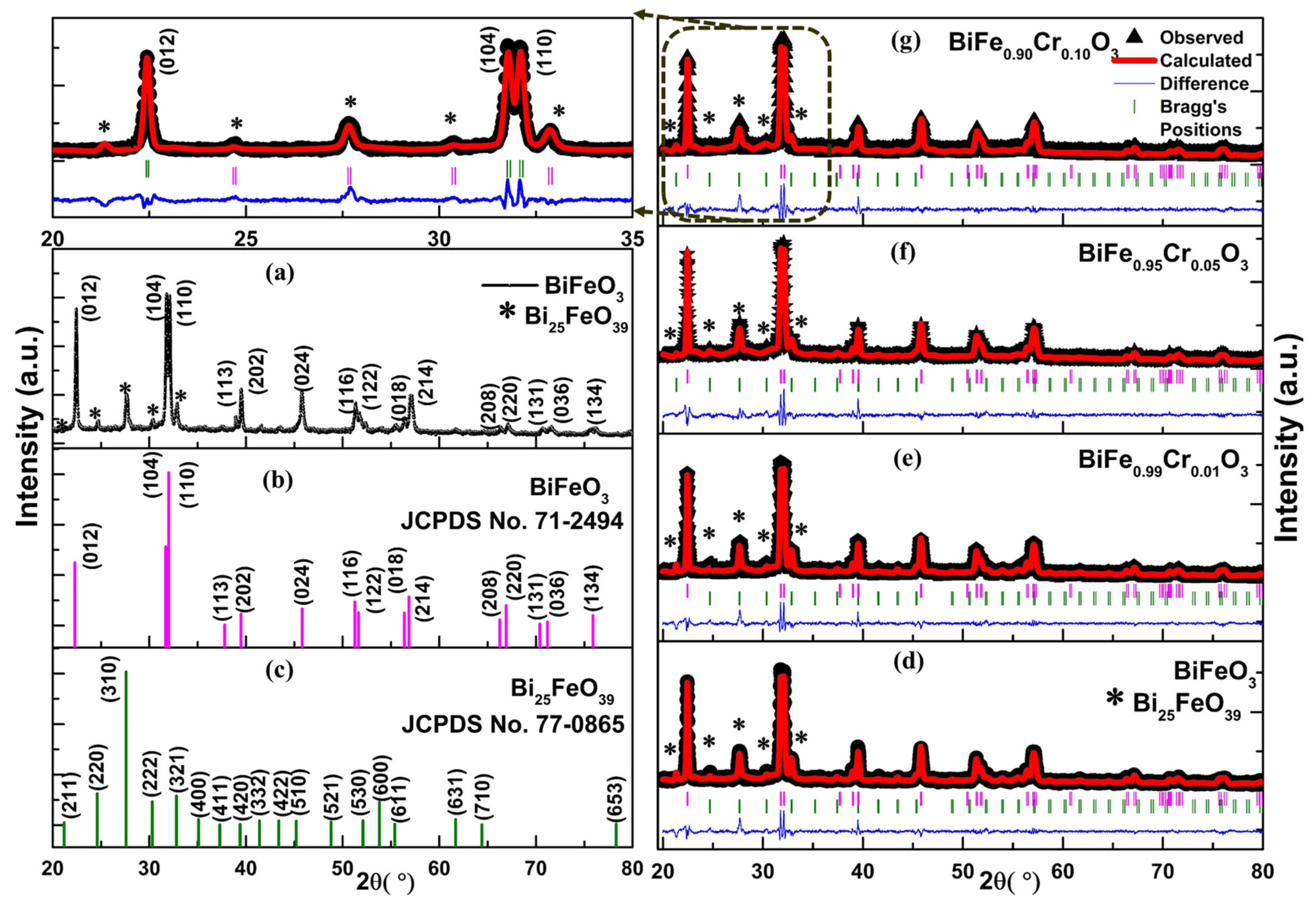
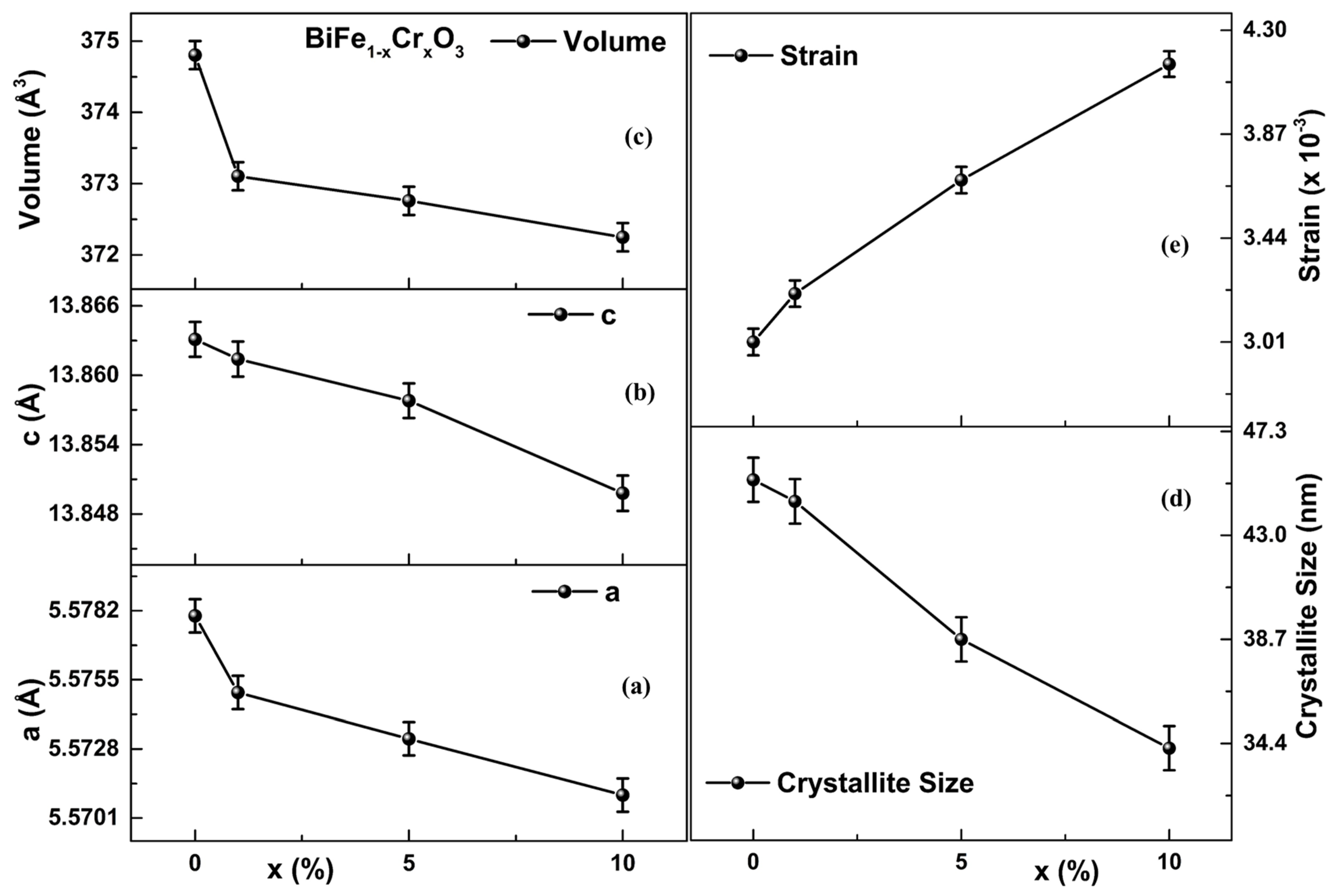
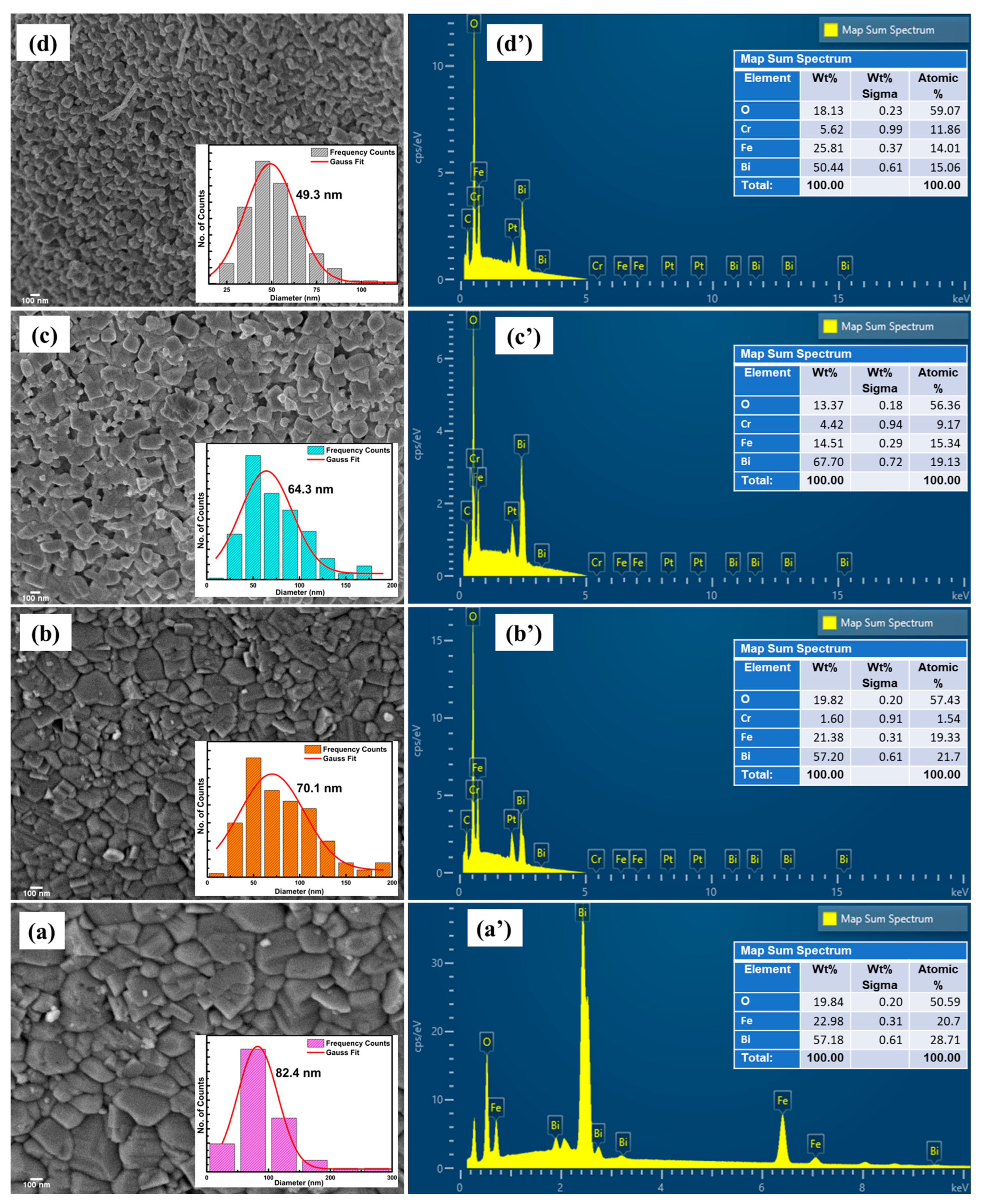
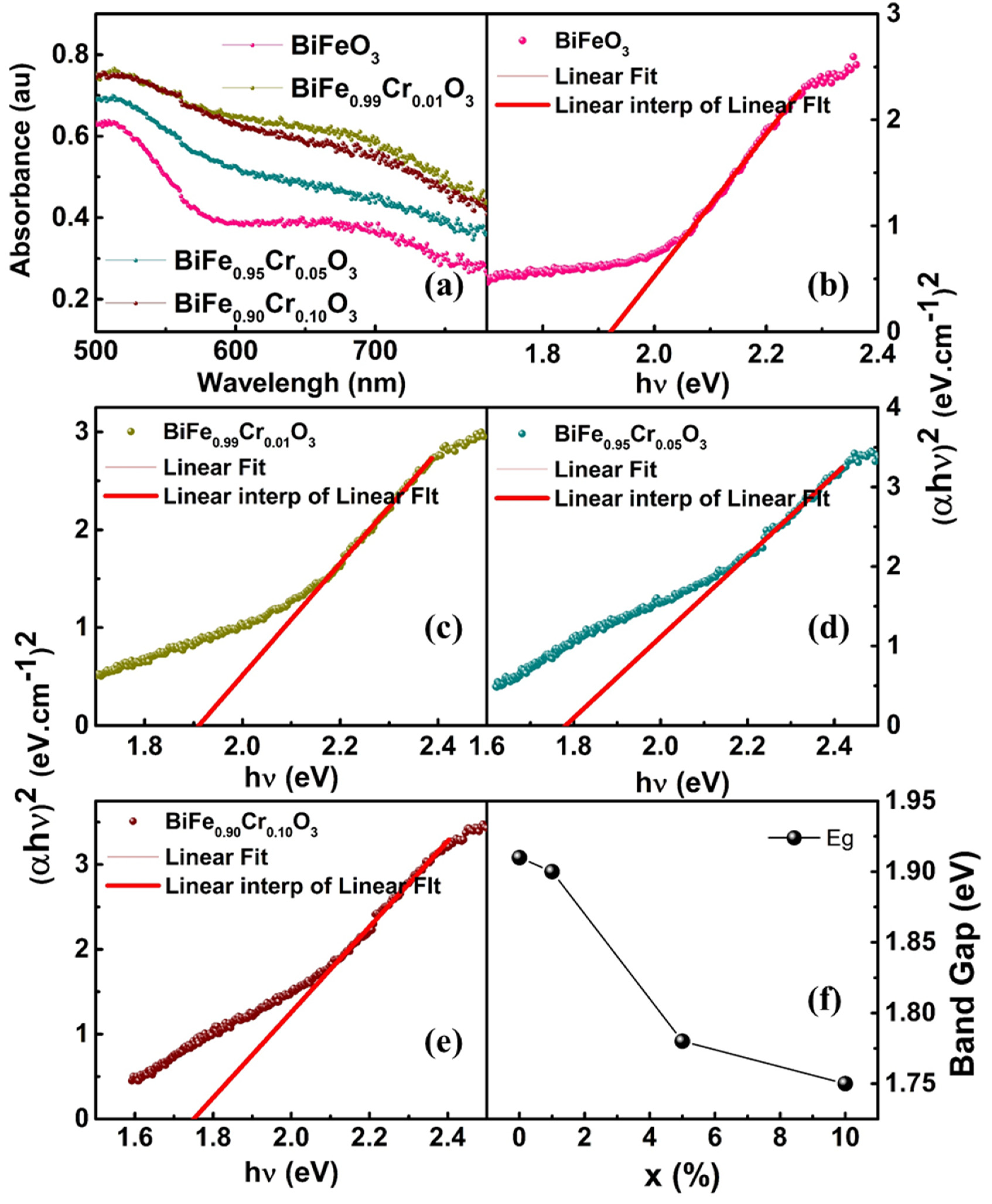


| Samples | Rp | Rwp | Rexp | χ2 | Density of the Compound (g/cm3) | Phase Fraction (%) R 3 c/I 2 3 |
|---|---|---|---|---|---|---|
| BiFeO3 | 6.55 | 8.81 | 5.48 | 2.54 | 7.862 | 83.15/16.85 |
| BiFe0.99Cr0.01O3 | 6.74 | 8.70 | 5.46 | 2.54 | 7.857 | 84.96/15.04 |
| BiFe0.95Cr0.05O3 | 6.96 | 8.80 | 5.29 | 2.77 | 7.733 | 92.72/7.28 |
| BiFe0.90Cr0.10O3 | 6.56 | 8.55 | 5.37 | 2.54 | 7.684 | 94.99/ 5.01 |
| Samples | x | y | CCT | CRI | LER |
|---|---|---|---|---|---|
| BiFeO3 | 0.3561 | 0.354 | 4749 | 88 | 239 |
| BiFe0.99Cr0.01O3 | 0.3581 | 0.3856 | 4696 | 89 | 234 |
| BiFe0.95Cr0.05O3 | 0.3603 | 0.3878 | 4636 | 90 | 230 |
| BiFe0.90Cr0.10O3 | 0.3527 | 0.3788 | 4835 | 89 | 229 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Ahmed, F.; Ahmad, N.; Shaalan, N.M.; Kumar, R.; Alshoaibi, A.; Arshi, N.; Dalela, S.; Albossed, M.; Chae, K.H.; et al. Role of Cr Doping on the Structure, Electronic Structure, and Electrochemical Properties of BiFeO3 Nanoparticles. Materials 2022, 15, 4118. https://doi.org/10.3390/ma15124118
Kumar S, Ahmed F, Ahmad N, Shaalan NM, Kumar R, Alshoaibi A, Arshi N, Dalela S, Albossed M, Chae KH, et al. Role of Cr Doping on the Structure, Electronic Structure, and Electrochemical Properties of BiFeO3 Nanoparticles. Materials. 2022; 15(12):4118. https://doi.org/10.3390/ma15124118
Chicago/Turabian StyleKumar, Shalendra, Faheem Ahmed, Naushad Ahmad, Nagih M. Shaalan, Rajesh Kumar, Adil Alshoaibi, Nishat Arshi, Saurabh Dalela, Mohammed Albossed, Keun Hwa Chae, and et al. 2022. "Role of Cr Doping on the Structure, Electronic Structure, and Electrochemical Properties of BiFeO3 Nanoparticles" Materials 15, no. 12: 4118. https://doi.org/10.3390/ma15124118
APA StyleKumar, S., Ahmed, F., Ahmad, N., Shaalan, N. M., Kumar, R., Alshoaibi, A., Arshi, N., Dalela, S., Albossed, M., Chae, K. H., Alvi, P. A., & Kumari, K. (2022). Role of Cr Doping on the Structure, Electronic Structure, and Electrochemical Properties of BiFeO3 Nanoparticles. Materials, 15(12), 4118. https://doi.org/10.3390/ma15124118













