Nanostructured Layer of Silver for Detection of Small Biomolecules in Surface-Assisted Laser Desorption Ionization Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Sample Preparation
2.3. Synthesis of SALDI Plates
2.4. SALDI TOF-MS Analysis
2.5. SEM Analysis
3. Results and Discussion
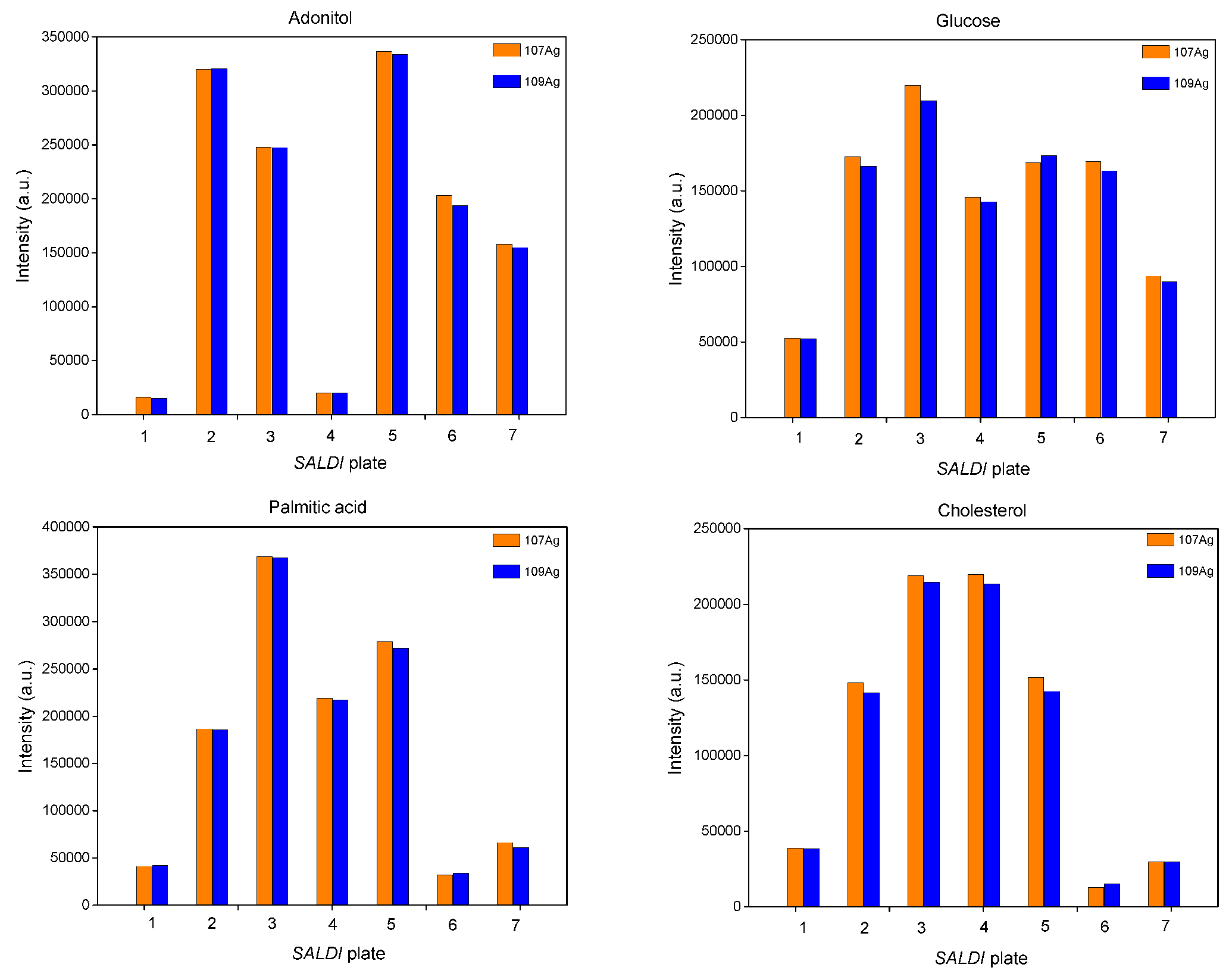
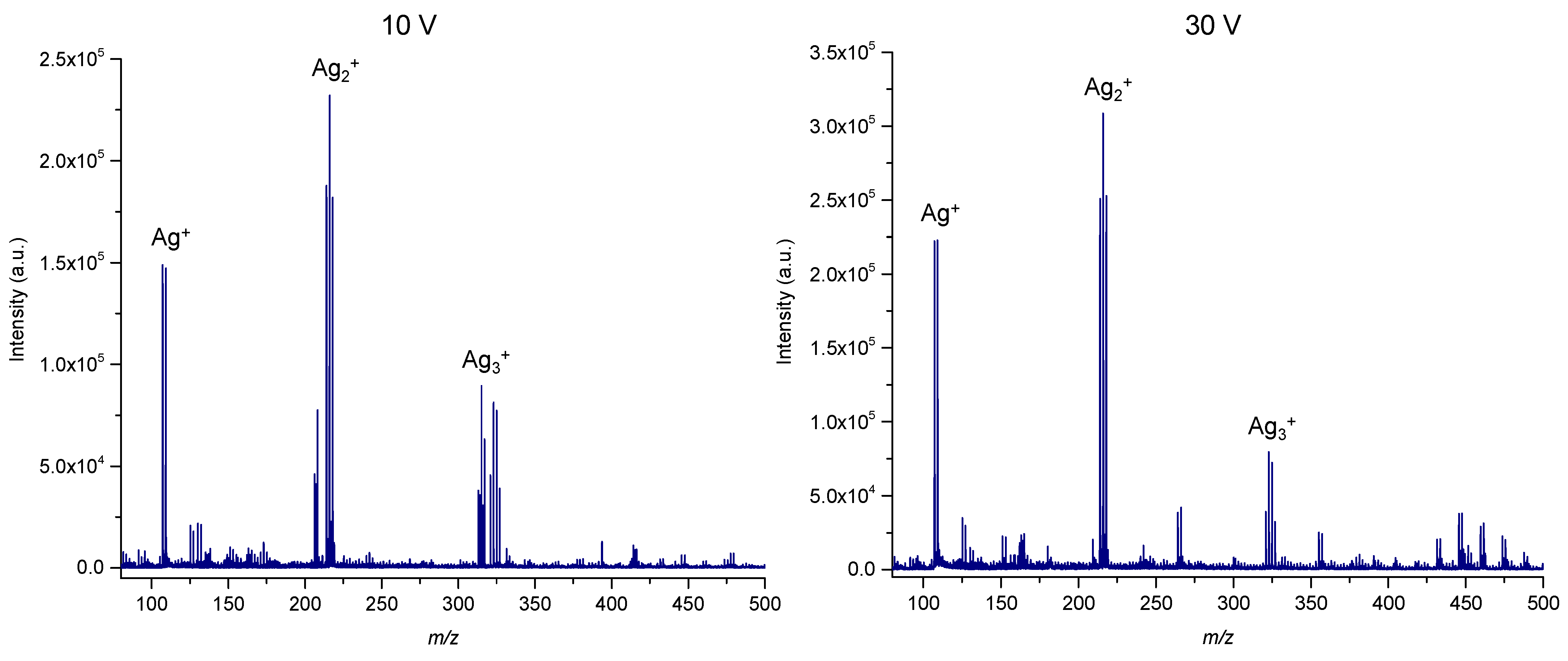
3.1. Effects of Precursor, Applied Voltage and Synthesis Time on SALDI Efficiency of Low-Molecular-Weight Compounds
3.2. SEM Analysis
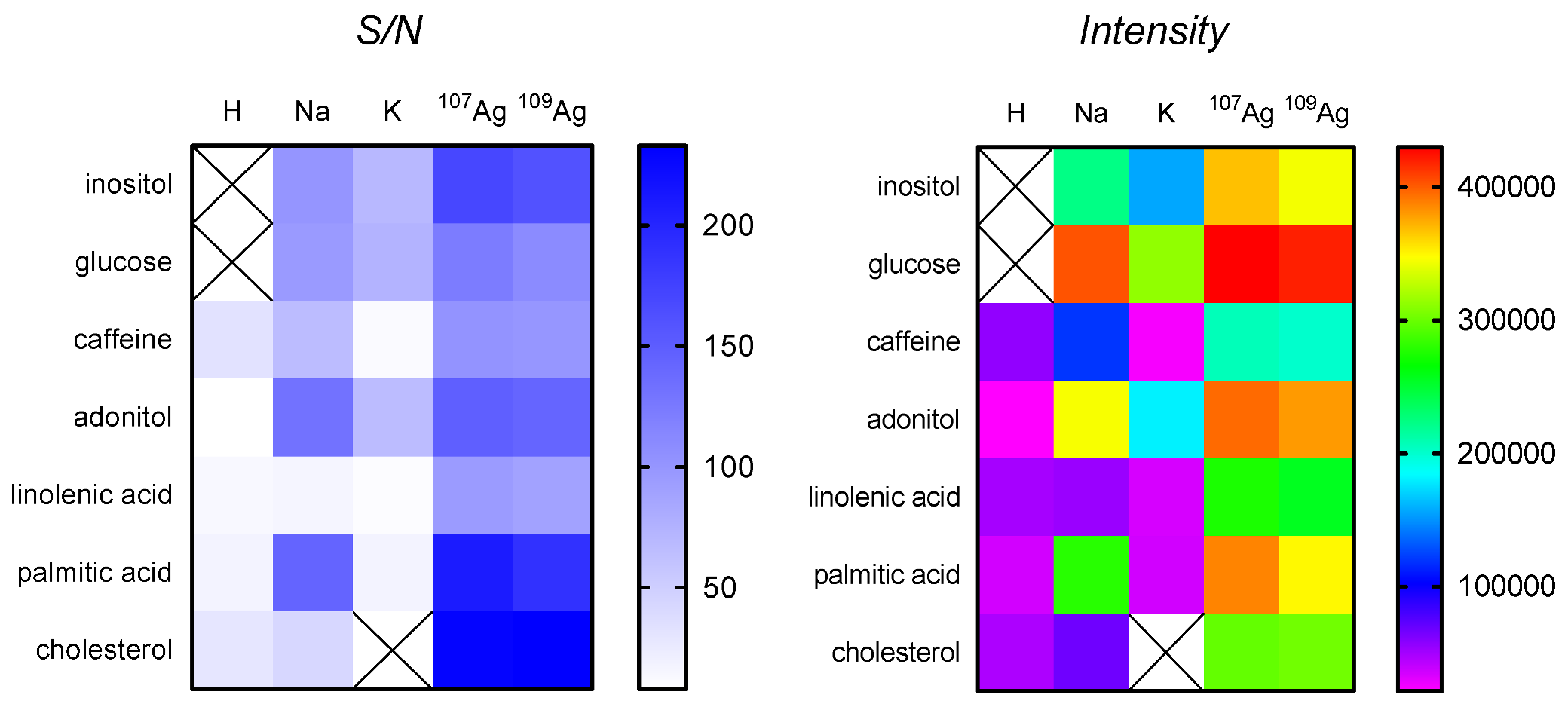
3.3. SALDI MS Performance
3.4. Determination of the Limit of Detection and Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauzon, N.; Chaurand, P. Detection of Exogenous Substances in Latent Fingermarks by Silver-Assisted LDI Imaging MS: Perspectives in Forensic Sciences. Analyst 2018, 143, 3586–3594. [Google Scholar] [CrossRef] [PubMed]
- Bronzel, J.L.; Milagre, C.D.F.; Milagre, H.M.S. Analysis of Low Molecular Weight Compounds Using MALDI- and LDI-TOF-MS: Direct Detection of Active Pharmaceutical Ingredients in Different Formulations. J. Mass Spectrom. 2017, 52, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.T.; Kandasamy, K.; Chen, Y.C. Magnetic Graphene Oxide-Based Affinity Surface-Assisted Laser Desorption/Ionization Mass Spectrometry for Screening of Aflatoxin B1 from Complex Samples. Anal. Chem. 2021, 93, 7310–7316. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Arima, K.; Takeshita, T.; Kunitake, Y.; Ohno, N.; Imamura, M.; Matsui, T. Laser Desorption Ionization-Mass Spectrometry with Graphite Carbon Black Nanoparticles for Simultaneous Detection of Taste- And Odor-Active Compounds. ACS Appl. Nano Mater. 2022, 5, 2187–2194. [Google Scholar] [CrossRef]
- Dévier, M.H.; Le Menach, K.; Viglino, L.; Di Gioia, L.; Lachassagne, P.; Budzinski, H. Ultra-Trace Analysis of Hormones, Pharmaceutical Substances, Alkylphenols and Phthalates in Two French Natural Mineral Waters. Sci. Total Environ. 2013, 443, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.C.; Laghi, L.; Parolin, C.; Foschi, C.; Marangoni, A.; Liberatore, A.; Dias, A.L.T.; Cricca, M.; Vitali, B. Metabolic Profiling of Candida Clinical Isolates of Different Species and Infection Sources. Sci. Rep. 2020, 10, 16716. [Google Scholar] [CrossRef] [PubMed]
- Amor, R.E.; Nakhleh, M.K.; Barash, O.; Haick, H. Breath Analysis of Cancer in the Present and the Future. Eur. Respir. Rev. 2019, 28, 190002. [Google Scholar] [CrossRef]
- Lee, P.Y.; Yeoh, Y.; Omar, N.; Pung, Y.F.; Lim, L.C.; Low, T.Y. Molecular Tissue Profiling by MALDI Imaging: Recent Progress and Applications in Cancer Research. Crit. Rev. Clin. Lab. Sci. 2021, 58, 513–529. [Google Scholar] [CrossRef]
- Arendowski, A.; Ossoliński, K.; Ossolińska, A.; Ossoliński, T.; Nizioł, J.; Ruman, T. Serum and Urine Analysis with Gold Nanoparticle-Assisted Laser Desorption/Ionization Mass Spectrometry for Renal Cell Carcinoma Metabolic Biomarkers Discovery. Adv. Med. Sci. 2021, 66, 326–335. [Google Scholar] [CrossRef]
- Dietel, M. Molecular Pathology: A Requirement for Precision Medicine in Cancer. Oncol. Res. Treat. 2016, 39, 804–810. [Google Scholar] [CrossRef]
- Murray, K.K.; Boyd, R.K.; Eberlin, M.N.; Langley, G.J.; Li, L.; Naito, Y. Definitions of Terms Related to Mass Spectrometry (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1515–1609. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.C.; Huang, M.F.; Chang, H.T. Quantitative Surface-Assisted Laser Desorption/Ionization-MS Approaches for Bioanalysis. Bioanalysis 2013, 5, 633–635. [Google Scholar] [CrossRef] [Green Version]
- Sunner, J.; Dratz, E.; Chen, Y.C. Graphite Surface-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry of Peptides and Proteins from Liquid Solutions. Anal. Chem. 1995, 67, 4335–4342. [Google Scholar] [CrossRef]
- Vedarethinam, V.; Huang, L.; Zhang, M.; Su, H.; Hu, H.; Xia, H.; Liu, Y.; Wu, B.; Wan, X.; Shen, J.; et al. Vanadium Core–Shell Nanorods Inspect Metabolic Changes of Diabetic Retinopathy. Adv. Funct. Mater. 2020, 30, 2002791. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Liu, C.; Pei, C.; Shu, W.; Zhang, C.; Liu, L.; Zhou, L.; Wan, J. Design of Multi-Shelled Hollow Cr2O3 Spheres for Metabolic Fingerprinting. Angew. Chem.-Int. Ed. 2021, 60, 12504–12512. [Google Scholar] [CrossRef]
- Dou, S.; Wang, Z.; Chen, Q.; Lu, N. One-Step Fabrication of High-Density Si Nanotips as SALDI-MS Substrate for Highly Sensitive Detection. Sens. Actuators B Chem. 2022, 359, 131578. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Jiang, J.; Zheng, J.; Yang, L.; Li, Y.; Zhou, J.; Liu, T.; Xu, J.; Lou, W.; et al. Multiplexed Nanomaterial-Assisted Laser Desorption/Ionization for Pan-Cancer Diagnosis and Classification. Nat. Commun. 2022, 13, 617. [Google Scholar] [CrossRef]
- Kołodziej, A.; Płaza-Altamer, A.; Nizioł, J.; Ruman, T. Infrared Pulsed Fiber Laser-Produced Silver-109-Nanoparticles for Laser Desorption/Ionization Mass Spectrometry of Carboxylic Acids. Int. J. Mass Spectrom. 2022, 474, 116816. [Google Scholar] [CrossRef]
- Su, H.; Li, X.; Huang, L.; Cao, J.; Zhang, M.; Vedarethinam, V.; Di, W.; Hu, Z.; Qian, K. Plasmonic Alloys Reveal a Distinct Metabolic Phenotype of Early Gastric Cancer. Adv. Mater. 2021, 33, 2007978. [Google Scholar] [CrossRef]
- Cao, J.; Shi, X.; Gurav, D.D.; Huang, L.; Su, H.; Li, K.; Niu, J.; Zhang, M.; Wang, Q.; Jiang, M.; et al. Metabolic Fingerprinting on Synthetic Alloys for Medulloblastoma Diagnosis and Radiotherapy Evaluation. Adv. Mater. 2020, 32, e2000906. [Google Scholar] [CrossRef]
- Mizoshita, N.; Yamada, Y.; Murase, M.; Goto, Y.; Inagaki, S. Nanoporous Substrates with Molecular-Level Perfluoroalkyl/Alkylamide Surface for Laser Desorption/Ionization Mass Spectrometry of Small Proteins. ACS Appl. Mater. Interfaces 2022, 14, 3716–3725. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, X.; Gao, Z.; Wang, H.; Liu, Y.; Guo, C.; Pan, Y. Pd Nanoparticles Decorated Thiol-Functionalized MOF as an Efficient Matrix for Differentiation and Quantitation of Oligosaccharide Isomers by Laser Desorption/Ionization Mass Spectrometry. Anal. Chim. Acta 2022, 1202, 339665. [Google Scholar] [CrossRef]
- Karas, M.; Hillenkamp, F. Laser Desorption Ionization of Proteins with Molecular Masses Exceeding 10 000 Daltons. Anal. Chem. 1988, 60, 2299–2301. [Google Scholar] [CrossRef]
- Hu, J.B.; Chen, Y.C.; Urban, P.L. Coffee-Ring Effects in Laser Desorption/Ionization Mass Spectrometry. Anal. Chim. Acta 2013, 766, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Sekuła, J.; Nizioł, J.; Rode, W.; Ruman, T. Silver Nanostructures in Laser Desorption/Ionization Mass Spectrometry and Mass Spectrometry Imaging. Analyst 2015, 140, 6195–6209. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, X.; Zhan, L.; Xue, J.; Wang, J.; Xiong, C.; Nie, Z. Hot Electron Transfer Promotes Ion Production in Plasmonic Metal Nanostructure Assisted Laser Desorption Ionization Mass Spectrometry. Chem. Commun. 2018, 54, 10905–10908. [Google Scholar] [CrossRef] [PubMed]
- Silina, Y.E.; Meier, F.; Nebolsin, V.A.; Koch, M.; Volmer, D.A. Novel Galvanic Nanostructures of Ag and Pd for Efficient Laser Desorption/Ionization of Low Molecular Weight Compounds. J. Am. Soc. Mass Spectrom. 2014, 25, 841–851. [Google Scholar] [CrossRef]
- Cioffi, N.; Colaianni, L.; Pilolli, R.; Calvano, C.D.; Palmisano, F.; Zambonin, P.G. Silver Nanofractals: Electrochemical Synthesis, XPS Characterization and Application in LDI-MS. Anal. Bioanal. Chem. 2009, 394, 1375–1383. [Google Scholar] [CrossRef]
- Niedermeyer, T.H.J.; Strohalm, M. MMass as a Software Tool for the Annotation of Cyclic Peptide Tandem Mass Spectra. PLoS ONE 2012, 7, e44913. [Google Scholar] [CrossRef] [Green Version]
- Patiny, L.; Borel, A. ChemCalc: A Building Block for Tomorrow’s Chemical Infrastructure. J. Chem. Inf. Model. 2013, 53, 1223–1228. [Google Scholar] [CrossRef] [Green Version]
- Reindl, W.; Northen, T.R. Rapid Screening of Fatty Acids Using Nanostructure-Initiator Mass Spectrometry. Anal. Chem. 2010, 82, 3751–3755. [Google Scholar] [CrossRef]
- Trimpin, S.; Keune, S.; Räder, H.J.; Müllen, K. Solvent-Free MALDI-MS: Developmental Improvements in the Reliability and the Potential of MALDI in the Analysis of Synthetic Polymers and Giant Organic Molecules. J. Am. Soc. Mass Spectrom. 2006, 17, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Chung, B.C. Simultaneous Measurement of Urinary Polyols Using Gas Chromatography/Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 831, 126–131. [Google Scholar] [CrossRef]
- Thomas, M.P.; Mills, S.J.; Potter, B.V.L. The “Other” Inositols and Their Phosphates: Synthesis, Biology, and Medicine (with Recent Advances in Myo-Inositol Chemistry). Angew. Chem.-Int. Ed. 2016, 55, 1614–1650. [Google Scholar] [CrossRef] [Green Version]
- Carrero-Carralero, C.; Mansukhani, D.; Ruiz-Matute, A.I.; Martínez-Castro, I.; Ramos, L.; Sanz, M.L. Extraction and Characterization of Low Molecular Weight Bioactive Carbohydrates from Mung Bean (Vigna Radiata). Food Chem. 2018, 266, 146–154. [Google Scholar] [CrossRef]
- Verhoeven, N.M.; Huck, J.H.J.; Roos, B.; Struys, E.A.; Salomons, G.S.; Douwes, A.C.; Van der Knaap, M.S.; Jakobs, C. Transaldolase Deficiency: Liver Cirrhosis Associated with a New Inborn Error in the Pentose Phosphate Pathway. Am. J. Hum. Genet. 2001, 68, 1086–1092. [Google Scholar] [CrossRef] [Green Version]
- Galant, A.L.; Kaufman, R.C.; Wilson, J.D. Glucose: Detection and Analysis. Food Chem. 2015, 188, 149–160. [Google Scholar] [CrossRef]
- Patrício, M.; Pereira, J.; Crisóstomo, J.; Matafome, P.; Gomes, M.; Seiça, R.; Caramelo, F. Using Resistin, Glucose, Age and BMI to Predict the Presence of Breast Cancer. BMC Cancer 2018, 18, 29. [Google Scholar] [CrossRef] [Green Version]
- Ghanavat, M.; Shahrouzian, M.; Deris Zayeri, Z.; Banihashemi, S.; Kazemi, S.M.; Saki, N. Digging Deeper through Glucose Metabolism and Its Regulators in Cancer and Metastasis. Life Sci. 2021, 264, 118603. [Google Scholar] [CrossRef]
- Kołodziej, A.; Ruman, T.; Nizioł, J. Gold and Silver Nanoparticles-Based Laser Desorption/Ionization Mass Spectrometry Method for Detection and Quantification of Carboxylic Acids. J. Mass Spectrom. 2020, 55, e4604. [Google Scholar] [CrossRef]

| Analyte | LOD a [ng/mL] | LLOQ b [ng/mL] | RSD c [%] | Regression Equation | r2 |
|---|---|---|---|---|---|
| Adonitol | 107.95 | 179.91 | 2.16 | y = 9.073x + 8.5833 | 0.998 |
| Glucose | 374.18 | 623.63 | 19.02 | y = 5.0072x + 7.0728 | 0.968 |
| Palmitic acid | 98.76 | 164.60 | 14.84 | y = 7.3103x + 18.045 | 0.980 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arendowski, A.; Sagandykova, G.; Mametov, R.; Rafińska, K.; Pryshchepa, O.; Pomastowski, P. Nanostructured Layer of Silver for Detection of Small Biomolecules in Surface-Assisted Laser Desorption Ionization Mass Spectrometry. Materials 2022, 15, 4076. https://doi.org/10.3390/ma15124076
Arendowski A, Sagandykova G, Mametov R, Rafińska K, Pryshchepa O, Pomastowski P. Nanostructured Layer of Silver for Detection of Small Biomolecules in Surface-Assisted Laser Desorption Ionization Mass Spectrometry. Materials. 2022; 15(12):4076. https://doi.org/10.3390/ma15124076
Chicago/Turabian StyleArendowski, Adrian, Gulyaim Sagandykova, Radik Mametov, Katarzyna Rafińska, Oleksandra Pryshchepa, and Paweł Pomastowski. 2022. "Nanostructured Layer of Silver for Detection of Small Biomolecules in Surface-Assisted Laser Desorption Ionization Mass Spectrometry" Materials 15, no. 12: 4076. https://doi.org/10.3390/ma15124076
APA StyleArendowski, A., Sagandykova, G., Mametov, R., Rafińska, K., Pryshchepa, O., & Pomastowski, P. (2022). Nanostructured Layer of Silver for Detection of Small Biomolecules in Surface-Assisted Laser Desorption Ionization Mass Spectrometry. Materials, 15(12), 4076. https://doi.org/10.3390/ma15124076









